Abstract
Background
Cognitive frailty (CF) featured as frailty plus cognitive impairment was deemed to be a novel target for dementia and disable prevention. The study was intended to investigate the epidemiology of CF and the association between CF and all-cause mortality.
Methods
The national representative cohort study was comprised of 1,103 community-living middle-aged and older adults. CF was defined as the co-existence of dynapenia (weakness and/or slowness) and cognitive impairment (1.5 standard deviations below the age-, sex- and education-matched norms in cognitive tests) without known neurodegenerative diseases. Dynapenia was defined by the Asian Working Group for Sarcopenia and cognitive function was assessed by the Short Portable Mental Status Questionnaire.
Results
The prevalence of CF was 8.6% in this study. Subjects with CF were older, more likely to be women, having less regular exercise, fewer educational years, more depressive symptoms and greater multimorbidity. Compared to robust individuals, CF was significantly associated with all-cause mortality (HR: 3.1, 95% CI:1.3–7.7, p = 0.012).
Conclusion
Dynapenia and cognitive impairment synergistically contribute to the mortality risk for the participants in this study. Further study is needed to explore the underlying pathophysiology and the reversibility of CF.
Introduction
The concept of cognitive frailty (CF) was firstly proposed by Panza, et al., in 2006 to capture a complex phenotype of people by the concomitant presence of physical frailty and cognitive impairment for disability and dementia prevention [1]. The consensus from International Academy on Nutrition and Aging (I.A.N.A) and the International Association of Gerontology and Geriatrics (I.A.G.G) proposed the operational definition of CF as the co-existence of physical frailty (defined by Fried’s criteria) and mild cognitive impairment (Clinical Dementia Rating (CDR) scale = 0.5), and without dementia, and other neurodegenerative diseases [2]. The operational definition was proposed conceptually without supporting epidemiological evidences. After the introduction of the IANA/IAGG definition of CF, two major controversies were reported. First, the prevalence of CF defined by IANA/IAGG criteria was low, ranged from 1.2% to 1.8, which could not identify meaningful numbers for intervention [3]. Second, many MCI patients (CDR = 0.5) eventually presented with a progressive and irreversible process to dementia [4], and extensive or irreversible neural damages may have occurred already [1]. Hence, MCI may not be an appropriate component in the diagnostic criteria for CF due to the lack of benefits for dementia prevention [5].
A consensus from the Subjective Cognitive Decline Initiative Working Group proposed research criteria for cognitive performance testing for pre-MCI subjective cognitive decline, which was defined as lower or equal to 1.5 standard deviation from age- gender- and education-adjusted norms on standardized cognitive tests [6,7]. On the other hand, pre-frailty may achieve better outcomes when timely intervention was introduced and has become the main target for primary prevention despite that physical frailty per se has been shown to be a reversible state [8]. Some researchers argued that clinical manifestations of frailty phenotypes were initiated by weakness and slowness [9], and proposed pre-frailty to be the diagnostic component of CF [10]. Previous studies have identified that dynapenia, i.e. slowness and/or weakness, was an important subtype in frailty development and was substantially associated with adverse clinical outcomes [11–13]. Dynapenia, either in pre-frail or frail state, was also significantly associated with cognitive impairment [12]. Moreover, results from clustering analysis of brain MRI images showed that slowness and weakness were both associated with reduced gray matter in the cerebellum [13]. Altogether, selecting dynapenia plus cognitive impairment (1.5 SD below normal age- sex- and education-adjusted norms on standardized cognitive tests) to constitute the diagnostic criteria of CF may be a better approach. Therefore, this study aimed to explore the combined effects of dynapenia and cognitive impairment on all-cause mortality.
Methods
Study population and study design
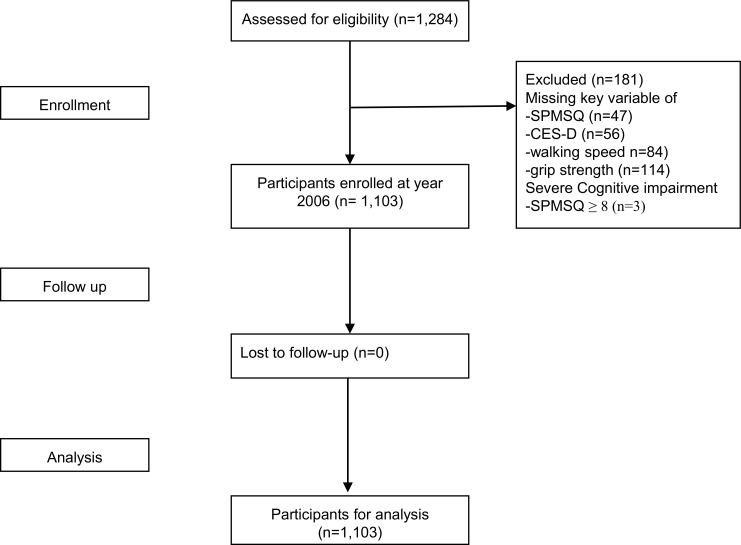
Data from the second wave of the Social Environment and Biomarkers of Aging Study (SEABAS) in 2006 were retrieved for this study. SEBAS intended to explore the interrelationship between biopsychosocial factors and aging, which used multi-stage proportional-to-size sampling strategies to ensure the national representativeness of enrollees. Details of the study design, and data collection procedures have been published previously [14]. Briefly, 1,284 subjects were enrolled for SEBAS 2006 from the original 1,659 participants of SEBAS 2000, and all participants received face-to-face interviews by well-trained nurses. Data of 181 participants with data incompleteness were excluded, which left data of 1,103 participants for analysis (Fig 1). Although the excluded subjects were significantly older (70.0 versus 65.1 years, p<0.001), having fewer educational years (4.7 versus 7.4 years, p<0.001), more multimorbidity (Charlson Comorbidity Index: 1.0 versus 0.7, p = 0.003), there were no sex-differences in their demographic characteristics.
Fig 1. Participants derived from the Social Environment and Biomarkers of Aging Study 2006.
The observational design and reporting format of this study followed STROBE guidelines [15]. A written informed consent was obtained from every participant. The Joint Institutional Review Board of Taiwan approved the study protocol. The design and procedures of the study were carried out in accordance with the principles of the Declaration of Helsinki.
Dynapenia and cognitive impairment
The North Coast™ hydraulic hand-dynamometer (NC70142, California, US) was used to measure dominant handgrip strength. The maximal reading of three trials was recorded, and weakness was defined by the Asian Working Group on Sarcopenia (AWGS) <26 kg for men and <18 kg for women [16]. Walking speed was measured by a 3-meter walking test, and slowness was defined as walking speed less than 0.8 m/s according to AWGS criteria [16]. Dynapnea was defined as the presence of slowness and/or weakness [12].
The Short Portable Mental Status Questionnaire (SPMSQ), an easy-handled and validated tool, was used to evaluate the cognitive performance for participants [17,18]. Participants had severe cognitive impairment (SPMSQ≥8) were excluded for analysis. Cognitive impairment was defined as the SPMSQ score less than 1.5 standard deviation or more below age-, sex-, and education adjusted norms in the same population, i.e. SPMSQ >1.7, and CF was defined as concomitant presence of dynapenia and cognitive impairment.
Outcomes and follow-up
The main outcomes of the study was mortality. The date of death was identified from the Taiwan national death registry between their original interview and December 31 of 2010.
Measurements for other covariates
Demographic characteristics of all participants, including age, sex and education years were collected. Smoking status was defined as tobacco consumption in the past six months. Participants who did exercise twenty minutes for twice or more per week were defined as those who carried on regular exercise. Multimorbidity was measured by the Charlson Comorbidity Index (CCI) [19]. Physical function was evaluated by the Katz Index of independence in activities of daily living (ADL) and the Lawton Instrumental Activities of Daily Living (IADL) [20,21]. Depressive symptoms was evaluated by the short version of Center for Epidemiological Studies Depression (CES-D) [22].
Statistical analysis
In this study, numerical variables were expressed as means ± standard deviation and categorical variables were expressed as proportions. One-way ANOVA test was used to compare numerical differences between various combinations of dynapenia and cognitive impairment, and Chi square or Fisher Exact test were used compare categorical variables when appropriate. Prevalence of cognitive frailty was stratified by ages and the Cochran-Armitage Trend Test was used to test the underlying trend. Schoenfeld residuals were used to test proportionality assumptions of Cox proportional hazard models. Age, sex and education attainment adjusted- and full adjusted- Cox proportional hazard model was used to explore the association between CF and mortality risk. Rothman synergetic index was used to examine the synergistic effect of dynapenia and cognitive impairment on mortality risk [23]. Sensitivity analysis was conducted by (1) excluding participants died in first year, who might commit serious illness and (2) excluding those with any disability of ADL.
All analyses were performed with the SAS statistical package, version 9.4 for windows (SAS Institute, Inc., Cary, NC, USA). A two-sided P-value <0.05 was considered statistically significant.
Results
Overall, the mean age of all participants was 65.1±9.5 years (from 53 to 85 years), and the prevalence of CF was 8.6% in the entire cohort, 2.2% in people aged 53–64 years, 10.2% in people aged 65–74 years and 22.7% in people aged 75 years and over. The prevalence of CF increased across age groups (p for trend <0.001). Table 1 summarized the baseline characteristics of the entire study cohort and compared differences between various CF conditions. Participants with CF were older, more likely to be women, less commonly to have regular exercise, having fewer educational years, more depressive symptoms and greater multimorbidity. Among those with dynapenia,179 (35.6%) posed both slowness and weakness.
Table 1. Baseline characteristics of all participants and those stratified by dyanpenia and cognitive impairment conditions.
| Total | Robust | Dynapenia(+) CI(-) | Dynapenia(-) CI(+) | Cognitive frailty | p value | |
|---|---|---|---|---|---|---|
| n | 1103 | 572(51.9) | 408(37.0) | 28(2.5%) | 95(8.6%) | |
| Age | 65.1±9.5 | 61.1±7.5 | 68.5±9.5 | 65.0±8.1 | 74.7±8.1 | <0.001 |
| Women | 510(46.2) | 220(38.5) | 210(51.5) | 16(57.1) | 64(67.4) | <0.001 |
| Smoke | 222(20.1) | 126(22.0) | 82(20.1) | 4(14.3) | 10(10.5) | 0.062 |
| Exercise | 444(40.2) | 257(44.9) | 155(38.0) | 11(39.3) | 21(22.1) | <0.001 |
| Education | 7.4±4.9 | 9.0±4.5 | 6.5±4.4 | 5.3±5.2 | 2.8±4.0 | <0.001 |
| SPMSQ | 0.5±1.0 | 0.2±0.4 | 0.3±0.5 | 2.4±0.9 | 2.9±1.3 | <0.001 |
| CES-D | 4.5±5.4 | 3.2±4.2 | 5.3±5.8 | 5.1±5.9 | 8.4±7.0 | <0.001 |
| Charlson Cormobility Index | 0.7±1.0 | 0.5±0.9 | 0.9±1.1 | 0.8±1.3 | 1.1±1.2 | <0.001 |
| Walking speed | 0.9±0.3 | 1.1±0.2 | 0.7±0.2 | 1.0±0.1 | 0.5±0.2 | <0.001 |
| Grip strength | 27.5±10.4 | 32.5±9.2 | 22.9±8.9 | 27.0±7.1 | 16.8±7.1 | <0.001 |
| ADL | 5.9±0.6 | 6.0±0.1 | 5.9±0.6 | 5.7±1.2 | 5.5±1.3 | <0.001 |
| IADL | 5.5±1.2 | 5.9±0.4 | 5.3±1.2 | 5.3±1.4 | 3.8±1.9 | <0.001 |
| any ADL disable | 52(4.7) | 3(0.5) | 24(5.9) | 3(10.7) | 22(23.2) | <0.001 |
| any IADL disable | 304(27.6) | 55(9.6) | 167(40.9) | 8(28.6) | 74(77.9) | <0.001 |
| Hypertension | 362(32.8) | 152(26.6) | 164(40.2) | 7(25.0) | 39(41.1) | <0.001 |
| Diabetes | 173(15.7) | 66(11.5) | 71(17.4) | 6(21.4) | 30(31.6) | <0.001 |
| Stroke | 40(3.63) | 7(1.2) | 22(5.4) | 2(7.1) | 9(9.5) | <0.001 |
| Heart disease | 184(16.7) | 55(9.6) | 98(24.0) | 4(14.3) | 27(28.4) | <0.001 |
| Kidney disease | 54(4.9) | 22(3.9) | 23(5.6) | 2(7.1) | 7(7.4) | <0.001 |
Plus-minus values are means ± standard deviation; n(%) are numbers(percentage); CI denotes cognitive impairment; SPMSQ denotes Short Portable Mental State Questionnaire; CES-D denotes Center for Epidemiologic Studies Depression Scale; ADL denotes activity of daily living; IADL denotes instrumental activity of daily living.
Survival analysis
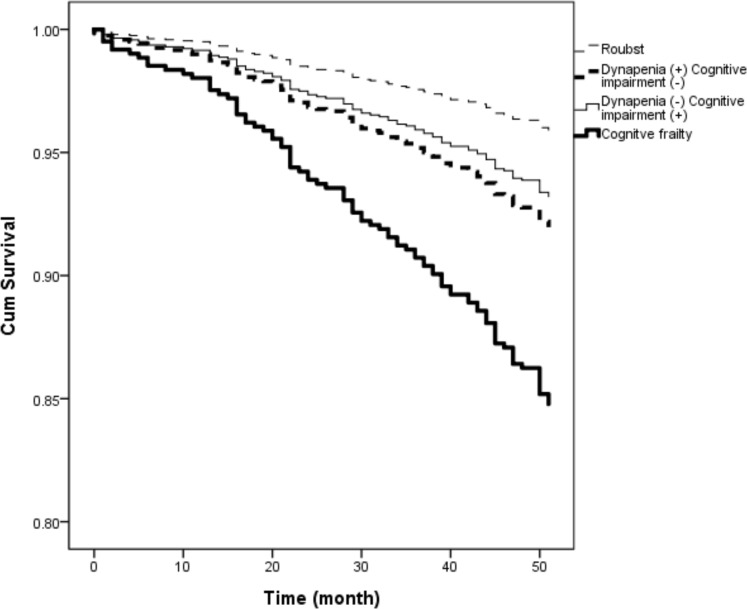
During the median follow-up for 4 years, 88 participants died (2.0 per 100 person-years). Age, sex and education attainment adjusted survival plot showed that CF had highest risk for 4-year mortality. (Fig 2) Table 2 showed the association between CF and all-cause in the multivariate Cox proportional hazard model. Compared to robust individuals, hazard ratio (HR) of CF for all-cause mortality were 3.1 (95% CI:1.3–7.7). Rothman synergetic index for all-cause mortality were 2.8 (95% CI: 0.1–72.3), which showed synergistic effects of dynapenia and cognitive impairment on mortality risk.
Fig 2. Age, sex and education adjusted survival plot for cognitive frailty.
Table 2. Cox proportional hazard model for all-cause deaths according to status of dynapnea and cognitive impairment.
| All causes | |||
|---|---|---|---|
| Model I | Model II | ||
| Death/Total | HR (95%CI),p | HR (95%CI),p | |
| 88/1103 | |||
| Robust | 22/572 | 1 | 1 |
| Dynapenia(+) CI(-) | 42/408 | 1.8(0.9–3.3),0.078 | 1.6(0.8–3.1),0.145 |
| Dynapenia(-) CI(+) | 2/28 | 1.6(0.2–12.3),0.640 | 1.1(0.1–8.9),0.900 |
| Cognitive frailty | 22/95 | 4.1(1.8–9.2),<0.001 | 3.1(1.3–7.7),0.012 |
| Exclude participants die within first year | |||
| 76/1091 | |||
| Robust | 20/570 | 1 | 1 |
| Dynapenia(+) CI(-) | 36/402 | 1.6(0.8–3.1),0.159 | 1.5(0.8–2.9),0.226 |
| Dynapenia(-) CI(+) | 1/27 | 0.0(0.0-.),0.988 | 0.0(0.0-.),0.985 |
| Cognitive frailty | 19/92 | 3.4(1.5–8.0),0.004 | 2.7(1.1–6.9),0.038 |
| 74/1051 | |||
| Robust | 20/569 | 1 | 1 |
| Dynapenia(+) CI(-) | 37/384 | 2.0(1.0–3.8),0.041 | 1.9(0.9–3.7),0.071 |
| Dynapenia(-) CI(+) | 2/25 | 2.1(0.3–16.4),0.462 | 2.2(0.3–16.7),0.452 |
| Cognitive frailty | 15/73 | 4.0(1.6–9.8),0.002 | 3.4(1.3–8.9),0.015 |
CI denotes cognitive impairment; HR denotes hazard ratios.
Model I adjusted for age, sex and educational years
Model II adjusted for Model I plus smoke, exercise, Center for Epidemiologic Studies Depression Scale, activity of daily living, instrumental activity of daily living, and Charlson comorbidity index
Sensitive analysis
The association between CF and mortality risk remained robust when participants who died in the first follow-up year were excluded. The HR of CF for all-cause were 2.7 (95% CI:1.1–6.9). Further analysis for exclusion of baseline physical disability also confirmed the association between CF and all-cause death (HR: 3.4, 95% CI: 1.3–8.9).
Discussion
In this study, the prevalence of CF was 8.6% based on the new diagnostic criteria and CF significantly predicted all-cause mortality. The association between CF and mortality risk remained strong after exclusion of baseline physical disability and those who died in the first-year follow-up period. Moreover, the dynapenia and cognitive impairment showed positive synergetic effect on all-cause mortality risk. The prevalence of CF was higher than that from previous studies using IANA/IAGG definition (ranging from 0.9% to 2.5%), but the demographic characteristics of CF subjects were in line with previous studies, i.e. older, less educated, and more often to be women [24–26]. Results of this study supported this new diagnostic criteria of CF by identifying reasonable numbers of people at risk for adverse health outcome, and may be early enough for disability and dementia prevention.
It has been reported that physical frailty and age-related cognitive impairment may share some common risk factors, such as depression and many cardiometabolic risk factors [27–29]. In this study, CF subjects had more depressive symptoms, higher prevalence of diabetes mellitus, hypertension, heart and kidney diseases, which was compatible with previous findings. In the Three-City Study, adding cognitive impairment to physical frailty improved the predictive validity for adverse health outcomes.[30] A study of 1,815 Mexican Americans showed that mortality risk of frail older people increased in the presence of cognitive impairment and vice versa [31]. Cumulative effects of cognitive impairment and frailty on mortality were reported in a Canadian cohort of 5-year follow-up and a Korean cohort of 3-year follow-up [32, 33].Results of this study extended previous findings to dynapenia with cognitive impairment in predicting all-cause mortality [26–34].
Ruan, et al., proposed to modify IANA/IAGG definition by using pre-frailty as a diagnostic component instead of physical frailty due to better intervention outcomes [10]. However, we proposed using dynapenia (slowness and/or weakness) instead of random combinations of other components of physical frailty to construct CF definition and aimed to explore the pathophysiology of CF [9, 11–13, 29]. It has been reported that weakness and slowness were the first emerging components of physical frailty [9], and results from the latent class analysis revealed that slowness and weakness was the commonest and strongest cluster of physical frailty, and was strongly associated adverse clinical outcomes [11]. This clustering phenomenon was also supported by the brain MRI imaging study [13]. For cognitive components of CF, researchers suggested using 1.5 standard deviation below the age- gender- and education- adjusted norms of cognitive tests instead of MCI [6,10,25]. Based on the definition, the prevalence of dynapenia without cognitive impairment was higher than the cognitive impairment without dynapenia in this study, which was in line with previous studies[35,36]. Therefore, the new operational definition of CF we used in this study was completely compatible with suggestions from previous studies, which also identified reasonable numbers of people and clearly demonstrated the association with adverse outcomes.
Despite all the efforts went into this study, there were still some limitations. First, data of other adverse health outcomes, e.g. new-onset disability, incident dementia, and healthcare utilization, were not available in this study, which may underestimate the diagnostic impact of CF. However, the survival status per se may sufficiently construct a conceptual model of CF. Second, no comprehensive neuropsychological and diagnostic evaluation of all neurodegenerative conditions were performed for study participants. However, participants with established diagnosis of neurodegenerative diseases would be excluded in the SEBAS recruitment process. Third, this study used only SPMSQ to identified subjects with cognitive impairment instead of measurements of individual cognitive domains. However, we believe this approach would be sufficient to identify cognitive impairment in various dimensions. Canevelli, et al., have indicated that there have been no robust/consistent operational definition of cognitive impairment in available studies [3]. Therefore, we believed we have done the best arrangement to identify early cognitive impairment for CF diagnosis.
Conclusions
In conclusion, the prevalence of CF among community-living milled-aged and older people in Taiwan was 8.6% from a national representative cohort, and the individuals with CF were at significant risk for all-cause mortality. Further study is needed to evaluate the benefits of CF intervention programs and to explore the underlying pathophysiology of CF.
Acknowledgments
The authors express their gratitude to the staff from Health Promotion Administration, Ministry of Health and Welfare, Taiwan for data collection and to all the participants for their assistance. This study was supported by the Aging and Health Research Center, National Yang Ming University; Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, as well as the Ministry of Science and Technology of Taiwan (MOST 105-3011-B-010-001).
Data Availability
Data are available from the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan and a public-use version is available on-line from the ICPSR database (http://doi.org/10.3886/ICPSR03792.v7). Due to legal restrictions imposed by the government of Taiwan in relation to the Personal Information Protection Act, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the ICPSR (http://www.icpsr.umich.edu/icpsrweb/ICPSR/).
Funding Statement
This study was supported by the Aging and Health Research Center, National Yang Ming University; Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, as well as the Ministry of Science and Technology of Taiwan (MOST 105-3011-B-010-001 and MOST107-2634-F-010-001).
References
- 1.Panza F, Seripa D, Solfrizzi V, Tortelli R, Greco A, Pilotto A, et al. Targeting Cognitive Frailty: Clinical and Neurobiological Roadmap for a Single Complex Phenotype. J Alzheimers Dis. 2015;47(4):793–813. 10.3233/JAD-150358 . [DOI] [PubMed] [Google Scholar]
- 2.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–34. 10.1007/s12603-013-0367-2 . [DOI] [PubMed] [Google Scholar]
- 3.Canevelli M, Cesari M. Cognitive Frailty: Far From Clinical and Research Adoption. Journal of the American Medical Directors Association. 2017. 10.1016/j.jamda.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 4.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18(8):674–83. 10.1097/JGP.0b013e3181cdee4f ; PubMed Central PMCID: PMCPMC2906673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aisen PS. Treatment for MCI: is the evidence sufficient? Neurology. 2008;70(22):2020–1. 10.1212/01.wnl.0000313380.89894.54 . [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844–52. 10.1016/j.jalz.2014.01.001 ; PubMed Central PMCID: PMCPMC4317324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–27. 10.1016/S1474-4422(10)70223-4 . [DOI] [PubMed] [Google Scholar]
- 8.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. 10.1016/S0140-6736(12)62167-9 ; PubMed Central PMCID: PMCPMC4098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–90. . [DOI] [PubMed] [Google Scholar]
- 10.Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Research Reviews. 2015;20:1–10. 10.1016/j.arr.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 11.Liu LK, Guo CY, Lee WJ, Chen LY, Hwang AC, Lin MH, et al. Subtypes of physical frailty: Latent class analysis and associations with clinical characteristics and outcomes. Sci Rep. 2017;7:46417 10.1038/srep46417 ; PubMed Central PMCID: PMCPMC5387710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CY, Hwang AC, Liu LK, Lee WJ, Chen LY, Peng LN, et al. Association of Dynapenia, Sarcopenia, and Cognitive Impairment Among Community-Dwelling Older Taiwanese. Rejuvenation Res. 2016;19(1):71–8. 10.1089/rej.2015.1710 . [DOI] [PubMed] [Google Scholar]
- 13.Chen WT, Chou KH, Liu LK, Lee PL, Lee WJ, Chen LK, et al. Reduced cerebellar gray matter is a neural signature of physical frailty. Hum Brain Mapp. 2015;36(9):3666–76. 10.1002/hbm.22870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornman JC, Glei DA, Goldman N, Chang MC, Lin HS, Chuang YL, et al. Cohort Profile: The Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan. Int J Epidemiol. 2014. Epub 2014/09/11. 10.1093/ije/dyu179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45(4):247–51. 10.1016/j.ypmed.2007.08.012 . [DOI] [PubMed] [Google Scholar]
- 16.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. 10.1016/j.jamda.2013.11.025 . [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–41. . [DOI] [PubMed] [Google Scholar]
- 18.Malhotra C, Chan A, Matchar D, Seow D, Chuo A, Do YK. Diagnostic performance of short portable mental status questionnaire for screening dementia among patients attending cognitive assessment clinics in Singapore. Ann Acad Med Singapore. 2013;42(7):315–9. . [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. . [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–9. . [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. . [PubMed] [Google Scholar]
- 22.Zhang W, O'Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793 10.1371/journal.pone.0040793 ; PubMed Central PMCID: PMCPMC3400644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortina-Borja M, Smith AD, Combarros O, Lehmann DJ. The synergy factor: a statistic to measure interactions in complex diseases. BMC Res Notes. 2009;2:105 10.1186/1756-0500-2-105 ; PubMed Central PMCID: PMCPMC2706251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solfrizzi V, Scafato E, Seripa D, Lozupone M, Imbimbo BP, D'Amato A, et al. Reversible Cognitive Frailty, Dementia, and All-Cause Mortality. The Italian Longitudinal Study on Aging. J Am Med Dir Assoc. 2017;18(1):89 e1–e8. 10.1016/j.jamda.2016.10.012 . [DOI] [PubMed] [Google Scholar]
- 25.Shimada H, Makizako H, Lee S, Doi T, Lee S, Tsutsumimoto K, et al. Impact of Cognitive Frailty on Daily Activities in Older Persons. J Nutr Health Aging. 2016;20(7):729–35. 10.1007/s12603-016-0685-2 . [DOI] [PubMed] [Google Scholar]
- 26.Feng L, Zin Nyunt MS, Gao Q, Feng L, Yap KB, Ng TP. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies (SLAS). J Am Med Dir Assoc. 2017;18(3):252–8. 10.1016/j.jamda.2016.09.015 . [DOI] [PubMed] [Google Scholar]
- 27.Lohman MC, Mezuk B. Frailty and depression: comorbidity in the context of imperfect measurement. J Am Geriatr Soc. 2013;61(3):474 10.1111/jgs.12138 ; PubMed Central PMCID: PMCPMC3602919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang AC, Liu LK, Lee WJ, Chen LY, Peng LN, Lin MH, et al. Association of Frailty and Cardiometabolic Risk Among Community-Dwelling Middle-Aged and Older People: Results from the I-Lan Longitudinal Aging Study. Rejuvenation Res. 2015;18(6):564–72. 10.1089/rej.2015.1699 . [DOI] [PubMed] [Google Scholar]
- 29.Lee WJ, Peng LN, Chiou ST, Chen LK. Physical Health Indicators Improve Prediction of Cardiovascular and All-cause Mortality among Middle-Aged and Older People: a National Population-based Study. Sci Rep. 2017;7:40427 10.1038/srep40427 ; PubMed Central PMCID: PMCPMC5227916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–61. 10.1111/j.1532-5415.2008.02136.x [DOI] [PubMed] [Google Scholar]
- 31.Cano C, Samper-Ternent R, Al Snih S, Markides K, Ottenbacher KJ. Frailty and cognitive impairment as predictors of mortality in older Mexican Americans. J Nutr Health Aging. 2012;16(2):142–7. Epub 2012/02/11. ; PubMed Central PMCID: PMCPMC3281306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St John PD, Tyas SL, Griffith LE, Menec V. The cumulative effect of frailty and cognition on mortality—results of a prospective cohort study. Int Psychogeriatr. 2017;29(4):535–43. Epub 2016/12/03. 10.1017/S1041610216002088 . [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Kim J, Chon D, Lee KE, Kim JH, Myeong S, et al. The effects of frailty and cognitive impairment on 3-year mortality in older adults. Maturitas. 2018;107:50–5. Epub 2017/11/25. 10.1016/j.maturitas.2017.10.006 . [DOI] [PubMed] [Google Scholar]
- 34.Roppolo M, Mulasso A, Rabaglietti E. Cognitive Frailty in Italian Community-Dwelling Older Adults: Prevalence Rate and Its Association with Disability. J Nutr Health Aging. 2017;21(6):631–6. 10.1007/s12603-016-0828-5 . [DOI] [PubMed] [Google Scholar]
- 35.Liu LK, Lee WJ, Wu YH, Hwang AC, Lin MH, Shimada H, et al. Cognitive Frailty and Its Association with All-Cause Mortality among Community-Dwelling Older Adults in Taiwan: Results from I-Lan Longitudinal Aging Study. Rejuvenation Res. 2018. Epub 2018/04/13. 10.1089/rej.2017.2038 . (in press) [DOI] [PubMed] [Google Scholar]
- 36.Feng L, Zin Nyunt MS, Gao Q, Feng L, Yap KB, Ng TP. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies (SLAS). J Am Med Dir Assoc. 2017;18(3):252–8. 10.1016/j.jamda.2016.09.015 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan and a public-use version is available on-line from the ICPSR database (http://doi.org/10.3886/ICPSR03792.v7). Due to legal restrictions imposed by the government of Taiwan in relation to the Personal Information Protection Act, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the ICPSR (http://www.icpsr.umich.edu/icpsrweb/ICPSR/).