Introduction
The primate visual system is functionally described in terms of two parallel processing pathways: the ventral “what” stream (form and color), and the dorsal “where” stream (motion and depth) (Livingstone & Hubel, 1988; Mishkin et al., 1983; Ungerleider & Haxby, 1994; Ungerleider & Mishkin, 1982; but see Merigan & Maunsell, 1993). Many aspects of the ventral visual system have been examined developmentally via neuroimaging studies comparing children and adults, such as object processing (Gathers et al., 2004, Golarai et al., 2007, 2010; Scherf et al., 2007), face processing (Aylward et al., 2005; Gathers et al., 2004; Golarai et al., 2007, 2015; Joseph et al., 2011; Passarotti et al., 2003), and visual word processing (Ben-Shachar et al., 2011; Brem et al., 2009, 2010; Martin et al., 2015; Olulade et al., 2013a; Schlaggar et al., 2002; Turkeltaub et al., 2003). Yet relatively little is known about the neural bases of the typical developmental trajectory of dorsal stream function, specifically those underlying coherent motion perception. An important first step is to apply the same approach used to characterize the neural substrates of coherent motion perception in adults (Braddick et al., 2001; Dupont et al., 1994; McKeefry et al., 1997; Paradis et al., 2000; Sunaert et al., 1999; Watson et al., 1993) to the study of children. This information will not only provide an understanding of the role that experience and development have in dorsal stream function (especially area V5/MT), but it will also provide a foundation upon which to understand a number of developmental disorders that have been associated with dorsal stream vulnerability, such as autism, Williams syndrome, and developmental dyslexia (Atkinson et al., 1997; Atkinson & Braddick, 2011; Boets et al., 2011; Braddick et al., 2003; Grinter et al., 2010; Milne et al., 2002; Pellicano & Gibson, 2008; Spencer et al., 2000; Stein, 2001).
Behavioral studies have investigated age-dependent changes for coherent motion perception. For example, Boets and colleagues (2011) reported the amount of coherence needed to detect direction of motion to be lower in adults than children and found that detection thresholds decreased in a group of children followed longitudinally from 5 to 6 years of age. From studies in older children, it has also been shown that coherent motion detection thresholds decrease (improve) with increasing age until children are 11 years (Gunn et al., 2002) or 12-14 years (Hadad et al., 2011) of age, at which point they reach adult levels. Gunn and colleagues (2002) examined dorsal stream motion coherence sensitivity together with ventral stream form coherence sensitivity in a large cross-sectional study of typical children (4-11 years of age) and adults. The motion coherence stimulus consisted of two adjacent random dot kinematograms, one of which was segregated into three horizontal strips, such that the varying proportion of coherently moving dots in the middle strip moved opposite to the coherent motion of the upper and lower strips. The form coherence stimulus was a static array of randomly oriented short line segments within which a target region on one side of the display contained a varying proportion of segments that were oriented to form static concentric circles. The investigators found age-dependent differences in performance for both tasks. They also showed a relative delay in development of motion coherence compared to form coherence in reaching adult-like levels. To ensure that these findings could not be attributed to differences in the stimuli, Atkinson and Braddick (2005) measured perception of motion and form coherence again, this time using visual stimuli that were more closely matched and found similar age-dependent differences as originally described by Gunn et al. (2002). A similar result was also found in another group of children examined as part of a brain imaging study (5-12 years), showing that improvement with age for global form occurs earlier, relative to global motion (Braddick et al, 2016). Taken together, despite varying methods and stimulus parameters (especially the spatial and temporal parameters of the stimulus; for review, see Hadad et al., 2015), there is a developmental curve for the perception of coherent motion. As such, one would expect differences in brain function for this task when comparing primary school-age children with adults.
The neurophysiology of motion processing development has been investigated with electroencephalography (EEG). For example, in a study comparing global motion processing between infants (4-5 months old) and adults, Wattam-Bell et al. (2010) observed a more lateral response in infants, whereas adults exhibited a more medial response to visual motion. Further work by these investigators suggests, however, that development of visual motion processing may not simply be a matter of changes in functional activation within visual cortex, but may be a matter of a developmental increase in communication between extrastriate and striate cortices (Wattam-Bell et al. 2012). With regards to the relationship between dorsal and ventral stream processing in development, Mitchell and Neville (2004) investigated visually-evoked event-related potentials (ERPs) in children, aged 6-7 and 8-10 years, and adults, and found that ventral stream ERPs in response to color stimuli differed minimally between subject groups (decreased amplitude with age), while dorsal stream ERPs in response to motion stimuli exhibited marked differences in wave shape, amplitude and latency with age. Similarly, Coch and colleagues (2005) found that ERPs in response to visual motion significantly differed between children (6-8 years old) and adults, whilst color processing did not. Together, these electrophysiological findings suggest that dorsal stream development is protracted, going into late childhood, and it has been proposed that this long period of development renders the dorsal stream more vulnerable to disorders of development in comparison to the ventral stream (Atkinson, 2017; Atkinson & Braddick 2011; Braddick et al., 2003, 2016; Stevens & Neville, 2006).
Further, studies of motion perception have utilized functional magnetic resonance imaging (fMRI). Klaver and colleagues (2008) used whole-brain and region of interest (ROI) approaches (using regions created from a prior study in adults; Murray et al., 2003) to compare children’s (5–6 years old) and adults’ responses to two different kinds of visual motion stimuli: random motion and shape-from-motion (in which contours defined by moving dots create a shape percept). For random visual motion (compared to static dots), there were no significant differences in activation between the two groups in the whole-brain analysis, but there were differences for the ROI analyses (left and right hemisphere ROIs were combined): adults had significantly greater activation compared to children in area V3a, with a similar effect trending toward significance in area V5/MT. When examining activation in response to the other motion stimulus, shape-from-motion (compared to random motion), in the same bilateral ROIs, the investigators found greater activation in parietal cortex in the same sample of adults compared to children. However, children showed greater activation in V5/MT compared to adults, reflecting an age-related decrease in V5/MT response to shape-from-motion perception. Further, in a study that utilized both EEG and fMRI, Bucher and colleagues (2006) compared ERPs and functional activation in adolescents (15-17 years old) and adults (20-30 years old) during a shape-from-motion task. While the investigators did not find a between-group difference in activation patterns, there was a delay in the adolescent group’s N1 component (attributed to area V5/MT). This suggests that the network of regions supporting visual motion processing may be established by adolescence, but that fine-tuning of the response characteristics continues to change developmentally. Overall, these findings suggest that the dorsal stream exhibits developmental changes in activation specific to different types of visual motion stimuli (random motion and shape-from-motion) and in different regions. However, despite its common usage in publications of adult participants (Aspell et al., 2005; Braddick et al., 2000, 2001; Cornette et al., 1998; Huk et al., 2002; Nakamura et al., 2003; Paradis et al. 2000; Watson et al., 1993; Wilms et al., 2005), coherent motion perception has not yet been investigated in elementary school-aged children with fMRI.
An important aspect of developmental studies is the difficulty of disentangling brain development from experience, especially those experiences afforded by formal education. In the last decade, it has become clear that visual system function is altered by the introduction of cultural activities such as learning to read. Behavioral work, for example, has shown that the acquisition of literacy can affect performance of early visual system function. Specifically, Szwed and colleagues (2012) compared age- and socioeconomically-matched adult literates, illiterates, and ex-illiterates (who learned to read as adults) on a task of contour integration and found that the illiterate adults’ performance was worse than both literate groups. From this the authors concluded that the extensive visual training undertaken during literacy acquisition not only influences higher-level visual processing in the ventral stream (as demonstrated in a separate study by the same investigators showing functional reorganization in object processing areas; Dehaene et al., 2010), but also results in marked changes in behavioral performance for low-level visual perception. The anatomical and physiological impact exerted by the formal learning of reading and writing has been shown to manifest in multiple brain regions, including visual, auditory, and motor areas (Carreiras et al., 2009; Dehaene et al., 2010). Finally, it has been suggested, based on a behavioral study of coherent motion detection in children, that learning to read in typical readers "mobilizes" the dorsal visual system (Boets et al., 2011). As such, a significant challenge is to determine whether the differences in dorsal visual stream function that are observed between children and adults are due to maturity or reading experience.
Of note is that the dorsal visual motion processing pathway has been described as “vulnerable” (Atkinson, 2017; Braddick et al., 2003), with altered performance attributed to this pathway observed in a range of developmental disorders such as autism (Spencer et al., 2000) and the reading disability developmental dyslexia (for review, see Stein, 2001). Notably, dyslexia has been associated with difficulties in tasks reliant on the dorsal steam, such as motion perception (Cornelissen et al., 1998; Hansen et al., 2001; Wilmer et al., 2004; Witton et al., 1998); and brain activity in area V5/MT during visual motion perception has been shown to be lower for adults (Demb et al., 1998; Eden et al., 1996) and children (Olulade et al., 2013b) with dyslexia compared to age-matched normal readers. However, it is unclear if the association between visual motion perception and reading ability is causal or consequential, a question that would benefit from greater knowledge of brain activity in area V5/MT, and its connectivity with other regions, in typically reading children.
The present study builds on prior work using fMRI to examine differences between children and adults in brain activity underlying visual motion processing of random and shape-from-motion stimuli (Klaver et al., 2008), and extends it to coherent motion processing. The present study also includes a longitudinal component to investigate the nature of visual area V5/MT activity and functional connectivity in early elementary school readers, thereby building on the behavioral study by Boets and colleagues (2011). Our first study utilized a cross-sectional design to compare children with adults, while our second study tracked a group of children longitudinally from 2nd to 3rd grade. In both studies, we examined activity within area V5/MT and functional connectivity between V5/MT and other brain regions using a coherent dot motion detection task contrasted with a static dot density detection task. In both studies, we asked if being older was associated with greater activity in and/or connectivity with area V5/MT. In the longitudinal study, we also examined the relationship of these measures with reading proficiency by testing for a relationship between the degree of change of both V5/MT activity and reading ability during children’s development over one year; and additionally, by testing for the predictive strengths of V5/MT activity for reading ability one year later.
Our analysis approach used both whole-brain and ROI analyses to allow comparison to the published literature. While the ROI approach is well-suited to our question given the reliability of the V5/MT signal in response to motion, the absence of pediatric studies of coherent visual motion in the published literature means that coordinates drawn from the literature are derived from adult data, as they were in Klaver at al., 2008. This leaves open the question of whether any differences between children and adults in a ROI analysis are driven by activity in the same brain areas, with a lesser degree of activity in children; or if they are the result of the ROI being placed in the optimal location of V5/MT for adults, while being unsuitably placed for pediatric V5/MT activity. As such, we examined activity not only within a ROI based on the adult published literature, but also by identifying and quantifying area V5/MT activation in each subject individually. Together these approaches should provide comprehensive characterization of the developmental differences of the dorsal visual motion-processing stream, especially area V5/MT, during coherent motion perception.
Materials and Methods
Participants
All participants were healthy, monolingual, right-handed, native speakers of English. The Cross-Sectional Study was based on 28 participants, 13 children (5 female; age range 7.0-8.4 years; avg. age 7.7 years) and 15 adults (7 female; age range 18.7-28.8 years; avg. age 22.5 years; see Table 1). The Longitudinal Study involved 17 children who had just completed 2nd grade (7 female; age range 7.9-9.3 years; avg. age 8.3 years at the first Time Point, T1, of the study) and who were re-tested one year later (T2). However, five subjects from this Longitudinal Study were excluded at the data analysis stage because of excessive head movement at either or both time points and the final sample thus consisted of 12 children (5 females; age range: 7.9-9.3 years; mean age: 8.4 years at T1; see Table 2). Demographic and behavioral information for the final groups are summarized in Tables 1 and 2.
Table 1.
Demographics, Neuropsychological Measures and In-Scanner Performance for the Cross-Sectional Study
| Children | Adults | p value | |
|---|---|---|---|
| N | 13 | 15 | |
| Sex (female/male) | 5/8 | 7/8 | |
| Age | 7.7 ± 0.4 | 22.5 ± 2.8 | |
| IQ | 122 ± 11 | 125 ± 7 | n.s. |
| Word Identification – SS | 116 ± 6* | 113 ± 5† | n.s. |
| Word Attack – SS | 117 ± 7* | 107 ± 6† | 0.003 |
| Mean Inter-Scan Displacement (mm) | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.012 |
| Motion Accuracy (%) | 94 ± 5 | 99 ± 1 | 0.001 |
| Static Accuracy (%) | 98 ± 3 | 100 ± 0 | n.s. |
| Motion - Static accuracy (%) | −5 ± 6 | −1 ± 1 | 0.03 |
| Motion reaction time (ms) | 1449 ± 315 | 1035 ± 335 | <0.001 |
| Static reaction time (ms) | 1081 ± 167 | 831 ± 163 | 0.002 |
| Motion - Static reaction time (ms) | 368 ± 299 | 204 ± 255 | n.s. |
SS – Standard Score
± standard deviation
WRMT-R
WJ III
Statistical significance: p < 0.05
Table 2.
Demographics, Neuropsychological Measures and In-Scanner Performance for the Longitudinal Study
| T1 | T2 | p value | |
|---|---|---|---|
| N | 12 | ||
| Sex (female/male) | 5/7 | ||
| Age | 8.4 ± 0.4 | 9.1 ± 0.3 | <0.001 |
| IQ | 119 ± 12 | ||
| Word Identification - SS* | 118 ± 10 | 114 ± 9 | 0.002 |
| Word Identification - RS* | 68 ± 10 | 72 ± 9 | 0.006 |
| Word Attack - SS* | 117 ± 12 | 115 ± 14 | n.s. |
| Word Attack - RS* | 30 ± 6 | 32 ± 7 | n.s. |
| Reading Fluency - SS | 113 ± 13 | 116 ± 13 | n.s. |
| Reading Fluency - RS | 39 ± 12 | 48 ± 12 | <0.001 |
| CTOPP-Elision - SS | 117 ± 6 | 114 ± 10 | n.s. |
| CTOPP-Elision - RS | 17 ± 1 | 17 ± 2 | n.s. |
| Mean Inter-Scan Displacement (mm) | 0.3 ± 0.2 | 0.2 ± 0.1 | n.s. |
| Motion Accuracy (%) | 91 ± 15 | 96 ± 5 | n.s. |
| Static Accuracy (%) | 97 ± 4 | 97 ± 3 | n.s. |
| Motion - Static accuracy (%) | −6 ± 12 | −1 ± 5 | n.s. |
| Motion reaction time (ms) | 1244 ± 282 | 1120 ± 231 | n.s. |
| Static reaction time (ms) | 978 ± 223 | 945 ± 140 | n.s. |
| Motion - Static reaction time (ms) | 267 ± 271 | 175 ± 209 | n.s. |
SS – Standard Score
RS – Raw Score
± standard deviation
WRMT
Statistical significance: p < 0.05
All participants underwent a behavioral battery that included the Wechsler Abbreviated Scale of Intelligence (IQ; Wechsler, 1999). Reading was evaluated using the Word Identification and Word Attack subtests to measure single real and pseudoword reading accuracy, respectively. For the Cross-Sectional Study, these subtests were measured in adults using the Woodcock-Johnson III Tests of Achievement (WJ III; Woodcock et al., 2001), and in children using the Woodcock Reading Mastery Test-Revised (WRMT-R; Woodcock, 1998). Since both are standardized and similar in nature, direct comparison is possible. The WRMT-R was used for the children in the Longitudinal Study. Additional reading measures for the Longitudinal Study included reading rate, using the Reading Fluency subtest of the WJ III Tests of Achievement (Woodcock et al., 2001), and phonemic awareness, measured via the Elision subtest of the Comprehensive Test of Phonological Processing (CTOPP-Elision; Wagner et al., 1999). To be included in either of the studies, all participants had to have PIQ, VIQ, Word ID, and Word Attack standard scores above 85. All subjects had greater than 37 weeks gestation, no major complications at birth, and no history of a learning disability, ADHD, neurological, or psychiatric disorders. The adult and pediatric groups in the Cross-Sectional Study were matched on IQ and reading (Word ID) standard scores.
The adults and children from the Cross-Sectional Study were drawn retrospectively from studies in the laboratory, with three of the 13 children in the Cross-Sectional Study also serving in the Longitudinal Study. Data for some participants was also included in a previously published report focused on dyslexia (Olulade et al., 2013b).
fMRI Task and Data Acquisition
fMRI Task
All participants received a training session before entering the MRI system to acquaint them with the tasks and give them an opportunity to perform and practice the tasks. As part of the training, the experimenter informed all participants about what to expect during the scanning session (emphasizing the importance of lying still throughout all scans) and had participants practice the two tasks on a computer. For children, this was followed by a session in which they lay inside a mock scanner where they were exposed to simulated scanner noises as they performed the tasks. Adults did not use the mock-scanner and simply practiced the tasks at a computer. Following this training, all participants entered the real MRI system and performed the task during two fMRI “runs”, each lasting 4 minutes and 27 seconds, as described below. Following the completion of these two runs, the subjects participated in a 6-minute structural scan (while watching a movie) and were then taken out of the scanner for a break.
We used a coherent visual motion detection task previously shown to activate area V5/MT of the dorsal stream (Olulade et al., 2013b). For the active task, Motion, participants viewed low-contrast, random dot kinematograms consisting of gray dots moving at a constant speed on a black background. While most dots moved randomly, with their direction changing constantly (left, right, up, down, or diagonally), a subset of dots (40%) moved coherently in either the left or right horizontal direction. Participants were asked to indicate the direction of the perceived coherent motion (towards the left or right) via button press (e.g., left-thumb button press for leftward motion), while maintaining fixation on a central cross. The overall stimulus was composed of 400 dots, with each dot moving at a constant speed of 3 deg/sec.
For the active control task, Static, participants performed a density judgment task during which static dots were presented on the screen with densities differing between the left and right visual fields (density differences ranged between 35% and 65%). Participants were asked to maintain fixation on a central cross and indicate via button press with the left or right thumb on which side of the screen the dot density was greater.
For both the coherent Motion and the Static conditions, stimuli were presented for 3 sec followed by a crosshair for 1.2 sec, with 10 stimuli presentations per block. Alternating blocks of Static and Motion stimuli (42 sec/block) were separated by blocks of Fixation (21 sec). Fixation consisted of a single crosshair presented at the center of the visual field. One run consisted of two blocks each of the Static and Motion conditions. Order of alternating Static and Motion blocks was the same across the two runs and across all subjects, with the Static task occurring first (after an initial 21 sec of Fixation). Each run began and ended with an additional 9 sec and 6 sec of Fixation, respectively, which were not included in analysis. Two runs per participant were entered into the analysis for both the Cross-Sectional and the Longitudinal Studies.
fMRI Task Performance Measures
Measures of in-scanner accuracy and reaction time were recorded for the Motion and Static tasks and compared between them (Motion > Static), thereby following the approach used for the fMRI data analysis. These behavioral measures were compared between the two groups (adults vs. children) in the Cross-Sectional Study using a two-sample t-test, and between the two time points (T1 vs. T2) in the Longitudinal Study using a paired t-test. For those comparisons for which accuracy or reaction time for the contrast of interest (Motion > Static) were significantly different, the behavioral data was included as a covariate of no interest in our fMRI between-group comparisons.
fMRI Data Acquisition
Images were acquired on a 3T Siemens Trio Scanner located at the Center for Functional and Molecular Imaging at the Georgetown University Medical Center. For each functional run, 89 images consisting of 50 contiguous axial slices (2.8 mm thickness with 0.2 mm interslice gap) covering the whole brain were acquired with the following parameters: Flip angle = 90°, TR = 3 sec, TE = 30 msec, FOV = 192 mm, in-plane resolution = 64 x 64, voxel size = 3 mm x 3 mm x 3 mm.
fMRI Data Analysis
Preprocessing
All fMRI data pre-processing and statistical analyses were carried out using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). The same pre-processing procedures were applied to data from both the Cross-Sectional and the Longitudinal Studies. After removing the first three scans of each run to account for T1 saturation effects, each subject’s fMRI data were slice-time corrected and realigned to the mean image. A magnetization-prepared rapid gradient-echo (MPRAGE) sequence structural scan acquired during the same scanning session was then co-registered to the mean functional image; structural scans were warped and segmented into gray matter, white matter, and CSF using the VBM8 Toolbox; and functional and structural images were warped to standard MNI stereotaxic space and smoothed with a Gaussian kernel of full width at half maximum 8 mm (see below for lesser smoothing for single-subject data analysis).
Head Movement
Data were examined for head movement artifacts. Participants whose overall data exhibited excessive head movement (>20% of images in the run exhibiting scan-to-scan movement beyond the 1.5 mm threshold) were entirely removed from the study. Even after this removal in the quality control process, children exhibited significantly greater head movement than adults (mean inter-scan displacement, two-sample t-test, p < 0.05, see Table 1). As such, six rigid-body head movement parameters and global signal were used as regressors of no interest in the fMRI analyses.
Given the risk of identifying differences in fMRI signal between children and adults because of head movement-related artifacts in the pediatric group, we took further steps at the second-level analysis (see below) to characterize the relationship between head movement and brain activity. Specifically, we searched for significant correlations between subjects’ inter-scan head movement over the duration of the runs with whole brain activity for the contrast of Motion > Static. This was carried out for the children and adults as a way to determine if brain activity during our task of interest was in any way related to the amount of head movement made during scanning (since it is possible that head movement was evoked during the task in children to a greater degree than in adults).
First-Level Analysis
Data were analyzed for each subject using the general linear model. Statistical analysis at the first level involved generating within-subject parametric activation maps for the contrasts of the Motion and Static conditions relative to baseline (Motion > Fixation, Static > Fixation) and for the direct contrast between the two conditions (Motion > Static). Stimulus onsets were modeled using the canonical SPM hemodynamic response function, and functional datasets were high-pass filtered with a cutoff of 128 sec. Second-level statistical analysis differed between the two studies, Cross-Sectional and Longitudinal, as outlined below.
Second-Level Analysis
The single-subject statistical maps for the voxel-wise contrast of Motion > Static from the first-level analysis were submitted to a one-sample t-test at the second level to generate a group map for each group: adults and children (Cross-Sectional Study), and children at T1 and T2 (Longitudinal Study). For between-group or between-time point comparisons at the level of the whole brain, single-subject statistical maps for the voxel-wise contrast of Motion > Static were submitted to a two-sample t-test (adults vs. children) or a paired t-test (T1 vs. T2) at the second level. The comparison of Motion > Static removes activation related to lower-level visual processing, decision making, and the button-press response, with only activation specific to coherent motion processing remaining. To test for the specificity of any differences observed for coherent motion processing (that these were not driven by signal decrease during the Static task), we examined the data for the contrast of Static > Fixation using the same methods used for Motion > Static. As described in more detail below, all analyses (between groups or between time points) were approached in several ways: (1) group data using (a) whole brain and (b) literature-based ROIs in area V5/MT; (2) single-subject data using functional ROIs in area V5/MT with the purposes of (a) characterizing each subject’s area V5/MT in terms of location, extent and amplitude of activation, and (b) testing for reliability of these same measures between the two runs. For all of these, the results are always presented for the contrast of Motion > Static.
(1a) Group Analyses: Whole-Brain
To characterize brain activity in response to coherent visual motion in all brain regions (i.e., beyond area V5/MT), and following published work looking at motion perception in children (Klaver et al., 2008), we first generated whole-brain within- and between-group statistical parametric maps.
(1b) Group Analyses: Literature-Based ROIs for Area V5/MT
Due to our inquiry being specific to area V5/MT and following the approach in Klaver et al., 2008, we applied a small-volume correction (SVC) to examine ROIs based on the coordinates published by Watson et al. (1993) for adults during coherent motion perception (MNI coordinates x, y, z: left -41, -72, -2; right 41, -69, -2). We defined two 5 mm radius spheres centered on these coordinates, much in the same way that Klaver and colleagues used the results from Murray et al., 2003, to generate their ROIs for random motion and shape-from-motion processing. However, unlike Klaver and colleagues, we did not combine the data from the left and right hemispheres, but treated them separately. For both types of group analyses, whole-brain and ROI (SVC), results for the contrast of Motion > Static are reported (voxel: p < 0.005 uncorrected, cluster: p < 0.05 FWE-corrected).
(2 a and b) Single-Subject Analyses: Functional ROIs for Area V5/MT
Since extra caution has to be taken when dealing with pediatric data, single-subject analyses were conducted to counter any concerns that variability in the nature of the activation in the children (between or within subjects) could account for any potential between-group differences in the Cross-Sectional Study or for between-time point differences in the Longitudinal Study. As such, single-subject analysis was used to: (a) characterize each subject’s area V5/MT in terms of location, extent and amplitude of activation and (b) test for reliability of the data throughout the study (i.e. between runs). For characterization, data were combined for both runs. The location was specified along the x-, y-, and z-axes, the extent was defined as the number of above-threshold voxels within a 5 mm radius of the peak, and the amplitude was measured as the percent signal change (PSC) within 5 mm radius spheres centered on each subject’s peaks. This information was compared between children and adults (Cross-Sectional Study) and between T1 and T2 (Longitudinal Study) to provide a more detailed investigation, in addition to the group level analyses (whole-brain and literature-based ROI) described above. For within-subject reliability, we compared these same variables (location, extent, and amplitude) between the two runs within each subject using a student t-test.
For these single-subject analyses, all pre-processing and analysis steps were identical to the ones described above for the whole group and ROI analyses, except that a 4 mm Gaussian kernel was used for smoothing. We identified foci of activity in each subject by examination of activation closest to the Watson coordinates, with peaks significant at the voxel-corrected level of at least p < 0.005 for Motion > Static.
Brain–Behavior Relationships
For the Longitudinal Study, we also examined brain–behavior correlations between the fMRI signal underlying visual motion processing and raw scores of reading ability. Specifically, we first investigated the relationships between the amount of increase in V5/MT activity between the two time points and the concomitant gains made in (a) reading and (b) chronological age over the same time span. This allowed us to test the hypothesis that increased V5/MT activity occurs as a function of learning to read or as a function of becoming older. Next, we examined whether activity in V5/MT at T1 predicted reading outcome at T2. This tested the hypothesis that integrity of the dorsal pathway subserving visual motion processing is needed for successful reading acquisition (similar to the prevailing notion that good phonological awareness skills predict later reading success). For all of these analyses, V5/MT activity was measured as PSC extracted from spheres of 5 mm radius centered on the average peak coordinates of the uncorrected clusters identified in our single-subject functional ROI analysis at T1 (left: -50, -74, 8; right: 51, -69, 7), using the MarsBaR toolbox (Brett et al., 2002). A linear regression was used to test whether change in V5/MT PSC from T1 to T2 was associated with change from T1 to T2 in raw reading scores (for Word ID, Word Attack, and Reading Fluency); or if change in V5/MT PSC was associated with change in age (months) over the same period (T1 to T2). The time duration between testing at T1 and T2 differed somewhat amongst the children, thus providing variability in the age increases within the group. Similarly, a linear regression was performed between V5/MT activity PSC at T1 and raw reading scores (Word ID, Word Attack, and Reading Fluency) at T2, to test for predictive power of V5/MT activity in reading outcome. Finally, we conducted a linear regression using raw scores of phonemic awareness (CTOPP-Elision) at T1 and reading (Word ID, Word Attack and Reading Fluency) at T2, to seek confirmation of phonemic awareness predicting reading outcome over this time period (Wagner et al., 1994; 1997).
Functional Connectivity Analysis
Connectivity analysis was performed using the CONN functional connectivity toolbox (15.e, Whitfield-Gabrieli & Nieto-Castanon, 2012). The same procedures were applied to the Cross-Sectional and Longitudinal Studies. Pre-processed data were submitted to CONN for seed-to-voxel analyses. First, data underwent several noise reduction steps, including (i) regression of white matter and CSF ROI time series using aCompCor with five principal components (Behzadi et al., 2007); (ii) regression of six rigid-body head movement parameters, as well as time points for which scan-to-scan motion exceeded 1.5 mm (50% of the voxel size); (iii) regression of the effects of our Fixation, Motion, and Static blocks; and iv) temporal high-pass filtering (f > 0.008 Hz) to reduce the effect of low-frequency drift while also reducing any contamination of signal between blocks.
For each subject, the residual time series associated with left and right area V5/MT, separately, was submitted to a bivariate correlation to generate Fisher-Z-transformed correlation maps, in which every voxel in the brain is assigned a normalized correlation value that represents the magnitude of the correlation between that voxel and the averaged time series of all voxels within our V5/MT seed (one for each hemisphere). Area V5/MT was identified as a ROI using each group’s location averaged from the single-subject data (i.e. the locations listed in Table 5 and Table 9 below) and growing a 5 mm sphere around it. The V5/MT ROIs for within-group and within-time point analyses were created in this way, while between-group and between-time-point analyses were centered on the average of the two locations (e.g. the average location for adults and children combined).
Table 5.
Single-Subject Analyses: Characterization of Average Location, Extent, and Amplitude of Activation of Functional ROIs for Area V5/MT in the Cross-Sectional Study
| MNI Coordinates
|
MNI Coordinates
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Voxels | Avg PSC (Mot > Stat) | x | y | z | Voxels | Avg PSC (Mot > Stat) | Coordinates p-value | Voxels p-value | Avg PSC p-value | |
| Adults | Children | Adults vs. Children | |||||||||||
|
|
|||||||||||||
| Left V5/MT | −46 | −74 | 6 | 86 | 1.37 ± 0.70 | −49 | −72 | 9 | 132 | 1.51 ± 0.61 | n.s. | < 0.001 | n.s. |
| Right V5/MT | 48 | −70 | 4 | 88 | 1.34 ± 0.52 | 50 | −72 | 8 | 135 | 1.64 ± 0.53 | n.s. | < 0.001 | n.s. |
Statistical significance: p < 0.05
PSC – percent signal change
± standard deviation
Table 9.
Single-Subject Analyses: Characterization of Average Location, Extent, and Amplitude of Activation of Functional ROIs for Area V5/MT in the Longitudinal Study
| MNI Coordinates
|
MNI Coordinates
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Voxels | Avg PSC (Mot > Stat) | x | y | z | Voxels | Avg PSC (Mot > Stat) | Coordinates p-value | Voxels p-value | Avg PSC p-value | |
| T1 | T2 | T1 vs. T2 | |||||||||||
|
|
|||||||||||||
| Left V5/MT | −50 | −74 | 8 | 127 | 1.56 ± 0.47 | −49 | −72 | 9 | 121 | 1.36 ± 0.48 | n.s. | n.s. | n.s. |
| Right V5/MT | 51 | −69 | 7 | 131 | 1.52 ± 0.38 | 52 | −68 | 5 | 121 | 1.53 ± 0.58 | n.s. | n.s. | n.s. |
Statistical significance: p < 0.05
PSC – percent signal change
± standard deviation
To capture the connectivity profile of each of our groups separately, single-subject statistical maps generated at the first level for the entire run (see Ganger et al., 2015) were submitted to a one-sample t-test. To test for between-group or for between-time point differences in whole-brain connectivity with area V5/MT, the single-subject statistical maps generated at the first level were submitted to a two-sample t-test (adults vs. children) for the Cross-Sectional Study, and a paired t-test (T1 vs. T2) for the Longitudinal Study at the second level. Results for the functional connectivity analyses are reported at voxel: p < 0.005 uncorrected, cluster: p < 0.05 FWE-corrected.
Results
Cross-Sectional Study
fMRI Task Performance
All participants performed with high accuracy on the in-scanner Motion and Static tasks. Accuracy and reaction time differed for the Motion task and reaction time differed for the Static tasks between the two groups, as shown in Table 1. However, there was no significant difference between adults and children for reaction time when considering Motion > Static, the comparison of interest for the associated fMRI data. However, performance accuracy for Motion > Static differed significantly between adults and children; thus, performance accuracy was included as a covariate of no interest in our fMRI between-group comparisons.
Head Movement
For the whole-brain group analysis of the Cross-Sectional Study, we did not observe any significant correlations between head movement and task-related activation in our adults. However, there were significant correlations between head movement and task-related activation in children. These were located in right angular gyrus and right cuneus/superior parietal lobule. These overlapped with the children’s within-group whole brain map for the contrast of Motion > Static in right angular gyrus (232 voxel overlap) and in right middle occipital gyrus (68 voxel overlap). However, they were not located in any areas reported in our between-group results described below.
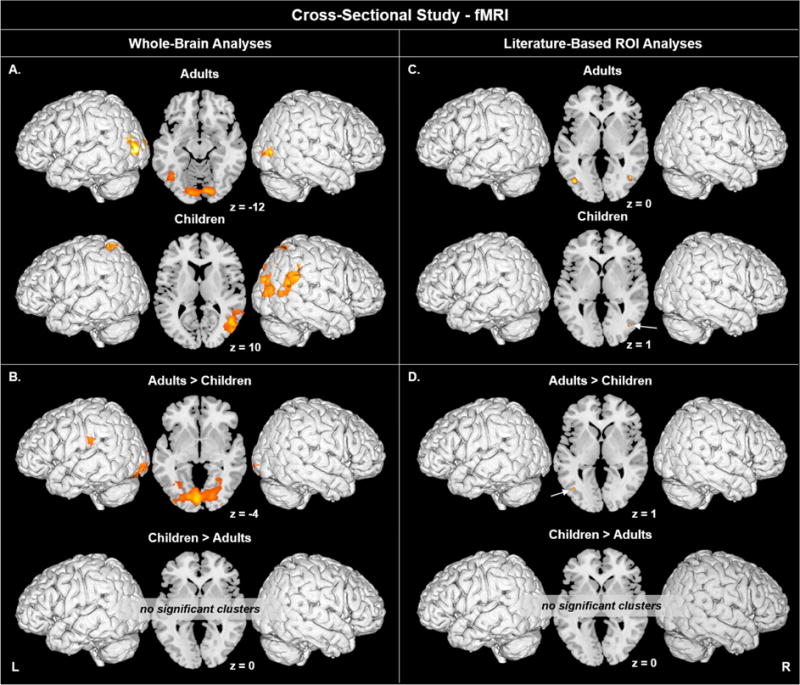
Group Analyses: Whole-Brain in Adults
Whole-brain analysis revealed six clusters of significant activation for Motion > Static in the adults (Table 3, Figure 1A). In the left hemisphere, clusters were found in middle occipital gyrus (BA 19) extending to superior and middle temporal gyri (area V5/MT, BA 19), in cuneus (BA 18), including area V3a, extending to bilateral lingual gyri, in fusiform gyrus (BA 37), and in cingulate gyrus (BA 24) extending to left superior parietal lobule. In the right hemisphere, we observed significant activation extending from middle (area V5/MT, BA 37) to superior temporal gyrus, as well as in cingulate cortex (BA 24) extending to precentral gyrus.
Table 3.
Whole-Brain Analyses: MNI Coordinates of Peak Functional Activation in the Cross-Sectional Study
| MNI Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Peak Anatomical Region | Voxels | Peak BA | Peak Z | |
| Adults | |||||||
| −40 | −74 | 4 | L Middle Occipital Gyrusa | 2244 | 19 | 4.87 | |
| −20 | −92 | 15 | L Cuneusb | 4012 | 18 | 4.90 | |
| −40 | −62 | −14 | L Fusiform Gyrus | 552 | 37 | 3.60 | |
| −6 | −20 | 40 | L Cingulate Gyrusc | 570 | 24 | 4.47 | |
| 46 | −68 | 2 | R Middle Temporal Gyrusd | 953 | 37 | 4.61 | |
| 12 | −16 | 38 | R Cingulate Cortexe | 617 | 24 | 4.51 | |
| Children | |||||||
| −28 | −54 | 56 | L Superior Parietal Lobulef | 1440 | 7 | 4.14 | |
| 40 | −64 | 18 | R Middle Temporal Gyrus | 4607 | 39 | 4.64 | |
| 8 | −46 | 54 | R Paracentral Lobuleg | 2134 | 5 | 3.97 | |
| Adults > Children | |||||||
| −4 | −87 | −6 | L Lingual Gyrush | 14288 | 18 | 5.27 | |
| −46 | −21 | 22 | L Insulai | 1282 | 13 | 4.36 | |
| Children > Adults | |||||||
| no significant clusters | |||||||
Adults
Extends into left superior and middle temporal gyri.
Extends into bilateral lingual gyri.
Extends into left superior parietal lobule.
Extends into right superior temporal gyrus.
Extends into right precentral gyrus.
BA – Brodmann Area
Children
Extends into left inferior parietal lobule.
Extends into left precuneus.
Adults > Children
Extends into right lingual and bilateral inferior occipital gyri.
Extends into left postcentral gyrus.
Figure 1.
A-B) Coherent visual motion-evoked activation in A) adults and children, and in B) adults versus children across the whole brain. C-D) Coherent visual motion-evoked activation within V5/MT in C) adults and children, and in D) adults versus children, using ROIs centered on literature-based coordinates. Motion > Static, voxel p < 0.005 uncorrected, cluster p < 0.05 corrected. Activation up to 5 mm beneath the cortical surface is displayed.
Group Analyses: Whole-Brain in Children
In children, whole-brain analysis for the contrast of Motion > Static revealed significant activation in left superior parietal lobule (BA 7) extending to inferior parietal lobule. Activity in left area V5/MT did not survive FWE correction (p = 0.071). In the right hemisphere, significant activation was found in middle temporal gyrus (BA 39), including area V5/MT (BA 19); and in paracentral lobule (BA 5) extending to left precuneus (Table 3, Figure 1A).
Group Analyses: Whole-Brain in Adults versus Children
Adults showed significantly greater activation for Motion > Static than children in bilateral lingual (BA 18) and extending into bilateral inferior occipital gyri (Table 3, Figure 1B), as well as in left posterior insula (BA 13) extending to postcentral gyrus. There were no differences in area V5/MT for either hemisphere. The opposite contrast (children > adults) did not identify any significant differences. There were no significant between-group results for the contrast of Static > Fixation.
Group Analyses: Literature-Based ROIs for Area V5/MT in Adults
The ROI analyses with SVC informed by the coordinates published by Watson and colleagues (1993) revealed significant activity in area V5/MT bilaterally in adults (Table 4, Figure 1C). In both the left and right hemispheres, activation extended from middle occipital gyrus (BA 19) to middle temporal gyrus (BA37).
Table 4.
Literature-Based ROI Analyses: MNI Coordinates of Peak Functional Activation in the Cross-Sectional Study
| MNI Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Peak Anatomical Region | Voxels | Peak BA | Peak Z | |
| Adults | |||||||
| −42 | −74 | 3 | L Middle Occipital Gyrus | 104 | 19 | 4.66 | |
| 45 | −68 | 0 | R Middle Occipital Gyrus | 49 | 37 | 4.14 | |
| Children | |||||||
| 39 | −70 | 3 | R Middle Occipital Gyrus | 6 | 19 | 2.79 | |
| Adults > Children | |||||||
| −42 | −69 | 2 | L Middle Occipital Gyrus | 15 | 37 | 2.95 | |
| 40 | −69 | −6 | R Inferior Occipital Gyrus | 5 | 19 | 2.76 | |
| Children > Adults | |||||||
| no significant clusters | |||||||
p = 0.051
BA – Brodmann Area
Group Analyses: Literature-Based ROIs for Area V5/MT in Children
ROI analyses revealed no above-FWE threshold clusters (nor trending) within the left hemisphere, but did reveal significant activation in right area V5/MT (BA 19) in children (Table 4, Figure 1C).
Group Analyses: Literature-Based ROIs for Area V5/MT in Adults versus Children
Analyses of literature-based ROIs for area V5/MT revealed significantly greater activation in adults than in children (Table 4, Figure 1D). In the left hemisphere this was in middle occipital gyrus (BA37), and in the right hemisphere, an activation difference of trending significance (= 0.051) was within inferior occipital gyrus (BA 19).
Single-Subject Analyses: Functional ROIs for Area V5/MT in Adults
Using the subject-specific data, of the 15 adults, all had significant activation in left, and 14 in right V5/MT at uncorrected p < 0.001 (the 15th participant had activation at p < 0.005, uncorrected). Location, extent and amplitude of activation of area V5/MT were characterized for each adult participant using both runs combined. The group averages of these are reported in Table 5.
To test for reliability of these same measures from one run to the next, paired t-tests were conducted between the first and the second run for the adult group. We tested for differences in location, extent or amplitude of activation between runs, and found none (p > 0.1; Table 6).
Table 6.
Single-Subject Analyses: Reliability of Average Location, Extent, and Amplitude of Activation of Functional ROIs for Area V5/MT in the Cross-Sectional Study
| MNI Coordinates
|
MNI Coordinates
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Voxels | Avg PSC (Mot > Stat) | x | y | z | Voxels | Avg PSC (Mot > Stat) | Coordinates p-value | Voxels p-value | Avg PSC p-value | |
| Adults | Children | Adults vs. Children | |||||||||||
|
|
|||||||||||||
| Left V5/MT | |||||||||||||
| Run 1 | −47 | −72 | 5 | 54 | 1.28 ± 0.32 | −51 | −73 | 7 | 82 | 1.47 ± 0.55 | n.s. | n.s. | n.s. |
| Run 2 | −47 | −73 | 6 | 54 | 1.27 ± 0.56 | −49 | −75 | 8 | 99 | 1.66 ± 0.73 | n.s. | n.s. | n.s. |
| Right V5/MT | |||||||||||||
| Run 1 | 51 | −68 | 3 | 51 | 1.26 ± 0.67 | 50 | −72 | 8 | 97 | 1.66 ± 0.64 | n.s. | n.s. | n.s. |
| Run 2 | 48 | −70 | 4 | 59 | 1.60 ± 0.73 | 49 | −73 | 7 | 113 | 1.78 ± 0.66 | n.s. | n.s. | n.s. |
Statistical significance: p < 0.05
PSC – percent signal change
± standard deviation
Single-Subject Analyses: Functional ROIs for Area V5/MT in Children
All of the 13 children had significant activation in left and right V5/MT at p < 0.001, uncorrected. Location, extent and amplitude of activation of area V5/MT were characterized for each child participant using both runs combined. Average values for these are reported for the group in Table 5.
For reliability, paired t-tests were conducted between the first and the second run within the pediatric group. This revealed no significant differences in location, extent or intensity of activation (p > 0.1; Table 6).
Single-Subject Analyses: Functional ROIs for Area V5/MT in Adults versus Children
Lastly, we tested for between-group differences in location, extent, and intensity of activation of single-subject V5/MT between the adults and children. Data from both runs were combined for each group and compared between the groups. This revealed no significant differences in peak location or intensity of activation. However, the children did exhibit a greater extent (p < 0.001) of activation than adults bilaterally, as measured by the number of voxels above threshold (Table 5).
To assess if there was a difference in reliability of the data between the two groups when comparing both runs, we conducted an ANOVA on the signal from the left and right functional ROI, and looked for a group × run interaction. This revealed no significant differences in location, extent or intensity of activation (p > 0.05).
In sum for the Cross-Sectional Study, independent of whether using whole-brain or ROI group analyses, or single-subject analyses, the adults showed bilateral activity within area V5/MT. In children, whole-brain and ROI group analyses revealed significant activity in right but not left area V5/MT, whereas activation was identified bilaterally for each child in the single-subject analysis. Group analyses at the level of the whole brain did not exhibit significant between-group differences in area V5/MT, whereas between-group analyses using a ROI approach revealed greater left (with right trending) area V5/MT activity in adults compared to children. Single-subject analyses revealed no significant differences between adults and children in terms of location or amplitude of activation, but children did exhibit significantly greater extent of activation than did adults.
Functional Connectivity
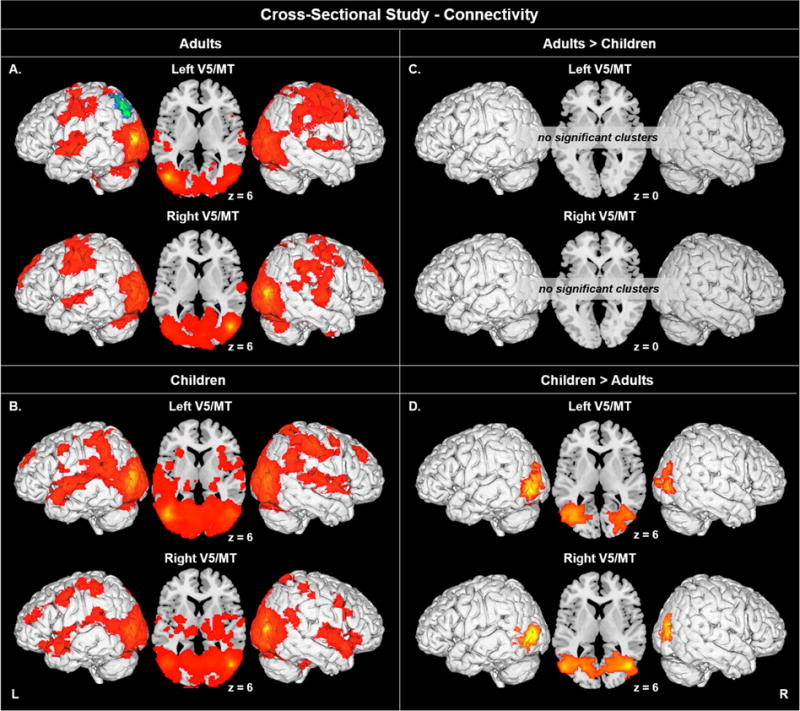
For both groups, the time courses of left and right area V5/MT activation exhibited significant correlations with bilateral posterior cortex, extending ventrally and dorsally. Figure 2A and 2B show the results for each of the two groups. For simplicity, we do not describe the results for each of the groups, but focus only on the results from the between-group comparisons.
Figure 2.
Bilateral V5/MT seed-to-voxel results for A) adults, B) children, C) adults > children, and D) children > adults. For the within-group maps, areas exhibiting positive correlations are indicated in orange/red, areas exhibiting negative correlations are indicated in green/blue. Voxel p < 0.005 uncorrected, cluster p < 0.05 corrected. Connectivity up to 5 mm beneath the cortical surface is displayed.
Group-Level Analyses: Seed-to-Voxel in Adults versus Children
The contrast of adults greater than children did not result in any significant findings. However, there was greater functional connectivity in children compared to adults (Table 7, Figure 2D). For left V5/MT, children exhibited significantly greater connectivity with left middle occipital gyrus (BA 19) extending to lateral occipital cortex, middle and inferior temporal gyri, lingual and fusiform gyri, and to cuneus and precuneus. There was also greater functional connectivity with contralateral middle temporal gyrus (BA 37) extending to right intracalcarine cortex, precuneus, and occipital pole. For right area V5/MT, children exhibited significantly greater connectivity with contralateral middle occipital gyrus (BA 19) extending to bilateral lateral occipital and intracalcarine cortices, to bilateral middle and inferior temporal gyri, and to bilateral fusiform and lingual gyri.
Table 7.
Seed-to-Voxel Analyses: MNI Coordinates of Peak Differences in Functional Connectivity Between Groups in the Cross-Sectional Study
| MNI Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Peak Anatomical Region | Voxels | Peak BA | Peak Z | |
| L V5/MT ROI Seed | |||||||
| Adults > Children | |||||||
| no significant clusters | |||||||
| Children> Adults | |||||||
| −36 | −68 | 0 | L Middle Occipital Gyrusa | 4068 | 19 | 6.49 | |
| 44 | −68 | 6 | R Middle Temporal Gyrusb | 519 | 37 | 4.50 | |
| R V5/MT ROI Seed | |||||||
| Adults > Children | |||||||
| no significant clusters | |||||||
| Children > Adults | |||||||
| 42 | −70 | 6 | L Middle Occipital Gyrusc | 7383 | 19 | 6.05 | |
Extends into left lateral occipital cortex, middle and inferior temporal gyri, lingual and fusiform gyri, cuneus, and precuneus.
Extends into right intracalcarine cortex, precuneus, and occipital pole.
Extends into bilateral lateral occipital and intracalcarine cortices, middle and inferior temporal gyri, fusiform and lingual gyri.
BA – Brodmann Area
Longitudinal Study
Behavior
As expected, from T1 to T2, a period spanning around 10 months, the children on average experienced gains in raw score measures of reading, significant for single real word reading (two-tailed paired t-test, T2 > T1: p = 0.006), reading fluency (p = 0.003), and trending toward significance for pseudoword reading (p = 0.053).
fMRI Task Performance
The children performed with high accuracy on the in-scanner Motion and Static tasks (see Table 2). While, for the Motion task, accuracy increased and reaction time decreased between T1 and T2, this change was not significant. There were also no significant changes for the Static task for accuracy or reaction time between T1 and T2. When the difference between conditions for the contrast of interest (Motion > Static) was considered, they did not differ between T1 and T2 either for accuracy or reaction time.
Head Movement
For the whole-brain group analysis in our Longitudinal Study, children at T1 exhibited significant correlations between head movement and brain activation in bilateral medial/superior frontal gyri; however, these did not overlap with the within-group result at T1 described below. At T2, the children did not exhibit significant correlations between head movement and brain activity. Based on these, we believe our results were not influenced by head movement.
Group Analyses: Whole-Brain in Children at T1
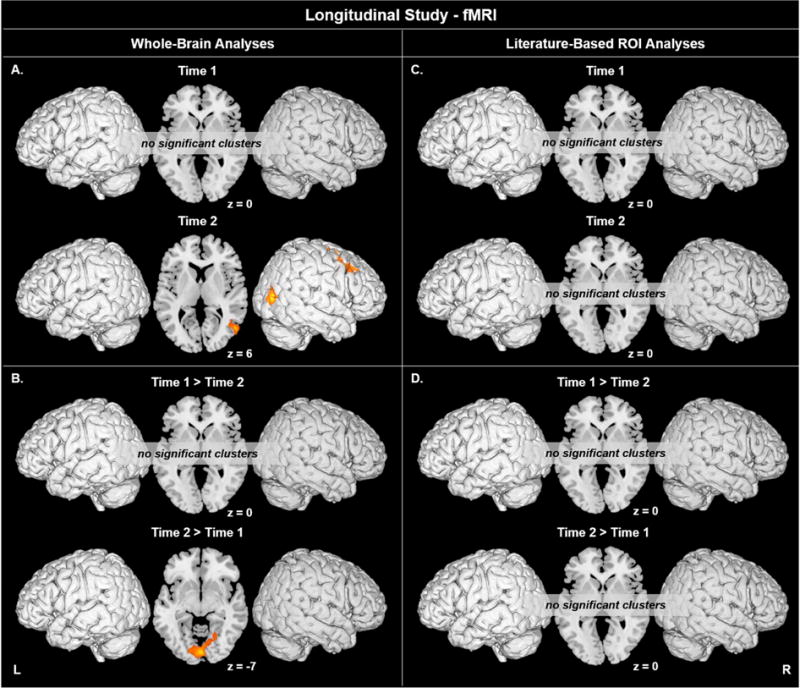
At T1, the children exhibited no activation above FWE threshold (nor trending) for the contrast of Motion > Static at the whole-brain level.
Group Analyses: Whole-Brain in Children at T2
Whole-brain analysis revealed significant activity in two right hemisphere clusters (Table 8, Figure 3A). Children at T2 exhibited significant activation in right area V5/MT in posterior middle temporal gyrus (BA 37) extending to superior temporal gyrus, and in right middle frontal gyrus (BA 9) extending to precentral gyrus. There was no significant (nor trending) left hemisphere V5/MT activation.
Table 8.
Whole-Brain Analyses: MNI Coordinates of Peak Functional Activation in the Longitudinal Study
| MNI Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Peak Anatomical Region | Voxels | Peak BA | Peak Z | |
| Time 1 | |||||||
| no significant clusters | |||||||
| Time 2 | |||||||
| 52 | −68 | 4 | R Middle Temporal Gyrusa | 1144 | 37 | 4.12 | |
| 36 | 2 | 39 | R Middle Frontal Gyrusb | 1281 | 9 | 4.79 | |
| Time 2 > Time 1 | |||||||
| −2 | −98 | 22 | L Cuneusc | 5393 | 19 | 4.49 | |
| Time 1 > Time 2 | |||||||
| no significant clusters | |||||||
Time 2
Extends into right superior temporal gyrus.
Extends into right precentral gyrus.
Time 2 > Time 1
Extends into right cuneus and bilateral lingual gyri.
BA – Brodmann Area
Figure 3.
A-B) Coherent visual motion-evoked activation at A) T1 and T2, and for B) T1 versus T2 across the whole brain. C-D) Coherent visual motion-evoked activation within V5/MT at C) T1 and T2, and for D) T1 versus T2, using ROIs centered on literature-based coordinates. Motion > Static, voxel p < 0.005 uncorrected, cluster p < 0.05 corrected. Activation up to 5 mm beneath the cortical surface is displayed.
Group Analyses: Whole-Brain in Children at T1 versus T2
For the contrast of T2 > T1, children at T2 showed significantly greater activation in left cuneus (BA 19) extending to right cuneus and bilateral lingual gyri (Table 8, Figure 3B). There were no significant findings for the opposite contrast, T1 > T2. Neither contrast revealed activation changes over time in area V5/MT. A paired -test at the second level for the voxel-wise contrast of Static > Fixation did not reveal any significant changes between T1 and T2.
Group Analyses: Literature-Based ROIs for Area V5/MT in Children at T1
ROI analyses found no significant activity in either left or right area V5/MT at Time 1.
Group Analyses: Literature-Based ROIs for Area V5/MT in Children at T2
ROI analyses found no significant activity in either left or right area V5/MT at Time 2.
Group Analyses: Literature-Based ROIs for Area V5/MT in Children at T1 versus T2
ROI analyses found no significant changes from T1 to T2 in left or right area V5/MT.
Single-Subject Analyses: Functional ROIs for Area V5/MT in Children at T1
All of the 12 children had significant activation in left and right V5/MT at p < 0.001, uncorrected. Location, extent and amplitude of activation of area V5/MT were characterized for every participant at T1 from data combined from both runs. Average location, extent and amplitude at T1 are reported in Table 9.
To test for reliability of the data between the two runs at Time 1, paired t-tests were conducted and revealed no significant differences for location, extent or amplitude of activation (p > 0.05) between runs (Table 10).
Table 10.
Single-Subject Analyses: Reliability of Average Location, Extent, and Amplitude of Activation of Functional ROIs for Area V5/MT in the Longitudinal Study
| MNI Coordinates
|
MNI Coordinates
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Voxels | Avg PSC (Mot > Stat) | x | y | z | Voxels | Avg PSC (Mot > Stat) | Coordinates p-value | Voxels p-value | Avg PSC p-value | |
| T1 | T2 | T1 vs. T2 | |||||||||||
|
|
|||||||||||||
| Left V5/MT | |||||||||||||
| Run 1 | −50 | −74 | 8 | 94 | 1.61 ± 0.41 | −49 | −72 | 9 | 89 | 1.49 ± 0.61 | n.s. | n.s. | n.s. |
| Run 2 | −52 | −68 | 7 | 72 | 1.43 ± 0.49 | −50 | −71 | 8 | 76 | 1.31 ± 0.43 | n.s. | n.s. | n.s. |
| Right V5/MT | |||||||||||||
| Run 1 | −50 | −74 | 8 | 94 | 1.61 ± 0.41 | −49 | −72 | 9 | 89 | 1.49 ± 0.61 | n.s. | n.s. | n.s. |
| Run 2 | −52 | −68 | 7 | 72 | 1.43 ± 0.49 | −50 | −71 | 8 | 76 | 1.31 ± 0.43 | n.s. | n.s. | n.s. |
Statistical significance: p < 0.05
PSC – percent signal change
± standard deviation
Single-Subject Analyses: Functional ROIs for Area V5/MT in Children at T2
Eleven of the 12 children had significant activation in left and right V5/MT at p < 0.001, uncorrected, at T2. The twelfth child had significant activation in left and right V5/MT at p < 0.005, uncorrected. Characteristics of average location, extent and amplitude at T2 are reported in Table 9.
To test for reliability of the data between the two runs at Time 2, paired t-tests were conducted and revealed no significant differences in location, extent or amplitude of activation (p > 0.05) between the two runs (Table 10).
Single-Subject Analyses: Functional ROIs for Area V5/MT in Children at T1 versus T2
Paired t-tests were used to compare the averages of the single-subject data between the two time points for location, extent and amplitude of activation. These revealed no significant differences in any of these measures (p > 0.1, Table 9).
To assess if there was a difference in reliability of the data between the two time points when comparing both runs, we conducted an ANOVA for each measurement in each hemisphere, and looked for a time point × run interaction. This revealed no significant differences for location, extent or amplitude of activation (p > 0.1).
In sum, for the Longitudinal Study, the whole-brain analyses revealed no activation in area V5/MT at T1 and significant activation in the right hemisphere only at T2. There were no changes observed in area V5/MT from T1 to T2. The literature-based ROI approach did not reveal V5/MT activation at T1 or T2, nor changes from T1 to T2. However, single-subject analysis identified bilateral peaks of V5/MT activation at T1 and T2 in each child, but no significant differences emerged in the location, extent, or amplitude of activation between the two time points for the group means.
Brain–Behavior Relationships: V5/MT Activity and Reading Performance
Correlations between change in V5/MT activation and change in raw reading scores for (i) real word and (ii) pseudoword reading accuracy as well as (iii) reading fluency from T1 to T2 yielded no significant results. Also, correlations between change in V5/MT activation and change in age (months) yielded no significant results.
Linear regression revealed that V5/MT activity at T1 did not predict reading outcome at T2 for real word reading accuracy, pseudoword reading accuracy, or reading fluency. On the other hand, phonemic awareness measured via sound elision at T1 predicted pseudoword reading accuracy outcome at T2 (r = 0.61, p = 0.04), and real word reading accuracy outcome at T2 at the trend level (r = 0.56, p = 0.06). Phonemic awareness at T1 did not predict reading fluency outcome at T2 (r = 0.47, p = 0.12).
Functional Connectivity
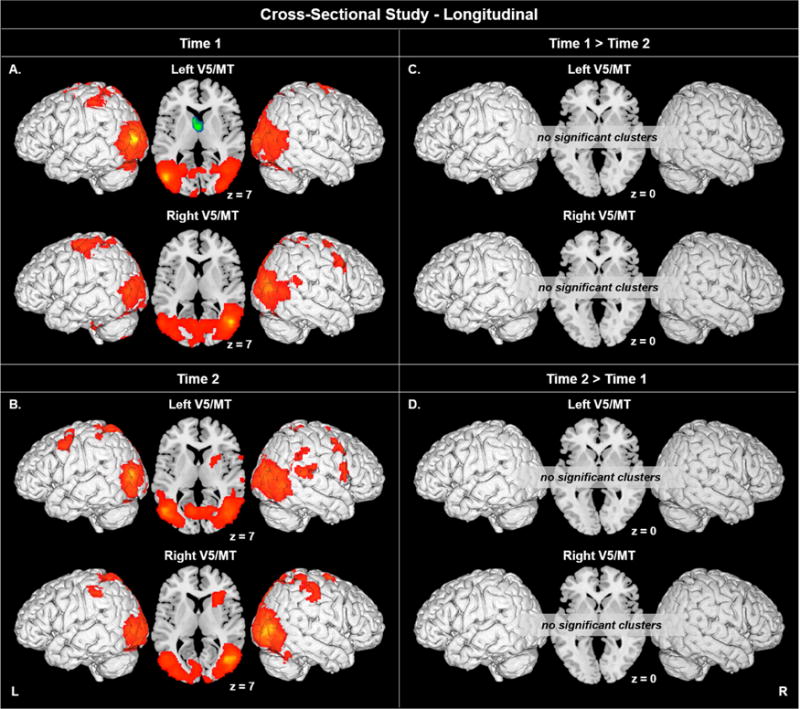
At both T1 and T2, the time courses of left and right area V5/MT activation exhibited significant correlations with bilateral posterior cortex, extending ventrally and dorsally. Figure 4A and 4B show the results for the children at T1 and T2. These will not be described in detail here; instead, the description will focus on the analysis comparing T1 and T2 data.
Figure 4.
Bilateral V5/MT seed-to-voxel results for A) T1, B) T2, C) T1 > T2, and D) T2 > T1. Areas exhibiting positive correlations are indicated in orange/red, areas exhibiting negative correlations are indicated in green/blue. Voxel p < 0.005 uncorrected, cluster p < 0.05 corrected. Connectivity up to 5 mm beneath the cortical surface is displayed.
Group Analyses: Seed-to-Voxel in Children at T1 versus T2
Pairwise connectivity analyses revealed no change in functional connectivity for left or right V5/MT from T1 to T2.
Discussion
This is the first study to compare V5/MT activation in children and adults during coherent motion processing as well as the functional connectivity of V5/MT to other brain regions in these two groups. Our Cross-Sectional Study of coherent visual motion processing extends the work of Klaver et al. (2008), who investigated differences in visual motion processing between children and adults for random motion stimuli and shape-from-motion stimuli, but who did not examine coherent motion stimuli. Similar to Klaver and colleagues, we used whole-brain analyses as well as a ROI analysis approach. As in the study by Klaver and colleagues, we found no differences in V5/MT activation between children and adults when considering group data for the whole brain; but we did find relatively more activity in adults in a between-group ROI analysis. While our findings are consistent with Klaver et al., who reported a difference of trending significance for their ROI combining left and right V5/MT, these ROI group differences may have been driven by the location of the ROI or the variability of V5/MT activation in children. Therefore, we conducted further analyses at the level of the single subject, and found that all 15 adults and all 13 children had bilateral activity in area V5/MT. We then generated group averages for location, extent and amplitude of activity identified in all individual participants and compared group averages of these measures between children and adults. There were no differences for these comparisons, other than that children exhibited relatively greater extent of V5/MT activation. Next, we examined the reliability of the data sets with regard to reproducibility from one run to the next, and found that children’s data was as reliable between the two runs as those of adults. Finally, we investigated functional connectivity and found children to have relatively greater local connectivity of area V5/MT bilaterally. As such, we conclude that children and adults are similar in intensity of activation, and only differ in the extent of activity, as well as in connectivity with other regions. Consistent with this, in our Longitudinal Study we found no changes in V5/MT activity from 2nd to 3rd grade for whole-brain, ROI group analyses or single-subject analyses. There was no change in functional connectivity of area V5/MT between these two time points. This suggests that the differences observed in the Cross-Sectional Study for extent of activity and functional connectivity represent a protracted process. Finally, we did not find evidence to support a relationship between change in brain activity in area V5/MT and change in reading ability from 2nd into 3rd grade; or between change in brain activity in area V5/MT and chronological age; or that brain activity in 2nd grade was predictive of reading in 3rd grade. In sum, we conclude that adults and children are very similar in their activation of area V5/MT for coherent motion perception and that there is a gradual developmental honing regarding the extent of activation of area V5/MT with increasing age, together with a gradual decrease in functional connectivity with surrounding extrastriate visual cortex.
Coherent Visual Motion Processing in Adults and Children: Group Analyses
Whole-brain analyses in the adult group revealed brain activity in response to coherent visual motion (compared to static dots) consistent with previous literature (Dupont et al., 1994; McKeefry et al., 1997; Sunaert et al., 1999; Watson et al., 1993), with significant activation in bilateral dorsal occipitotemporal cortex, including V5/MT. Coordinates of V5/MT activation were similar in location to those reported in prior studies (Dupont et al., 1994; McKeefry et al., 1997; Sunaert et al., 1999; Watson et al., 1993), as was activity observed in primary and secondary visual cortex (Dupont et al., 1994; McKeefry et al, 1997; Sunaert et al., 1999; Watson et al., 1993). Activation within these regions has been shown to be greater for coherent motion than random motion perception (Braddick et al., 2001; Paradis et al., 2000; but see McKeefry et al., 1997). The children in our study also exhibited significant functional activation in right area V5/MT, whereas activation in left V5/MT did not survive FWE correction.
When directly compared to adults, children exhibited less activation in bilateral early visual cortex (V1/V2), as well as in the left postcentral gyrus. These between-group differences were driven by differences in coherent visual motion processing rather than being due to any decrease during static dot processing (i.e., we observed no significant differences when comparing adults and children for the contrast of Static > Fixation). There were, however, no between-group differences in area V5/MT for the contrast of Motion > Static. In sum, like Klaver and colleagues, whose children (n=10) were 6 to 7 years of age (ours were 7 to 8 years of age), we found no differences between the children and adults in area V5/MT for the whole-brain group analysis. The only difference observed by Klaver and colleagues for a whole-brain analysis was for random motion in the left precentral gyrus (children > adults).
Turning to the group analyses using a ROI approach to examine V5/MT-specific activity, we placed spheres centered on the left and right coordinates reported by Watson and colleagues (1993) in a study of adults. Klaver and colleagues (2008) created spheres around coordinates reported by Murray et al. (2003), a study of shape-from-motion visual processing in adults. Using this approach, we found activation within the area V5/MT ROIs bilaterally in adults but only in the right hemisphere in children. Direct comparison between the two groups revealed greater activity in adults relative to children in the left V5/MT ROI, with a right hemisphere difference of trending significance. These findings make it tempting to conclude that there is a developmental shift of V5/MT activation, increasing with age, and that this is mostly driven by an increase in activity in left area V5/MT. Our between-group results using a ROI approach are similar to those from Klaver and colleagues, who reported that adults exhibit greater activation in area V5/MT relative to children during perception of random motion stimuli. However, it should be noted that they combined the data from the left and right ROIs and that significance was only trending. When our data from left and right V5/MT ROIs are collapsed to provide a comparison to Klaver et al., 2008, the results reveal significant between-group differences, with adults having greater activation than children.
While our findings are generally consistent with those of Klaver and colleagues (2008) and suggest age-specific differences in left (or left and right combined) V5/MT activity when using a ROI approach, there is reason to be cautious of the results. First, the ROIs were derived from coordinates published in a study of adults (Watson et al., 1993), leading to the question of whether they adequately captured activation within our pediatric group. Second, children may show more variability in location and extent of activation, which could inadvertently produce between-group differences in group ROI-based analyses. As such, we went on to conduct single-subject analyses, discussed next, which led us to conclude that the group results from the literature-based ROI are not reliable.
Coherent Visual Motion Processing in Adults and Children: Single-Subject Analyses
Within each subject, we searched foci of activity closest to the locations where V5/MT is typically observed. This individual subject analysis revealed that every participant, child and adult, had activity in area V5/MT. When examining group means for location, extent and amplitude of activation, there were no differences in location or amplitude between our child and adult groups. However, we did find that our pediatric participants exhibited a greater extent of V5/MT activation than adults in both left and right area V5/MT. Children’s clusters of activation contained ~50% more above-threshold voxels than adults, leading to larger extent of activation. In consideration of this observation in the single-subject data, it is notable that the whole-brain group map (Figure 1) also indicates more widespread right hemisphere activation in the children. At the same time, given this finding of greater extent, it seems paradoxical that left V5/MT in children did not meet statistical threshold in the group analysis, and that the ROI analysis found left V5/MT to be less active in children relative to adults. However, as noted in the Results section of the group analyses, a less stringent threshold did show activity in children in left V5/MT, and as already discussed, the ROI approach may be inaccurate for other reasons. Importantly, the single-subject analysis was helpful in capturing the individual profiles for all participants.
One question that arises is whether head movement is responsible for the more widespread pattern of activation in children than adults in this single-subject analysis. While the whole brain group analysis included a step to address the role of head movement (by generation of maps of correlations between inter-scan head movement and brain activity for Motion > Static), the single-subject analysis did not include an analogous procedure. To address this we conducted post-hoc analyses to rule out the potential concern that head movement in children could be responsible for the greater extent of V5/MT activation. First we computed correlations between in-scanner head movement with area of activation of V5/MT and found there was no significant positive relationship for adults or children (separately or when combined) in neither left nor right V5/MT. Secondly, we removed some subjects from the pediatric and adult groups so as to generate subgroups that were matched on the amount of head movement. When these were contrasted for the single-subject analysis, the finding of greater area of activity for children relative to adults remained. Specifically, for a subset of 8 children and 12 adults, the between-group t-test for head movement was no longer significant (p > 0.1) and yet there was greater area of activity for children: right V5/MT p < 0.001, and left V5/MT, p = 0.001. As such, while it would be reasonable to expect that head movement might be a contributing factor to the more widespread pattern of activation observed in children, the data do not support this.
A developmental paring down or ‘focalization’ of functional activation (fewer areas exhibiting significant activation) has been observed across cognitive domains, including tasks of language (Booth et al., 2001; see Berl et al., 2006, for review), motor (Müller et al., 1998), and visual processing (Passarotti et al., 2003; see Grill-Specter et al., 2008, for review). However, reports of changes in the extent of significant activation within the same specific region across development are mixed. Passarotti and colleagues (2003) reported a developmental shift (based on comparing children with adults) in focalization of activation during face- and location-matching tasks (to interrogate the ventral and dorsal visual systems, respectively). They reported a greater extent of activation in adults than in children in parietal cortex for location matching, but a smaller extent of activation in adults in fusiform gyrus as well as middle temporal gyrus during the face-matching task. Further, in a study of retinotopic organization of children (7-12 years old) and adults, Conner and colleagues (2004) observed larger extrastriate regions (i.e., V1, V2, V3 and V4) in adults than in children. Developmental differences in extent of activation of area V5/MT have not previously been reported. Whether areas exhibit greater or smaller extents of activation with age may vary as a function of brain region and task. Additionally, as Grill-Specter and colleagues (2008) suggest, reports may vary due to experimental design and methodological factors, in that different tasks require different strategies, which may further vary between children and adults; or in that analyses conducted at the group level do not take into account potential variability at the single-subject level.
To address this potential variability, we used single-subject data to examine the reliability of the results. For every subject we compared the first and second run with regards to location, extent and intensity of activation and found that children, like adults, had reproducible results. This is not too surprising, given that the task was both easy and engaging for young participants, but it is nevertheless an important observation and one that allows us to feel confident about any between-group differences.
Coherent Visual Motion Processing in Adults and Children: Functional Connectivity
Both groups showed extensive functional connectivity from area V5/MT to other regions, as has been previously reported. These regions include thalamus (Gaglianese, 2012), primary visual and extrastriate visual cortex, ventral temporal cortex, inferior and middle occipital gyri, (Hampson et al., 2004) superior parietal lobule, precentral sulcus/gyrus and the frontal eye fields (Yeo et al., 2011). We found greater functional connectivity in children than in adults between left and right V5/MT and surrounding ipsilateral and contralateral homotopic inferior temporal cortices. While this may be related to the greater extent of V5/MT activation observed in children (i.e. greater extent of active voxels result in more functionally connected voxels), such a relationship would need to be tested empirically. Observation of greater local and within-network connectivity in children relative to adults is consistent with previous reports of developmental changes in functional connectivity (Betzel et al., 2014; Fair et al., 2007, 2009; Farrant & Uddin, 2015; Rubia, 2013). However, in these reports, greater local or short-range and within-network connectivity in children was observed in tandem with greater long-range or ‘distributed’ connectivity in adults. For instance, Farrant and Uddin (2015) used resting state fMRI to compare functional connectivity within the dorsal attention network (DAN, in which V5/MT participates) between children (7-12 years old) and adults. Using the frontal eye fields (FEF) as their seed region, the investigators found children to exhibit greater functional connectivity within the DAN, whereas adults exhibited greater connectivity between the FEF seed region and extra-network regions. While we report greater short-range and local connectivity in our group of children, we did not find any regions (long- or short-range) where adults exhibited greater functional connectivity with our V5/MT seed regions. This could be due to the simple nature of the functional architecture of visual motion perception.
Coherent Visual Motion Processing in Children Measured Longitudinally at Two Time Points: Group and Single-Subject Analyses of Activity and Group Analysis of Functional Connectivity
We had anticipated, based on prior work, that adults and children would differ in the amount of brain activity in area V5/MT and included a longitudinal study to examine a more fine-grained time scale of such potential change within a group of children over the course of a year. However, we only observed significant changes in low-level visual cortex across this time span for the whole-brain group analysis. There were no other changes for this analysis or any of the other analyses (ROI group data or single-subject data). We also did not observe a difference between the two time points for functional connectivity. This indicates that there are no fine-grained developmental changes over the period of one year in area V5/MT that are measurable with fMRI under the experimental conditions described in this study. As such, the difference observed between children and adults in the Cross-Sectional Study (described above) in extent of activity is likely to take place over a protracted time period.
Following from the above discussion regarding the different outcomes depending on the analysis employed, at T1 of the Longitudinal Study we did not find activity in area V5/MT for the group using either a whole-brain or a ROI analysis approach. At T2, activity was found for right, but not left, area V5/MT for the whole-brain analysis. While this whole-brain analysis result is consistent with the children from the Cross-Sectional Study, it is odd that the right hemisphere activity was not observed when using the ROI analysis (the Cross-Sectional Study revealed right V5/MT activity using both whole brain and ROI analyses). This result of right V5/MT activity from the whole-brain but not ROI analysis again indicates that the use of ROI was not optimal for pediatric data. Notably, the single-subject analyses revealed that all 12 children had bilateral activity in area V5/MT at both time points (albeit one child just short of threshold at T2). These results once again speak to the advantage of using a single-subject approach, as our individually defined V5/MT analyses were most successful in showing brain activity during coherent motion processing in all children.
Few Age-Dependent Differences in Area V5/MT
As outlined in the Introduction, differences in motion perception thresholds between children at different ages and between children and adults (Boets et al., 2011) raise the possibility of age-dependent differences in activity in area V5/MT as well as differences in functional connectivity. Indeed, the adults in our study performed significantly better than did children on measures of accuracy and reaction time for the Motion task. The children in the longitudinal study improved their accuracy on the Motion task from 2nd to 3rd grade, but this change was not significant. Age-dependent difference in performance may have been greater if the task had not been so easy, with subjects performing close to ceiling, something that is not uncommon in fMRI studies where the goal is to try to equate the groups for performance inside of the scanner (Price et al., 2006). Our fMRI results show, however, that few differences exist between adults and children in terms of brain activity underlying coherent motion perception, and that these are constrained to the spatial extent and functional connectivity of activation. The subtleness of these differences, while surprising in the context of the existing behavioral literature (Atkinson & Braddick, 2005; Boets et al., 2011; Braddick et al., 2016; Gunn et al., 2002; Hadad et al., 2011), is not unexpected from the anatomical perspective. Area V5/MT is heavily myelinated at birth (Flechsig 1920; Watson et al., 1993) and has been shown with fMRI to be active in 7-week old infants (Biagi et al., 2015). Area V5/MT is typically found in the same location across individuals (Annese et al., 2005; Wilms et al., 2005; though see Large et al., 2016), most commonly at the intersection of two sulci (Annese et al., 2005; Dumoulin et al., 2000). Recent studies have directly linked a functionally defined V5/MT to areas that are heavily myelinated compared to surrounding cortex (Glasser & Van Essen, 2011; Sereno et al., 2013). Hence, the anatomical evidence suggests that area V5/MT is early to develop and, as such, may not be likely to show substantial developmental differences past a very young age. However, this does not preclude that developmental differences in visual motion processing skills may be driven by brain areas beyond extrastriate visual cortex. For example, recent work by Braddick and colleagues (2016) investigating the relationship between visual motion processing and brain anatomy (measured as local cortical surface area), suggests that strong global motion sensitivity in children is positively related to greater parietal cortex surface area. More recently, Braddick and colleagues (2017) reported an association between children’s motion coherence thresholds and functional anisotropy of the superior longitudinal fasciculus. This raises the possibility that other aspects of the dorsal stream, beyond area V5/MT, may have a contributing role determining motion processing ability over development.
Our work also illustrates the problems in using independent adult coordinates obtained from a previously published study, which led to between-group differences that, following our single-subject analyses, we deemed to be erroneous. The use of a stereotactic atlas, derived in adults, may only serve to compound this problem. However, the use of a stereotactic atlas is common in developmental imaging studies, with work by Burgund, Kang and colleagues (Burgund et al., 2002; Kang et al., 2003) reassuring us that comparisons between adults and children using MRI and fMRI are valid.
No Role for V5/MT Activity in Predicting Reading Outcome
Our longitudinal study failed to provide evidence of gains in reading being associated with changes in V5/MT activation. This was unexpected, as recent studies have suggested that increased experience in reading may be yoked with increased activity in area V5/MT (Boets et al., 2011; Olulade et al., 2013b). We also tested the hypothesis that V5/MT integrity may lay the groundwork for successful reading outcome by investigating the relationship between 2nd grade V5/MT activation and 3rd grade reading scores. Whereas a behavioral measure of phonemic awareness predicts reading outcome in our sample, as in previous studies (Schatschneider et al., 2004; Wagner & Torgesen, 1987), a similar predictive power for V5/MT activity at T1 and reading outcome at T2 was not supported. We cannot rule out the possibility, though, that our fMRI signal may have lacked statistical power to bear out a relationship between 2nd grade V5/MT activation and 3rd grade reading. Additionally, while behavioral studies into the relationship between reading and coherent motion processing have been conducted in pre-reading children, our participants had already learned to read. As a result of this, any reading-related changes in V5/MT activation may have already occurred in our participants. However, it is important to note that brain activity during a task of phonological processing (real word rhyming task) did not predict later reading in 8- to 12-year-old children who were typical readers (Hoeft et al., 2007). In that study, predictive powers for fMRI measures were only observed in a group of children with dyslexia, where activity in right inferior frontal gyrus was predictive of later reading gains.
Of note is that our results are also consistent with those of Kevan and Pammer (2009), who found that a behavioral measure of coherent motion sensitivity did not predict later reading. They found instead that a measure of pre-reading frequency doubling sensitivity predicted early reading measures of real and pseudoword reading. Their suggestion for why one dorsal stream measure predicted reading outcome but not the other (i.e., coherent motion perception) was that perhaps the higher-order neuroanatomical substrates of coherent motion sensitivity, as opposed to lower-level substrates of frequency doubling sensitivity, are only “assimilated” into the reading network once reading skills become more sophisticated. Whereas the authors hypothesized that perhaps their early readers (avg. age 7 years) were not yet at a level of reading advanced enough to require areas subserving coherent motion perception (e.g., V5/MT) to have been incorporated into the reading network, this explanation does not apply to our children, who were older and have had more complex reading experiences. Our results are also consistent with a study by Braddick and colleagues (2016), who did not find correlations between global motion sensitivity and single word reading. Interestingly, they did find a significant association with this measure and performance on a task of numerical cognition.
Conclusion
Together, our two studies provide the first insight into area V5/MT activity measured with fMRI during coherent visual motion perception in elementary school-aged children. Foremost, our findings illustrate that the method for defining and interrogating V5/MT is critical for characterizing this region. We observed significant differences in V5/MT activation between children and adults when using a ROI identified in adults. When using a single-subject approach, V5/MT no longer differed between the adults and children, other than in extent of activation. Further, we did not observe any significant changes in V5/MT activation from 2nd to 3rd grade, regardless of which analysis was performed. Reading development did not appear to be driving MT/V5 activity. To conclude, we offer a comprehensive examination of coherent visual motion processing in children in area V5/MT.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (P50HD40095 and R01HD056107) and the National Science Foundation (SBE0541953 Science of Learning Center). We are grateful to the staff at the Center for Functional and Molecular Imaging, and the support of the Intellectual and Development Disorders Research Center (IDDRC5 P30 HD040677). We thank the following for aiding in the acquisition of the data: Eileen Napoliello, Emma Cole, Iain DeWitt, Alison Merikangas, Jenni Rosenberg, Ashley Wall-Piche; and Lynn Flowers for oversight on the behavioral testing and Diana Alkire for editing the manuscript. We thank each of our participants for their time, Maximilian Riesenhuber for input on the data analysis, and two anonymous reviewers for their constructive feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annese J, Gazzaniga MS, Toga AW. Localization of the human cortical visual area MT based on computer aided histological analysis. Cerebral Cortex. 2005;15(7):1044–1053. doi: 10.1093/cercor/bhh205. [DOI] [PubMed] [Google Scholar]
- Aspell JE, Tanskanen T, Hurlbert AC. Neuromagnetic correlates of visual motion coherence. European Journal of Neuroscience. 2005;22(11):2937–2945. doi: 10.1111/j.1460-9568.2005.04473.x. [DOI] [PubMed] [Google Scholar]
- Atkinson J. The Davida Teller Award Lecture, 2016: Visual Brain Development: A review of “Dorsal Stream Vulnerability”—motion, mathematics, amblyopia, actions, and attention. Journal of Vision. 2017;17(3):26. doi: 10.1167/17.3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O. Dorsal stream vulnerability and autistic disorders: the importance of comparative studies of form and motion coherence in typically developing children and children with developmental disorders. Cahiers de Psychologie Cognitive. 2005;23(1-2):49–58. [Google Scholar]
- Atkinson J, Braddick O. From genes to brain development to phenotypic behavior: “dorsal-stream vulnerability” in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Progress in Brain Research. 2011;189:261–283. doi: 10.1016/B978-0-444-53884-0.00029-4. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. NeuroReport. 1997;8(8):1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, Meltzoff AN. Brain activation during face perception: evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17(2):308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. NeuroImage. 2006;30(3):679–691. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102:345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Biagi L, Crespi SA, Tosetti M, Morrone MC. BOLD response selective to flow-motion in very young infants. PLoS Biology. 2015;13(9):e1002260. doi: 10.1371/journal.pbio.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Cornelissen P, Wouters J, Ghesquière P. Coherent motion sensitivity and reading development in the transition from prereading to reading stage. Child Development. 2011;82(3):854–869. doi: 10.1111/j.1467-8624.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Santen FWV, Harasaki Y, Gitelman DR, Parrish TB, Mesulam MM. The development of specialized brain systems in reading and oral-language. Child Neuropsychology. 2001;7(3):119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Akshoomoff N, Newman E, Curley LB, Gonzalez MR, Brown T, Dale A, Jernigan T. Individual differences in children’s global motion sensitivity correlate with TBSS-based measures of the superior longitudinal fasciculus. Vision Research. 2017;141:145–156. doi: 10.1016/j.visres.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Newman E, Akshoomoff N, Kuperman JM, Bartsch H, Chen CH, Dale AM, Jernigan TL. Global visual motion sensitivity: Associations with parietal area and children’s mathematical cognition. Journal of Cognitive Neuroscience. 2016;28(12):1897–1908. doi: 10.1162/jocn_a_01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41(13):1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception-London. 2001;30(1):61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Current Biology. 2000;10(12):731–734. doi: 10.1016/s0960-9822(00)00540-6. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proceedings of the National Academy of Sciences. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Human Brain Mapping. 2009;30(6):1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. NeuroImage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. [Google Scholar]
- Bucher K, Dietrich T, Marcar VL, Brem S, Halder P, Boujraf S, Summers P, Brandeis D, Martin E, Loenneker T. Maturation of luminance-and motion-defined form perception beyond adolescence: a combined ERP and fMRI study. NeuroImage. 2006;31(4):1625–1636. doi: 10.1016/j.neuroimage.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461(7266):983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Coch D, Skendzel W, Grossi G, Neville H. Motion and color processing in school-age children and adults: an ERP study. Developmental Science. 2005;8(4):372–386. doi: 10.1111/j.1467-7687.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- Conner IP, Sharma S, Lemieux SK, Mendola JD. Retinotopic organization in children measured with fMRI. Journal of Vision. 2004;4(6):10. doi: 10.1167/4.6.10. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Hansen PC, Hutton JL, Evangelinou V, Stein JF. Magnocellular visual function and children’s single word reading. Vision Research. 1998;38(3):471–482. doi: 10.1016/s0042-6989(97)00199-5. [DOI] [PubMed] [Google Scholar]
- Cornette L, Dupont P, Rosier A, Sunaert S, Van Hecke P, Michiels J, Mortelmans L, Orban GA. Human brain regions involved in direction discrimination. Journal of Neurophysiology. 1998;79(5):2749–2765. doi: 10.1152/jn.1998.79.5.2749. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. Journal of Neuroscience. 1998;18(17):6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Le Goualher G, Pike GB, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cerebral Cortex. 2000;10(5):454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Dupont P, Orban GA, De Bruyn B, Verbruggen A, Mortelmans L. Many areas in the human brain respond to visual motion. Journal of Neurophysiology. 1994;72(3):1420–1424. doi: 10.1152/jn.1994.72.3.1420. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;382(6586):66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. Asymmetric development of dorsal and ventral attention networks in the human brain. Developmental Cognitive Neuroscience. 2015;12:165–174. doi: 10.1016/j.dcn.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Anatomie des menschlichen Gehirns und Rückenmarks auf Myelogenetischer Grundlage. Lepizig (DE): Thieme; 1920. [Google Scholar]
- Gaglianese A, Costagli M, Bernardi G, Ricciardi E, Pietrini P. Evidence of a direct influence between the thalamus and hMT+ independent of V1 in the human brain as measured by fMRI. Neuroimage. 2012;60(2):1440–1447. doi: 10.1016/j.neuroimage.2012.01.093. [DOI] [PubMed] [Google Scholar]
- Ganger S, Hahn A, Küblböck M, Kranz GS, Spies M, Vanicek T, Seiger R, Sladky R, Windischberger C, Kasper S, Lanzenberger R. Comparison of continuously acquired resting state and extracted analogues from active tasks. Human Brain Mapping. 2015;36(10):4053–4063. doi: 10.1002/hbm.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers AD, Bhatt R, Corbly CR, Farley AB, Joseph JE. Developmental shifts in cortical loci for face and object recognition. NeuroReport. 2004;15(10):1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1-and T2-weighted MRI. Journal of Neuroscience. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Grill-Spector K. Experience Shapes the Development of Neural Substrates of Face Processing in Human Ventral Temporal Cortex. Cerebral Cortex. 2015;27(2):1229–1244. doi: 10.1093/cercor/bhv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Liberman A, Yoon JM, Grill-Spector K. Differential development of the ventral visual cortex extends through adolescence. Frontiers in Human Neuroscience. 2010;3:80. doi: 10.3389/neuro.09.080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Golarai G, Gabrieli J. Developmental neuroimaging of the human ventral visual cortex. Trends in Cognitive Sciences. 2008;12(4):152–162. doi: 10.1016/j.tics.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter EJ, Maybery MT, Badcock DR. Vision in developmental disorders: is there a dorsal stream deficit? Brain Research Bulletin. 2010;82(3):147–160. doi: 10.1016/j.brainresbull.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002;13(6):843–847. doi: 10.1097/00001756-200205070-00021. [DOI] [PubMed] [Google Scholar]
- Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Developmental Science. 2011;14(6):1330–1339. doi: 10.1111/j.1467-7687.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- Hadad BS, Schwartz S, Maurer D, Lewis TL. Motion perception: a review of developmental changes and the role of early visual experience. Frontiers in Integrative Neuroscience. 2015;9:49. doi: 10.3389/fnint.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. NeuroReport. 2004;15(8):1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hansen PC, Stein JF, Orde SR, Winter JL, Talcott JB. Are dyslexics’ visual deficits limited to measures of dorsal stream function? NeuroReport. 2001;12(7):1527–1530. doi: 10.1097/00001756-200105250-00045. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. The Journal of Neuroscience. 2002;22(16):7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Bhatt RS. Progressive and regressive developmental changes in neural substrates for face processing: Testing specific predictions of the interactive specialization account. Developmental Science. 2011;14(2):227–241. doi: 10.1111/j.1467-7687.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kevan A, Pammer K. Predicting early reading skills from pre-reading measures of dorsal stream functioning. Neuropsychologia. 2009;47(14):3174–3181. doi: 10.1016/j.neuropsychologia.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Klaver P, Lichtensteiger J, Bucher K, Dietrich T, Loenneker T, Martin E. Dorsal stream development in motion and shape-from-motion perception. NeuroImage. 2008;39(4):1815–1823. doi: 10.1016/j.neuroimage.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Large I, Bridge H, Ahmed B, Clare S, Kolasinski J, Lam WW, Miller KL, Dyrby TB, Parker AJ, Smith JE, Daubney G, Sallet J, Bell AH, Krug K. Individual differences in the alignment of structural and functional markers of the V5/MT complex in primates. Cerebral Cortex. 2016;26(10):3928–3944. doi: 10.1093/cercor/bhw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeefry DJ, Watson JDG, Frackowiak RSJ, Fong K, Zeki S. The activity in human areas V1/V2, V3, and V5 during the perception of coherent and incoherent motion. Neuroimage. 1997;5(1):1–12. doi: 10.1006/nimg.1996.0246. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16(1):369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends in Neurosciences. 1983;6:414–417. [Google Scholar]
- Mitchell TV, Neville HJ. Asynchronies in the development of electrophysiological responses to motion and color. Journal of Cognitive Neuroscience. 2004;16(8):1363–1374. doi: 10.1162/0898929042304750. [DOI] [PubMed] [Google Scholar]
- Müller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT. Developmental changes of cortical and cerebellar motor control: a clinical positron emission tomography study with children and adults. Journal of Child Neurology. 1998;13(11):550–556. doi: 10.1177/088307389801301105. [DOI] [PubMed] [Google Scholar]
- Murray SO, Olshausen BA, Woods DL. Processing shape, motion and three-dimensional shape-from-motion in the human cortex. Cerebral Cortex. 2003;13(5):508–516. doi: 10.1093/cercor/13.5.508. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kashii S, Nagamine T, Matsui Y, Hashimoto T, Honda Y, Shibasaki H. Human V5 demonstrated by magnetoencephalography using random dot kinematograms of different coherence levels. Neuroscience Research. 2003;46(4):423–433. doi: 10.1016/s0168-0102(03)00119-6. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden GF. Developmental differences for word processing in the ventral stream. Brain and Language. 2013a;125(2):134–145. doi: 10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Napoliello EM, Eden GF. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 2013b;79(1):180–190. doi: 10.1016/j.neuron.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis AL, Cornilleau-Peres V, Droulez J, Van De Moortele PF, Lobel E, Berthoz A, Le Bihan D, Poline JB. Visual perception of motion and 3-D structure from motion: an fMRI study. Cerebral Cortex. 2000;10(8):772–783. doi: 10.1093/cercor/10.8.772. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6(1):100–117. [Google Scholar]
- Pellicano E, Gibson LY. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46(10):2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. Journal of Magnetic Resonance Imaging. 2006;23:816–826. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. European Child & Adolescent Psychiatry. 2013;22(12):719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatschneider C, Fletcher JM, Francis DJ, Carlson CD, Foorman BR. Kindergarten prediction of reading skills: A longitudinal comparative analysis. Journal of Educational Psychology. 2004;96(2):265–282. [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–F30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Lutti A, Weiskopf N, Dick F. Mapping the human cortical surface by combining quantitative T1 with retinotopy. Cerebral Cortex. 2013;23(9):2261–2268. doi: 10.1093/cercor/bhs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, O’brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. NeuroReport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7(1):12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stevens C, Neville H. Neuroplasticity as a double-edged sword: deaf enhancements and dyslexic deficits in motion processing. Journal of cognitive neuroscience. 2006;18(5):701–714. doi: 10.1162/jocn.2006.18.5.701. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Experimental Brain Research. 1999;127(4):355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- Szwed M, Ventura P, Querido L, Cohen L, Dehaene S. Reading acquisition enhances an early visual process of contour integration. Developmental Science. 2012;151(1):139–149. doi: 10.1111/j.1467-7687.2011.01102.x. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101(2):192–212. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Development of reading-related phonological processing abilities: New evidence of bidirectional causality from a latent variable longitudinal study. Developmental Psychology. 1994;30(1):73–87. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive test of phonological processing. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, Hecht SA, Barker TA, Burgess SR, Donahue J, Garon T. Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: a 5-year longitudinal study. Developmental Psychology. 1997;33(3):468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cerebral Cortex. 1993;3(2):79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J, Birtles D, Nystrm P, von Hofsten C, Rosander K, Anker S, Atkinson J, Braddick O. Reorganization of global form and motion processing during human visual development. Current Biology. 2010;20(5):411–5. doi: 10.1016/j.cub.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J, Chiu M, Kulke L. Developmental Reorganisation of Visual Motion Pathways. i-Perception. 2012;3(4):230. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation, Harcourt Brace and Company; San Antonio, Texas: 1999. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilmer JB, Richardson AJ, Chen Y, Stein JF. Two visual motion processing deficits in developmental dyslexia associated with different reading skills deficits. Journal of Cognitive Neuroscience. 2004;16(4):528–540. doi: 10.1162/089892904323057272. [DOI] [PubMed] [Google Scholar]
- Wilms M, Eickhoff SB, Specht K, Amunts K, Shah NJ, Malikovic A, Fink GR. Human V5/MT+: comparison of functional and cytoarchitectonic data. Anatomy and Embryology. 2005;210(5-6):485–495. doi: 10.1007/s00429-005-0064-y. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott JB, Hansen PC, Richardson AJ, Griffiths TD, Rees A, Stein JF, Green GG. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Current Biology. 1998;8(14):791–7. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Tests-Revised (WRMT-R/NU) Circle Pines, Minnesota: American Guidance Service; 1998. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: The Riverside Publishing Company; 2001. [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner R. The organization of the human Cerebral Cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


