Abstract
In 120 Stage I–IV testicular diffuse large B-cell lymphoma (DLBCL) patients treated from 1964 to 2015, we assessed the benefits of prophylactic contralateral testicular radiation (RT) and prophylactic central nervous system (CNS) therapy on overall, progression free, testicular relapse free, and CNS relapse free survival (OS, PFS, TRFS, and CRFS, respectively). Seventy percent of patients received RT, 53% received anthracyclines and rituximab (modern therapy), and 61% received CNS prophylaxis. On univariate analysis RT was associated with improved TRFS, PFS, and trended toward improved OS. On multivariate analysis (MVA), RT was significantly associated with improved OS and PFS; the PFS benefit persisted among patients receiving modern therapy. CNS prophylaxis was associated with improved OS, PFS, and TRFS, but not CRFS on univariate analysis, and was not significant on MVA. RT is associated with improved survival, and should be considered for all testicular DLBCL patients, but additional strategies are needed to prevent CNS relapse.
Keywords: Testicular, lymphoma, radiation therapy, intrathecal chemotherapy, rituximab
Introduction
Primary testicular diffuse large B-cell lymphoma (DLBCL) represents 80–90% of all testicular lymphomas and is the most common testicular malignancy in men over the age of 50 years [1]. Nevertheless, testicular DLBCL is quite rare, representing 0.6% of all non-Hodgkin lymphomas [2]. Overall, the prognosis is poor, with a continuous risk of late relapses at extranodal sites, including the central nervous system (CNS) and contralateral testis.
Evidence regarding the optimal treatment of patients with testicular DLBCL is limited. The modern standard of care is radical unilateral orchiectomy followed by doxorubicin-based chemotherapy. Since the addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy resulted in a survival benefit in DLBCL patients, this regimen was also adopted for the management of testicular DLBCL [3–5]. Because of the risk of CNS relapse, CNS prophylaxis with intrathecal chemotherapy or high-dose systemic methotrexate has also become a component of standard therapy [6].
Another sanctuary site at risk for relapse is the contralateral testis, with one series showing a risk of 15% at 3 years and 42% at 15 years in the absence of radiation therapy (RT) [7]. Prophylactic RT to the contralateral testis is thus often recommended but has not been well studied, particularly in the modern era of rituximab and anthracycline-based chemotherapy [8].
In this study, we sought to examine the influence of prophylactic testicular RT and prophylactic CNS therapy on survival outcomes and patterns of relapse among patients with primary testicular DLBCL at a single institution over the course of several decades.
Materials and methods
Patient selection
After approval by our institutional review board, we retrospectively identified patients with testicular DLBCL between 1964 and 2015. All patients had a pathological diagnosis of DLBCL (all pathologic specimens reviewed at MD Anderson Cancer Center), and no prior treatment. Patients with late testicular involvement, no follow-up information, or who had disease progression during induction chemotherapy were excluded. Institutional records were used to obtain data on clinical, pathological and imaging characteristics, radiation treatment plans, recurrence and survival.
Between 1964 and 2015, 147 patients presented to MD Anderson for newly diagnosed testicular DLBCL. Thirteen patients were excluded for having not completed induction chemotherapy, and 14 patients were excluded for lack of follow-up, leaving 120 patients who met inclusion criteria and were included in this analysis.
Disease staging
Initial staging evaluations varied over time. Before 1993, staging evaluation included bone marrow aspirate and biopsy, lymphangiography, intravenous pyelography, gallium scanning, and/or computed tomography (CT) scans of the abdomen and pelvis. From 1993 to 2002, patients generally underwent bone marrow aspirate and biopsy, chest radiography, and CT of the chest, abdomen, and pelvis. Starting around 2002, positron emission tomography (PET) CT scans were consistently included in the staging evaluation. Information on Ann Arbor disease stage, performance status, and serum lactate dehydrogenase (LDH) levels was obtained from the pretreatment records. The International Prognostic Index (IPI) was calculated for all patients (retroactively for patients treated prior to the development of the IPI).
Treatment
RT was delivered after completion of chemotherapy. Prophylactic RT was typically given with a high-energy appositional electron beam targeting the entire scrotum, with custom lead skin collimation to limit dose to the surrounding structures. The most commonly used dose was 30.6 Gy in 17 daily fractions. CNS prophylaxis was typically administered during induction chemotherapy, and included either intrathecal chemotherapy, and/or high-dose methotrexate.
Follow-up and response
After therapy completion, patients were typically followed every 3–4 months for 2–3 years, then at 6-month intervals, and yearly thereafter. Surveillance radiographic studies were obtained at the discretion of the treating physician. Patients who were not followed at our institution were contacted annually to obtain information about survival, disease and treatment status. We used the definitions recommended by the International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas to classify response [9]. For patients without PET/CT scans, CT scans alone were used to determine response.
Statistical analysis
Endpoints assessed included overall survival (OS), progression-free survival (PFS), testicular relapse-free survival (TRFS), and CNS relapse-free survival (CRFS). For PFS, relapse and death from any cause were considered events, and patients were censored at last followup. For TRFS and CRFS, testicular relapse and CNS relapse, respectively, were counted as events, and patients were censored at death or last follow-up. Survival rates were calculated from date of diagnosis. A subgroup analysis was done for patients who received modern systemic therapy (i.e. anthracyclineand rituximab-based chemotherapy).
Univariate associations between patient/tumor characteristics and therapy were tested using the chi-squared test and Fisherˊs exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. Kaplan-Meier curves were estimated and stratified by RT and CNS prophylaxis. The log-rank test was used to test the survival difference between the patient and treatment characteristic groups. Univariate and multivariate Cox proportional hazard models were used to determine the effects of patient and treatment factors on survival outcomes, adjusted for covariates. The variables with p values of ≤.2 in the univariate analysis were included in the full multivariable model. RT was forced in the multivariate model for OS and PFS, and CNS prophylaxis was forced into the multivariate model for CRFS. The reduced multivariate model was obtained using a backward selection approach, removing the least significant covariate from the full model one at a time, and p values of <.05 was used as the limit for inclusion in this analysis. All tests are twosided. p Values less than .05 are considered statistically significant. All analyses were conducted using SAS 9.4 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) software.
Results
Patient and treatment characteristics
Clinical and treatment characteristics are summarized in Table 1. Ann Arbor clinical stage was Stage I in 57 patients (49%), II in 26 (22%), III in 7 (6%), and IV in 27 (23%). Nearly all (97%) patients received orchiectomy; 105 (87%) received anthracycline-based chemotherapy; 64 (53%) received rituximab; and 102 (85%) received CHOP chemotherapy. Seventy-three (61%) patients received CNS prophylaxis, 72 with intrathecal chemotherapy, and 1 patient with high-dose methotrexate. Among the 84 patients (70%) who received RT, the median dose was 30.6 Gy (range, 24–40 Gy), at a median 1.8 Gy per fraction. Twenty-six (22%) patients who received testicular RT also received RT to other involved nodal regions, and 3 (3%) patients received nodal RT but not testicular RT. Patients receiving RT were more likely to have limited stage versus advanced stage (p = .004), to have received rituximab (p = .038), and to have received CNS prophylaxis (p < .001; Table 2). Patients treated with CNS prophylaxis were more likely to have a normal LDH level (p = .002), received anthracycline therapy (p < .001), CHOP chemotherapy (p < .001) and RT (p < .001; Table 2).
Table 1.
Baseline patient and treatment characteristics among 120 patients.
| Characteristic | Number | % |
|---|---|---|
| Stage | ||
| I | 57 | 49% |
| II | 26 | 22% |
| III | 7 | 6% |
| IV | 27 | 23% |
| Age | ||
| Median (range) | 63 (22–96) years | |
| ≥60 | 73 | 61% |
| <60 | 47 | 39% |
| LDH | ||
| Elevated | 24 | 20% |
| Not elevated | 59 | 49% |
| Missing | 37 | 31% |
| IPI risk category | ||
| Low (0 or 1) | 59 | 67% |
| Intermediate (2 or 3) | 24 | 27% |
| High (4 or 5) | 5 | 6% |
| Orchiectomy | ||
| Yes | 116 | 97% |
| No | 4 | 3% |
| Anthracycline | ||
| Yes | 105 | 87% |
| No | 13 | 11% |
| Missing | 2 | 2% |
| Rituximab | ||
| Yes | 64 | 53% |
| No | 56 | 47% |
| CHOP | ||
| Yes | 102 | 85% |
| No | 16 | 13% |
| Missing | 2 | 2% |
| CNS prophylaxis | ||
| Yes | 73 | 61% |
| No | 47 | 39% |
| Testicular radiation | ||
| Yes | 84 | 70% |
| No | 36 | 30% |
| Median dose (range) | 30.6 Gy (24–40 Gy) | |
IPI: international prognostic index; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; LDH: lactate dehydrogenase; CNS: central nervous system.
Table 2.
Patient characteristics stratified by receipt of modern therapy (rituximab and anthracycline chemotherapy), RT and CNS prophylaxis.
| Characteristic | Modern therapy (n = 64) Number (%) | Non-modern therapy (n = 56) Number (%) | p Value | RT (n = 84) Number (%) | No RT (n = 36) Number (%) | p Value | CNS Tx (n = 73) Number (%) | No CNS Tx (n = 47) Number (%) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Stage | |||||||||
| I | 27 (42) | 30 (57) | .183 | 46 (55) | 11 (33) | .020 | 35 (49) | 22 (49) | .062 |
| II | 13 (20) | 13 (25) | 20 (24) | 6 (18) | 11 (15) | 15 (33) | |||
| III | 5 (8) | 2 (4) | 5 (6) | 2 (6) | 6 (8) | 1 (2) | |||
| IV | 19 (30) | 8 (15) | 13 (16) | 14 (42) | 20 (28) | 7 (16) | |||
| Age | |||||||||
| ≥60 | 38 (59) | 35 (63) | .726 | 52 (62) | 21 (58) | .364 | 42 (58) | 31 (66) | .356 |
| <60 | 26 (41) | 21 (38) | 32 (38) | 15 (42) | 31 (43) | 16 (34) | |||
| LDH | |||||||||
| Elevated | 16(25) | 8 (14) | .281 | 13 (16) | 11 (31) | .093 | 19 (26) | 5 (11) | .002 |
| Not elevated | 31 (48) | 28 (50) | 46 (55) | 13 (36) | 40 (55) | 19 (40) | |||
| Missing | 17 (27) | 20 (36) | 25 (30) | 12 (33) | 14 (19) | 23 (49) | |||
| IPI Risk category | |||||||||
| Low (0 or 1) | 38 (55) | 31 (84) | .008 | 47 (72) | 12 (52) | .086 | 37 (60) | 22 (85) | .068 |
| Intermediate | 18 (35) | 6 (16) | 16 (25) | 8 (35) | 20 (32) | 4 (15) | |||
| (2 or 3) | |||||||||
| High (4 or 5) | 5 (10) | 0 | 2 (3) | 3 (13) | 5 (8) | 0 | |||
| Orchiectomy | |||||||||
| Yes | 61 (95) | 55 (98) | .622 | 82 (98) | 34 (94) | .582 | 70 (96) | 46 (98) | 1.000 |
| No | 3 (5) | 1 (2) | 2 (2) | 2 (6) | 3 (4) | 1 (2) | |||
| Anthracycline | |||||||||
| Yes | 64 (100) | 41 (76) | .011 | 74 (89) | 31 (89) | .926 | 71 (99) | 34 (74) | <.001 |
| No | 0 | 13 (24) | 9 (11) | 4 (11) | 1 (1) | 12 (26) | |||
| Missing | 2 | 1 | 1 | 1 | 1 | ||||
| Rituximab | |||||||||
| Yes | 64 (100) | 0 | <.001 | 50 (60) | 14 (39) | .038 | 56 (77) | 8 (17) | <.001 |
| No | 0 | 56 (100) | 34 (41) | 22 (61) | 17 (23) | 39 (83) | |||
| CHOP | |||||||||
| Yes | 64 (100) | 38 (70) | <.001 | 74 (89) | 28 (78) | .185 | 71 (99) | 31 (67) | <.001 |
| No | 0 | 16 (30) | 9 (11) | 7 (19) | 1 (1) | 15 (33) | |||
| Missing | - | 2 | 1 | 1 | 1 | 1 | |||
| Intrathecal chemotherapy | |||||||||
| Yes | 56 (88) | 17 (30) | <.001 | 60 (71) | 13 (36) | <.001 | - | - | |
| No | 8 (13) | 39 (70) | 24 (29) | 23 (64) | |||||
| Testicular radiation |
|||||||||
| Yes | 50 (78) | 34 (61) | .038 | - | - | 60 (82) | 24 (51) | <.001 | |
| No | 14 (22) | 22 (39) | - | - | 13 (18) | 23 (49) |
Abbreviations: IPI: international prognostic index; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; LDH: lactate dehydrogenase; CNS: central nervous system; RT: radiation therapy.
Survival and relapse
The median follow-up time was 5.1 years (range 0.3–39.7 years). The median PFS time was 5.3 years [95% confidence interval (CI) 3.9–6.5; Figure 1]. Patients who received RT had a significantly higher PFS time [median 6.3 years (95% CI 5.7–9.6) and 5-year PFS rate 65%] compared to those who did not [median 2.1 years (95% CI 1.1 −5.2) and 5-year PFS rate 30%] (p=.001; Figure 2). The median OS time was 7.7 years for all patients (95% CI 6.5–12.4; Figure 1). Patients who received RT had a median OS 8.3 years (95% CI 6.9–16.9; 5-year OS rate 73%) compared with a median OS of 5.9 years for those who did not (95% CI 2.6–13.3; 5-year OS rate 52%) (p = .065; Figure 2).
Figure 1.
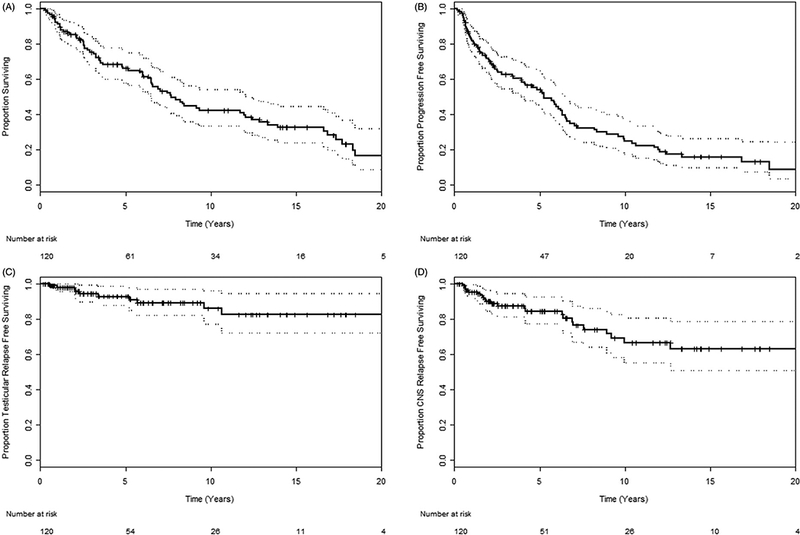
(A) Overall survival, (B) progression free survival, (C) testicular relapse free survival, and (D) CNS relapse free survival among all 120 patients.
Figure 2.
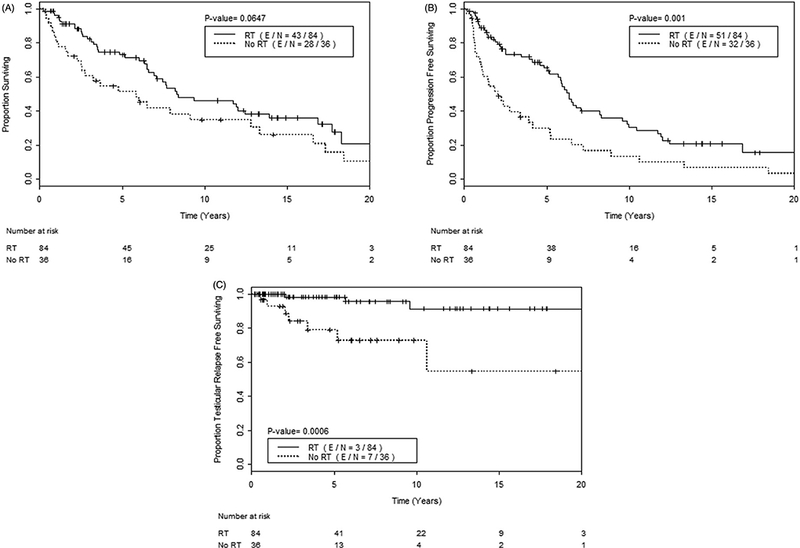
(A) Overall survival, (B) progression free survival, and (C) testicular relapse free survival, among all 120 patients, comparing those who received testicular RT (solid line) versus those who did not (dashed line).
Ten (8%) patients experienced a testicular relapse. The median TRFS time was not reached; the 5-year TRFS rate was 93%, and the 10-year TRFS rate was 86% (Figure 1). Patients who received RT had a higher TRFS (5- and 10-year TRFS rates of 98% and 91%, respectively) compared to those who did not (5- and 10-year TRFS rates of 79% and 73%, respectively) (p = .001; Figure 2).
Twenty-three (19%) patients experienced CNS relapse, including 8 CNS parenchymal failures, and 2 orbital failures. The median CRFS time was not reached; the 5-year CRFS rate was 85% and the 10-year CRFS rate was 67% (Figure 1). Patients who received CNS prophylaxis had a longer median OS time (9.3 years; 95% CI 6.6 to not reached) compared to those that did not (median OS 5.9 years; 95% CI 3.3–12.4) (p = .017; Figure 3). Patients who received CNS prophylaxis had a higher median PFS time (6.3 years; 95% CI 5.2–10.0) versus those who did not (3.4 years; 95% CI 1.9–5.9) (p = .009; Figure 3). CRFS was no different among patients who did or did not receive CNS prophylaxis (5-year CRFS rate 86% vs. 83%) (p = .845; Figure 3).
Figure 3.
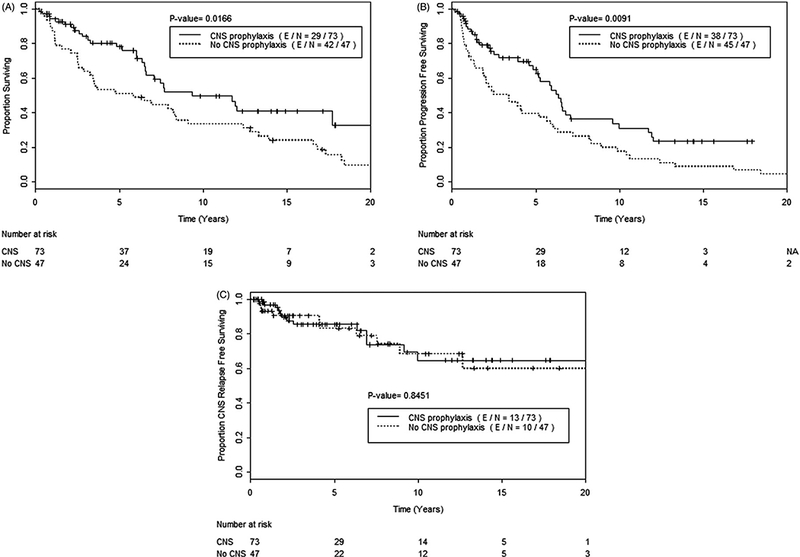
(A) Overall survival, (B) progression free survival, and (C) CNS relapse free survival, among all 120 patients, comparing those who received CNS prophylaxis (solid line) versus those who did not (dashed line).
Univariate analysis
Several patient and treatment factors were evaluated in univariate analyses for potential associations with OS, PFS, TRFS, and CRFS (Table 3). For PFS, three factors were identified: LDH at diagnosis, CNS prophylaxis, and RT. Having an initial abnormal serum LDH level was associated with a higher risk of progression (HR 1.85, 95% CI 1.02–3.38, p = .045), whereas CNS prophylaxis (HR 0.56, 95% CI 0.36–0.87, p = .010), and RT (HR 0.48, 95% CI 0.31–0.75, p = .001) were associated with improved PFS. Increasing age, as a continuous variable, was of borderline significance for association with worse PFS (HR 1.02, 95% CI 0.99–1.03, p = .064).
Table 3.
Univariate analyses of prognostic factors with PFS, OS, TRFS.
| PFS |
OS |
TRFS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Stage (advanced vs. early) | 1.39 | 0.86–2.27 | .183 | 1.43 | 0.83–2.44 | 0.195 | 0.96 | 0.20–4.66 | .961 |
| Age (continuous) | 1.02 | 0.99–1.03 | .064 | 1.04 | 1.02–1.06 | <0.001 | 0.98 | 0.94–1.02 | .333 |
| LDH (elevated vs. normal) | 1.85 | 1.02–3.38 | .045 | 1.69 | 0.87–3.27 | 0.122 | 3.19 | 0.64–15.9 | .157 |
| IPI risk group (int. vs. low) | 1.09 | 0.56–2.12 | .800 | 1.82 | 0.89–3.69 | 0.099 | 0.94 | 0.10–8.69 | .956 |
| Anthracycline (yes vs. no) | 0.64 | 0.34–1.20 | .164 | 0.45 | 0.24–0.84 | 0.013 | - | - | - |
| Rituximab (yes vs. no) | 0.78 | 0.50–1.21 | .266 | 0.78 | 0.48–1.29 | 0.333 | 0.39 | 0.10–1.52 | .174 |
| CNS Prophylaxis (yes vs. no) | 0.56 | 0.36–0.87 | .010 | 0.56 | 0.35–0.91 | 0.018 | 0.16 | 0.03–0.75 | .020 |
| Testicular radiation (yes vs. no) | 0.48 | 0.31–0.75 | .001 | 0.64 | 0.40–1.03 | 0.067 | 0.13 | 0.03–0.52 | .004 |
Abbreviations: IPI: international prognostic index; int.: intermediate; PFS: progression free survival; OS: overall survival; TRFS: testicular relapse free survival.
p Values in bold indicate statistical significance.
On univariate analysis for OS, RT was of borderline significance for improved survival (HR 0.64, 95% CI 0.40–1.03, p = .067; Table 3). Three factors were significantly associated with OS on univariate analysis: increasing age (as a continuous variable) was associated with worse OS (HR 1.04, 95% CI 1.02–1.06, p < .001), whereas anthracycline chemotherapy and CNS prophylaxis were associated with improved OS (HR for anthracycline 0.45, 95% CI 0.24–0.84, p = .013; and HR for CNS prophylaxis 0.56, 95% CI 0.35–0.91, p = .018).
Patients who received RT were at lower risk of testicular relapse (HR 0.13, 95% CI 0.03–0.52, p = .004; Table 3). Receipt of CNS prophylaxis was also associated with decreased testicular relapse risk (HR 0.16, 95% CI 0.03–0.75, p = .020; Table 3). No factors were significantly associated with CRFS on univariate analysis, although there was a trend for an association with advanced stage (vs. limited stage) and worse CRFS [HR 2.22, 95% CI 0.92–5.36, p = .077].
Multivariate analysis
In multivariate analysis (MVA) for PFS, RT remained a significant predictor of improved PFS (HR 0.39, 95% CI 0.24–0.64, p < .001; Table 4), after adjusting for anthracycline chemotherapy (HR 0.39, 95% CI 0.20–0.76, p = .005), stage (HR 1.93, 95% CI 1.12–3.34, p = .018), and age (HR 1.03, 95% CI 1.01–1.05, p = .013). In MVA for OS, RT was associated with improved OS (HR 0.47, 95% CI 0.27–0.83, p = .009), in addition to anthracycline chemotherapy (HR 0.23, 95% CI 0.12–0.46, p < .001); whereas advanced disease stage (vs. limited stage) and increasing age (as a continuous variable) were associated with worse OS (HR for advanced disease 2.45, 95% CI 1.3–4.49, p = .004; HR for age 1.06, 95% CI 1.03–1.08, p < .001). The number of events was insufficient to analyze TRFS. No factors were significantly associated with CRFS in MVA, including CNS prophylaxis.
Table 4.
Multivariate models of prognostic factors for PFS and OS, for all patients, and for those who received modern therapy (rituximab and anthracycline).
| PFS |
OS |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| All patients (n = 120) | ||||||
| Age (continuous) | 1.03 | 1.01–1.05 | .013 | 1.06 | 1.03–1.08 | <.001 |
| Stage (advanced vs. early) | 1.93 | 1.12–3.34 | .018 | 2.45 | 1.33–4.49 | .004 |
| Anthracycline | 0.39 | 0.20–0.76 | .006 | 0.23 | 0.12–0.46 | <.001 |
| Testicular radiation | 0.39 | 0.24–0.64 | <.001 | 0.47 | 0.27–0.83 | .009 |
| Modern therapy patients (n = 64) | ||||||
| Age (continuous) | - | - | - | 1.05 | 1.02–1.09 | .004 |
| CNS Prophylaxis | 0.28 | 0.12–0.69 | .005 | - | - | - |
| Testicular radiation | 0.29 | 0.13–0.64 | .002 | 0.50 | 0.21–1.18 | .114 |
Subgroup analysis of patients given modern systemic therapy
To assess the added value of RT in the era of modern systemic therapy agents, we analyzed a subgroup of 64 patients who received rituximab and anthracycline chemotherapy (Table 2). The median follow-up time for these patients was 3.93 years (range 0.7–20.2 years). Compared with patients who did not receive modern systemic therapy, those who did were more likely to have intermediate- and high-risk IPI risk groups (p = .008), more likely to be advanced stage (p = .027), and more likely to have received CNS prophylaxis (p < .001) and RT (p = .038).
Among the 64 patients treated with modern therapy, on univariate analysis for PFS, RT was significantly associated with improved PFS (HR 0.23, 95% CI 0.11–0.50, p < .001), as was CNS prophylaxis (HR 0.21, 95% CI 0.09–0.48, p < .001). Advanced stage was marginally associated with worse PFS (HR 1.97, 95% CI 1.00–3.88, p = .050). On MVA, RT remained a significant predictor of improved PFS (HR 0.29, 95% CI 0.13–0.64, p = .002), after adjusting for CNS prophylaxis (HR 0.28, 95% CI 0.12–0.69, p = .005; Table 4).
On univariate and multivariate analyses for potential predictors of OS, increasing age was the only significant factor associated with worse OS (multivariate HR 1.05, 95% CI 1.02–1.09, p = .004). No treatment factors were associated with improved CRFS on univariate or multivariate analyses; advanced stage was the only predictor of worse CRFS (multivariate HR 3.31, 95% CI 1.07–10.26, p = .038). The number of events among patients who received modern systemic therapy was insufficient for a stable analysis of TRFS.
Patients who received RT had significantly improved median PFS (6.5 years vs. 2.2 years, p < .001) (Figure 4) and also had a significantly higher 5-year TRFS rate of 100% (vs. 82%, p = .033; Figure 4). Patients who received RT had a higher 5-year OS rate (80% vs. 69% in those who did not), but this difference was not significant (p = .187; Figure 4).
Figure 4.
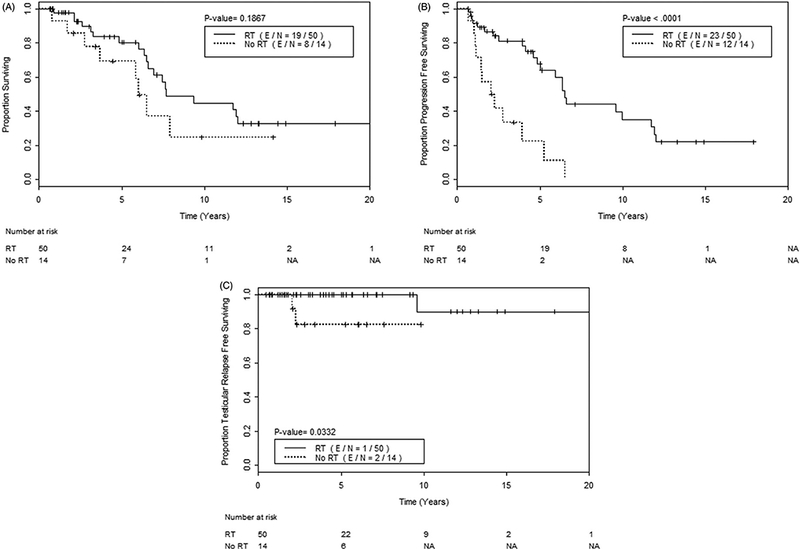
(A) Overall survival, (B) progression free survival, and (C) testicular relapse free survival, among 64 patients who received modern systemic therapy, comparing those who received testicular RT (solid line)
Discussion
In this large cohort of stage I–IV patients with testicular DLBCL, prophylactic testicular RT was associated with a decreased risk of testicular relapse and improved PFS and OS. The benefit in PFS remained among patients treated with modern-era rituximab and anthracycline-based chemotherapy. These results underscore the importance of treating this patient group with testicular RT, particularly as the natural history of this disease, in contrast to nodal DLBCL, has a propensity for late and sanctuary site relapses that are difficult to salvage with systemic therapy.
Our finding of an association between RT and improved survival is supported by other studies [7,8,10]. In one multi-institutional study of 373 patients with primary testicular DLBCL (79% stage I-II) treated in the pre-rituximab era, RT was associated with longer OS on MVA [7]. Another population-based study showed that patients who were not treated with surgical excision and RT had an inferior disease-specific survival [10]. Similar to the study by Zucca et al., we found that the OS benefit of RT was significant in a MVA accounting for stage, age, and anthracycline chemotherapy. In addition, RT was an independent predictor of improved PFS in all patients, including those receiving modern systemic therapy. Although RT could be a surrogate for other favorable factors (patients receiving RT were more likely to have limited stage disease, and to have received rituximab and CNS prophylaxis), RT was statistically significant in multivariate analyses for OS and PFS accounting for several other patient and treatment characteristics, including stage. We also show that RT reduces testicular relapse, which is important given the continuous risk for late testicular relapse without RT, which can reach up to 42% at 15 years [7]. Because salvage therapies for relapse of testicular DLBCL are limited and prognosis after relapse is poor, with one study showing a median survival time of only 10 months [11], treatments to prevent relapse are imperative. Although data from prospective randomized studies are lacking, current evidence led to the inclusion of testicular RT in the recent International Extranodal Lymphoma Study Group (IELSG) phase II trial [6]. Despite increasing evidence in favor of RT, the rates of RT use have not changed over the past several decades, remaining at approximately 30–40% [10].
Prophylactic scrotal RT has limited acute toxicity. One expected side effect, dermatitis, can present as moist desquamation and typically resolves within a few weeks. The location of the treatment field far from critical structures and the relatively low RT dose make scrotal RT generally tolerable. At our institution, use of an appositional electron field with custom lead skin collimation also limits toxicity to nearby structures. A potential late effect of RT is hypogonadism, with continued declines in testosterone levels several years after treatment [12]; however, many patients are already at risk after orchiectomy and with increasing age. Patients should have their testosterone levels checked during follow-up and be referred to endocrinologists when necessary. Although secondary malignancies are a serious toxicity for other lymphomas affecting younger patients, this is considered of little concern given the generally older age of testicular DLBCL patients [13].
Interestingly, we found that CNS prophylaxis was not associated with decreased CNS relapse, but was associated with improved OS, superior PFS, and reduced testicular relapse (on univariate analysis in all patients), as well as improved PFS on MVA in the modern systemic therapy subgroup. Although our findings are limited by the retrospective design and long time span of this study, one possible explanation is that CNS prophylaxis is a surrogate for better overall treatment, as those patients who received CNS prophylaxis were also more likely to have received rituximab, anthracycline chemotherapy, CHOP, and RT. These findings suggest that CNS prophylaxis could be a surrogate for other treatment factors, further supported by the finding that receipt of CNS therapy reduced the testicular relapse risk in our dataset. Although intrathecal chemotherapy prophylaxis continues to be considered standard of care for patients with testicular DLBCL, several studies have not shown a benefit for intrathecal treatment with regard to CNS relapses, although there is an association with improved PFS. Zucca et al. reported a CNS relapse rate of 15%, most of which were brain parenchymal relapses. However, prophylactic intrathecal chemotherapy, although associated with improved PFS, did not have a significant effect on CNS relapse [7]. Also, in the recent IELSG phase II trial, which included intrathecal methotrexate, the rate of CNS relapse was 6% at 5 years. The lack of benefit from intrathecal chemotherapy for CNS relapse was confirmed by a report from the International Primary Testicular Lymphoma Consortium on 280 patients with DLBCL and testicular lymphoma treated at several institutions (p = .918) [11]. Further, in a retrospective study of DLBCL patients treated in the rituximab era, receipt of CNS prophylaxis (with various regimens including both intrathecal and high-dose methotrexate) did not affect the rate of CNS relapse [14]. Because CNS relapses in testicular DLBCL more often occur in the brain parenchyma rather than the meninges, it is possible that intrathecal chemotherapy has inadequate parenchymal penetration. Another CNS prophylaxis option is systemic high-dose methotrexate; however, its use is limited by toxicity, especially among older patients. In our study, 72 patients had intrathecal chemotherapy, and only 1 patient received high-dose methotrexate, precluding analysis of differences between these two prophylaxis types. A newer prophylaxis type, liposomal cytarabine, maintains elevated drug levels in the cerebrospinal fluid; its safety and efficacy in high-risk DLBCL patients was recently reported in a prospective trial [15]. The currently ongoing IELSG 30 study includes both intrathecal liposomal cytarabine and systemic intermediate-dose methotrexate for CNS prophylaxis in an effort to decrease CNS relapses.
The adoption of anthracycline chemotherapy, in particular the CHOP regimen, based on standard treatment for patients with nodal DLBCL, has resulted in 5-year survival rates of 30–75% [1]. Because the addition of rituximab to CHOP resulted in a survival benefit relative to CHOP alone in trials of nodal DLBCL [3–5], an international prospective phase II trial of 53 patients with primary testicular lymphoma assessed the use of R-CHOP, along with intrathecal methotrexate and testicular RT for most patients, and reported a 5-year PFS rate of 74% and a 5-year OS rate of 85% [6]. However, in our analysis, rituximab was not associated with improved outcomes, a finding also noted in a large population-based study [10]. Possible explanations are that the biological characteristics of testicular DLBCL may make it less responsive to rituximab, or that rituximab does not effectively penetrate the extranodal sites commonly involved in the natural history of testicular DLBCL. The latter theory is supported by a large study of DLBCL patients with osseous involvement that showed that rituximab did not improve outcomes, although RT doubled their event-free survival [16]. If, in fact, rituximab does not have the same magnitude of benefit in testicular DLBCL as it does for nodal DLBCL, the role of RT may be even more important.
Our study has limitations, particularly its retrospective design and the heterogeneity of patient, diagnostic and treatment strategies over the long study period, which are important to note when considering our findings. Nevertheless, this report is one of the largest single-institution series to date with prolonged followup. The broad inclusion of patients allows us to study the general benefits of different treatment strategies in a rare disease where prospective randomized trials are unlikely to be feasible.
In conclusion, there is a continuous risk of late relapses in patients with testicular DLBCL often involving sanctuary sites such as the contralateral testis and CNS. We observed that the use of prophylactic testicular RT reduced the risk of contralateral testicular relapse, which seemed to translate into an improved OS and PFS. The improvement in PFS was also maintained even in the modern era of rituximab therapy. Therefore, given the aggressive nature of testicular DLBCL, the difficulty of salvaging failures, and the relatively low morbidity of RT, prophylactic testicular RT should be offered to all patients with this disease.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2017.1312381
The results of this study were presented in part at the American Society for Radiation Oncology (ASTRO) Annual Meeting in Boston, MA, 25–28 September 2016.
References
- [1].Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Crit Rev Oncol Hematol. 2008;65:183–189. [DOI] [PubMed] [Google Scholar]
- [2].Moller MB, d’Amore F, Christensen BE. Testicular lymphoma: a population-based study of incidence, clinicopathological correlations and prognosis. The Danish Lymphoma Study Group, LYFO. Eur J Cancer. 1994;30A:1760–1764. [DOI] [PubMed] [Google Scholar]
- [3].Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. [DOI] [PubMed] [Google Scholar]
- [4].Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. [DOI] [PubMed] [Google Scholar]
- [5].Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105–116. [DOI] [PubMed] [Google Scholar]
- [6].Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29: 2766–2772. [DOI] [PubMed] [Google Scholar]
- [7].Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20–27. [DOI] [PubMed] [Google Scholar]
- [8].Mazloom A, Fowler N, Medeiros LJ, et al. Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: the M. D. Anderson Cancer Center experience. Leuk Lymphoma. 2010;51:1217–1224. [DOI] [PubMed] [Google Scholar]
- [9].Cheson BD, Fisher RI,Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gundrum JD, Mathiason MA, Moore DB, et al. Primary testicular diffuse large B-cell lymphoma: a population based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. 2009;27:5227–5232. [DOI] [PubMed] [Google Scholar]
- [11].Deng L, Xu-Monette ZY, Loghavi S, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30:361–372. [DOI] [PubMed] [Google Scholar]
- [12].Petersen PM, Giwercman A, Daugaard G, et al. Effect of graded testicular doses of radiotherapy in patients treated for carcinoma-in-situ in the testis. J Clin Oncol. 2002;20:1537–1543. [DOI] [PubMed] [Google Scholar]
- [13].Sacchi S, Marcheselli L, Bari A, et al. Second malignancies after treatment of diffuse large B-cell non-Hodgkin’s lymphoma: a GISL cohort study. Haematologica. 2008;93:1335–1342. [DOI] [PubMed] [Google Scholar]
- [14].Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol. 2012;159: 39–49. [DOI] [PubMed] [Google Scholar]
- [15].Gonzalez-Barca E, Canales M, Salar A, et al. Central nervous system prophylaxis with intrathecal liposomal cytarabine in a subset of high-risk patients with diffuse large B-cell lymphoma receiving first line systemic therapy in a prospective trial. Ann Hematol. 2016;95:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol. 2013;31:4115–4122. [DOI] [PubMed] [Google Scholar]


