Abstract
Purpose:
Gadoxetate-disodium (Gd-EOB-DTPA)-enhanced 3D T1- weighted (T1w) MR cholangiography (MRC) is an efficient method to evaluate biliary anatomy due to T1 shortening of excreted contrast in the bile. A method that exploits both T1 shortening and T2* effects may produce even greater bile duct conspicuity. The aim of our study is to determine feasibility and compare the diagnostic performance of two-dimensional (2D) T1w multi-echo (ME) spoiled gradient-recalled-echo (SPGR) derived R2* maps against T1w MRC for bile duct visualization in living liver donor candidates.
Materials and methods:
Ten potential living liver donor candidates underwent pretransplant 3T MRI and were included in our study. Following injection of Gd-EOBDT-PA and a 20-min delay, 3D T1w MRC and 2D T1w ME SPGR images were acquired. 2D R2* maps were generated inline by the scanner assuming exponential decay. The 3D T1w MRC and 2D R2* maps were retrospectively and independently reviewed in two separate sessions by three radiologists. Visualization of eight bile duct segments was scored using a 4-point ordinal scale. The scores were compared using mixed effects regression model.
Results:
Imaging was tolerated by all donors and R2* maps were successfully generated in all cases. Visualization scores of 2D R2* maps were significantly higher than 3D Tlw MRC for right anterior (p = 0.003) and posterior (p = 0.0001), segment 2 (p < 0.0001), segment 3 (p = 0.0001), and segment 4 (p < 0.0001) ducts.
Conclusions:
Gd-EOB-DTPA-enhanced 2D R2* mapping is a feasible method for evaluating the bile ducts in living donors and may be a valuable addition to the living liver donor MR protocol for delineating intrahepatic biliary anatomy.
Keywords: T1w MR Cholangiogram, Biliary anatomy, R2* map, Living donor imaging
Variations in biliary anatomy are common and noted up to 40%−50% of livers [1]. Accurate preoperative imaging of donor biliary anatomy to identify both the presence and the specific type of anatomic variation is critical for successful living donor liver transplantation planning and to help reduce postoperative complications [1]. Standard preoperative MRI protocols rely on T2-weighted (T2w) MR cholangiography (MRC), but these sequences can be limited for visualization of non-dilated intrahepatic ducts [2]. Even with routine use of preoperative and intraoperative imaging, biliary complications remain the most common cause of morbidity, accounting for 20%−30% of all postoperative complications in living donor liver transplantation [1].
Recent studies have suggested that the addition of gadoxetate-disodium (Gd-EOB-DTPA)-enhanced three-dimensional (3D) T1-weighted (T1w) MRC to T2w MRC improves depiction of biliary anatomy in living donors [3, 4]. Gd-EOB-DTPA is taken up by hepatocytes and excreted into bile with resultant T1 shortening of the bile (i.e., the bile ducts appear hyperintense). In addition to T1 shortening, however, Gd-EOB-DTPA also shortens T2* of bile which may counteract the positive effect of T1 shortening and reduce the visual conspicuity of bile ducts relative to background liver. We hypothesize that two-dimensional (2D) Gd-EOB-DTPA-enhanced T1w multi-echo (ME) spoiled gradient-recalled-echo (SPGR) images with parametric R2* maps (subsequently referred to as 2D R2* maps) may improve visualization of bile ducts through the additive effects of T1 and T2* shortening.
The aim of this preliminary study was to determine in potential liver donor candidates the feasibility of visualizing bile ducts on 2D R2* images and secondarily to compare bile duct visualization with 3D T1w MRC.
Methods
Patient population
Following Institutional Review Board approval, we retrospectively identified all potential liver donor candidates imaged for pretransplant evaluation with Gd-EOB-DTPA-enhanced 3T MRI from 2013 to 2016. During this time period, the standard living donor MR protocol at our institution included 2D T1w ME SPGR from which R2* maps were generated and 3D T1w MRC following Gd-EOB-DTPA intravenous administration (detailed below).
MRI acquisition
Ten potential liver donors (6 females, 4 males; mean age of 39.1 ± 6.8 years) were imaged using a 3T MRI system (Signa EXCITE HDxt scanner, GE Medical Systems, Waukesha, WI) in supine position with an eight-channel torso phased-array coil centered over the liver. Intravenous Gd-EOB-DTPA (Eovist, Bayer Healthcare, Whippany, NJ) 0.1 mL/kg was injected at a rate of 1.0 mL/s. Axial breath-hold 3D SPGR and 2D ME SPGR sequences (parameters summarized in Table 1) were obtained through the liver 20 min after contrast injection. For the 2D ME SPGR sequence, six echoes were obtained per repetition time (TR) at nominally out-of-phase and in-phase echo times (TEs). 2D R2* maps were generated inline by the scanner computer using a custom algorithm that assumes exponential decay and corrects for fat-water or fat-fat signal interference effects [5].
Table 1.
MRI parameters
| Series description | 3D T1w SPGR | 2D ME SPGR |
|---|---|---|
| TR (ms) | 2.9–5.6 | 150 |
| TE (ms) | 1.4—1.8 | 1.15, 2.3, 3.45, 4.6, 5.75, 6.9 |
| Flip angle (degrees) | 15 | 50 |
| Receiver bandwidth (kHz) | 125 | 166 |
| Number of echoes | 1 | 6 |
| Matrix | 320–384 × 224–256 | 288 × 224 |
| Field of view (cm) | 44 | 44 |
| Slice thickness (mm) | 2–4 | 6 |
| Acceleration factor for parallel imaging | 1 | 1.25 |
| Breath-hold time | ~20 s | ~20 s |
3D T1w SPGR, three-dimensional T1-weighted spoiled gradient-recalled-echo; 2D ME SPGR, two-dimensional multi-echo spoiled gradient-recalled-echo. The flip angle of 15° for 3D T1w SPGR was the maximum allowed by our scanner. The rectangular field of view was adjusted depending on the subject’s body habitus and breath-hold capacity
Image interpretation and scoring
Three radiologists independently reviewed images on a digital monitor and, using a previously published fourpoint ordinal scale [3], scored the visualization of the following eight bile duct segments: common hepatic, right hepatic, left hepatic, right anterior, right posterior, segment 2, segment 3, and segment 4. Readers also scored the presence and type of anatomic variants if identified. 3D T1w MRC and 2D R2* maps were reviewed in two separate sessions performed 1–2 weeks apart.
Statistical analysis
For each bile duct segment, visualization scores of 3D T1w MRC vs. 2D R2* maps were compared using mixed effects regression model (R version 3.3.1). Bonferroni’s adjustment for multiple comparisons was applied and a p value < 0.00625 (= 0.05/8) was considered significant to ensure family-wise significance level of 0.05.
Results
3D T1w cholangiograms and R2* maps were obtained successfully in all ten subjects. Common hepatic and first-order bile ducts were visualized equally well on both sequences. Second-order bile ducts were visualized with greater signal contrast relative to liver (Fig. 1) and received higher visualization scores on R2* maps than 3D T1w cholangiograms (Fig. 2). Due to blooming from R2* decay, the bile ducts appeared larger on R2* maps than 3D T1w cholangiograms. No biliary anatomical variants were identified on R2*maps or 3D T1w cholangiograms.
Fig. 1.
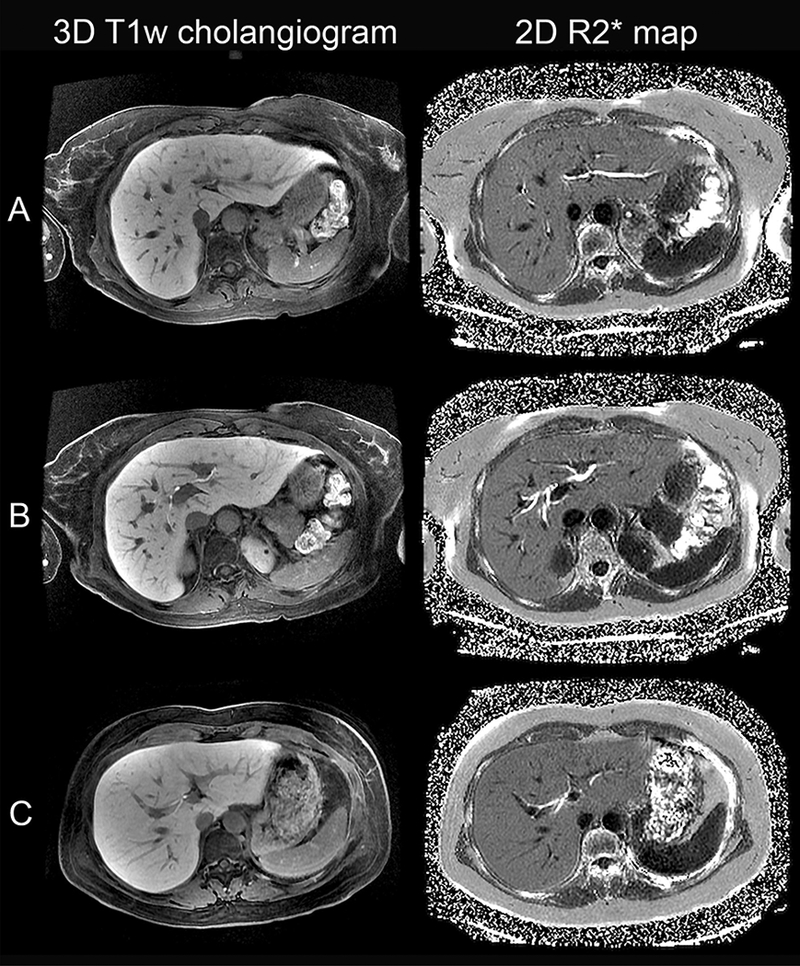
3D T1w cholangiograms and 2D R2* maps of three living liver donors demonstrating A segment 2 duct in a 57-year-old female, B segment 4, right anterior, and right posterior ducts in a 56-year-old female, and C right anterior and segment 3 ducts in a 36-year-old female. Note the more complete visualization of second-order bile ducts on R2* maps compared to Gd-EOB-DTPA-enhanced 3D T1w MRC.
Fig. 2.
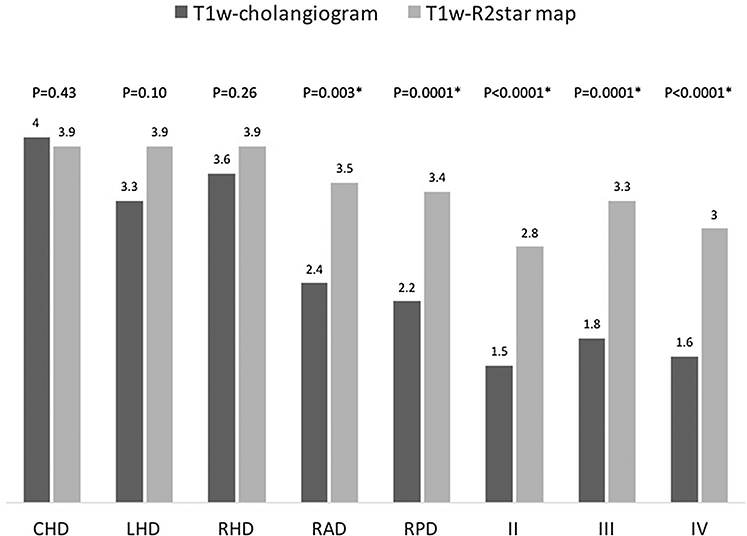
Mean visualization scores for 3D T1w cholangiograms and 2D R2* maps. Note The 4-point visualization scale is from 1 = absent visualization to 4 = excellent visualization; asterisk represents statistically significant difference between 3D T1w cholangiogram and 2D R2* map visualization scores; p values are considered significant at * < 0.00625 to ensure a family-wise p value of 0.05; CHD, common hepatic duct; RHD, right hepatic duct; LHD, left hepatic duct; RAD, right anterior duct; RPD, right posterior duct; II, segment 2 duct; III, segment 3 duct; IV, segment 4 duct.
Discussion
In this preliminary study, we found that 2D R2* imaging is a feasible method for visualizing bile ducts in living liver donor patients. Visualization scores for second order bile ducts were significantly higher on 2D R2* imaging than the 3D T1w MRC. Recent studies have shown that Gd-EOB-DTPA-enhanced 3D T1w MRC is superior to T2w MRC for visualization of second-order bile ducts [3, 4]. In the current proof-of-concept study, we implemented a T1w ME SPGR technique to exploit both T1 shortening and T2* effect and we showed proof-of-concept that R2* maps may provide better visualization of ducts than T1w MRC.
The results of our study may have important clinical implications. Biliary complications are the most common cause of morbidity in living donor liver transplantation and have been reported in 6%−10% of living liver donors [6, 7]. Improved preoperative imaging of the bile ducts is essential not only for the safety of the liver donors but also for the success of the living donor program. One of the most common bile duct variants is the right posterior duct draining into the left hepatic duct occurring in up to 16% of the population [1, 8]. In the presence of this variant, donor candidates are typically excluded from living donor program. In 9 of 10 liver donor candidates in our study, 2D R2* maps provided better visualization of the right posterior duct than 3D T1w MRC. Figure 3 demonstrates a case example of a 41-year-old female donor. Note that the right posterior duct is not discernible on the T1w cholangiogram, even on a maximum projection intensity (MIP) image, but is well visualized on the R2* map. These findings suggest that 2D R2* mapping may be preferable to 3D T1w MRC as an adjunct to T2 imaging for bile duct evaluation. While 2D R2* mapping depicts hepatic ducts, however, it does not allow reliable measurement of their diameter due to blooming as mentioned earlier.
Fig. 3.
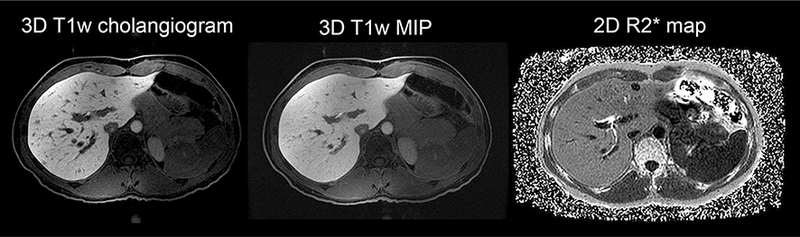
3D T1w cholangiogram, 3D T1w MIP, and 2D R2* map of a 41-year-old female donor candidate. Note better visualization of right posterior and right anterior ducts on the R2* map. The slice thickness is 6 mm in 3D T1w MIP image; MIP, maximum intensity projection.
A limitation of this study was that we only compared 2D R2* mapping to the 3D T1w MRC, and not the T2w MRC, a comparison that warrants further research. Additionally, our study was retrospective and the 2D ME SPGR sequence was not optimized for the purpose of bile duct evaluation. Future efforts aimed at optimization of R2* mapping sequences such as use of thinner slices, higher flip angle, and 3D acquisition may further improve biliary imaging. Finally, our small sample size and the retrospective nature of our study did not allow proper assessment of reader repeatability, reproducibility, or accuracy for detecting biliary variants.
Despite these limitations, our preliminary results show that Gd-EOB-DTPA-enhanced 2D R2* mapping is a feasible method for evaluating the bile ducts in living donors and may be a valuable addition to the living liver donor MR protocol for delineating intrahepatic biliary anatomy. Future studies are needed to validate our findings in larger prospective cohorts evaluating the effectiveness of the donor MR protocol with regards to all aspects including vascular, biliary, and other anatomic considerations.
Acknowledgments
Funding National Institutes of Health Grant T32 EB005970–09.
SFD was supported by a T32 Grant from the NIH (4T32EB005970–09) at the time of the study.
Footnotes
Conflicts of interest KF declares that she has no conflict of interest. TW declares that she has no conflict of interest. SI declares that she has no conflict of interest. CLP declares that she has no conflict of interest. JH declares that he has no conflict of interest. CH declares that he has no conflict of interest. AM declares that she has no conflict of interest. AG declares that he has no conflict of interest. AH declares that he has no conflict of interest. CS has received research grants from Siemens, GE, and Guerbet. CS also consults and advises for Alexion, AstraZeneca, Bioclinica, BMS, Fibrogen, Galmed, Genzyme, Gilead, Fibrogen, Icon, Intercept, Isis, Janssen, NuSirt, Perspectum, Pfizer, Profil, Sanofi, Shire, Synageva, Tobira, Takeda, Virtual Scopics.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Due to retrospective and observational nature of our study, patient consent was not required by the IRB.
Compliance with ethical standards
References
- 1.Catalano OA, Singh AH, Uppot RN, et al. (2008) Vascular and biliary variants in the liver: implications for liver surgery. RadioGraphics 28:359–378. doi: 10.1148/rg.282075099 [DOI] [PubMed] [Google Scholar]
- 2.Lim JS, Kim M-J, Myoung S, et al. (2008) MR cholangiography for evaluation of hilar branching anatomy in transplantation of the right hepatic lobe from a living donor. Am J Roentgenol 191:537–545. doi: 10.2214/AJR.07.3162 [DOI] [PubMed] [Google Scholar]
- 3.Cai L, Yeh BM, Westphalen AC, et al. (2016) 3D T2-weighted and Gd-EOB-DTPA-enhanced 3D T1-weighted MR cholangiography for evaluation of biliary anatomy in living liver donors. Abdom Radiol. doi: 10.1007/s00261-016-0936-z [DOI] [PubMed] [Google Scholar]
- 4.Mangold S, Bretschneider C, Fenchel M, et al. (2012) MRI for evaluation of potential living liver donors: a new approach including contrast-enhanced magnetic resonance cholangiography. Abdom Imaging 37:244–251. doi: 10.1007/s00261-011-9736-7 [DOI] [PubMed] [Google Scholar]
- 5.Yokoo T, Shiehmorteza M, Hamilton G, et al. (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258:749–759. doi: 10.1148/radiol.10100659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RS, Russo MW, Lai M, et al. (2003) A survey of liver transplantation from living adult donors in the United States. N Engl J Med 348:818–825. doi: 10.1097/01.sa.0000119084.92230.f7 [DOI] [PubMed] [Google Scholar]
- 7.Lo C-M (2003) Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation 75:S12-S15. doi: 10.1097/01.TP.0000046534.45645.47 [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Byun JH, Lee SS, et al. (2010) Biliary tract depiction in living potential liver donors: intraindividual comparison of MR cholangiography at 3.0 and 1.5 T. Radiology 254:469–478. doi: 10.1148/radiol.09090003 [DOI] [PubMed] [Google Scholar]


