Abstract
Objective:
Recent international guidelines suggest renal imaging to detect occult urolithiasis in all patients with asymptomatic primary hyperparathyroidism (PHPT), but data regarding their prevalence and associated risk factors are limited. We evaluated the prevalence and risk factors for occult urolithiasis.
Methods:
Cross-sectional analysis of 96 asymptomatic PHPT patients from a university hospital in the United States with and without occult nephrolithiasis.
Results:
Occult urolithiasis was identified in 21% of patients. Stone formers had 47% higher 24-hour urinary calcium excretion (p = 0.002). Although available in only a subset of patients (n = 28), activated vitamin D [1,25(OH)2D] was 29% higher (p = 0.02) in stone formers. There was no difference in demographics, BMI, calcium or vitamin D intake, other biochemistries, renal function, BMD, or fractures. Receiver operating characteristic curves indicated that urinary calcium excretion and 1,25(OH)2D had an area under the curve of 0.724 (p = 0.003) and 0.750 (p = 0.04), respectively. A urinary calcium threshold of >211mg/day provided a sensitivity of 84.2% and a specificity of 55.3% while a 1,25(OH)2D threshold of >91pg/mL provided a sensitivity and specificity of 62.5% and 90.0% respectively for the presence of stones.
Conclusion:
Occult urolithiasis is present in about one-fifth of patients with asymptomatic PHPT and is associated with higher urinary calcium and 1,25(OH)2D. Given that most patients will not have occult urolithiasis, targeted imaging in those most likely to have occult stones rather than screening all asymptomatic PHPT patients may be useful. The higher sensitivity of urinary calcium versus 1,25(OH)2D suggests screening those with higher urinary calcium may be an appropriate approach.
Keywords: Asymptomatic, imaging, occult nephrolithiasis, predictors, primary, hyperparathyroidism, stones
Precis:
Given the low prevalence of occult urolithiasis in primary hyperparathyroidism, targeted imaging in those with high urine calcium rather than screening all asymptomatic patients may be useful.
Introduction:
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by hypercalcemia and elevated or inappropriately normal parathyroid hormone (PTH) levels.1–3 Today most patients (~80%) with PHPT are asymptomatic and incidentally discovered in areas of the world where calcium is routinely measured.4–6 Prior to the routine measurement of serum calcium in the 1970’s, PHPT most often presented with overt end-organ manifestations, including nephrolithiasis and osteitis fibrosa cystica. Additionally, polyuria, renal dysfunction, and nephrocalcinosis were commonly observed.7–9 As the disease presentation has evolved, the incidence of symptomatic nephrolithiasis has declined over the last four decades.10 Currently, symptomatic nephrolithiasis occurs in approximately 17–20% of PHPT patients in the United States (U.S.) and Western Europe compared to more than 50% before 1981.5,7,11 There has been recent recognition, however, that a higher percentage of patients have nephrolithiasis if radiographic screening for occult stones is undertaken. Therefore, the most recent international guidelines (2014) for the management of asymptomatic PHPT recommend obtaining renal imaging to detect occult nephrolithiasis and a 24-hour urine biochemical risk profile.12 Guidelines further recommend referral for parathyroidectomy, not only for symptomatic nephrolithiasis, but also for the presence of occult stone disease, nephrocalcinosis, hypercalciuria (>400 mg/24hr), or other urinary biochemical risk factors that place the patient at increased risk for nephrolithiasis or nephrocalcinosis.
To our knowledge, there are no data regarding the prevalence of occult stones in PHPT from a cohort in the U.S., where asymptomatic PHPT is the predominant phenotype. Given the variability in the presentation of PHPT by geographic location, prevalence data on occult nephrolithiasis from other countries are not necessarily applicable to the U.S.6 Further, risk factors for stone formation in PHPT, both symptomatic and occult, remain unclear. Hypercalciuria has variably been associated with stone formation (symptomatic and silent) in PHPT.13–15 The etiology is thought to be multifactorial with some data indicating younger age, male sex and higher BMI are risk factors for nephrolithiasis in PHPT.16–19 Other studies suggest that nephrolithiasis is associated with higher serum 1,25(OH)2D, calcium, PTH, urine hydroxyproline levels, and higher estimated glomerular filtration rate (eGFR).13,15,20,21 With the change in paradigm calling for screening for occult nephrolithiasis in all PHPT patients without a clinical stone history, information about the prevalence of silent stones and risk factors for them is needed. Such data could provide valuable information regarding the pathophysiology of stones in PHPT and may also help select patients who are most likely to have nephrolithiasis on imaging. The purpose of this study was to evaluate the prevalence of occult nephrolithiasis in patients with PHPT and to determine clinical and biochemical risk factors associated with occult stones.
Methods
Study design and participants
This is a cross-sectional study assessing the prevalence of and risk factors for occult urinary stones among 96 patients with asymptomatic PHPT. The term “asymptomatic” PHPT is defined in the literature to refer to the absence of classical PHPT manifestations (osteitis fibrosa cystica and clinically symptomatic kidney stones). We conducted a chart review of patients with PHPT who had renal imaging performed as part of their clinical evaluation at Columbia University Medical Center (CUMC), a tertiary care academic center, from November 2013 through August 2017. All participants enrolled gave written informed consent before their inclusion. Over the enrollment period, 254 patients with PHPT were screened for participation. Of those screened, 158 did not meet inclusion criteria or refused to participate. This study was approved by the Institutional Review Board of CUMC.
Patients with PHPT were included in the study if they had no clinical history of urinary stones and had renal imaging including abdominal or renal ultrasound, radiograph, computed tomography, or magnetic resonance imaging performed as part of their clinical evaluation for PHPT. Renal imaging is routinely obtained by metabolic bone specialists at CUMC in PHPT patients without a clinical history of urinary stones. Participants were directly recruited by study physicians and represent consecutively enrolled patients with PHPT who qualified for enrollment and agreed to participate. The diagnosis of PHPT was made by the presence of hypercalcemia (patients with normocalcemic PHPT were not included) and elevated or inappropriately normal PTH levels. Patients taking calcium and vitamin D supplementation were included. Exclusion criteria were presence of kidney stones within 4 years before the diagnosis of PHPT, familial hypocalciuric hypercalcemia (diagnosed by family history and urinary calcium excretion), other causes of hypercalcemia (history of active granulomatous disease and current use of lithium and thiazide diuretics), and use of cinacalcet. Cinacalcet users were excluded because cinacalcet alters serum calcium and PTH, which were included in the assessment of predictors of nephrolithiasis.
Demographic data (age, race, sex) and medical history (PHPT disease duration, fracture history, kidney stone history, calcium and vitamin D supplementation, bisphosphonate use, BMI and presence of surgical criteria for parathyroidectomy) were abstracted from participants’ medical records. Duration of PHPT was defined by patient history as the time from initial onset of hypercalcemia. The following biochemical parameters were abstracted from the patient’s records: serum 25-hydroxyvitamin D (25OHD), 1,25(OH)2D, phosphorus (P), creatinine (Cr), total serum calcium (Ca), albumin, PTH, and 24-hour urinary calcium excretion. Due to the nature of this study (chart review), laboratory data were analyzed in several clinical laboratories. Additionally, some participants had missing biochemical data. Activated vitamin D levels were available in 28 participants. Areal bone mineral density (BMD) measurements by dual energy x-ray absorptiometry and T-scores at the lumbar spine (LS; n = 96), total hip (TH; n = 95), femoral neck (FN; n = 95), and distal one-third radius (1/3-radius; n = 89) were collected when available. Osteoporosis was defined by T-score ≤ −2.5 at any site as described by the World Health Organization (WHO).22 Biochemical data closest to the date of renal imaging were utilized.
Statistical analysis
Descriptive statistics were expressed as absolute (n) and relative (%) frequency for categorical variables. For continuous variables, mean and standard deviation (SD) were used to describe normally distributed variables and median and interquartile range were used for non-normal distributions. Participants were categorized as those with or without urinary stones based on the reading radiologist’s report from ultra-sound, radiograph, CT scan, or MRI. Between-group differences in continuous variables were assessed by unpaired student’s t test or Mann Whitney U test, as appropriate. Between-group comparisons in categorical variables were assessed by χ2 test or Fischer’s exact test. Multiple logistic regression was used to examine the association between potential predictors and the likelihood of a positive screen for occult urinary stones. Potential predictors included in the model were selected on the basis of pathophysiologic plausibility and literature review. 1,25(OH)2D was not included in the multiple regression model because it was not available in most patients (n = 68). The ability of various predictors to identify the presence of occult urinary stones was evaluated with receiver operating characteristic curves, calculation of area under the curve (AUC), and determination of sensitivity and specificity. Pearson’s coefficient (r) was used for bivariate correlation between 24-hour urinary calcium excretion and 1,25(OH)2D. A twotailed p < 0.05 was considered to indicate statistical significance. SPSS 23.0 for Windows (IBM Corp., Armonk, NY) was used for the analyses.
Results
As shown in Table 1, participants were predominantly Caucasian (81.3%) and postmenopausal women (71.9%). They had evidence of mild PHPT: mean serum calcium 10.5 ± 0.5 mg/dL (range: 9.9–11.8 mg/dl); PTH 86.9 ± 42 pg/mL (range: 26–290 pg/ml); 25OHD 31.1 ± 12 ng/dL. In the cohort, 34.4% had osteoporosis by DXA criteria, 20.8% had a history of fracture, 8.3% had an estimated glomerular filtration rate <60 ml/min (all 8 with stage 3 CKD), 10.4% were <50 years old, 12.4% had 24-hour urinary calcium excretion >400 mg/day (but no clinical stones) and 92.7% met ≥ 1 criterion for parathyroidectomy based on 2014 guidelines. No one had osteitis fibrosa cystica or nephrocalcinosis. Mean T-scores were in the osteopenic range at the femoral neck and distal 1/3- radius while they were normal at the spine and total hip (Table 1). Twenty- three percent of participants were taking calcium supplements while 61.5% were taking vitamin D supplements. The average doses are shown in Table 1.
Table 1.
Demographic and clinical factors by presence of occult urinary stones.
| Variable | Whole cohort (n = 96) |
Occult urinary stones present (n = 20) |
Occult urinary stones absent (n = 76) |
p-value |
|---|---|---|---|---|
| Age, years | 63.9 ± 10 | 63.5 ± 9 | 64.1 ± 11 | 0.83 |
| Sex, % female | 81.3 | 70.0 | 84.2 | 0.15 |
| Race/ethnicity, % white | 81.3 | 90.0 | 78.9 | 0.63 |
| Duration of PHPT, years | 4.64 ± 5.1 | 4.17 ± 3.8 | 4.76 ± 5.4 | 0.64 |
| Weight, kg | 74.4 ± 18 | 74.8 ± 12 | 74.3 ± 19 | 0.92 |
| Height, cm | 163.9 ± 8 | 167.8 ± 11 | 162.9 ± 7 | 0.06 |
| BMI (kg/m2) | 27.7 ± 6 | 26.7 ± 5 | 28.0 ± 7 | 0.43 |
| Calcium Supplementation, % | 22.9 | 10.0 | 26.3 | 0.12 |
| Calcium Supplementation, mg/day* | 615 (430–1031) | 750 (500–1000) | 615 (290–1094) | 0.98 |
| Vitamin D supplementation, % | 61.5 | 50.0 | 64.5 | 0.24 |
| Vitamin D Supplementation, IU/day* | 1,600 (1,000–2,067) | 1,714 (900–2,100) | 1,600 (1000–2,133) | 0.49 |
| Meet Criteria for PTX, %** | 92.7 | 90.0 | 93.4 | 0.63 |
| Age < 50 years, % | 10.4 | 10.0 | 10.5 | 0.95 |
| GFR < 60 ml/min, % | 8.3 | 0.0 | 10.5 | 0.20 |
| Calcium >1mg/dl, % | 18.8 | 30.0 | 15.8 | 0.15 |
| Osteoporosis by DXA, % | 34.4 | 40.0 | 32.9 | 0.55 |
| Urine calcium >400 mg/24 hours, % | 12.4 | 31.6 | 7.1 | 0.01 |
| Lumbar spine T-score | −0.84 ± 1.5 | −0.81 ± 1.4 | −0.84 ± 1.5 | 0.93 |
| Femoral Neck T-score | −1.37 ± 1.0 | −1.48 ± 0.6 | −1.34 ± 1.1 | 0.47 |
| Total Hip T-score | −0.85 ± 1.1 | −0.88 ± 0.7 | −0.84 ± 1.1 | 0.89 |
| 1/3-Radius T-score | −1.27 ± 1.3 | −1.62 ± 1.5 | −1.28 ± 1.3 | 0.21 |
| Previous bisphosphonate use, % | 21.9 | 10.0 | 25.0 | 0.23 |
Values represent mean ± SD, percentage or
median (interquartile range)
Excluding presence of urinary stones as a criterion
The majority of participants had renal imaging by ultrasound (50.0%). The remainder of the cohort had imaging by abdominal radiograph (34.4%), abdominal computed tomography (12.5%) or magnetic resonance imaging (3.1%). Urinary stones were identified in 20.8% (n = 20) of the participants. Of all stone formers, 65.0% had just one stone while 15.0% had two stones and 20.0% had more than 2 stones. Of those with 2 or more stones, all but one (87.5%) had stones found bilaterally. Mean stone size was approximately 4.7 ± 4 mm (range 1–21mm). Stones were located in the renal pelvis, ureter and bladder. 15% of the stone formers had urinary stones that were not limited to the renal pelvis. Participants who had CT were more likely to have stones compared to those imaged by other modalities (50% vs. 17%, p = 0.008).
Comparing those with versus without occult urinary stones (Table 1–2 and Figure 1), stone formers had higher urine calcium excretion whether expressed as milligrams per day (327 ± 142 vs 223 ±126 mg/day, p = 0.002) or milligrams per kilogram per day (p = 0.005; Table 2). Urine creatinine was higher and urinary volume tended to be slightly higher in those with occult stones (Table 2, p = 0.01 and 0.05 respectively). Subjects with occult renal stones had higher 1,25(OH)2D (90.7 ± 20 vs 70.1 ± 20 pg/mL, p = 0.02). Although 1,25(OH)2D levels were only available on a subset of patients (n = 28), those with versus without 1,25(OH)2D measurements did not differ in terms of age (p = 0.33), sex (p = 0.26), serum calcium (p = 0.37), or PTH level (p = 0.55). Stone formers were not more likely to have 1,25(OH)2D measured (p = 0.23).
Table 2.
Biochemical factors associated with occult urinary stones.
| Variable | N | Normal Range | Whole Cohort | Occult urinary stones present |
Occult urinary stones absent |
p-value |
|---|---|---|---|---|---|---|
| Serum calcium, mg/dl | 96 | 8.4–9.8 | 10.5 ± 0.5 | 10.6 ± 0.5 | 10.4 ± 0.5 | 0.26 |
| Parathyroid hormone, pg/ml* | 96 | 14–64 | 78.5 (61.5–106.0) | 85.5 (53.4–106.1) | 78.0 (63.5–106.0) | 0.92 |
| Phosphorus, mg/dl | 96 | 2.5–4.5 | 3.2 ± 0.5 | 3.1 ± 0.6 | 3.3 ± 0.5 | 0.44 |
| 25-hydroxyvitamin D, ng/dl | 95 | 30–100 | 31.1 ± 12 | 31.3 ± 10 | 31.0 ± 12 | 0.91 |
| 1,25-dihydroxyvitamin D, pg/ml | 28 | 16–65 | 76.0 ± 22 | 90.7 ± 20 | 70.1 ± 20 | 0.02 |
| Urine calcium excretion (mg/24 hours) | 89 | Female <250 Male <300 | 245 ± 136 | 327 ± 142 | 223 ± 126 | 0.002 |
| Urine calcium excretion (mg/kg/24 hours) | 89 | <4mg/kg/day | 3.47 ± 1.89 | 4.54 ± 2.09 | 3.18 ± 1.73 | 0.005 |
| 24 hour urine creatinine (g/24 hours) | 81 | 0.8–1.8 | 1.19 ± 0.40 | 1.41 ± 0.44 | 1.13 ± 0.38 | 0.01 |
| 24 hour urine volume (ml/24 hours) | 88 | 2173 ± 1145 | 2618 ± 1803 | 2014 ± 882 | 0.05 | |
| Estimated glomerular filtration rate, ml/min | 96 | >60 | 80.9 ± 16 | 80.2 ± 13 | 81.1 ± 17 | 0.83 |
Values represent mean ± SD;
PTH is shown as median and interquartile range
Figure 1.
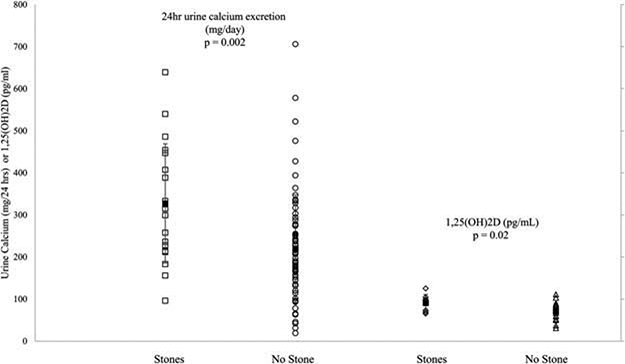
Scatter plots showing mean differences in 24-hour urinary calcium excretion and 1,25(OH)2D levels between stone formers and non-stone formers. The error bars denote ± standard deviation.
Excluding the criterion of urinary stones, the proportion of stone formers and non-stone formers meeting guidelines for recommending parathyroidectomy was not different (90.0% vs. 93.4%, p = 0.63). Nevertheless, stone formers were more likely to meet the specific surgical criterion of urinary calcium >400mg/24 hours (31.6% vs. 7.1%, p = 0.01) and 54.5% of those with urine calcium >400mg/ 24 hours had stones. Other commonly clinically used urinary thresholds were assessed as well. Among females, 64.3% of stone formers as compared to 37.9% of the non-stone formers had urinary calcium exceeding 250 mg/day, the upper limit of normal for women (p = 0.07). Among males, 60.0% of the stone formers as compared to 16.7% of the non-stone formers had urinary calcium exceeding 300 mg/day, the upper limit of normal for men (p = 0.08). There were no between-group differences in age, gender, height, weight, BMI, race/ethnicity, duration of PHPT, daily calcium or vitamin D intake, bisphosphonate use, serum calcium, PTH, phosphate, 25OHD, renal function, BMD, or fracture history.
In a logistic model, higher urine calcium excretion (ß = 0.008, p = 0.01) was associated with occult urinary stones adjusting for age, race, ethnicity, sex, height, BMI, calcium and vitamin D usage, urine volume, and urine creatinine (Table 3). None of the other covariates was significant in the model. Receiver operator curves indicated that urinary calcium and 1,25(OH)2D had an area under the curve (AUC) of 0.724 (CI: 0.598–0.850, p = 0.003) and 0.750 (CI: 0.548–0.952, p = 0.042), respectively (Figure 2). A 24-hour urinary calcium excretion threshold of >211 mg/24 hours maximized sensitivity (84.2%) and specificity (55.3%) for correctly identifying those with stones. 84.2% of the stone formers had a urinary calcium exceeding 211mg/day as compared to 44.3% of non-stone formers (p = 0.002). Aserum 1,25(OH)2D threshold of >91 pg/ml provided a sensitivity of 62.5% and a specificity of 90.0%.
Table 3.
Multivariable logistic model - predictors of occult urinary stones.
| Variable | B | SE | p-value |
|---|---|---|---|
| Age (per year) | 0.090 | 0.050 | 0.07 |
| Race (white vs. non-white) | 0.708 | 1.284 | 0.58 |
| Ethnicity (non-Hispanic vs. Hispanic) | −1.063 | 1.206 | 0.38 |
| Sex (male vs. female) | −1.268 | 1.194 | 0.29 |
| Height (per meter) | 11.137 | 6.631 | 0.09 |
| BMI (per kg/m2) | −0.058 | 0.071 | 0.41 |
| Calcium supplementation (user vs. non-user) | −1.299 | 1.168 | 0.27 |
| Vitamin D supplementation (user vs. non-user) | 0.028 | 0.749 | 0.97 |
| Urinary calcium excretion (per mg/24 hours) | 0.008 | 0.003 | 0.01 |
| 24 hour urine volume (per ml/24 hours) | −0.0002 | 0.0004 | 0.70 |
| 24 hour urine creatinine (per mg/24 hours) | 1.696 | 1.212 | 0.16 |
Figure 2.
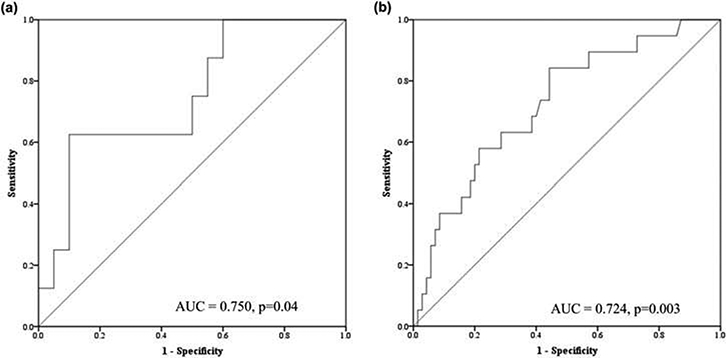
ROC curves show the diagnostic performance of 1,25(OH)2D (A) and 24-hour urinary calcium excretion (B). ROC curve plots the true positive rate (sensitivity) against the false positive rate (1-specificity) for different cut-offs. The curved line represents the AUC for the biochemical variable(s) of interest while the straight diagonal line represents chance (AUC of 0.5).
Applying both thresholds of >211 mg/24hr urinary calcium excretion and >91 pg/ml serum 1,25(OH)2D provided a sensitivity of 62.5% and specificity of 94.7%. A sensitivity of 100% was achieved with a urinary calcium value of 96 mg/day but with specificity of only 13%. Likewise, a sensitivity of 100% was achieved with a 1,25(OH)2D of 63 pg/ml with a specificity of 40%. The urinary calcium threshold used to define “hypercalciuria” in women (250mg/day) had a sensitivity of 63% and specificity of 67%. There was a moderate correlation between 24-hour urinary calcium excretion and 1,25(OH)2D (r = 0.58, p = 0.001; Figure 3). Limiting the ROC analysis to those who had both urine calcium and 1,25(OH)2D values (n = 23) available did not improve the discriminatory ability [AUC 0.697 (CI: 0.463–0.931), p = 0.11].
Figure 3.
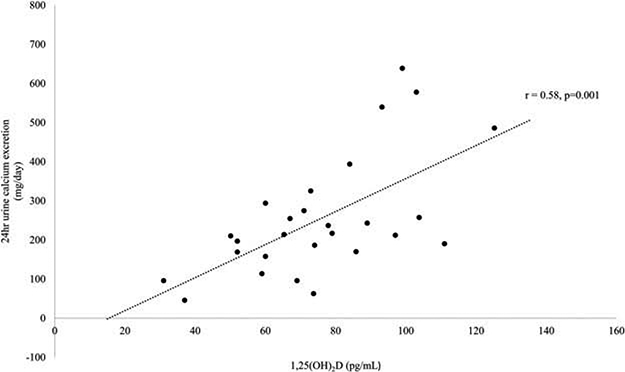
Scatter plot indicating the correlation between serum 1,25(OH)2D levels and 24-hour urinary calcium excretion (r = 0.58, p = 0.001).
Discussion
Given recent international guidelines suggesting screening for occult nephrolithiasis in all asymptomatic PHPT patients, we investigated the prevalence and clinical as well as biochemical risk factors for urolithiasis. To our knowledge, this is the first study to assess the prevalence of occult nephrolithiasis in a U.S. cohort with PHPT or to assess the accuracy of clinical or biochemical factors for correctly classifying silent stone formers. Our results indicate that occult urinary stones are present in about one-fifth of patients with asymptomatic PHPT. The prevalence of asymptomatic nephrolithiasis among PHPT patients in our investigation is, however, many times higher than prevalence rates reported in the general population, which ranges from 1.6 to 3%.12,23,24 Taking into account typical rates of symptomatic urinary stones in patients with PHPT in the United States and Western Europe (17–20%), our results suggest that close to 40% of patients with PHPT overall may have urinary stones, both symptomatic and asymptomatic.13,25,26
Since the majority of asymptomatic patients will not, however, have occult urinary stones when screened by imaging, a targeted screening approach, rather than routine renal imaging of all asymptomatic patients may be more appropriate. Thus, the results imply that a potential streamlining of imaging recommendations may be warranted. Such an approach is important to consider in order to control health care costs, reduce radiation exposure and unnecessary testing in those unlikely to have stones. To this end, we assessed both clinical as well as serum and urinary factors associated with occult urinary stones in PHPT. We found that higher urinary calcium excretion and higher serum 1,25(OH)2D levels were both associated with the presence of occult urinary stones. Our results are similar to that of Starup-Linde et al. who found renal stones/calcifications in 25% and an association with 24- hour urine excretion expressed as calcium/creatinine ratio but not as mg of calcium/24 hours.27 Starup-Linde also found a trend toward higher activated vitamin D levels in stone formers. Biochemical factors, however, were not good predictors of the presence of occult nephrolithiasis in this previous study.
In the current study, only a minority (31.6%) of stone formers had evidence of hypercalciuria exceeding the threshold of 400 mg/day used to recommend parathyroidectomy and only 54% of those with urine calcium >400mg/day had stones, indicating this threshold has poor sensitivity. Further analyses indicated both urinary calcium excretion and 1,25(OH) 2D using the thresholds of 211 mg/day and 91pg/ml respectively had fairly good discriminative power (AUC 0.7–0.8) for correctly classifying those with and without silent stones, comparable to the AUC of DXA for predicting future fracture. Utilizing thresholds that maximized sensitivity and specificity, a 24-hour urine calcium excretion threshold of >211 mg/day provided fairly high sensitivity but low specificity while a 1,25(OH)2D threshold of 91 pg/ml had low sensitivity but high specificity. The combination of both factors also had high specificity but low sensitivity. Therefore, it may be most appropriate to recommend imaging in those with high urinary calcium (>211 mg/day) in order to maximize sensitivity. This threshold minimizes the false negative rate (i.e. missing stones in those who have them) at the expense of having a higher false positive rate.
The exact pathogenesis of urinary stone formation in PHPT has remained unclear. Many but not all studies have implicated hypercalciuria as a predisposing factor.15–17,29,31–36 However, the overlap of 24-hour urinary calcium excretion between stone and non-stone formers in our study suggests that the etiology of renal stones is likely multifactorial. The reasons for the variable association of urinary calcium and stones in prior studies are unclear, but most studies have assessed risk factors for symptomatic stones in PHPT. Because renal imaging was not done in the majority of such investigations, many studies have likely misclassified those with occult stones as non-stone formers, which may have led to the inability to detect urinary calcium as a risk factor. Additionally, those with symptomatic stones may have been instructed to modify behaviors that increase the risk of kidney stones. Changes in dietary calcium, vitamin D, sodium or protein intake and fluid consumption prior to study enrollment may have modulated urinary calcium excretion. In contrast, because we studied patients with occult stones found by imaging, our study offers improved classification. Furthermore, because we included patients who were unaware of their stone status prior to enrollment, they were perhaps less likely to change behaviors influencing calcium excretion.
In this study, we further showed an association between 1,25(OH)2D and stones as well as a positive correlation between 24-hour urinary calcium excretion and 1,25(OH)2D. Other PHPT studies have shown a similar association between urinary calcium and 1,25(OH)2D.34–36 It is unclear why stone-forming PHPT patients have high 1,25(OH)2D levels, since PTH levels are similar between groups. One possible mechanism is that stone formers have dysregulation of the 1-alpha hydroxylase or 1,25-dihydroxyvitamin D 24-hydroxylase enzymes. This pattern of increased activated vitamin D is similar to the biochemical profile observed in patients with “idiopathic hypercalciuria”37 and suggests that stone formers with PHPT could have risk factors unrelated to their parathyroid disease.
While causality cannot be definitively established by this study, it is plausible that higher 1,25(OH)2D levels could lead to higher urinary calcium excretion through several physiological mechanisms. High 1,25 (OH)2D levels may increase intestinal calcium absorption or bone resorption which could predispose toward a greater filtered load of calcium, hypercalciuria and subsequent renal stones20,35,36 Another plausible mechanism linking elevated 1,25(OH)2D and hypercalciuria may relate to calcium sensing receptors (CaSR) or calcium channels in the kidney.41 Physiologically, 1,25(OH)2D regulates CaSR and renal calcium channel expression which influences calcium reabsorption in the distal renal tubules.39–41 Our results suggest that routine measurement of 1,25 (OH)2D levels in the clinical evaluation PHPT could be useful if this association is confirmed in other studies.
In addition, polymorphisms in the CaSR gene itself may account for hypercalciuria in PHPT. CaSR variants with different sensitivities to extracellular calcium may result in varying renal reabsorption and urinary calcium excretion.42 For example, a CaSR polymorphism which increases the sensitivity to extracellular calcium would result in decreased renal reabsorption and increased urinary calcium excretion.43 Such a variant in the CaSR (R990G) was associated with higher urinary calcium excretion and increased prevalence of renal stones in PHPT.44 Unfortunately, no information regarding CaSR genotype is available in this study.
Our study has several limitations. First, our 24- hour urine data are limited. We do not have information on urinary factors other than urine calcium, creatinine, and volume nor do we have information from multiple urine collections. It is possible that other factors such as dietary or urinary sodium alter risk of hypercalciuria or nephrolithiasis in PHPT. As calcium excretion may vary depending on dietary factors and from collection to collection, it is important that future studies aim to collect these data. Second, the mode of imaging used to screen participants was selected by participants’ clinicians. Several recent studies suggest that the prevalence of occult urolithiasis ranges from 7–36% as assessed by ultrasound.11,12,30,45,46 Rates may be higher when CT is used because ultrasound can miss small stones.46 Most of our patients had renal ultrasound rather than abdominal CT. The higher prevalence of stones in the group screened with CT suggests that the prevalence of occult renal stones could be underestimated by this study. However, routine use of CT in asymptomatic patients, most of whom do not have nephrolithiasis, may be less acceptable to both physicians and patients due to its high radiation exposure and cost. The 4th International workshop guidelines recommend renal imaging to detect occult stones with either abdominal x-ray, ultrasound or CT but suggest that “physicians may not want to subject their patients to the radiation associated with this technique [CT] in the absence of clinical symptoms”.6 Thus, our study does offer meaningful real-world data on recommended and prevailing clinical practice.
Our study is also limited by the fact that biochemistries were assessed in clinical laboratories and laboratory assessment was incomplete in some patients. Only 29% of participants had 1,25(OH)2D levels measured. While our data suggest that those who had 1,25(OH)2D levels measured did not differ from those without measurements, we cannot completely exclude potential bias nor that 1,25(OH)2D may have higher sensitivity than our study indicates. These data suggest, however, that 1,25(OH)2D measurements are indicated in future studies and may be important in the clinical evaluation of patients with PHPT. Lastly, as we do not have demographic or biochemical information on PHPT patients at our center who did not qualify for the study or declined participation, we are unable to assess how representative this cohort is of our greater population of PHPT patients. However, the similar characteristics of this cohort to prior cohorts enrolled in earlier studies at our center is re-assuring in this regard.47–50.
Our study also has several strengths. We studied a relatively large cohort of patients with PHPT, assessed stone status by imaging in patients unaware of their stone category, and assessed the discriminatory ability of various clinical and biochemical predictors, including urine volume and creatinine, to correctly classify stone formers. These results advance our knowledge of stone formation in PHPT and offer a potential framework for screening PHPT patients who are most likely to have occult stones. In summary, assessment for subclinical nephrolithiasis as suggested by the 2014 International Guidelines reveals the presence of occult stones in about one-fifth of patients. Based on these results and others, close to 40% of all PHPT patients may have stones (20% clinical and 21% subclinical). However, most asymptomatic PHPT patients will not have occult stones when screened. Our results suggest that a screening approach targeting those with higher urine calcium may be an appropriate algorithm for detecting occult stones rather than imaging all patients with asymptomatic PHPT.
Acknowledgments
Funding
This work was supported by NIH grant K24 DK074457 and the Joseph Weintraub Family Foundation.
Footnotes
Declaration of interest
No potential conflicts of interest were disclosed.
References
- 1.Press DM, Siperstein AE, Berber E, et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from theelectronic medical record. Surgery. 2013;154:1232–1237. discussion 1237–1238. doi: 10.1016/j.surg.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 2.Yeh MW, Ituarte PHG, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser WD. Hyperparathyroidism. Lancet. 2009;374: 145–158. [DOI] [PubMed] [Google Scholar]
- 4.Brasileira De Sociedade Endocrinologia EM, Bandeira F, Griz L, et al. Diagnosis and management of primary hyperparathyroidism-a scientific statement from the Department of Bone Metabolism, the Brazilian Society for Endocrinology and Metabolism. Arq Bras Endocrinol Metabol. 2013;57:406–424. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg SJ, Clarke BL, Peacock M, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism:proceedings of the Fourth International Workshop. J Clin Endocrinol Metab 2014; 99:3580–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope O The study of hyperparathyroidism at the Massachusetts General Hospital. N Engl J Med. 1966;274:1174–1182. [DOI] [PubMed] [Google Scholar]
- 8.Albright F, Aub J, Bauer W. Hyperparathyroidism: common and polymorphic condition as illustrated by seventeen proven cases in one clinic. Jama. 1934;102:1276. [Google Scholar]
- 9.Pallan S, Rahman MO, Khan AA. Diagnosis and management of primary hyperparathyroidism. Bmj. 2012;344:e1013. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg SJ, Asymptomatic Primary BJ. Hyperparathyroidism In: eds., Bilezikian J, Marcus R, Ma L, Marcocci C, Sj S, Jt P Jr. The Parathyroids. 3rd ed. USA: Elsevier; 2015. [Google Scholar]
- 11.Scholz DA, Purnell DC. Asymptomatic primary hyperparathyroidism. 10-year prospective study. Mayo Clin Proc. 1981;56:473–478. [PubMed] [Google Scholar]
- 12.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg SJ, Shane E, Jacobs TP, et al. Nephrolithiasis and bone involvement in primary hyperparathyroidism. Am J Med. 1990;89:327–334. [DOI] [PubMed] [Google Scholar]
- 14.Odvina CV, Sakhaee K, Heller HJ, et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res. 2007;35:123–128. [DOI] [PubMed] [Google Scholar]
- 15.Corbetta S, Baccarelli A, Aroldi A, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinol Invest. 2005;28:122–128. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Duh QY, Stackhouse GB, Stoller ML. Oral calcium supplementation associated with decreased likelihood of nephrolithiasis prior to surgery for hyperparathyroidism. Int Journal Urology: Official Journal Jpn Urol Assoc. 2007;14:1113–1115. [DOI] [PubMed] [Google Scholar]
- 17.Mollerup CL, Vestergaard P, Frokjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. Bmj. 2002;325:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran H, Grange JS, Adams-Huet B, et al. The impact of obesity on the presentation of primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99:2359–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazeh H, Sippel RS, Chen H. The role of gender in primary hyperparathyroidism: same disease, different presentation. Ann Surg Oncol. 2012;19:2958–2962. [DOI] [PubMed] [Google Scholar]
- 20.Patron P, Gardin JP, Paillard M. Renal mass and reserve of vitamin D: determinants in primary hyperparathyroidism. Kidney Int. 1987;31:1174–1180. [DOI] [PubMed] [Google Scholar]
- 21.Castellano E, Attanasio R, Latina A, Visconti GL, Cassibba S, Borretta G. Nephrolithiasis in primary hyperparathyroidism: a comparison between silent and symptomatic patients. Endocr Pract. 2017;23:157–162. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporosis Int J Estab Res Coop Eur Found Osteoporosis National Osteoporosis Found USA. 1994;4:368–381. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz NP, Abbas F, Afzal M, Khan R, Rizvi I, Talati J. The prevalence of silent kidney stones-an ultrasonographic screening study. JPMA J Pakistan Med Assoc. 2003;53:24–25. [PubMed] [Google Scholar]
- 24.Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Sonographic evaluation of renal appearance in 665 adult volunteers. Correlation with age and obesity. Acta Radiologica. 1993;34:482–485. [PubMed] [Google Scholar]
- 25.Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger AD,Wu W, Eisner BH, Cooperberg MR, Duh QY, Stoller ML Patients with primary hyperparathyroidism–why do some form stones? J Urol. 2009;181:2141–2145. [DOI] [PubMed] [Google Scholar]
- 27.Starup Linde J, Waldhauer E, Rolighed L, Mosekilde L, Vestergaard P. Renal stones and calcifications in patients with primary hyperparathyroidism: associations with biochemical variables. Eur J Endocrinol. 2012. doi: 10.1530/EJE-12-0032 [DOI] [PubMed] [Google Scholar]
- 28.Söreide JA, van Heerden JA, Grant CS, Lo CY, Ilstrup DM. Characteristics of patients surgically treated for primary hyperparathyroidism with and without renal stones. Surgery. 1996;120:1033–1038. [DOI] [PubMed] [Google Scholar]
- 29.Walker MD, Nickolas T, Kepley A, et al. Predictors of renal function in primary hyperparathyroidism. J Clin Endocrinol Metabolism. 2014;99:1885–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viccica G, Cetani F, Vignali E, Miccoli M, Marcocci C Impact of vitamin D deficiency on the clinical and biochemical phenotype in women with sporadic primary hyperparathyroidism. Endocrine. 2017;55:256–265. [DOI] [PubMed] [Google Scholar]
- 31.Elkoushy MA, Yu AX, Tabah R, Payne RJ, Dragomir A, Andonian S. Determinants of urolithiasis before and after parathyroidectomy in patients with primary hyperparathyroidism. Urology. 2014;84:22–26. [DOI] [PubMed] [Google Scholar]
- 32.Pak CYC, Nicar MJ, Peterson ROY, Zerwekh JE, Snyder W. A lack of unique pathophysiologic background for nephrolithiasis of primary hyperparathyroidism*. J Clin Endocrinol Metabolism. 1981;53:536–542. [DOI] [PubMed] [Google Scholar]
- 33.Frakjaer VG, Mollerup CL. Primary hyperparathyroidism: renal calcium excretion in patients with and without renal stone disease before and after parathyroidectomy. World J Surg. 2002;26:532–535. [DOI] [PubMed] [Google Scholar]
- 34.Locker FG, Silverberg SJ, Bilezikian JP. Optimal dietary calcium intake in primary hyperparathyroidism. Am J Med. 1997;102:543–550. [DOI] [PubMed] [Google Scholar]
- 35.Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25- Dihydroxyvitamin D in the pathogenesis of hypercal-ciuria and renal-stone formation in primary hyperparathyroidism. New England J Med. 1980;302:421–426. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan RA, Haussler MR, Deftos LJ, Bone H, Pak CY. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercal-ciuria. J Clin Invest. 1977;59:756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemann J Jr., Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 1991;17:386–391. [DOI] [PubMed] [Google Scholar]
- 38.Bai S, Favus MJ. Vitamin D and calcium receptors: links to hypercalciuria. Curr Opin Nephrol Hypertens. 2006;15:381–385. [DOI] [PubMed] [Google Scholar]
- 39.Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol. 2005;16:15–26. [DOI] [PubMed] [Google Scholar]
- 40.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Ren Physiol. 2010;298:F485–F499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egbuna O, Quinn S, Kantham L, et al. The full-length calcium-sensing receptor dampens the calcemic response to 1alpha,25(OH)2 vitamin D3 in vivo independently of parathyroid hormone. Am J Physiol Renal Physiol. 2009;297:F720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scillitani A, Guarnieri V, Battista C, et al. Primary hyperparathyroidism and the presence of Kidney stones are associated with different haplotypes of the calcium-sensing receptor. J Clin Endocrinol Metabolism. 2007;92:277–283. [DOI] [PubMed] [Google Scholar]
- 43.Vezzoli G, Terranegra A, Soldati L. Calcium-sensing receptor gene polymorphisms in patients with calcium nephrolithiasis. Curr Opin Nephrol Hypertens. 2012;21:355–361. [DOI] [PubMed] [Google Scholar]
- 44.Corbetta S, Eller-Vainicher C, Filopanti M, et al. R990G polymorphism of the calcium-sensing receptor and renal calcium excretion in patients with primary hyperparathyroidism. Eur J Endocrinol. 2006;155:687–692. [DOI] [PubMed] [Google Scholar]
- 45.Cipriani C, Biamonte F, Costa AG, et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J Clin Endocrinol Metab. 2015;100:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selberherr A, Hörmann M, Prager G, Riss P, Scheuba C, Niederle B. “Silent” kidney stones in “asymptomatic” primary hyperparathyroidism—a comparison of multidetector computed tomography and ultrasound. Langenbeck’s Arch Surg. 2017;402:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker MD, Cong E, Kepley A, et al. Association between serum 25-hydroxyvitamin D level and subcli-nical cardiovascular disease in primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker MD, Cong E, Lee JA, et al. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos Int. 2015;26:2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker MD, Fleischer J, Rundek T, et al. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:3849–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker MD, Fleischer JB, Di Tullio MR, et al. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2010;95:2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]


