Abstract
Although sleep appears to be broadly conserved in animals, the physiological functions of sleep remain unclear. In this study, we sought to identify a physiological defect common to a diverse group of short-sleeping Drosophila mutants, which might provide insight into the function and regulation of sleep. We found that these short-sleeping mutants share a common phenotype of sensitivity to acute oxidative stress, exhibiting shorter survival times than controls. We further showed that increasing sleep in wild-type flies using genetic or pharmacological approaches increases survival after oxidative challenge. Moreover, reducing oxidative stress in the neurons of wild-type flies by overexpression of antioxidant genes reduces the amount of sleep. Together, these results support the hypothesis that a key function of sleep is to defend against oxidative stress and also point to a reciprocal role for reactive oxygen species (ROS) in neurons in the regulation of sleep.
Author summary
Most animals sleep; humans sleep nearly a third of their lives. Yet the fundamental functions of sleep remain unknown. Here, we used short-sleeping Drosophila mutants to uncover a role for sleep in resistance to oxidative stress. Oxidative stress is an imbalance of reactive oxygen species and antioxidant responses. Although these short-sleeping mutants have defects in diverse pathways, they all exhibit sensitivity to oxidative stress. Moreover, increasing sleep in wild-type flies increased resistance to oxidative stress. This suggests that one function of sleep is to defend against oxidative stress. Finally, reducing oxidative stress in neurons of wild-type flies reduces their sleep, suggesting that oxidative stress also regulates sleep. Taken together, our results support an intriguing hypothesis for a bidirectional relationship between sleep and oxidative stress: oxidative stress triggers sleep, which then acts as an antioxidant for both the body and the brain. These results have implications for human patients suffering from chronic sleep restriction and diseases associated with oxidative stress.
Introduction
A sleeping animal is vulnerable to predators and other dangers in its environment for a large portion of the day. Despite these daily risks, sleep is an evolutionarily conserved behavior throughout the animal kingdom [1–3], suggesting that sleep serves important functions. In support of this, prolonged episodes of acute sleep deprivation in both rodents and invertebrates cause an increased need to sleep [4–7], cognitive impairment [8,9], increased metabolic rate [6,10], and death [6,10,11]. It remains unclear whether these effects are due to loss of sleep or due to the intense stress associated with acute sleep deprivation. Epidemiological studies have revealed that chronic sleep restriction, or shortened sleep duration, in humans is associated with metabolic disorders [12], cardiovascular disease [13], inflammation [14,15], psychiatric disorders [16], and even premature mortality [17,18]. Similar to experimental results involving acute sleep deprivation, it is unclear whether these defects are due to the loss of sleep itself, to associated disruptions in circadian rhythm, or from the very factors that cause sleep loss, such as shift work, aging, or psychological stress. Thus, while current research in both humans and model organisms has demonstrated an important role for sleep in learning and memory [19–22], it has been difficult to identify underlying functions for sleep essential to the organism’s survival or fitness.
Sleep is thought to be regulated by two distinct types of mechanisms: those that control the timing of sleep, such as the circadian system, and those that control the duration of sleep, also called sleep homeostasis mechanisms [23,24]. While the molecular mechanisms underlying circadian regulation have been well characterized, molecular mechanisms regulating sleep homeostasis are less well defined but are thought to be neuronally based [24–29] and context dependent—that is, sleep deprivation or other stress conditions may induce different homeostasis pathways than baseline sleep. Because acute sleep deprivation increases sleep need and results in extended sleep duration at the animal’s next opportunity to sleep, many models of sleep homeostasis propose a feedback mechanism in which the wake state increases sleep-promoting factors, such as adenosine or overall synaptic strength [24,29]. The sleep state then clears or abrogates these factors to allow the wake state.
A controversial hypothesis for the function of sleep is the free radical flux theory of sleep, proposed in a theoretical paper by Reimund in 1994. Reimund proposed that reactive oxygen species (ROS) accumulate in neurons during the wake state and that sleep allows for the clearance of ROS in the brain [30]. ROS are chemically reactive by-products of metabolism, which, when not properly neutralized, cause damaging covalent modifications that inhibit the function of proteins, lipids, and DNA and can lead to cell death. Thus, the free radical flux hypothesis proposed that the core function of sleep is to act as an antioxidant for the brain. Despite the appeal of this hypothesis, data to support it are conflicting. While some groups have reported decreased antioxidant capacity and oxidative damage in the brains of sleep-deprived rats and mice [31–34], other reports have contradicted these findings [35–37]. As a result, the Reimund hypothesis has fallen out of favor as a model for sleep function. Notably, all studies testing the Reimund hypothesis focused on the effects of acute sleep deprivation. In contrast to acute sleep deprivation, the relationship between chronic sleep restriction and oxidative stress has not been thoroughly investigated, despite the physiological relevance of chronic sleep restriction widespread in modern society [38].
In recent years, the fruit fly has become a powerful, genetically tractable model system for the study of sleep [39,40]. Forward genetic screens have identified a number of Drosophila mutants that are short sleeping and retain intact circadian rhythms. Loss-of-function mutations in ion channels and ion-channel regulators, including sleepless, which regulates the potassium channel Shaker and nicotinic acetylcholine receptors (nAChRs), have been shown to reduce sleep [20,26,41,42]. Other short sleep–causing mutations include the redeye allele of the nAChRα4 subunit [43], the fumin allele of the dopamine transporter (DAT) [44], and loss of function of the putative ubiquitin ligase adaptor encoded by insomniac (inc) [45,46]. It has been hypothesized that these mutations cause short sleep by increasing neuronal excitability [24]. These mutants allow researchers to investigate the effects of chronic short sleep independent of circadian defects. While the specific genes affected vary widely and it is not clear whether these mutants sleep less than controls because of reduced sleep need or an inability to sleep, the common phenotype of these diverse mutants is chronic short sleep. Thus, together these mutants provide a system for identifying a “core” or essential function of sleep; we hypothesized that if chronic short sleep has negative effects on health, these diverse short-sleeping Drosophila mutants might share a common physiological defect independent of the specific mechanism driving their short sleep.
In this study, we sought to identify a physiological defect common to short-sleeping flies that might provide insight into the function and regulation of sleep. We found that diverse short-sleeping mutants are sensitive to acute oxidative stress, exhibiting shorter survival times than controls, and that increasing total sleep duration of wild-type flies promotes survival after oxidative challenge. We further showed that neuronal overexpression of antioxidant genes in wild-type flies reduces sleep. Our data demonstrate that one function of sleep is to increase the organism’s resistance to oxidative stress and support the hypothesis that sleep abrogates neuronal oxidative stress; these results also point to a role for neuronal ROS in the homeostatic regulation of sleep.
Results
Neuronal knockdown of inc does not compromise lifespan, metabolism, or immunity
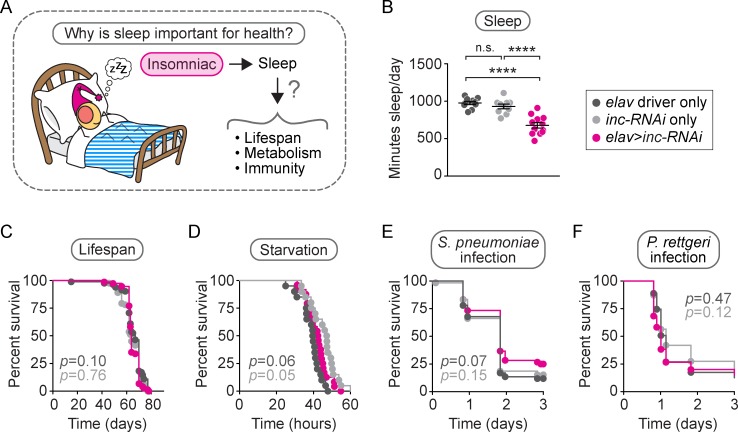
To identify specific physiological functions of sleep (Fig 1A), we first focused on neuron-specific RNA interference (RNAi) of the inc gene, which has been shown to cause short sleep [45,46]. inc encodes a putative adaptor protein for Cullin-3 (Cul3), an E3 ubiquitin ligase expressed in both the brain and the body. Cul3 is involved in a number of crucial biological processes, and inc null mutants have reduced lifespan [45]. In contrast, neuron-specific RNAi of inc was reported to cause short sleep without affecting lifespan [45], suggesting that reduction of Inc activity in nonneuronal tissues affects lifespan in a sleep-independent manner. For this reason, we used flies expressing neuron-specific inc-RNAi as our initial model of short sleep.
Fig 1. Neuronal inc-RNAi reduces sleep without affecting lifespan, metabolism, or immunity.
We investigated the importance of sleep in the health of neuronal inc-RNAi flies by examining three specific health parameters: lifespan, metabolism, and immunity (A). Relative to genetic controls, neuronal inc-RNAi flies slept 30% less than controls (B, p < 0.0001 compared to either control, n = 10–12 flies/genotype), displayed a normal lifespan (C, p > 0.05 compared to either control, n = 74–82 flies/genotype), died from starvation at an intermediate rate (D, p > 0.05 compared to driver control, p = 0.05 compared to inc-RNAi control, n = 20–24 flies/genotype), and died at the same rate as controls after injection with Streptococcus pneumoniae (E, p > 0.05 compared to either control, n = 59–60 flies/genotype) or Providencia rettgeri (F, p > 0.05 compared to either control, n = 60–63 flies/genotype). For the scatterplot in (B), each data point represents the average sleep in minutes/day, measured across 4–5 days for an individual animal. Data are shown as mean ± SEM. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test when significance was detected (B) or by log-rank analysis (C–F). Data from representative experiments are shown. Lifespans were performed twice. All other experiments were performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. inc, insomniac; n.s., not significant p > 0.05; RNAi, RNA interference.
We verified that animals expressing an upstream activation sequence (UAS)-inc-RNAi construct via the pan-neuronal driver elav-GAL4, hereafter referred to as neuronal inc-RNAi flies, exhibited a 30% reduction in total sleep time relative to isogenic controls carrying one copy of either the inc-RNAi construct or elav driver alone (Fig 1B, p < 0.0001 relative to either control; S1A Fig). We further confirmed that neuronal inc-RNAi flies exhibit normal lifespan compared to controls (Fig 1C, p > 0.5 compared to either control), consistent with a previous report [45] and with recent findings on inbred short-sleeping Drosophila lines that have normal lifespan [47]. This result confirms earlier findings that chronic short sleep does not itself shorten lifespan.
Changes in sleep are often associated with altered metabolic energy storage. In humans and mice, sleep loss is associated with metabolic dysfunction such as obesity [48,49], and in flies, starvation suppresses sleep behavior [50] and prolonged sleep is associated with increased starvation resistance [51]. We tested whether neuronal inc-RNAi flies have altered starvation resistance, which reflects altered metabolic energy stores. We found that the mortality rate of inc-RNAi flies after starvation was intermediate between normally sleeping control flies containing either the elav driver or the UAS-inc-RNAi construct alone (Fig 1D, p = 0.0592 compared to elav control, p = 0.0493 compared to inc-RNAi control), suggesting that short sleep does not affect metabolic energy storage in neuronal inc-RNAi animals.
Acute sleep deprivation has also been associated with immune dysfunction in humans, rats, and mice [52–55]. Work in flies has shown that acute sleep deprivation can also augment the immune response [56]. To assay for defects or enhancement in immunity because of chronic short sleep, we injected neuronal inc-RNAi flies with different bacterial pathogens, including Streptococcus pneumoniae, a gram-positive pathogen that has been well characterized in Drosophila (Fig 1E), Providencia rettgeri, a gram-negative natural pathogen found in wild-caught Drosophila (Fig 1F), Listeria monocytogenes, and Staphylococcus aureus (S1B and S1C Fig). In each case, neuronal inc-RNAi flies died at the same rate as one or both of their genetic controls. To further test whether chronically reduced sleep causes deficits in immune function, we examined the response of short-sleeping fumin mutants that lack a functional DAT [44]. We confirmed earlier findings that fumin mutants exhibit short sleep (an approximately 95% reduction in sleep relative to controls) (S1D Fig). We found that fumin mutants responded variably to these pathogens (S1E–S1H Fig). The lack of a consistent immunity defect across different pathogens in both neuronal inc-RNAi flies and fumin mutants suggests that chronic short sleep does not have a dramatic or common impact on immune function in Drosophila.
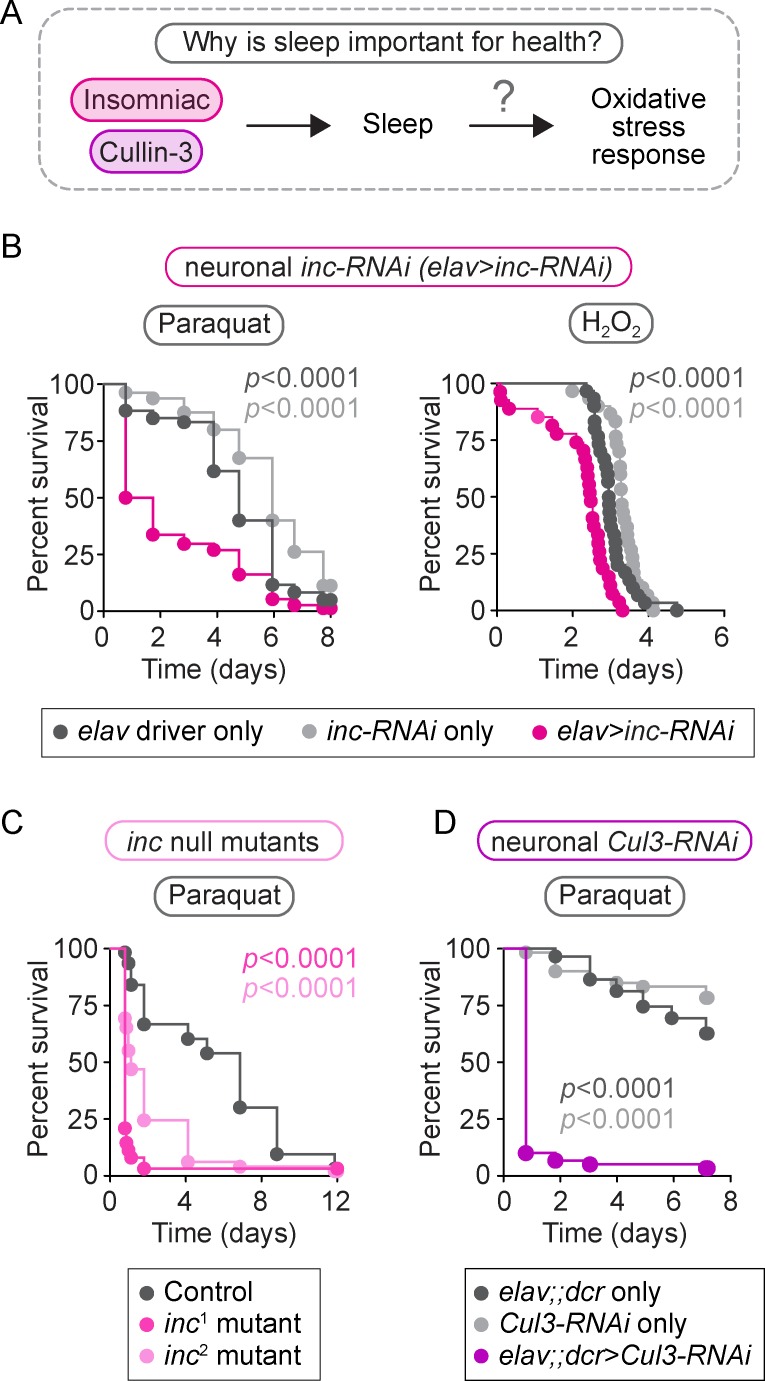
Short sleep via reduction of inc causes sensitivity to oxidative stress
We next set out to test whether sleep is required to defend against oxidative stress (Fig 2A) [30]. We compared the survival of neuronal inc-RNAi flies relative to controls when subjected to two different treatments that induce oxidative stress by increasing ROS levels (Fig 2B). We first injected neuronal inc-RNAi flies with a lethal dose of paraquat, an herbicide that catalyzes the production of superoxide anions [57]. We found that neuronal inc-RNAi flies died at a significantly faster rate after paraquat injection than controls (Fig 2B, left panel, p < 0.0001 relative to either control). To determine whether neuronal inc-RNAi flies have a specific sensitivity to superoxide anions or if they are also sensitive to other forms of oxidative stress, neuronal inc-RNAi flies and controls were fed hydrogen peroxide (H2O2), an oxidant that produces highly reactive hydroxyl radicals and has been shown to alter locomotor activity when fed to flies [58]. Similar to paraquat injection, neuronal inc-RNAi flies were sensitive to H2O2 feeding compared to controls (Fig 2B, right panel, p < 0.0001 relative to either control). These results indicate that short-sleeping neuronal inc-RNAi flies are susceptible to oxidative stress.
Fig 2. Reducing inc or Cul3 expression results in sensitivity to oxidative stress.
We investigated whether reduction of inc or Cul3, either of which causes short sleep, affects the oxidative stress response (A). Neuronal inc-RNAi flies died faster than controls after paraquat injection (B, left panel, p < 0.0001 compared to either control, n = 60–80 flies/genotype) and H2O2 feeding (B, right panel, p < 0.0001 compared to either control, n = 27–30 flies/genotype). Similar sensitivity to paraquat was observed in inc1 and inc2 null mutants (C, p < 0.0001 for both mutants compared to control, n = 49–63 flies/genotype) and neuronal Cul3-RNAi flies (D, p < 0.0001 compared to either control, n = 59–60 flies/genotype). p-values were obtained by log-rank analysis. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; dcr, UAS-Dicer; inc, insomniac; RNAi, RNA interference.
To verify that oxidative stress sensitivity is caused by the reduction in inc expression, rather than an off-target effect of RNAi, we next tested inc null mutants for paraquat sensitivity. We confirmed that inc null mutants exhibit a 50% reduction in sleep (S2A Fig, p < 0.0001 for both inc1 and inc2 mutants, relative to controls), as previously reported [45]. Consistent with neuronal inc-RNAi flies, inc null mutants died faster than controls when injected with paraquat (Fig 2C, p < 0.0001 for both inc1 and inc2 mutants, relative to controls). Furthermore, because Inc is a putative adaptor for the Cul3 ubiquitin ligase, we predicted that reduction of neuronal Cul3 activity would also cause paraquat sensitivity. As previously reported [45], neuronal Cul3-RNAi flies exhibit a 60% reduction in sleep (S2B Fig, p < 0.0001 relative to either control); here we found that neuronal Cul3-RNAi flies were also sensitive to paraquat injection (Fig 2D, p < 0.0001 relative to either control). Thus, chronic short-sleeping inc null mutants and Cul3-RNAi flies are sensitive to oxidative stress induced by elevated ROS levels, similar to neuronal inc-RNAi flies.
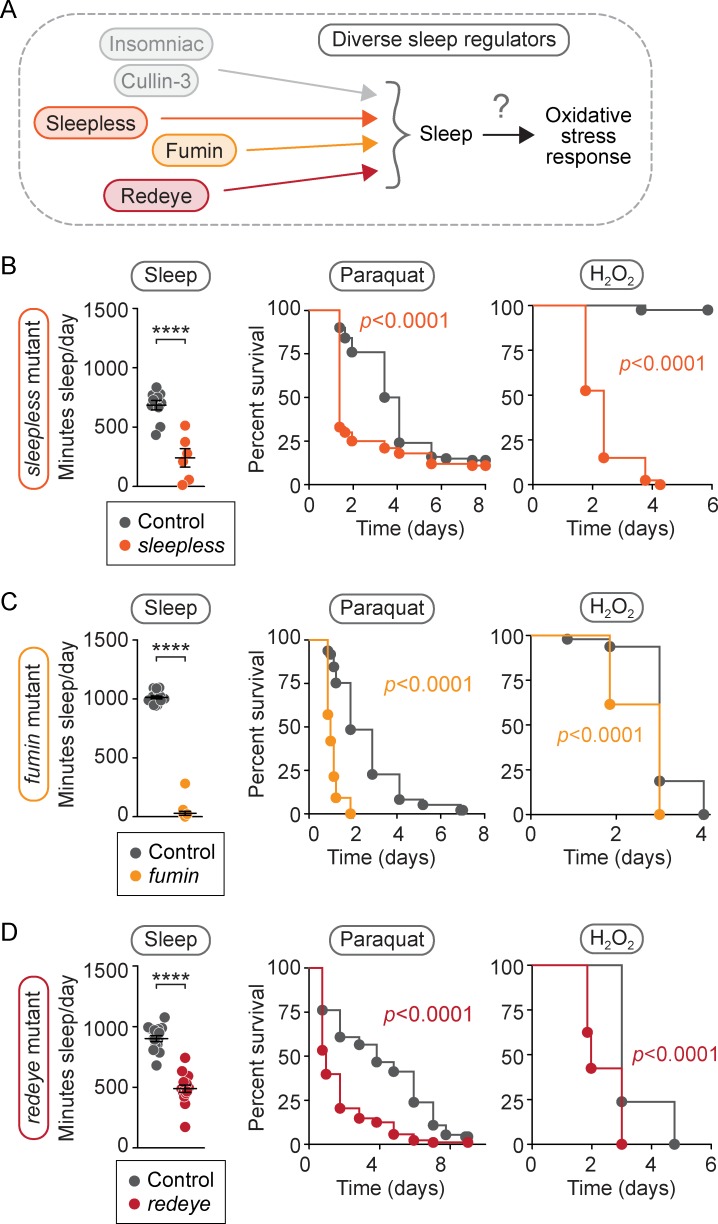
Sensitivity to oxidative stress is common to a diverse group of short-sleeping mutants
To determine whether sensitivity to oxidative stress is caused specifically by the reduction in inc or Cul3 activity or whether it is more broadly associated with loss of sleep, we next tested for sensitivity to oxidative stress in three different short-sleeping mutants, each carrying mutations in different genes with varied functions: sleeplessΔ40 (sleepless), DATfumin (fumin), and nAChRα4rye (redeye) (Fig 3A). We first confirmed, as previously reported [42–44], that each mutant spends significantly less time sleeping than its isogenic control (Fig 3B–3D, left panels, p < 0.0001 for each; S1C, S3A and S3B Fig). We next tested these short-sleeping mutants for sensitivity to oxidative stress. Relative to controls, we found that each mutant was sensitive to both paraquat injection (Fig 3B–3D, middle panels, p < 0.0001 for each) and H2O2 feeding (Fig 3B–3D, right panels, p < 0.0001 for each). Thus, our finding that this molecularly diverse set of short-sleeping mutants has a common susceptibility to oxidative challenge raises the possibility that sleep itself is required for proper response to oxidative stress.
Fig 3. A diverse group of short-sleeping mutants is sensitive to oxidative stress.
We asked (A) whether other sleep mutants unrelated to inc or Cul3 share the same sensitivity to oxidative stress. (B–D, left panels) We found that sleepless mutants slept 65% less than controls (B, p < 0.0001, n = 6–10 flies/genotype), fumin mutants slept 95% less than controls (C, p < 0.0001, n = 15–16 flies/genotype), and redeye mutants slept 50% less than controls (D, p < 0.0001, n = 16 flies/genotype). (B–D, middle panels) When injected with paraquat, sleepless mutants (B, p < 0.0001, n = 100 flies/genotype), fumin mutants (C, p < 0.0001, n = 97–98 flies/genotype), and redeye mutants (D, p < 0.0001, n = 88–92 flies/genotype) died faster than controls. (B–D, right panels) Faster death kinetics were also observed after H2O2 feeding relative to controls for sleepless mutants (B, p < 0.0001, n = 40 flies/genotype), fumin mutants (C, p < 0.0001, n = 39–40 flies/genotype), and redeye mutants (D, p < 0.0001, n = 39–42 flies/genotype). For scatterplots (B–D), each data point represents the average sleep in minutes/day measured across 4–5 days for an individual animal. Data are shown as mean ± SEM and p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test when significance was detected. For survival curves (B–D), p-values were obtained by log-rank analysis. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; inc, insomniac.
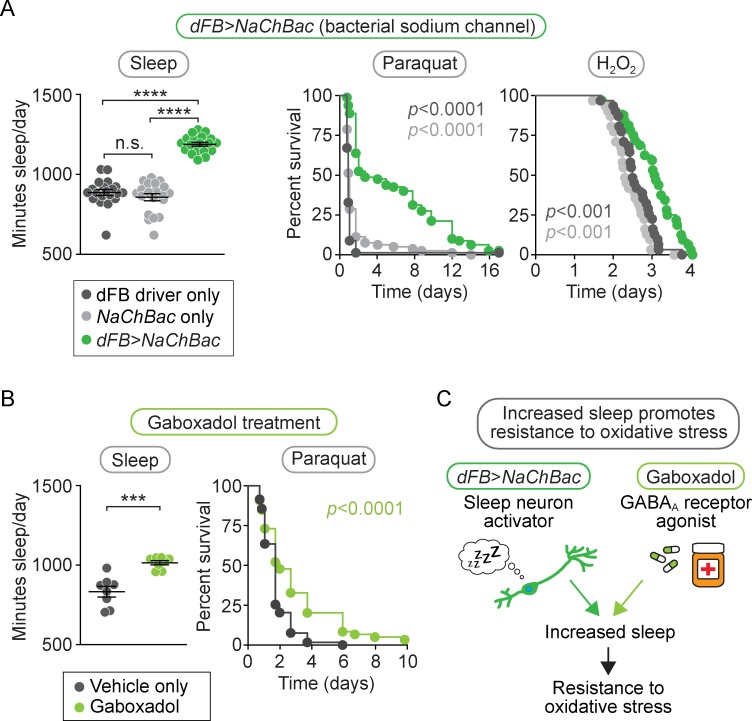
Increasing sleep confers resistance to oxidative stress
Because short-sleeping mutants exhibit sensitivity to oxidative stress, we next tested whether extending sleep duration promotes resistance to oxidative stress. We increased sleep by either genetic manipulation or pharmacological treatment and measured the effect on survival after oxidative challenge. For the genetic approach, we used transgenic flies in which sleep-inducing neurons were activated by the expression of a neuron-activating bacterial sodium channel [21]. For the pharmacological approach, we treated wild-type animals with the sleep-inducing drug Gaboxadol [19,59].
It was previously shown that total sleep time is increased by constitutively activating neurons in the dorsal Fan-shaped Body (dFB), a sleep-promoting region in the fly brain [21]. We verified this phenotype using a previously established dFB driver (23E10-GAL4) [60] to drive expression of the neuron-activating bacterial sodium channel construct UAS-NaChBac [61] and observed a 40% increase in sleep duration in dFB>NaChBac flies (Fig 4A, left panel, p < 0.0001 relative to either control; S3C Fig). We then subjected dFB>NaChBac flies to oxidative stress by either paraquat injection or H2O2 feeding. In both cases, dFB-activated flies died at a slower rate than controls (Fig 4A, middle and right panels, p < 0.001 for each). Thus, genetically activating the dFB to increase sleep promotes resistance to oxidative stress.
Fig 4. Inducing sleep increases resistance to oxidative stress.
(A) dFB>NaChBac flies slept 40% more than controls (left panel, p < 0.0001 compared to either control, n = 20 flies/genotype) and died slower than controls after either paraquat injection (middle panel, p < 0.0001 compared to either control, n = 79–80 flies/genotype) or H2O2 feeding (right panel, p < 0.001 compared to either control, n = 31–32 flies/genotype). (B) Flies fed the GABAA agonist Gaboxadol slept 25% more than controls (left panel, p < 0.001, n = 8 flies/condition) and died slower than controls after paraquat injection (right panel, p < 0.0001, n = 118–119 flies/condition). These data support the conclusion (C) that inducing sleep by either genetic or pharmacological means confers oxidative stress resistance. For scatterplots (A–B, left panels), each data point represents average sleep in minutes/day measured across 4–5 days in an individual animal; data are shown as mean ± SEM. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test when significance was detected (A–B, left panels) or by log-rank analysis (A–B, middle and right panels). Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. dFB, dorsal Fan-shaped Body; GABAA, γ-aminobutyric acid-A.
To further test whether extended sleep duration can increase survival of acute oxidative stress, we used an independent pharmacological method of sleep induction. Wild-type animals were fed the GABAA receptor agonist Gaboxadol, which induces sleep in Drosophila [19,59]. We observed a 25% increase in total sleep time in Gaboxadol-treated animals (Fig 4B, left panel, p < 0.001; S3D Fig) and a corresponding increase in resistance to paraquat injection relative to vehicle-fed controls (Fig 4B, right panel, p < 0.0001). Together, these results demonstrate that two different methods of increasing sleep both promote resistance to oxidative stress, consistent with the idea that oxidative stress resistance is a physiological function of sleep (Fig 4C).
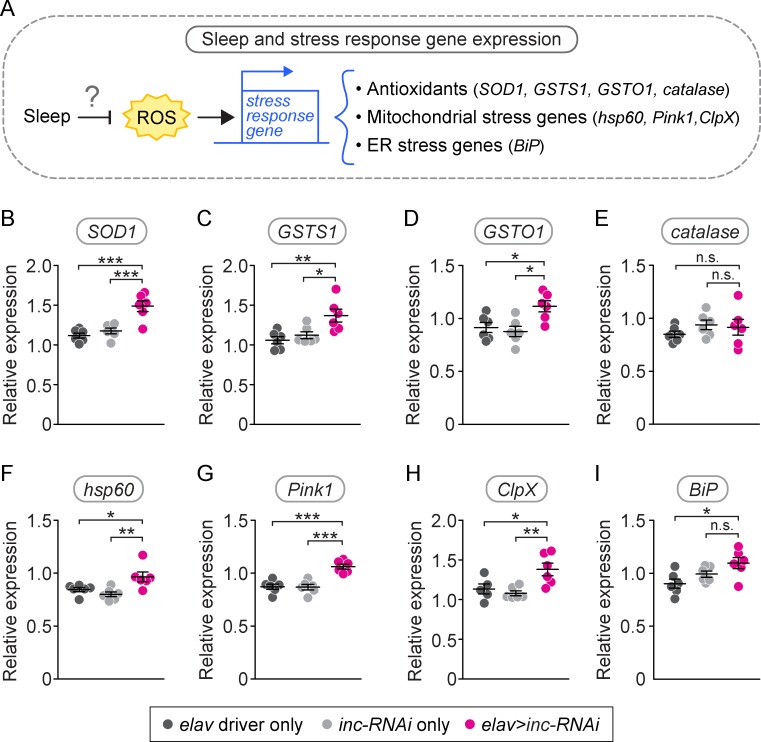
Neuronal knockdown of inc causes altered expression of stress response genes
If sleep clears ROS from neurons, one would expect short-sleeping flies to exhibit higher baseline levels of ROS in the brain. Quantitation of ROS in live brains is extremely difficult, possibly due to tight feedback control of ROS levels via the induction of antioxidant gene expression. As an indirect measure of ROS, we measured the expression of genes known to be activated by high levels of ROS by performing quantitative reverse transcription polymerase chain reaction (qRT-PCR) on the heads of neuronal inc-RNAi flies and controls (Fig 5A). These genes include the antioxidant genes superoxide dismutase 1 (SOD1), catalase, the glutathione-S-transferases GSTS1 and GSTO1, and; the mitochondrial stress response genes hsp60, ClpX, and Pink1; and the endoplasmic reticulum stress response gene BiP, which was previously shown to be induced by sleep deprivation [40,62–64]. We found that neuronal inc-RNAi flies exhibited increased expression of all of these genes except catalase and BiP (Fig 5B–5I). While neuronal inc-RNAi flies had modestly elevated BiP expression in the head (Fig 5I), the difference was not significant. Thus, the increased baseline expression of antioxidant genes and mitochondrial stress genes in neuronal inc-RNAi flies is consistent with short sleep causing increased ROS levels in the brain.
Fig 5. Neuronal inc-RNAi heads have increased expression of stress response genes.
We investigated whether short sleep affects the expression of three main groups of stress response genes: antioxidant genes, mitochondrial stress genes, and one ER stress gene (A). Neuronal inc-RNAi flies exhibited increased baseline head expression of antioxidant genes SOD1 (B, p < 0.001 compared either control, n = 6 biological replicates per genotype), GSTS1 (C, p < 0.05 compared to either control, n = 6 biological replicates per genotype), and GSTO1 (D, p < 0.05 compared to either control, n = 6 biological replicates per genotype), but normal expression of catalase (E, p > 0.05 compared to either control, n = 6 biological replicates per genotype). Neuronal inc-RNAi flies also exhibited increased basal head expression of mitochondrial stress genes hsp60 (F, p < 0.05 compared to either control, n = 6 biological replicates per genotype), Pink1 (G, p < 0.001 compared to either control, n = 6 biological replicates per genotype), and ClpX (H, p < 0.05 compared to either control, n = 5–6 biological replicates per genotype). The ER chaperone gene BiP was elevated compared to one, but not both, controls (p < 0.05 compared to elav control, p > 0.05 compared to inc-RNAi control, n = 6 biological replicates per genotype). Expression was normalized to actin. Data are shown as mean ± SEM. Each data point represents an independent biological replicate with 15–20 individual fly heads per biological replicate. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test when significance was detected. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. ER, endoplasmic reticulum; GST, glutathione-S-transferase; hsp60, heatshock protein 60; Pink1, PTEN-induced putative kinase 1; inc, insomniac; RNAi, RNA interference; SOD1, superoxide dismutase 1.
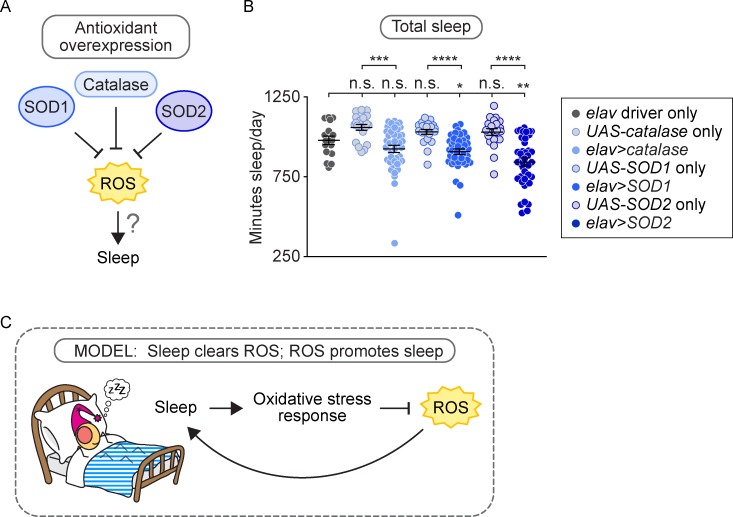
Overexpression of antioxidant genes in neurons reduces sleep
If one function of sleep is to clear ROS from the brain, then it is plausible that ROS itself may be a factor that triggers sleep, perhaps when it reaches a certain critical threshold. To determine whether neuronal ROS levels play a role in the regulation of sleep, we reduced ROS levels in the brains of otherwise wild-type flies by driving neuronal overexpression of the antioxidant genes catalase, SOD1, or SOD2 using the elav-Gal4 driver (Fig 6A). SOD1 or SOD2 overexpression resulted in a significant reduction in the total amount of sleep, with an average decrease in total sleep of 10% and 16%, respectively (Fig 6B, p < 0.05 compared to either control; S3F and S3G Fig). catalase overexpression resulted in a similar trend but did not reach significance compared to the driver control (Fig 6B, S3E Fig). Our observation that reducing neuronal ROS levels reduces sleep amount suggests that ROS levels reflect sleep need and play a role in the regulation of sleep (Fig 6C).
Fig 6. Neuronal overexpression of antioxidants reduces sleep, suggesting a role for ROS in sleep regulation.
(A) Neuronal overexpression of the antioxidant genes SOD1 and SOD2 reduced sleep by 10% (B, p < 0.05 compared to either control, n = 16–40 flies/genotype) and 16% (p < 0.01 compared to either control, n = 16–38 flies/genotype), respectively. Neuronal overexpression of catalase also reduced sleep, but the decrease was not statistically significant compared to the driver control (p > 0.05 compared to elav control, p < 0.001 compared to catalase control, n = 16–40 flies/genotype). Each data point represents average sleep in minutes/day measured across 5 days in an individual animal; data are shown as mean ± SEM. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test when significance was detected. Pooled data from two independent experiments are shown. (B) Model: high ROS levels promote sleep, which in turn clears ROS to promote wake. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. ROS, reactive oxygen species; SOD, superoxide dismutase.
Discussion
Although sleep appears to be evolutionarily conserved across all animal species [1–3], the physiological function of sleep remains unclear. Our data show that chronic sleep restriction sensitizes flies to two types of oxidative stress: paraquat injection and H2O2 feeding (Figs 2 and 3). Conversely, increasing sleep through either genetic or pharmacological methods promotes resistance to oxidative stress (Fig 4). Thus, our data suggest that one important function of sleep is defense against oxidative stress.
The molecular mechanisms underlying the susceptibility of short-sleeping mutants to acute oxidative stress and whether this susceptibility is due to the effects of oxidative stress on the brain or other, nonneuronal tissues of the body remains unclear. It is possible that increased baseline ROS levels in neurons or other tissues sensitize short sleepers to acute oxidative stress. Other investigators have found that accumulation of cellular ROS was associated with susceptibility to acute oxidative challenge [65,66]. Chronic sleep loss may lead to accumulated mitochondrial damage that, in the presence of an acute oxidative stress, triggers cell death pathways. Another possibility is that short sleepers are less able to detect or respond to acute oxidative challenge in specific tissues. Testing these hypotheses will be an important focus for future investigation.
Our data also suggest that short-sleeping animals accumulate higher baseline ROS levels in the brain. While ROS levels in the brain are difficult to measure directly, we observed increased expression of antioxidant and mitochondrial stress response genes in the heads of short-sleeping neuronal inc-RNAi flies, consistent with increased ROS levels in the brain. Other studies have similarly observed that sleep-deprived animals display increased expression of genes induced by high ROS levels. Induction of the antioxidant regulator cap’n’collar (cnc) was observed in fly heads when flies were exposed to recurrent sleep fragmentation [67], and its mammalian homolog Nuclear factor (erythroid-derived 2)-like 2 (nrf2) was reported to be induced in the cerebral cortex of mice after 6 hours of sleep deprivation [68]. Sleep deprivation has also been associated with activation of the unfolded protein response in the ER in fly heads and mouse brains [40,62–64]. Because both the ER- and mitochondrial unfolded protein responses can be induced by high levels of ROS, we hypothesize that both genetic and environmental sleep loss increase baseline ROS levels that, depending on the specific method of sleep deprivation, genetic background, and tissue tested, are reflected in the activation of different response pathways.
Finally, we found that increasing antioxidant gene expression in the brain causes short sleep, suggesting that decreasing neuronal ROS levels will promote the wake state. Emerging evidence demonstrates that ROS can act as crucial signaling molecules in a number of biological processes [69,70], and it has been demonstrated that injecting an oxidant into the rat brain induces sleep [71]. One study showed modest effects of lifelong, low-dose paraquat feeding on sleep in flies [72]. Thus, ROS levels, either directly or indirectly through the activation of oxidative stress responses, appear to induce sleep.
Taken together, our results support a model for a bidirectional relationship between sleep and oxidative stress, in which one function of sleep is to act as an antioxidant for both the body and the brain, increasing the organism’s resistance to acute oxidative challenge and reducing ROS levels in the brain; moreover, neuronal ROS play a role in the regulation of sleep and wake states (Fig 6C). Thus, with chronic sleep restriction, the animal accumulates higher ROS levels in the brain and is sensitive to acute oxidative stress.
Identifying the physiological functions and key regulators of sleep is critical to understanding the negative effects on health associated with chronic sleep restriction. In the United States, average sleep time is steadily decreasing [73], and one third of adults sleep less than the recommended 7 hours per night [38]. Sleep restriction is correlated with a variety of diseases [12,13], many of which are also associated with oxidative stress [74–78]. Sleep disturbances have been implicated as a predictor for Alzheimer, Parkinson, and Huntington’s diseases [79–82], and in all of these diseases, oxidative damage has been reported in the brains of patients postmortem [83–85]. Because oxidative stress can induce protein misfolding and aggregation through protein damage, neuronal accumulation of ROS is a plausible contributing factor in the pathogenesis of neurodegenerative diseases. Thus, understanding the role of sleep in defense against oxidative stress and the role of ROS in regulating sleep could provide much-needed insight into the pathology and treatment of neurodegenerative diseases.
Materials and methods
Results from all experiments are summarized in S1 Table in the Supporting information, and raw data are available upon request.
Fly strains and rearing conditions
The following flies were used to manipulate inc and Cul3 as described previously [45]: UAS-inc-RNAi (VDRC stock #18225), elavC155-Gal4, UAS-Dicer (dcr) (Bloomington stock #24651), inc1 deletion mutant, and inc2 transposon insertion mutant (CG32810f00285), all in the same genetic background (w1118 iso31 or Bloomington stock #5905), along with the isogenic iso31 strain used for outcrossing. UAS-Cul3-RNAi (NIG stock #11861R-2) was in the NIG w1118 background and compared to its isogenic control. For neuronal Cul3 knockdown experiments, the UAS-Dicer line (Bloomington stock #24651) was crossed into the elavC155-Gal4 line. Parental controls used for experiments were obtained by crossing expression driver (e.g., elav-Gal4) and RNAi construct (e.g., UAS-inc-RNAi) lines to the outcrossed wild-type line (e.g., iso31) for heterozygous controls, accounting for differences in complex phenotypes affected by genetic background. In case the absence of the white gene, which encodes an ABC transporter, has an effect on survival after paraquat or H2O2 exposure, red-eyed controls were used with the red- and orange-eyed inc1 and inc2 mutants; these w+ controls were generated by outcrossing w+ from an Oregon-R background for eight generations with the iso31 stock (Bloomington stock #5905).
redeye, sleeplessΔ40 (imprecise excision mutants), and their corresponding background-matched controls were obtained from Amita Sehgal (University of Pennsylvania). sleeplessΔ40 was used instead of sleeplessP1 because sleeplessP1 flies were sensitive to CO2, which made paraquat injection experiments difficult to interpret. Male sleeplessΔ40 flies also exhibited some wounding sensitivity, whereas females did not, so female sleeplessΔ40 flies were used in the paraquat injection experiments (S4 Fig). Male sleeplessΔ40 were used in H2O2 feeding experiments. fumin mutants and their background-matched controls were obtained from Rob Jackson (Tufts University).
UAS-NaChBac [61] was obtained from Paul Shaw (Washington University, St. Louis, MO) and 23E10-Gal4 [60] was obtained from Jeffrey Donlea (University of Oxford); both were outcrossed for eight generations with the iso31 stock. As described above, parental controls used for experiments were obtained by crossing expression driver (23E10-Gal4) and transgene construct (UAS-NaChBac) lines to the outcrossed wild-type line (iso31) for heterozygous controls.
The following stocks were obtained from the Bloomington Stock Center (BDSC, Bloomington, IN) and outcrossed 6–8 generations into the iso31 background: UAS-SOD1 (#24754), UAS-SOD2 (#24492), and UAS-cat (#24621).
All flies were raised at room temperature on standard molasses food (5.85% cornmeal, 2.675% yeast, 0.575% agar, 3% v/v blackstrap molasses, 0.14% methylparaben, 0.5% v/v propionic acid) and kept on cornmeal food (4% cornmeal, 2.15% yeast, 9% dextrose, 0.75% agar, 0.095% methylparaben) post-eclosion in a temperature- (25°C) and humidity- (55%) controlled incubator with a 12-hour light–dark cycle. Four- to ten-day-old males were used for all experiments, unless otherwise noted.
Sleep analysis and starvation assay
Individual flies were loaded into plastic tubes containing cornmeal food and allowed to acclimate for 1 day. Sleep was monitored for 4–5 days using Drosophila Activity Monitors (either DAM2s or DAM5s, an older model of DAM5M with a single beam per tube) (Trikinetics, Waltham, MA). Activity was recorded as beam-breaks in 1-minute bins and analyzed using PySolo software [86] or Microsoft Excel, with sleep defined as a 5-minute period of inactivity. Graphing and statistical analysis were performed using GraphPad Prism (survival assays and scatterplots) and PySolo (24-hour sleep profiles). When comparing two groups: an unpaired t test was performed when standard deviations were similar, and an unpaired t test with Welch’s correction was performed when standard deviations were not similar (F test p < 0.5). When comparing three groups, a one-way ANOVA was performed and followed by a post hoc Tukey test to compare means when significance was detected.
For starvation assays, flies were transferred to tubes containing 1% agar and loaded into Drosophila Activity Monitors. Time of death was determined by complete loss of movement.
Lifespan
Flies were collected on the day of eclosion and allowed to mate overnight. Total flies per genotype ranged from 74 to 225. Numbers were roughly equivalent for each group within different trials. Males were separated into groups of 20 per vial. Flies were transferred to new vials every 2–7 days and scored for death at time of transfer. Lifespan experiments were performed in at least two independent trials.
Bacterial and paraquat injections
Injections were carried out with a pulled glass capillary needle. A custom-made microinjector (Tritech Research, Los Angeles, CA) was used to inject 50 nL of liquid into the abdomen of each fly. Volume was calibrated by measuring the diameter of the expelled drop under oil. Death was assayed visually at least daily, with a typical n = 60 for both bacterial infections and paraquat injections. For each experiment, a smaller set of flies was injected with vehicle alone to ensure that wounding caused minimal death.
The following bacterial strains were used for injections: S. pneumoniae (strain SP1, a streptomycin-resistant variant of D39) obtained from Elizabeth Joyce (University of California, San Francisco, CA) was grown standing in Brain Heart Infusion media (BHI, Teknova, Hollister, CA) at 37°C with 5% CO2, frozen into aliquots with 10% glycerol, pelleted and resuspended upon thawing, and injected at an OD600 of 0.015–0.05; P. rettgeri (strain Dmel, a natural pathogen isolated from wild-caught D. melanogaster [87]) obtained from Brian Lazzaro (Cornell University) was grown shaking in LB at 37°C and injected at an OD600 of 0.003–0.005; L. monocytogenes (strain 10403S) obtained from Julie Theriot (Stanford University) was grown standing in BHI at 37°C and injected at an OD600 of 0.075–0.2; and S. aureus strain 12600 (ATCC) was grown shaking in BHI at 37°C and injected at an OD600 of 0.0001–0.001. Postinjection, flies were kept in a 29°C incubator for the remainder of the experiment to allow for optimal infection, with the exception of P. rettgeri injection, in which case optimal infection was achieved at 25°C. All OD600 measurements were made using a Genesys 10S Vis Spectrophotometer (ThermoScientific, Waltham, MA), blanked against the corresponding sterile media for the given culture. Cultures were then diluted in sterile media to the desired OD.
For paraquat injections, paraquat (methyl viologen hydrate, Fisher Scientific, Hampton, NH) was dissolved in water to a concentration of 3–5 mM. Paraquat solution was either stored at 4°C for up to 1 month or frozen in aliquots and thawed as needed. For every experimental genotype treated with paraquat injection, we conducted mock injections with ddH2O to control for wounding sensitivity (S4 Fig).
H2O2 feeding assays
These assays were performed in two ways. In one method, flies were transferred to vials containing a folded Kimwipe soaked with 1.5 mL of a 5% sucrose, 1%–4% H2O2 solution. Thirty percent H2O2 (Sigma-Aldrich, St. Louis, MO) was diluted in ddH2O to a concentration of 1%–4% depending on the death rate for the given genotype, titrated to complete death within several days. Flies were flipped onto a freshly soaked Kimwipe every 2 days and death was assayed visually and recorded daily. This method allows very rapid setup (typical experiment used 40 flies/genotype) but provides relatively low-resolution survival kinetics. In the second method, flies were transferred to 5 mm tubes containing a piece of a soaked Kimwipe and loaded into Drosophila Activity Monitors, in which case death was determined by a complete loss of movement. Control flies were kept on 5% sucrose alone to ensure that death did not occur by starvation or desiccation. This method provides high-resolution survival kinetics but requires more time-intensive setup (typical experiment used 30 flies/genotype). We found that all our results for short-sleeping mutants were consistent between the two methods.
Survival curves
Survival curves for starvation assays, lifespan experiments, bacterial infections, paraquat injections, and H2O2 feeding assays are all plotted as Kaplan-Meier graphs. Log-rank analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). All experiments were performed with a minimum of three independent trials and yielded statistically similar results, except where noted. Graphs and p-values in figures are from representative trials.
qRT-PCR
Age-matched, 6–8-day-old flies were anesthetized on ice and decapitated between ZT2 and ZT5. Fifteen to twenty heads per sample were homogenized in TRIzol (Invitrogen), and a phenol-chloroform extraction was performed to isolate nucleic acids. Samples were treated with DNAse (Invitrogen, Carlsbad, CA) to isolate RNA and then diluted to a concentration of about 60 ng/μL. RevertAid First Strand cDNA synthesis kit (ThermoFisher, Waltham, MA) was used to convert RNA to cDNA. Quantitative RT-PCR was performed using a Bio-Rad CFX Connect Real-Time qPCR machine, with Express Sybr GreenER qPCR SuperMix (Invitrogen, Carlsbad, CA) and the following primer sets:
SOD1:
For: GGAGTCGGTGATGTTGACCT
Rev: GGAGTCGGTGATGTTGACCT
GSTS1:
For: CACCAGAGCATTTCGATGGCT
Rev: ACGACTGCAATTTTTAGACGGA
GSTO1:
For: ACGACTGCAATTTTTAGACGGA
Rev: CCGATCGCCGGGAGTTCATGTAT
catalase:
For: TTCTGGTTATCCCGTTGAGC
Rev: GGTAATGGCACCAGGAGAAA
hsp60:
For: TGATGCTGATCTCGTCAAGC
Rev: TACTCGGAGGTGGTGTCCTC
ClpX:
For: AAAATGCTCGAAGGCACAGT
Rev: TTGAGACGACGTGCGATAAG
Pink1:
For: TCGGTGGTCAATGTAGTGC
Rev: CCACTCGGAAGATTCCACTGC
BiP:
For: GCTATTGCCTACGGTCTGGA
Rev: CATCACACGCTGATCGAAGT
actin:
For: TTGTCTGGGCAAGAGGATCAG
Rev: ACCACTCGCACTTGCACTTTC
Analysis was performed using the Standard Curve method. Total cDNA concentration was normalized to actin expression. Data are represented as mean ± SEM. Five to six biological replicates (containing 15–20 heads each) per experiment.
Gaboxadol and antioxidant feeding
Gaboxadol hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in water and added to melted cornmeal food to a final concentration of 0.1–0.2 mg/mL. Flies were flipped onto Gaboxadol-containing food for 3 days prior to paraquat injection and remained on Gaboxadol-containing food postinjection. Control food was made by adding the appropriate amount of vehicle alone (H2O) to melted cornmeal food.
Supporting information
(A) Twenty-four-hour sleep plot for neuronal inc-RNAi flies and controls. Neuronal inc-RNAi flies died at the same or a slightly slower rate than genetic controls after injection with Listeria monocytogenes (B, p = 0.09 compared to elav control, p = 0.04 compared to inc-RNAi control, n = 62–63 flies/genotype) and died at the same rate as controls after injection with Staphylococcus aureus (C, p > 0.05 compared to either control, n = 19–21 flies/genotype). (D) Twenty-four-hour sleep plot for fumin mutants and controls. fumin mutants died slower than controls after injection with Streptococcus pneumoniae (E, p < 0.01, n = 96–98 flies/genotype), died faster than controls after injection with Providencia rettgeri (F, p < 0.0001, n = 89–91 flies/genotype), died slower than controls after injection with L. monocytogenes (G, p < 0.01, n = 77–79 flies/genotype), and died at the same rate as controls after injection with S. aureus (H, p > 0.05, n = 94–100 flies/genotype). p-values were obtained by log-rank analysis. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. inc, insomniac; RNAi, RNA interference.
(TIF)
inc1 and inc2 null mutants slept about 50% less than controls (A, p < 0.0001 for both mutants, n = 20–22 flies/ genotype). elav;;dcr>Cul3-RNAi flies slept about 60% less than controls (B, p < 0.0001 compared to either control, n = 40–42 flies/genotype). Each data point in scatterplots (left) represents average sleep in minutes/day measured across 4–5 days in an individual animal. Data are shown as mean ± SEM. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test. Twenty-four-hour sleep plots (right) show sleep profiles for mutants and controls averaged over a 4–5-day period. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; dcr, UAS-Dicer; inc, insomniac; RNAi, RNA interference.
(TIF)
Shown here are the 24-hour sleep plots, averaged over 4–5 days, for the indicated short-sleeping flies, with their relevant controls. (A) sleepless mutants and controls; relates to Fig 3B. (B) redeye mutants and controls; relates to Fig 3D. (C) dFB>NaChBac flies and controls; relates to Fig 4A. (D) Gaboxadol-fed flies compared with vehicle only; relates to Fig 4B. (E–G) Neuronal overexpression of Catalase, SOD1, and SOD2, compared with controls; relates to Fig 6. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. dFB, dorsal Fan-shaped Body; SOD, superoxide dismutase.
(TIF)
Shown here are representative H2O-injected wounding controls for each of the genotypes subjected to paraquat injection: (A) neuronal inc-RNAi (relates to Fig 2B); (B) inc null mutants (relates to Fig 2C); (C) neuronal Cul3-RNAi (relates to Fig 2D); (D) sleepless mutants (relates to Fig 3B); (E) fumin mutants (relates to Fig 3C); (F) redeye mutants (relates to Fig 3D); (G) dFB>NaChBac flies (relates to Fig 4A); and (H) iso31 controls (relates to Fig 4B). In all cases, flies injected with paraquat died significantly faster (p < 0.5 by log-rank analysis) than H2O-injected controls. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; dFB, dorsal Fan-shaped Body; inc, insomniac; RNAi, RNA interference.
(TIF)
Summary of results from experimental trials. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request.
(XLSX)
Raw data from representative experiments are organized here by figure panel; raw data from all trials are available upon request.
(XLSX)
Acknowledgments
We thank Shirasu-Hiza and Canman lab members for support, productive discussions, and feedback on this manuscript; Charlotte Wayne for technical support; and Amita Sehgal, Rob Jackson, Paul Shaw, and Jeff Donlea for fly lines. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Abbreviations
- BHI
Brain Heart Infusion media
- Cul3
Cullin-3
- DAT
dopamine transporter
- dcr
Dicer
- dFB
dorsal Fan-shaped Body
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- inc
insomniac
- nAChR
nicotinic acetylcholine receptor
- RNAi
RNA interference
- ROS
reactive oxygen species
- SEM
standard error of the mean
- UAS
upstream activation sequence
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SOD
superoxide dismutase
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Amgen Scholars Program (G.B.S., summer undergraduate research fellowship). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. I.I. Rabi Scholars Program (I.S.I., undergraduate research funding). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NIH (grant number R01GM117407) (J.C.C.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NIH (grant number DP2OD008773) (J.C.C.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Hirschl Foundation (M.S.H.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NIH (grant number R01GM105775 and R35 GM127049) (M.S.H.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NIH (grant number R01AG045842) (M.S.H.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. NIH (grant number 2T32GM007367-42) (R.M.O., MSTP training grant). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. G. Harold & Leila Y. Mathers Foundation and Irma T. Hirschl Career Scientist Award from the Irma T. Hirschl / Weill-Caulier Trust Grant (N.S.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Campbell SS, Tobler I. Animal sleep: A review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8: 269–300. 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- 2.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6: 1605–1611. 10.1371/journal.pbio.0060216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joiner WJ. Unraveling the Evolutionary Determinants of Sleep. Curr Biol. Elsevier Ltd; 2016;26: R1073—R1087. 10.1016/j.cub.2016.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27: 628–639. 10.1093/sleep/27.4.628 [DOI] [PubMed] [Google Scholar]
- 5.Sauer S, Herrmann E, Kaiser W. Sleep deprivation in honey bees. J Sleep Res. 2004;13: 145–152. 10.1111/j.1365-2869.2004.00393.x [DOI] [PubMed] [Google Scholar]
- 6.Stephenson R, Chu KM, Lee J. Prolonged deprivation of sleep-like rest raises metabolic rate in the Pacific beetle cockroach, Diploptera punctata (Eschscholtz). J Exp Biol. 2007;210: 2540–2547. 10.1242/jeb.005322 [DOI] [PubMed] [Google Scholar]
- 7.Vyazovskiy V V, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050: 64–71. 10.1016/j.brainres.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 8.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science (80-). 2006;313: 1775–1781. 10.1126/science.1130408 [DOI] [PubMed] [Google Scholar]
- 9.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23: 2739–2748. 10.1111/j.1460-9568.2006.04808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25: 18–24. [DOI] [PubMed] [Google Scholar]
- 11.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417: 287–291. 10.1038/417287a [DOI] [PubMed] [Google Scholar]
- 12.Nedeltcheva A V, Scheer FAJL. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21: 293–298. 10.1097/MED.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappuccio FP, Cooper D, Delia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal. 2011. pp. 1484–1492. 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 14.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. Elsevier Ltd; 2012;16: 137–149. 10.1016/j.smrv.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, et al. Associations Between Change in Sleep Duration and Inflammation: Findings on C-reactive Protein and Interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178: 956–961. 10.1093/aje/kwt072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011. pp. 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: A population-based 22-year follow-up study. Sleep. 2007;30: 1245–1253. 10.1093/sleep/30.10.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallicchio L, Kalesan B. Sleep duration and mortality: A systematic review and meta-analysis. J Sleep Res. 2009;18: 148–158. 10.1111/j.1365-2869.2008.00732.x [DOI] [PubMed] [Google Scholar]
- 19.Dissel S, Angadi V, Kirszenblat L, Suzuki Y, Donlea J, Klose M, et al. Sleep restores behavioral plasticity to drosophila mutants. Curr Biol. Elsevier Ltd; 2015;25: 1270–1281. 10.1016/j.cub.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic Mutants Have Reduced Sleep and Impaired Memory. J Neurosci. 2007;27: 5384–5393. 10.1523/JNEUROSCI.0108-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing Sleep by Remote Control Facilitates Memory Consolidation in Drosophila. Science (80-). 2011;332: 1571–1576. 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gais S. Sleep after learning aids memory recall. Learn Mem. 2006;13: 259–262. 10.1101/lm.132106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982. pp. 195–204. 10.1111/jsr.12371 [DOI] [PubMed] [Google Scholar]
- 24.Allada R, Cirelli C, Sehgal A. Molecular mechanisms of sleep homeostasis in flies and mammals. Cold Spring Harb Perspect Biol. 2017;9: 1–20. 10.1101/cshperspect.a027730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441: 757–760. 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Robinson JE, Joiner WJ. SLEEPLESS is a bifunctional regulator of excitability and cholinergic synaptic transmission. Curr Biol. Elsevier Ltd; 2014;24: 621–629. 10.1016/j.cub.2014.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidner G, Robinson JE, Wu M, Worden K, Masek P, Roberts SW, et al. Identification of Neurons with a Privileged Role in Sleep Homeostasis in Drosophila melanogaster. Curr Biol. Elsevier Ltd; 2015;25: 2928–2938. 10.1016/j.cub.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Liu Q, Tabuchi M, Wu MN. Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell. Elsevier Inc.; 2016;165: 1347–1360. 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donlea JM, Alam MN, Szymusiak R. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol. Elsevier Ltd; 2017;44: 228–235. 10.1016/j.conb.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 30.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43: 231–233. 10.1016/0306-9877(94)90071-X [DOI] [PubMed] [Google Scholar]
- 31.D’Almeida V, Lobo LL, Hipólide DC, de Oliveira a C, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9: 2853–2856. 10.1097/00001756-199808240-00031 [DOI] [PubMed] [Google Scholar]
- 32.Eiland MM, Ramanathan L, Gulyani S, Siegel JM, Eiland MM, Ramanathan L, et al. Increases in amino-cupric-silver staining of the supraoptic nucleus after sleep deprivation. Brain Res. 2002;945: 1–8. 10.1016/S0006-8993(02)02448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13: 1387–1390. 10.1097/00001756-200208070-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46: 895–903. 10.1016/j.neuropharm.2003.11.032 [DOI] [PubMed] [Google Scholar]
- 35.D’Almeida V, Hipolide DC, Azzalis LA, Lobo LL, Junqueira VB, Tufik S. Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci Lett. 1997;235: 25–28. Available: http://www.ncbi.nlm.nih.gov/pubmed/9389587 [DOI] [PubMed] [Google Scholar]
- 36.Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Res. 1999;840: 184–193. 10.1016/S0006-8993(99)01768-0 [DOI] [PubMed] [Google Scholar]
- 37.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep J Sleep Sleep Disord Res. 2004;27: 27–35. Available: http://ezproxy.lib.uh.edu/login?url= http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2004-17199-006&site=ehost-live%5Cn http://ccirelli@wisc.edu [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65: 137–141. 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 39.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, et al. Rest in Drosophila Is a Sleep-like State. Neuron. 2000;25: 129–138. 10.1016/S0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- 40.Shaw PJ. Correlates of Sleep and Waking in Drosophila melanogaster. Science (80-). 2000;287: 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- 41.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434: 1087–1092. 10.1038/nature03486 [DOI] [PubMed] [Google Scholar]
- 42.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a Sleep-Promoting Factor. Science (80-). 2008;321: 372–376. 10.1126/science.1155942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi M, Yue Z, Kuryatov A, Lindstrom JM, Sehgal A. Identification of Redeye, a new sleepregulating protein whose expression is modulated by sleep amount. Elife. 2014;2014: 1–17. 10.7554/eLife.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume K. Dopamine Is a Regulator of Arousal in the Fruit Fly. J Neurosci. 2005;25: 7377–7384. 10.1523/JNEUROSCI.2048-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stavropoulos N, Young MW. Insomniac and cullin-3 regulate sleep and wakefulness in drosophila. Neuron. Elsevier Inc.; 2011;72: 964–976. 10.1016/j.neuron.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffenberger C, Allada R. Cul3 and the BTB Adaptor Insomniac Are Key Regulators of Sleep Homeostasis and a Dopamine Arousal Pathway in Drosophila. PLoS Genet. 2012;8 10.1371/journal.pgen.1003003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harbison ST, Negron YLS, Hansen NF, Lobell AS. Selection for long and short sleep duration in Drosophila melanogaster reveals the complex genetic network underlying natural variation in sleep. PLoS Genet. 2017;13: e1007098 10.1371/journal.pgen.1007098 eCollection 2017 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1: 210–217. 10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Carreras A, Lee S, Hakim F, Zhang SX, Nair D, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity. 2014;22: 758–762. 10.1002/oby.20616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami K, Yurgel ME, Stahl BA, Masek P, Mehta A, Heidker R, et al. Translin Is Required for Metabolic Regulation of Sleep. Curr Biol. 2016;26: 972–980. 10.1016/j.cub.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masek P, Reynolds LA, Bollinger WL, Moody C, Mehta A, Murakami K, et al. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J Exp Biol. 2014;217: 3122–3132. 10.1242/jeb.103309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally Assessed Sleep and Susceptibility to the Common Cold. Sleep. 2015;38: 1353–1359. 10.5665/sleep.4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irwin MR, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10: 643–653. [DOI] [PubMed] [Google Scholar]
- 54.Lungato L, Gazarini ML, Paredes-Gamero EJ, Tufik S, D’Almeida V. Paradoxical sleep deprivation impairs mouse survival after infection with malaria parasites. Malar J. 2015;14 10.1186/s12936-015-0690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zager a, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293: R504—9. 10.1152/ajpregu.00105.2007 [DOI] [PubMed] [Google Scholar]
- 56.Kuo T-H, Williams JA. Increased sleep promotes survival during a bacterial infection in Drosophila. Sleep. 2014;37: 1077–86, 1086A—1086D. 10.5665/sleep.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283: 1786–1798. 10.1074/jbc.M708597200 [DOI] [PubMed] [Google Scholar]
- 58.Grover D, Ford D, Brown C, Hoe N, Erdem A, Tavaré S, et al. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS ONE. 2009;4 10.1371/journal.pone.0007580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berry JA, Cervantes-Sandoval I, Chakraborty M, Davis RL. Sleep Facilitates Memory by Blocking Dopamine Neuron-Mediated Forgetting. Cell. Elsevier Inc.; 2015;161: 1656–1667. 10.1016/j.cell.2015.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, et al. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep. 2012;2: 991–1001. 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luan H. Functional Dissection of a Neuronal Network Required for Cuticle Tanning and Wing Expansion in Drosophila. J Neurosci. 2006;26: 573–584. 10.1523/JNEUROSCI.3916-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92: 1150–1157. 10.1111/j.1471-4159.2004.02952.x [DOI] [PubMed] [Google Scholar]
- 63.Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep horneostasis. Sleep. 2007;30: 557–565. [DOI] [PubMed] [Google Scholar]
- 64.Brown MK, Strus E, Naidoo N. Reduced sleep during social isolation leads to cellular stress and induction of the unfolded protein response. Sleep. 2017;40 10.1093/sleep/zsx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang S, Le PK, Tse S, Wallace DC, Huang T. Heterozygous mutation of Opa1 in Drosophila shortens lifespan mediated through increased reactive oxygen species production. PLoS ONE. 2009;4 10.1371/journal.pone.0004492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102: 13670–13675. 10.1073/pnas.0504610102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams MJ, Perland E, Eriksson MM, Carlsson J, Erlandsson D, Laan L, et al. Recurrent sleep fragmentation induces insulin and neuroprotective mechanisms in middle-aged flies. Front Aging Neurosci. 2016;8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikonova E V, Naidoo N, Zhang L, Romer M, Cater JR, Scharf MT, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33: 889–900. 10.1093/sleep/33.7.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. Elsevier; 2014;24: R453—R462. 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: The new wave? Trends Plant Sci. Elsevier Ltd; 2011;16: 300–309. 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 71.Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, et al. Brain oxidation is an initial process in sleep induction. Neuroscience. 2005;130: 1029–1040. 10.1016/j.neuroscience.2004.09.057 [DOI] [PubMed] [Google Scholar]
- 72.Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci. 2006;103: 13843–13847. 10.1073/pnas.0605903103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47: 921–927. 10.1016/S0006-3223(99)00169-9 [DOI] [PubMed] [Google Scholar]
- 74.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010. pp. 1058–1070. 10.1161/CIRCRESAHA.110.223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, et al. Diabetic cardiovascular disease induced by oxidative stress. International Journal of Molecular Sciences. 2015. pp. 25234–25263. 10.3390/ijms161025234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91: 7–11. 10.1016/S0002-9149(02)03144-2 [DOI] [PubMed] [Google Scholar]
- 77.Youn J-YY, Siu KL, Lob HE, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63: 2344–2355. 10.2337/db13-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carvajal K, Balderas-Villalobos J, Bello-Sanchez MD, Phillips-Farfán B, Molina-Muñoz T, Aldana-Quintero H, et al. Ca2+ mishandling and cardiac dysfunction in obesity and insulin resistance: Role of oxidative stress. Cell Calcium. 2014. pp. 408–415. 10.1016/j.ceca.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 79.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep Disturbance is Associated with Incident Dementia and Mortality. Curr Alzheimer Res. 2013;10: 767–775. 10.2174/15672050113109990134 [DOI] [PubMed] [Google Scholar]
- 80.Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36: 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med. 2013;14: 744–748. 10.1016/j.sleep.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 82.Lazar AS, Panin F, Goodman AOG, Lazic SE, Lazar ZI, Mason SL, et al. Sleep deficits but no metabolic deficits in premanifest Huntington’s disease. Ann Neurol. 2015;78: 630–648. 10.1002/ana.24495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allan Butterfield D, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiology of Aging. 2002. pp. 655–664. 10.1016/S0197-4580(01)00340-2 [DOI] [PubMed] [Google Scholar]
- 84.Dexter DT, Carter CJ, Wells FR, Javoy‐Agid F, Agid Y, Lees A, et al. Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease. J Neurochem. 1989;52: 381–389. 10.1111/j.1471-4159.1989.tb09133.x [DOI] [PubMed] [Google Scholar]
- 85.Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med. 2008;45: 667–678. 10.1016/j.freeradbiomed.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 86.Gilestro GF, Cirelli C. PySolo: A complete suite for sleep analysis in Drosophila. Bioinformatics. 2009;25: 1466–1467. 10.1093/bioinformatics/btp237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galac MR, Lazzaro BP. Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect. 2011;13: 673–683. 10.1016/j.micinf.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Twenty-four-hour sleep plot for neuronal inc-RNAi flies and controls. Neuronal inc-RNAi flies died at the same or a slightly slower rate than genetic controls after injection with Listeria monocytogenes (B, p = 0.09 compared to elav control, p = 0.04 compared to inc-RNAi control, n = 62–63 flies/genotype) and died at the same rate as controls after injection with Staphylococcus aureus (C, p > 0.05 compared to either control, n = 19–21 flies/genotype). (D) Twenty-four-hour sleep plot for fumin mutants and controls. fumin mutants died slower than controls after injection with Streptococcus pneumoniae (E, p < 0.01, n = 96–98 flies/genotype), died faster than controls after injection with Providencia rettgeri (F, p < 0.0001, n = 89–91 flies/genotype), died slower than controls after injection with L. monocytogenes (G, p < 0.01, n = 77–79 flies/genotype), and died at the same rate as controls after injection with S. aureus (H, p > 0.05, n = 94–100 flies/genotype). p-values were obtained by log-rank analysis. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. inc, insomniac; RNAi, RNA interference.
(TIF)
inc1 and inc2 null mutants slept about 50% less than controls (A, p < 0.0001 for both mutants, n = 20–22 flies/ genotype). elav;;dcr>Cul3-RNAi flies slept about 60% less than controls (B, p < 0.0001 compared to either control, n = 40–42 flies/genotype). Each data point in scatterplots (left) represents average sleep in minutes/day measured across 4–5 days in an individual animal. Data are shown as mean ± SEM. p-values were obtained by ordinary one-way ANOVA followed by a post hoc Tukey test. Twenty-four-hour sleep plots (right) show sleep profiles for mutants and controls averaged over a 4–5-day period. Data from representative experiments are shown. Each experiment was performed at least three times. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; dcr, UAS-Dicer; inc, insomniac; RNAi, RNA interference.
(TIF)
Shown here are the 24-hour sleep plots, averaged over 4–5 days, for the indicated short-sleeping flies, with their relevant controls. (A) sleepless mutants and controls; relates to Fig 3B. (B) redeye mutants and controls; relates to Fig 3D. (C) dFB>NaChBac flies and controls; relates to Fig 4A. (D) Gaboxadol-fed flies compared with vehicle only; relates to Fig 4B. (E–G) Neuronal overexpression of Catalase, SOD1, and SOD2, compared with controls; relates to Fig 6. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. dFB, dorsal Fan-shaped Body; SOD, superoxide dismutase.
(TIF)
Shown here are representative H2O-injected wounding controls for each of the genotypes subjected to paraquat injection: (A) neuronal inc-RNAi (relates to Fig 2B); (B) inc null mutants (relates to Fig 2C); (C) neuronal Cul3-RNAi (relates to Fig 2D); (D) sleepless mutants (relates to Fig 3B); (E) fumin mutants (relates to Fig 3C); (F) redeye mutants (relates to Fig 3D); (G) dFB>NaChBac flies (relates to Fig 4A); and (H) iso31 controls (relates to Fig 4B). In all cases, flies injected with paraquat died significantly faster (p < 0.5 by log-rank analysis) than H2O-injected controls. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request. Cul3, Cullin-3; dFB, dorsal Fan-shaped Body; inc, insomniac; RNAi, RNA interference.
(TIF)
Summary of results from experimental trials. Raw data from representative experiments are available in S1 Data; raw data from all trials are available upon request.
(XLSX)
Raw data from representative experiments are organized here by figure panel; raw data from all trials are available upon request.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.