Abstract
The study aim was to identify the timing of sensitive windows for ototoxicity related to perinatal exposure to PCBs. A total of 351 and 214 children from a birth cohort in eastern Slovakia underwent otoacoustic testing at 45 and 72 months, respectively, and distortion product otoacoustic emissions (DPOAEs) at 11 frequencies were recorded. Cord and child 6-, 16-, 45-, and 72- month blood samples were analyzed for PCB 153 concentration. The PCB 153 concentration-time profiles were approximated with a system model to calculate area under the PCB*time curves (AUCs) for specific time intervals (3 and 6 months for 45 and 72 months data, respectively). DPOAE amplitudes were correlated (Spearman) with cord serum PCB and AUCs, markers of prenatal and postnatal exposure, respectively. Two exposure critical windows were identified in infants, the first related to prenatal and early postnatal and the second to postnatal exposure to PCBs. Our data have shown tonotopicity, sexual dimorphism, and asymmetry in ototoxicity of PCBs.
Keywords: Ototoxicity, Polychlorinated biphenyls, Critical windows of exposure, Otoacoustic emissions
1. Introduction
Organochlorine compounds (OCs) in the environment include a wide range of chemicals, such as polychlorobiphenyls (PCBs), di-oxins, β-hexachlorocyclohexane (β-HCH), and 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (p,p′-DDT) and its metabolite 1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene (p,p′-DDE) or hexachlorobenzene (HCB). They are lipophilic, synthetic chemicals and belong to the family of persistent organic pollutants (POPs) because they persist in the environment for years and biomagnify through the food chain in human and animal fatty tissues (Carpenter, 2011). They are ubiquitous and can be found in animal and human tissues (Bergonzi et al., 2011; Lopez-Espinosa et al., 2011; Patayová et al., 2013; Vested et al., 2014). A large spectrum of health effects have been associated with prenatal and early post-natal exposures to organochlorine compounds (Haugen et al., 2015; Grandjean et al., 2008). Among these health outcomes, hearing impairment was described in animals after exposure to PCBs (Crofton and Zoeller, 2005; Powers et al., 2006) and organo-halogens as HCB (Hadjab et al., 2004), hexabromocyclododecane (Lilienthal et al., 2009) and polybrominated diphenyl ethers (Poon et al., 2011). Animal observations on ototoxicity of organochlorines have been extended to humans exposed to PCBs (Trnovec et al., 2008, 2010; Jusko et al., 2014; Min et al., 2014), organochlorine pesticides (Sisto et al., 2015) and furans (Li et al., 2015). Recently, the introduction of distortion product otoacoustic emission (DPOAE) measurements (Lasky et al., 2002) has helped increase the ability to detect potential ototoxicity, since the primary purpose of OAE tests is to determine cochlear status, specifically hair cell function. Using this approach, we found that DPOAEs measured in children at 45 months of age are related to prenatal and postnatal exposure to PCB 153, however in an opposite manner (Sisto et al., 2015). In the latter study the exposure was characterized by several single time point measurements of organochlorine serum concentration. Such study design did not allow to assess more finely the critical time of exposure of the human cochlea showing increased sensitivity to extraneous impacts. The importance of the exposure time in view of developmental damages has been accented repeatedly (Grandjean et al., 2008; Selevan et al., 2000; Palanza et al., 2016). The aim of the current study was to identify more exactly the exposure windows for these endpoints. Moreover, to expand on our previously published data (Sisto et al., 2015; Jusko et al., 2014) we presently analyze sex and side differences and tonotopicity in the auditory response.
2. Materials and methods
2.1. Study design
The current study is an extension of the previous ones (Jusko et al., 2014; Sisto et al., 2015) in which the study population, blood collection, chemical measurement, lipid measurement, exposure and outcome assessment, otologic and audiological assessments, assessment and analysis of DPOAEs, assessment of potential mixture effect were thoroughly described. The study protocol was approved by the Institutional Review Board at the Slovak Medical University.
2.2. Audiological examination
In brief, children were selected from a cohort of 811 children (Hertz-Picciotto et al., 2003). At 45 months, 351 children contributed audiological data, and at 72 months, 214 children. The number of participants in the study is a result of attrition by families who refused participation or could not be contacted. PCB 153 was measured in cord blood and at 6, 16, 45 and 72 months. Auditory function was evaluated at 45 and 72 months by determination of DPOAEs. Otologic and audiological assessments were performed at the Department of Otorhinolaryngology of the Michalovce district hospital. The otoscopic examination was conducted with the aim of excluding children with ear infections or obstructions of the ear canal. The tympanometric test (GSI 38 Auto Tymp; Grason-Stadler Inc., Milford, NH, USA) was used to test the middle ear function. Tympanograms were scored following the Jerger’s classification (Jerger, 1970). A signal of middle ear pathology mostly was type B tympanogram indicating fluid or infection behind the ear drum. These pathological states were mostly temporary and vanished after proper treatment and DPOAE response could be obtained.
In most children we have obtained valid DPOAE data from both ears. However, in many children we have obtained DPOAEs from one ear only. Examination at the age of 45 months, provided basic information compared to 72 months and exact data on the number of boys, girls and ear side examined are in Table 1.
Table 1.
Data on sex and ear side of examined ears at the age of 45 months.
| Children | % of children | Boys | % of ears | % of boys | Girls | % of ears | % of girls | |
|---|---|---|---|---|---|---|---|---|
| Both ears | 193 | 55% | 88 | 45.60% | 52.40% | 105 | 54.40% | 57.40% |
| Left ear only | 93 | 26.50% | 47 | 50.50% | 28% | 46 | 49.50% | 25.10% |
| Right ear only | 65 | 18.50% | 33 | 50.80% | 19.60% | 32 | 49.20% | 17.50% |
| Sum | 351 | 168 | 47.90% | 183 | 52.10% |
DPOAEs were recorded by using an Echoport ILO 292 USB-I Otodynamics Ltd. (Hatfield, Herts, UK) instrument connected to a personal computer equipped with ILO V6 software. DPOAEs were measured in response to pairs of primary tones (f2 > f1), with f2 set at default frequencies varied in one fourth of octave steps between 1000 and 5657 Hz. The f2/f1 ratio was 1.22 for each primary pair. Both the f1 and f2 levels were set to 70 dB SPL.
2.3. Chemical analysis
Fifteen PCB congeners [IUPAC (International Union of Pure and Applied Chemistry) numbers 28, 52, 101, 105, 114,118, 123 + 149, 138 + 163, 153, 156 + 171, 157, 167, 170, 180, and 189 were determined in child blood serum samples. The procedure for quantification of PCB concentrations was described previously (Conka et al., 2005; Kočan et al., 1994). The analysis was focused on the PCB 153 only. The reason for this choice is that the PCB 153 is highly correlated with total PCB concentration, and it is easily detectable in all child samples. For individual PCB measurements with concentrations below the limit of detection (LOD), we took the LOD value divided by the square root of 2. All PCB 153 serum concentration values were lipid adjusted (ng PCB 153/g lipid).
2.4. Exposure assessment
For integrative exposure assessment in time, expressed as an area under the PCB*time curve (AUC), we approximated the PCB 153 serum concentration-time profile for each subject with a mathematical function, taking into account mean residence time of PCB 153 molecules in the body, duration of breast feeding, hypothetical PCB 153 concentration in steady-state without breast feeding and alternately without normal food intake (Trnovec et al., 2011). Such analysis enabled the computation of definite integrals for consecutive time periods. Two different limits for definite integrals were chosen. When analyzing the hearing data recorded at 45 months, the difference between the upper and lower limit was 3 months and, with data recorded at 72 months, the difference was 6 months. The individual AUCs of series obtained in this way (independent variable) were Spearman Rank-Order correlated against the amplitudes of the DPOAEs (dependent variable).
2.5. Grouping of DPOAE amplitudes
The DPOAE levels in fourth-octave steps (11 default frequencies) were grouped for more operability into 5 half-octave bands according to the formula:
This grouping algorithm halves the number of bands by summing energy, which is the physical quantity that can be meaningfully summed, over neighboring bands. The factor −3 dB (more exactly, −10log10(2)) preserves the numerical value of the spectral level in dB.
The reason for grouping the bands is that the typical frequency scale A/(dA/df), where A is the signal amplitude in linear units, of both the OAE level and audiometric threshold variations is generally much larger than one fourth of octave, so neighboring bands are not statistically independent from each other, and an excessive number of bands decreases the statistical power of the analysis. Besides evaluation of DPOAEs at the reduced group of 5 f2 frequencies, we calculated the correlations for the complete set of 11 default frequencies when analyzing cord blood (3364 Hz was omitted for technical reasons).
2.6. Statistical analysis
Spearman’s rank correlation coefficients we estimated between the base 10 logarithms of the AUCs of PCB 153 blood serum concentration and the amplitude of DPOAEs. Analyses were fit stratifying by ear side and by ear side and sex of child. A significance criterion of p < 0.05 was conventionally adopted. All statistical analyses were performed using the statistical software SPSS 16 (Softonic International S.L., Barcelona, Spain). To visualize the direction and strength of the linear association between the concentration-time measures and the amplitude of the DPOAEs, we constructed plots in which on the horizontal axis are shown AUCs and on the vertical axis, the Spearman correlation ρ and its p value.
3. Results
3.1. PCB 153 exposure
The median cord serum concentration of PCB 153 was 130 ng/g lipid (IQR, 84–208 ng/g lipid). Median child serum concentrations were 141 ng/g lipid at 6 months of age (IQR, 41–265), 138 ng/g lipid at16 months of age (IQR, 41–341 ng/g lipid), 121 ng/g lipid at 45-months of age (IQR, 45–268 ng/g lipid) and 48 ng/g lipid at 72-months of age (IQR, 23–110 ng/g lipid).
3.2. Relationship between PCB153 exposure and grouped amplitudes of DPOAEs
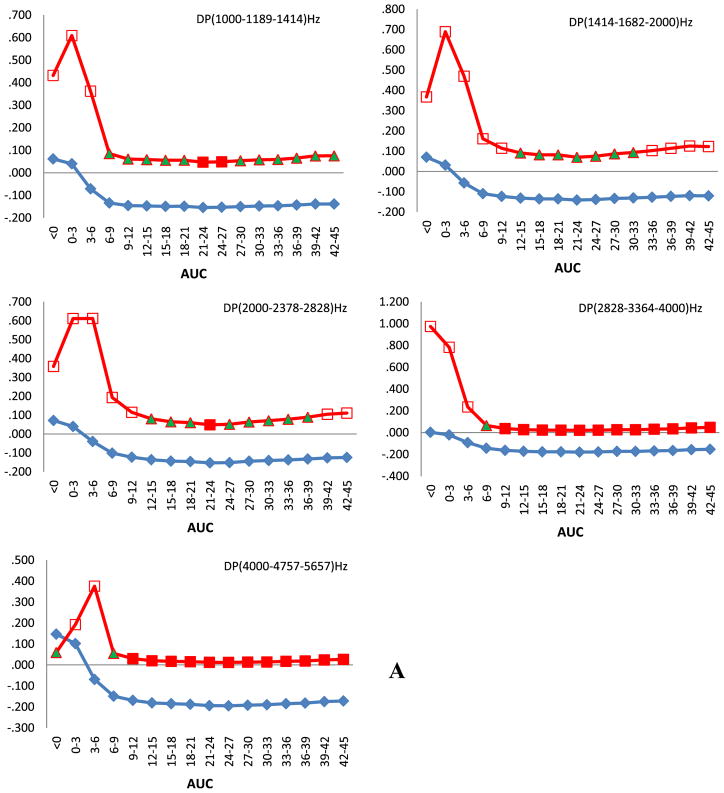
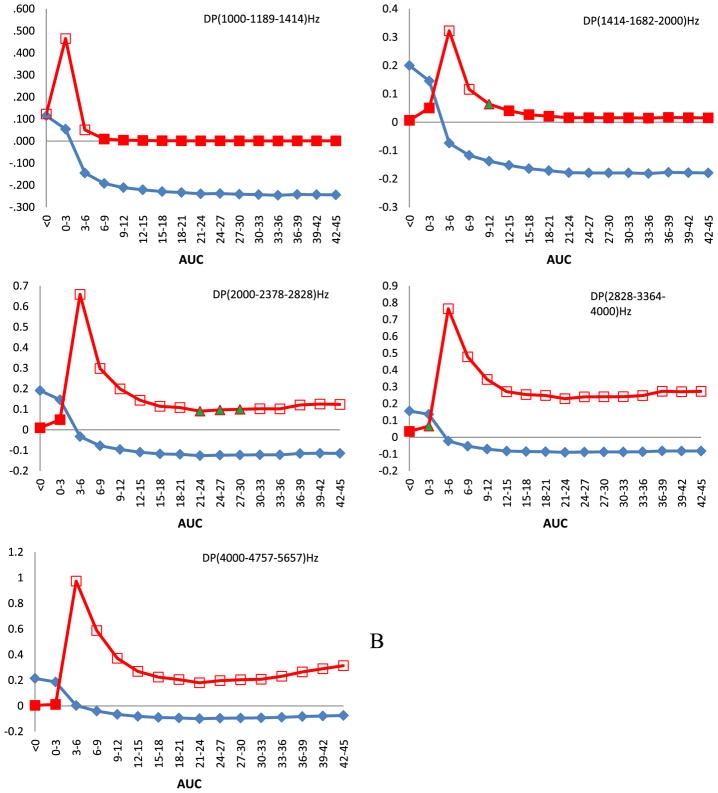
We observed a relationship between PCB 153 serum concentration expressed as AUCs and the amplitude of DPOAEs among children in this cohort. From Fig. 1A which depicts the response for the right ears of all children it can be seen that prenatal exposure is related to an increase of DPOAE amplitudes for all grouped frequencies (p < 0.05), however a borderline statistical significance (p < 0.1) was obtained only for the highest frequencies centered at 4757 Hz. The direction of the response changed postnatally during the first semester of life. Most statistically significant correlations were observed for the two upper frequencies centered at 3364 and 4757 Hz and they started to relate approximately at the age of 1 year. For the left ear (Fig. 1B), at the same settings, the prenatal concentration including the earliest AUC (0–3 months) correlated significantly (p < 0.05) with an amplitude increase for centered frequencies at 1682, 2378, and 4757 Hz. Similar to right side we observed a negative relationship between AUCs and DPOAE amplitudes from the time of about the age of one year, for 1189 and 1682 (p < 0.05) and for 2378 (p < 0.1) centered frequencies.
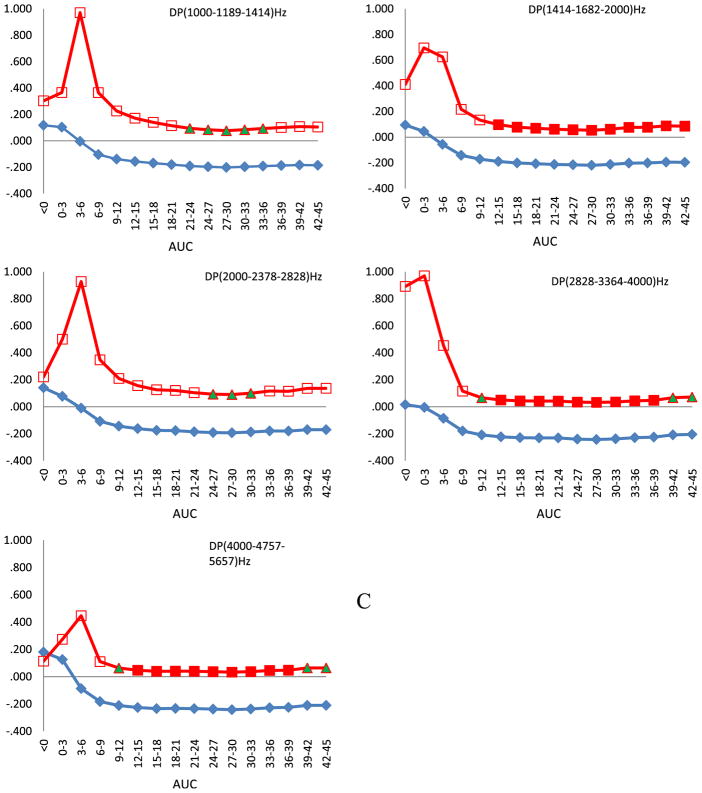
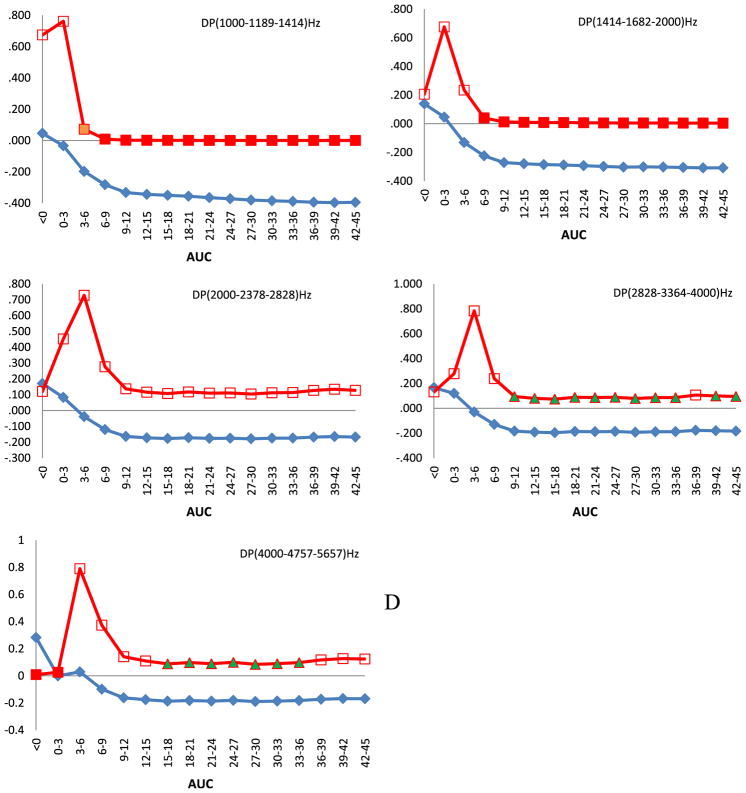
Fig. 1.
Spearman correlation of amplitudes of DPOAEs against areas under the curve (AUCs) calculated for 3 months time intervals after approximation of single time point PCB 153 serum concentration data with mathematical function. Children were audiologically examined at the age of 45 months. On horizontal axis are shown AUCs and on the vertical parameters of Spearman correlation ρ and its p value. Squares denote Spearman correlation coefficient and diamonds its direction. Full squares denote statistical significance at p < 0.05, full triangles at p < 0.1, empty squares p > 0.1. A. All children, right ears. B. All children, left ears. C. Boys, right ears. D. Boys, left ears.
For girls, we did not observe any significant relationships between DPOAEs amplitudes and PCB serum concentration (data not shown), nevertheless the directional trend was similar. The pattern of changes for the right ears of boys (Fig. 1C) was similar to the pattern for all children. Significant correlations (p < 0.05) between DPOAE amplitudes and AUCs were observed between 12 and 36 months for frequencies centered at 3364 and 4757 Hz. The. strength of correlation decreased with AUCs 36–39 months. For lower frequencies, marginal statistical significances appeared approximately between 21 and 36 months. The response of the left ears of boys (Fig. 1D) largely agreed with that for all children. The cord serum concentration, a marker of prenatal exposure, and the early postnatal exposure (AUC 0–3 months) correlated significantly with increased DPOAE amplitudes in the highest frequency region. On the other hand, the amplitudes were inversely related to AUCs between 6 and 45 months for the two lowest frequencies.
3.3. Evaluation of all 11 frequencies
From Fig. 2 it can be seen the consistent positive regression coefficients when DPOAE amplitudes from left ears were regressed against cord blood PCB 153 concentration for f2 > 1414 Hz.
Fig. 2.
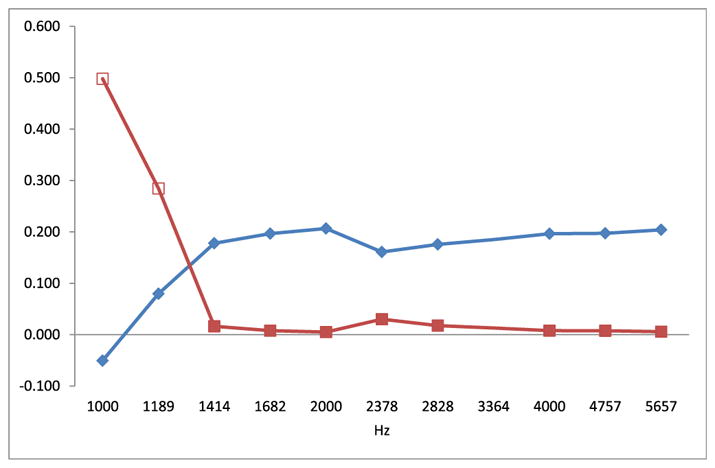
Spearman correlation of amplitudes of DPOAEs at 11 default frequencies for left ears of all children against cord serum PCB 153 concentration. On the vertical axis were plotted parameters of Spearman correlation ρ (red squares) and its p value (blue diamonds). Full squares denote statistical significance at p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Relationship between PCB 153 exposure and grouped amplitudes of DPOAEs at age 72 months
At the age of 72 months the Spearman’s rank correlation co-efficients have shown that the DPOAE amplitudes were not systematically related to perinatal exposures either in boys or girls in a similar way as with 45 months data. For this reason we did not extend the correlation analysis to AUCs for time intervals >45 months.
4. Discussion
From the examination at 45 months we observed two exposure sensitive windows, the first one linked to the relatively constant PCB cord serum concentration of prenatal period and the other one coinciding with increasing and peaking PCB serum concentration in breast fed infants (Lancz et al., 2015). It is difficult to explain the mechanism of the increase of DPOAE amplitudes during the first sensitive exposure window. To the best of our knowledge, a similar event has not been described either in animals or in humans. In spite of various molecular studies concerning PCB toxicity, the mechanism of how PCBs affect outer hair cells is still unknown or speculative. Some analogy represent the data on infants perinatally exposed to dioxins. Magnetoencephalography and electroencephalography in 41 healthy 7–12 year old children with documented perinatal dioxin exposure, revealed the first (N2a) and second (N2b) motion component yielding an increased amplitude effect, suggesting a developmental maturation delay of several years (ten Tusscher, 2002). A parallel to our observation represent data on alleviation of the ototoxic effect, documented by DPOAE amplitude decrease, by coexposure to another toxic agent. The ototoxicity of PCB exposure in rats appeared to be attenuated by coexposure to MeHg (Powers et al., 2009), demonstrating the great plasticity of the sensorineural hearing. Finally, PCBs behave in the human body as an endocrine disruptor (Bell, 2014; Parent et al., 2011) exhibiting antiestrogenic effect (Plísková et al., 2005) that may interfere with development of auditory system (Al-Mana et al., 2008).
The conclusions of the current study apparently contradict those from Jusko et al. (2014) in which we stated that maternal serum levels and cord blood levels did not affect auditory function. This contradiction may be a result of pooling the data in a different way in the previous and the current study. While previously (Jusko et al., 2014) we computed hearing outcomes for all children pooling data for both sexes, both ear sides and 11 frequencies, currently we calculated correlations separately for boys, girls, right and left ears and 5 frequencies. The discrepancy however appears to be less pronounced when considering data in Table 3 of Jusko et al., 2014. It is showing regression coefficients for the relationship between cord and maternal serum and DPOAE amplitudes with a positive sign, indicating amplitude increase, however not reaching statistical significance.
The pathophysiology of currently observed auditory deficiencies is unclear. Animal studies have shown that developmental exposure to PCBs causes temporary hypothyroxinemia (Goldey et al., 1995; Morse et al., 1996; Poon et al., 2011) leading to deficits in the development of the cochlea. However, with regard to different timing of development of cochlea in rats and humans, this mechanism in humans may be questioned (Crofton and Zoeller, 2005). Even the role of interaction with the aryl hydrocarbon receptor (AHR) is not fully understood at present. DPOAEs were not affected following TCDD exposure in any of the mouse strains studied (C57BL6, BalbC, and CBA). While TCDD exposure did not affect outer hair cell function the c57Bl/6 mice, carrying the Ah b-1subtype of the high-affinity Ah receptor, when prenatally exposedto TCDD exhibited significant ABR threshold shifts, with no concomitant change in DPOAE responses or cochlear structure (Safe and Luebke, 2016).
We described the sexual dimorphism in DPOAEs generation and its asymmetry in Slovak adolescents previously (Pavlovčinová et al., 2010). The background DPOAE amplitudes were higher in girls and on the right side. This however does not predict the side and gender differences in sensitivity towards chemical toxicants. Nevertheless, the relative resistance of DPOAEs originating in right ears of females against PCB exposure may be in line with this observation.
In the literature there is much information on asymmetry in noise-induced hearing loss (NIHL) (Nageris et al., 2007; Dobie, 2014) consistent with physiological differences as the primary cause of asymmetric hearing loss, with greater susceptibility to NIHL in the left ear of men (Berg et al., 2014). Data on asymmetry in ototoxic effect of environmental or industrial chemicals are scarce. Merely, a study of a PCB-exposed population in the Faroe Islands found increased hearing thresholds at 250 Hz and 12,000 Hz associated with higher prenatal PCB exposure, but only in the left ear (Grandjean et al., 2001). A clear side effect, observed in our study, has not been yet reported (EU-OSHA, 2009 – European Agency for Safety and Health at Work). In the extant literature, we have only been able to locate asymmetry in ototoxicity for cisplatin, preferring left ears (Schmidt et al., 2008).
Our data revealed a relation between the tonotopicity and ear side. From Fig. 1 it can be seen that in left ears the lower frequencies, 1189 and 1682 Hz, were impacted, while in right ears the middle frequencies, 3364, 4757 and 5657 Hz. High frequencies lead to maximum vibrations at the basal end of the cochlear coil, whereas low frequencies lead to maximum vibrations at the apical end of the cochlear coil. For some ototoxic compounds the base of the cochlea is more vulnerable to trauma than the apex, as seen in the pattern of hair cell damage by cisplatin or aminoglycosides (Sha et al., 2001). On the other hand, exposure to styrene caused outer hair cell losses in the apical cochlear region, which discriminates low frequencies (Venet et al., 2015). In rats exposed to PCBs, DPOAE deficits were observed at all. frequencies examined (2–12 kHz) (Powers et al., 2006). On the contrary, developmental exposure to A1254 in rats in earlier studies has been shown to result in low-frequency hearing loss (Crofton et al., 2000; Goldey et al., 1995; Herr et al., 1996).
We have observed much similarity in ototoxic behavior of organochlorinated pesticides (OPs) and PCB 153 (Sisto et al., 2015). With regard to the wide-spread presence of OPs and PCBs in the environment, the question arises on relative toxic potency of these pollutants. It follows from a recently published review (Mrema et al., 2013) that the adverse effects of the OPs are extremely complex and still unrecognized. We consider the present data on their ototoxicity as preliminary, needing confirmation in an animal model.
In conclusion we state that compared with previous studies on ototoxic effect of PCBs in humans (Trnovec et al., 2008, 2010; Jusko et al., 2014; Min et al., 2014; Sisto et al., 2015) in the current analysis we paid attention to hearing response in PCB exposed subjects in relation to three important factors: tonotopicity, sex, and ear side. Such approach, in combination with an attempt to assess critical exposure and outcome windows in time-to-event analysis, is contributing to better understanding the mechanism of the ototoxic effect of PCBs.
However it should be stressed that any kind of statistically significant relationship between sensory neural hearing response, positive or negative, and exposure to an environmental toxicant is evidence of a rude intervention into a closed, still only partly understood, extremely fine and intricate hearing process.
5. Conclusions
We have confirmed and extended our previous observation on existence of two sensitive windows for perinatal environmental exposure to PCBs. The first critical window is linked to prenatal exposure period and is marked by DPOAE amplitudes increase, whilst the second one is related to peaking PCB serum levels during breast feeding and is characterized by amplitude decrease. Differences were observed between the response pattern in boys and girls and ear sides. Likewise the response exhibited frequency specificity. Our data stress the importance of both prenatal and postnatal exposure to organochlorines for hearing impairment.
HIGHLIGHTS.
Amplitudes of DPOAE were used as a marker of cochlear status in children.
Relations between DPOAEs and perinatal exposures to organochlorines were confirmed.
Prenatal exposure to PCB 153 was linked to increased DPOAE amplitudes.
Regressions with postnatal PCB exposures indicated poorer DPOAE performance.
Any association of serum organochlorines with DPOAE pattern is considered harmful.
Acknowledgments
Funding
This work was supported by the National Institutes of Health grants R01 CA096525, R03 TW007152, P30 ES001247, and K12 ES019852; Slovak Research and Development Agency grants APVT-21-016804, APVV-0571-12, APVV-0444-11, SK-IT-0040-08.
Footnotes
Competing interests
The authors have declared that no competing interests exist.
References
- Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Hormones and the auditory system: a review of physiology and pathophysiology. Neuroscience. 2008;153:881–900. doi: 10.1016/j.neuroscience.2008.02.077. [DOI] [PubMed] [Google Scholar]
- Bell MR. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr Opin Pharmacol. 2014;19:134–144. doi: 10.1016/j.coph.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RL, Pickett W, Linneman JG, Wood DJ, Marlenga B. Asymmetry in noise-induced hearing loss: evaluation of two competing theories. Noise Health. 2014;16:102–107. doi: 10.4103/1463-1741.132092. [DOI] [PubMed] [Google Scholar]
- Bergonzi R, De Palma G, Specchia C, Dinolfo M, Tomasi C, Frusca T, Apostoli P. Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators of fetal growth and health. Sci Total Environ. 2011;409:2888–2893. doi: 10.1016/j.scitotenv.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Health effects of persistent organic pollutants: the challenge for the Pacific Basin and for the world. Rev Environ Health. 2011;26:61–69. doi: 10.1515/reveh.2011.009. [DOI] [PubMed] [Google Scholar]
- Conka K, Drobná B, Kočan A, Petrik J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organo-chlorine pesticides in human serum. J Chromatogr A. 2005;1084:33–38. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Widholm JJ, Crofton KM, Schantz SL. Perinatal exposure to Aroclor 1254 impairs distortion product otoacoustic emissions (DPOAEs) in rats. Toxicol Sci. 2002;68:458–464. doi: 10.1093/toxsci/68.2.458. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development hearing lossresulting from exposure to PHAHs. Crit Rev Toxicol. 2005;35:757–769. doi: 10.1080/10408440591007304. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Ding DL, Padich R, Taylor M, Henderson D. Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear Res. 2000;144:196–204. doi: 10.1016/s0378-5955(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Dobie RA. Does occupational noise cause asymmetric hearing loss? Ear Hear. 2014;35:577–579. doi: 10.1097/AUD.0000000000000043. [DOI] [PubMed] [Google Scholar]
- EU-OSHA – European Agency for Safety and Health at Work. Combined Exposure to Noise and Ototoxic Substances. 2009 doi: 10.2802/16028. [DOI]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H, Ellefsen P, Budtz-Jørgensen E, Keiding N, White RF. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Bellinger D, Bergman A, Cordier S, Davey-Smith G, Eskenazi B, Gee D, Gray K, Hanson M, van den Hazel P, Heindel JJ, Heinzow B, Hertz-Picciotto I, Hu H, Huang TT, Jensen TK, Landrigan PJ, McMillen IC, Murata K, Ritz B, Schoeters G, Skakkebaek NE, Skerfving S, Weihe P. The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102:73–75. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Hadjab S, Maurel D, Cazals Y, Siaud P. Hexachlorobenzene, a dioxin-like compound, disrupts auditory function in rat. Hear Res. 2004;191:125–134. doi: 10.1016/j.heares.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6:55–64. doi: 10.1017/S2040174414000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr D, Goldey ES, Crofton KM. Developmental exposure to Aroclor 1254 produces low-frequency alterations in adult rat brainstem auditory evoked responses. Fundam Appl Toxicol. 1996;33:120–128. doi: 10.1006/faat.1996.0149. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kočan A, Charles MJ, Čižnár P, Langer P, Šovčíková E, James R. PCBs and early childhood development in Slovakia: study design and background. Fresenius Environ Bull. 2003;12:208–214. [Google Scholar]
- Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol. 1970;92:311–324. doi: 10.1001/archotol.1970.04310040005002. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Sisto R, Iosif AM, Moleti A, Wimmerová S, Lancz K, Tihányi J, Sovčíková E, Drobná B, Palkovičová L, Jurečková D, Thevenet-Morrison K, Verner MA, Sonneborn D, Hertz-Picciotto I, Trnovec T. Prenatal and postnatal serum PCB concentrations and cochlear function in children at 45 months of age. Environ Health Perspect. 2014;122:1246–1252. doi: 10.1289/ehp.1307473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kočan A, Petrik J, Drobná B, Chovancová J. Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. I Blood Chemosphere. 1994;29:2315–2325. doi: 10.1016/0045-6535(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Lancz K, Hertz-Picciotto I, Jusko TA, Murínová L, Wimmerová S, Sovčíková E, Dedík L, Strémy M, Drobná B, Farkašová D, Trnovec T. Duration of breastfeeding and serum PCB 153 concentrations in children. Environ Res. 2015;136:35–39. doi: 10.1016/j.envres.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MC, Wu HP, Yang CY, Chen PC, Lambert GH, Leon Guo Y. Gestational exposure to polychlorinated biphenyls and dibenzofurans induced asymmetric hearing loss: Yucheng children study. Environ Res. 2015;137:65–71. doi: 10.1016/j.envres.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, van der Ven LT, Piersma AH, Vos JG. Effects of the brominated flame retardant hexabromocyclododecane (HBCD) on dopamine-dependent behavior and brainstem auditory evoked potentials in a one-generation reproduction study in Wistar rats. Toxicol Lett. 2009;185:63–72. doi: 10.1016/j.toxlet.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Murcia M, Iñiguez C, Vizcaino E, Llop S, Vioque J, Grimalt JO, Rebagliato M, Ballester F. Prenatal exposure to organo-chlorine compounds and birth size. Pediatrics. 2011;128(1):e127–34. doi: 10.1542/peds.2010-1951. [DOI] [PubMed] [Google Scholar]
- Min JY, Kim R, Min KB. Serum polychlorinated biphenyls concentrations and hearing impairment in adults. Chemosphere. 2014;102:6–11. doi: 10.1016/j.chemosphere.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol Appl Pharmacol. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Nageris BI, Raveh E, Zilberberg M, Attias J. Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol. 2007;28:434–437. doi: 10.1097/mao.0b013e3180430191. [DOI] [PubMed] [Google Scholar]
- Palanza P, Nagel SC, Parmigiani S, Vom Saal FS. Perinatal exposure to endocrine disruptors: sex, timing and behavioral endpoints. Curr Opin Behav Sci. 2016;7:69–75. doi: 10.1016/j.cobeha.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS, Naveau E, Gerard A, Bourguignon JP, Westbrook GL. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus and cerebral cortex. J Toxicol Environ Health B Crit Rev. 2011;14:328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patayová H, Wimmerová S, Lancz K, Palkovičová L, Drobná B, Fabišiková A, Kováč J, Hertz-Picciotto I, Jusko TA, Trnovec T. Anthropometric, socioeconomic, and maternal health determinants of placental transfer of organochlorine compounds. Environ Sci Pollut Res Int. 2013;20:8557–8566. doi: 10.1007/s11356-013-1786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovčinová G, Jakubíková J, Trnovec T, Lancz K, Wimmerová S, Sovcíková E, Palkovicová L. A normative study of otoacoustic emissions, ear asymmetry, and gender effect in healthy schoolchildren in Slovakia. Int J Pediatr Otorhinolaryngol. 2010;74:173–177. doi: 10.1016/j.ijporl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Plísková M, Vondrácek J, Canton RF, Nera J, Kocan A, Petrík J, Trnovec T, Sanderson T, van den Berg M, Machala M. Impact of polychlorinated biphenyl contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Powers BE, McAlonan RM, Ferguson DC, Schantz SL. Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicol Sci. 2011;124:161–168. doi: 10.1093/toxsci/kfr214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Poon E, Sable HJ, Schantz SL. Developmental exposure to PCBs, MeHg, or both: long-term effects on auditory function. Environ Health Perspect. 2009;117:1101–1107. doi: 10.1289/ehp.0800428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Widholm JJ, Lasky RE, Schantz SL. Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicol Sci. 2006;89:415–422. doi: 10.1093/toxsci/kfj051. [DOI] [PubMed] [Google Scholar]
- Safe TM, Luebke AE. Prenatal low dosage dioxin (TCDD) exposure impairs cochlear function resulting in auditory neuropathy. Hear Res. 2016;331:7–12. doi: 10.1016/j.heares.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CM, Knief A, Lagosch AK, Deuster D, Am Zehnhoff-Dinnesen A. Left-right asymmetry in hearing loss following cisplatin therapy in children–the left ear is slightly but significantly more affected. Ear Hear. 2008;29:830–837. doi: 10.1097/AUD.0b013e31818005a4. [DOI] [PubMed] [Google Scholar]
- Selevan S, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Epidemiology. 2000;11:S72. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Sisto R, Moleti A, Palkovičová Murínová Ľ, Wimmerová S, Lancz K, Tihányi J, Čonka K, Šovčíková E, Hertz-Picciotto I, Jusko TA, Trnovec T. Environmental exposure to organochlorine pesticides and deficits in cochlear status in children. Environ Sci Pollut Res Int. 2015;22:14570–14578. doi: 10.1007/s11356-015-4690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnovec T, Sovčíková E, Hust’ák M, Wimmerová S, Kočan A, Jurečková D, Langer P, Palkovičová L, Drobná B. Exposure to polychlorinated bi-phenyls and hearing impairment in children. Environ Toxicol Pharmacol. 2008;25:183–187. doi: 10.1016/j.etap.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Trnovec T, Sovčíková E, Hust’ák M, Wimmerová S, Kočan A, Jurečková D, Langer P, Palkovičová L, Drobná B. Serum PCB concentrations and cochlear function in 12-year-old children. Environ Sci Technol. 2010;44:2884–2889. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnovec T, Dedík L, Jusko TA, Lancz K, Palkovičová L, Kočan A, Šovčíková E, Wimmerová S, Tihányi J, Patayová H, Hertz-Picciotto I. Assessment of exposure to PCB 153 from breast feeding and normal food intake in individual children using a system approach model. Chemosphere. 2011;85:1687–1693. doi: 10.1016/j.chemosphere.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Tusscher GW. Neurodevelopmental Influences of Perinatal Dioxin Exposure as Assessed with Magnetoencephalography, Electroencephalography, Psychological and Neuromotor Tests. PhD thesis. 2002 http://hdl.handle.net/11245/1.198869.
- Venet T, Campo P, Thomas A, Cour C, Rieger B, Cosnier F. The tonotopicity of styrene-induced hearing loss depends on the associated noise spectrum. Neurotoxicol Teratol. 2015;48:56–63. doi: 10.1016/j.ntt.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Støvring H, Kristensen SL, Halldorsson TI, Rantakokko P, Kiviranta H, Ernst EH, Toft G. In utero exposure to persistent organochlorine pollutants and reproductive health in the human male. Reproduction. 2014;148:635–646. doi: 10.1530/REP-13-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]