Abstract
Age-related depletion of androgens in men results in functional impairments in androgen-responsive tissues, such as the brain, resulting in increased risk for Alzheimer’s disease. To investigate the relationship between normal age-related hormone loss and Alzheimer’s disease risk, we evaluated the brain and serum levels of androgens and estrogen in aging male rats. We observed that increasing age was associated with a significant reduction in brain levels of the potent androgen dihydrotestosterone and a trend toward decreased testosterone. Brain levels of soluble β-amyloid were observed to increase with age. Collectively, these findings highlight differences in brain and circulating levels of androgens during aging, and identify an inverse correlation with β-amyloid levels that may be relevant to Alzheimer’s disease risk.
Keywords: aging, Alzheimer’s disease, β-amyloid, dihydrotestosterone, estrogen, testosterone
Introduction
During normal aging, men experience a significant decline in circulating levels of their primary sex steroid hormone, testosterone [1]. Age-related testosterone depletion in men is associated with increased risk of disease and dysfunction in androgen-responsive tissues including brain [2]. As testosterone induces both androgenic and estrogenic actions, the senescent effects of testosterone loss may reflect androgen and or estrogen deficits. Induction of androgen and estrogen actions of testosterone is determined in large part by its enzymatic conversion in tissues. Testosterone is reduced to the potent androgen dihydrotestosterone (DHT) by the enzyme 5α-reductase and converted to the estrogen 17β-estradiol (E2) by aromatase. Thus, understanding how age-related testosterone loss affects the brain requires knowledge of androgen and estrogen levels in brain.
Although tissue levels of sex steroid hormones can parallel circulating levels, factors including the presence of sex hormone-binding globulin in blood and steroidconverting enzymes in tissues often yield differences between serum and tissue levels [3,4]. Therefore, levels of hormones in specific tissues are generally thought to provide a more accurate measure of bioavailable hormones than serum levels [4]. Serum levels of testosterone, but neither DHT nor E2 are decreased in aging men [1]. How normal male aging affects tissue levels of sex steroid hormones has not been well studied, although there is evidence of age-related decreases in androgen levels in heart, lung, and pubic skin [5]. Earlier study from our laboratory found that brain levels of testosterone [6] and DHT [7] decrease in aging men.
In brain, age-related testosterone depletion in men is associated with impaired cognition [8] and increased risk of Alzheimer’s disease [9]. Androgens have numerous protective actions in brain, the loss of which may contribute to the senescent effects of low testosterone. Of particular relevance to Alzheimer’s disease, androgens are implicated in reducing levels of amyloid-β (Aβ) [10–13], a protein implicated as a causal factor in Alzheimer’s disease pathogenesis [14]. For example, experimental depletion of androgens in male rodents significantly increases Aβ accumulation in brain, an effect prevented by androgen treatment [15,16]. However, the relationships between normal aging, Aβ accumulation, and circulating and brain levels of sex steroid hormones remain undefined. In this study, we investigated these issues in aging male Brown Norway (BN) rats, a model of male reproductive aging [17].
Materials and methods
Animals
Male BN rats (National Institute on Aging; Bethesda, Maryland, USA) were housed in individual cages with ad libitum access to food and water under a 12 h on/12 h off light cycle. Rats were anesthetized and killed by decapitation at ages 3, 13, and 23 months (N = 7/group). At the time of killing (i) trunk blood was collected and separated into the serum fraction; (ii) seminal vesicles were dissected, blotted, and weighed as a bioassay of androgen activity; and (iii) brains were rapidly dissected, bisected in the sagittal plane, and snap-frozen for Aβ and hormone determinations.
Hormone assays
Steroid hormones were purified from frozen brain tissue and serum using Celite chromatography [18,19], and then quantified by radioimmunoassay as described earlier [7]. Briefly, frozen (−80°C) brain tissue was thawed, weighed, and homogenized in 1 ml of ice-cold PBS. Trace levels of 3H-labeled testosterone, DHT, and E2 were included as internal standards to correct for procedural losses in both the tissue homogenates and serum samples. Samples were extracted with hexane/ethyl acetate (3 : 2) and separated from interfering steroids by Celite column partition chromatography with ethylene glycol as stationary phase. DHT and testosterone were eluted with 10 and 35% toluene in isooctane, respectively, whereas elution of E2 was accomplished with 40% ethyl acetate in isooctane. Levels of purified hormones were determined by validated radioimmunoassays. These assays have been shown to be sensitive, accurate, precise, and specific; interassay and intra-assay coefficient of variation were less than 12% [18,19]. The sensitivity of the radioimmunoassays is 15 pg/ml for testosterone, 8 pg/ml for DHT, and 3 pg/ml for E2; all collected data exceeded these detection limits. Data from triplicate readings are presented as a fraction of starting tissue weight or per unit volume serum. Data were statistically compared using analysis of variance followed by between-group comparisons using the Fischer’s Least Significant Difference test.
Amyloid-β enzyme-linked immunosorbent assay
Brain levels of soluble Aβ1-40 were determined by enzyme-linked immunosorbent assay (ELISA) as described earlier [20]. In brief, hemi-brains were processed for Aβ ELISA after extracting soluble protein by homogenization in buffer (0.2% diethylamine, 50 mM NaCl; 1 ml/100 mg tissue) using a polytron for 1 min on ice. Homogenates were centrifuged at 100 000g at 4°C for 1 h and the supernatants collected and neutralized (1/10th volume of 0.5 M Tris–HCl pH 6.8). This soluble protein fraction was used to measure Aβ with a sandwich ELISA using 13.1.1 as the Aβ40 specific capture antibody and 32.4.1 as the rodent-specific detection antibody. Raw data (expressed as pmol Aβ/g protein) were statistically analyzed by analysis of variance followed by between-group comparisons using the Fischer’s Least Significant Difference test.
Results
Circulating and brain levels of sex steroid hormones during aging
To investigate the effects of normal male aging on sex steroid levels, we measured circulating and brain levels of testosterone, DHT, and E2 in male BN rats at ages 3, 13, and 23 months. Serum levels of testosterone decreased by nearly 50% between 3 and 13 months of age and by 63% at the age of 23 months (Table 1). In contrast, we observed no age-related changes in circulating levels of DHT (Table 1). The estrogen E2 showed a nonsignificant increase in middle age (Table 1). Analysis of sex steroid hormone levels in hemi-brain homogenates showed a different pattern of age-related changes. Brain levels of testosterone showed a statistically nonsignificant trend of reduction with an approximately 40% decrease between ages 3 and 23 months. In comparison, the androgen metabolite DHT showed a relatively stronger age-related decrease. In comparison with brain levels in young adult 3-month-old rats, DHT decreased by 80% at the age of 13 months and more than 90% at the age of 23 months (Table 1). Brain levels of E2 showed a statistically nonsignificant trend of age-related depletion (Table 1). The observation of significantly decreased DHT in brain, but not serum suggests the possibility of age changes in testosterone metabolism. To investigate this possibility, we evaluated precursor/product ratios for testosterone and its metabolites DHT and E2. The testosterone/DHT ratio in brain increased from 3.57 at the age of 3 months to 9.29 at the age of 13 months and 9.71 at the age of 23 months (F = 5.6, P <0.02). In comparison, the testosterone/E2 ratio in brain did not significantly change with age (F = 0.4, P = 0.68). Similarly, the correlation between brain levels of testosterone and DHT decreased from r = 0.80 (P < 0.04) at the age of 3 months to r = 0.04 (P = 0.65) at the age of 13 months and r = 0.23 (P = 0.66) at the age of 23 months. Brain levels of testosterone and E2 were not significantly correlated at any age.
Table 1.
Age-related changes in serum and brain levels (mean ±standard error) of testosterone, DHT, and estrogen (E2) in male Brown Norway rats
| Age of male rats |
||||
|---|---|---|---|---|
| 3 Months | 13 Months | 23 Months | P value | |
| Serum testosterone (ng/ml) |
2.9 ± 0.9 | 1.5 ± 0.4 | 1.1 ± 0.2 | 0.07 |
| Serum DHT (pg/ml) | 57.2 ± 10.1 | 50.0 ± 9.4 | 68.0 ± 10.1 | 0.45 |
| Serum E2 (pg/ml) | 5.0 ± 8.0 | 21.0 ± 8.7 | 7.6 ± 8.7 | 0.41 |
| Brain testosterone (ng/g WT) |
2.1 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.6 | 0.50 |
| Brain DHT (ng/g WT) | 0.65 ± 0.1 | 0.13 ± 0.1* | 0.08 ± 0.1* | 0.004 |
| Brain E2 (pg/g WT) | 79.6 ± 15.9 | 50.7 ± 15.9 | 27.7 ± 17.1 | 0.11 |
DHT, dihydrotestosterone; WT, wet tissue.
P<0.05 relative to 3 months.
Age-related changes in androgen-sensitive measures
To evaluate whether androgen function was impaired by age-related hormone losses, we first measured a biomarker of androgen levels, size of the seminal vesicles. We found a significant, age-related decrease in seminal vesicle weight normalized to body weight (F = 59.1, P =0.001). In comparison with the age of 3 months (1.74 ±0.13 × 103), normalized seminal vesicle weight decreased by approximately 35% at the age of 13 months (1.12 ± 0.13 × 103) and by nearly 50% at the age of 23 months (0.93 ± 0.15 × 103). Next, we measured brain levels of Aβ, a protein negatively regulated by androgens [9]. We observed that brain levels of Aβ1–40 significantly increased (F = 4.8, P = 0.03) by approximately 300% at the age of 13 months and nearly 400% by the age of 23 months in comparison with levels observed in young adult 3-month-old rats (Fig. 1).
Fig. 1. Brain levels of soluble β-amyloid protein increase during normal male aging. Data show mean levels (±SEM) of Aβ1–40 protein as measured by enzyme-linked immunosorbent assay from hemi-brain homogenates of male Brown Norway rats at ages 3, 13, and 23 months (N = 7/group).
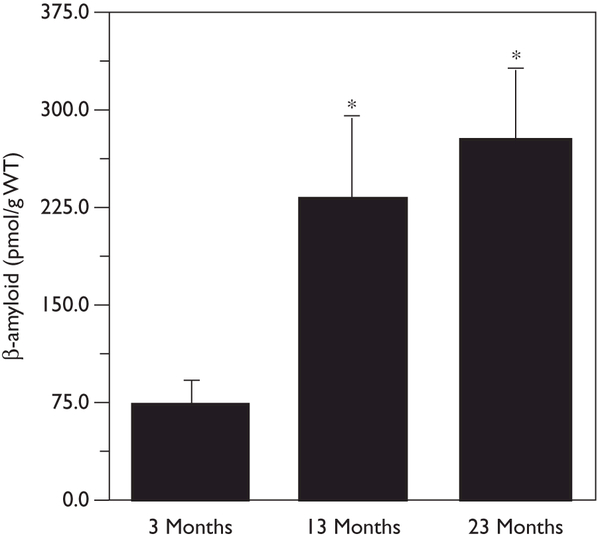
*P<0.05 relative to the 3 month group. WT, wet tissue.
Discussion
In this study, we examined age-related changes in circulating and brain levels of androgens and estrogen. We found that increasing age was associated with a nonsignificant decrease in circulating testosterone, no obvious changes in circulating levels of DHT, and a transient increase in E2 at middle age. In contrast, brain levels of all the three hormones showed evidence of reduction, although only the robust depletion of DHT achieved statistical significance. In parallel, significant impairments were observed in androgen-sensitive seminal vesicle weight and brain levels of Aβ, suggesting functional consequences of age-related androgen loss.
Our results are consistent with reported age changes in circulating levels of sex steroids in male rats. Testosterone levels in young adult male rats are fairly consistent across strains with mean levels normally between 2 and 3 ng/ml [17,21,22]. An approximately 50% or greater age-related reduction in testosterone has been reported in Sprague–Dawley [23], Wistar [21], and Fischer [22] rat strains. Similarly, earlier study in male BN rats showed a significant, approximately 75% loss in circulating levels of testosterone between 6 and 28 months of age [17]. Consistent with this established literature, we observed a trend of decreased testosterone levels with aging (although this effect did not meet statistical significance) and no evidence of age changes in DHT. Although testosterone levels can be affected by illness and chronic disorders, age-related depletion of circulating testosterone in male BN rats is a normal consequence of aging resulting from testicular atrophy as well as dysregulation of the hypothalamic–pituitary–gonadal axis [17,24]. The effects of aging on circulating level of E2 are less clear, with reports indicating no change [24] or increased levels [22]. Interestingly, in a recent report, circulating E2 levels in male Sprague–Dawley rats were observed to increase during middle age followed by a decrease at the age of 22 months, whereas circulating testosterone levels progressively declined across ages [25]. Our findings in male BN rats are most consistent with the latter observations of transient increases in serum E2 during middle age.
An important new finding in our study is that age changes in sex steroid hormones differ between brain and blood. Earlier studies of sex steroid hormones in animals largely focused on serum rather than brain levels and have not investigated the effects of age on brain levels in either males or females. Our findings suggest that the male brain is particularly vulnerable to age-related changes in sex steroid hormone levels. Specifically, we observe evidence of decreased levels of testosterone, DHT, and E2 in brain. In recent studies, we examined levels of sex steroid hormones in the frontal cortex of aged men and found that brain levels of testosterone decreased with increasing age, however, there was no change in brain levels of E2 [6]. Similar to our findings with the male BN rats, we also found an age-related decrease in brain levels of DHT in men [7]. Together, these data suggest that the male brain is particularly vulnerable to age-related androgen depletion although the underlying factors are unknown. Two potential causes are age-related changes in sex hormone binding globulin, which could reduce neural accumulation of testosterone, and altered activity of converting enzymes [19,26]. Our data show changes in the precursor/product ratios between testosterone and DHT as well as changes in the correlation between these two androgens, both of which suggest decreased neural conversion of testosterone to DHT with increasing age. Interestingly, we did not observe a relationship between testosterone and E2 levels, suggesting that brain levels of E2 may be the result of another prohormone, such as estrone. Further research is needed to investigate the roles of hormone-converting enzymes and other factors contributing to the age-related decrease in brain levels of androgens.
Earlier study from our lab suggests that androgens may modulate risk for the development of Alzheimer’s disease through regulation of Aβ [15,16]. In humans, age-related androgen depletion is inversely correlated with elevated Aβ levels [7,12]. In this study, we also observe that aging is associated with both a decrease in brain levels of androgens and an increase in brain levels of the Alzheimer’s-related protein Aβ, however, the design of this study does not permit for the assessment of a potential causal relationship between these factors. Although advancing age is the greatest risk factor for idiopathic Alzheimer’s disease, the age-related changes underlying this risk are unknown. The present data are consistent with the theory that age-related loss of androgens in brain may increase Alzheimer’s disease risk in men by facilitating the Aβ accumulation.
Conclusion
This study shows that normal aging in male BN rats is characterized by decreased brain levels of sex steroid hormones and increased levels of the Alzheimer’s-related protein Aβ. These findings may be important for understanding the role of sex steroid hormones in brain aging and age-related neurological disorders including Alzheimer’s disease.
Acknowledgements
This study was supported by the NIA grants R01 AG23739 (C.J.P.) and P01 AG005119 (M.M.P.).
References
- 1.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005; 26:833–876. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE. Androgens and aging. Maturitas 2001; 38:61–71; discussion 71–73. [DOI] [PubMed] [Google Scholar]
- 3.Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, et al. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab 1985; 61:705–710. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab 1986; 15:259–278. [DOI] [PubMed] [Google Scholar]
- 5.Deslypere JP, Vermeulen A. Influence of age on steroid concentrations in skin and striated muscle in women and in cardiac muscle and lung tissue in men. J Clin Endocrinoi Metab 1985; 61:648–653. [DOI] [PubMed] [Google Scholar]
- 6.Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA 2004; 292:1431–1432. [DOI] [PubMed] [Google Scholar]
- 7.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobioi Aging 2009. [Epub ahead of print 8 May 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience 2006; 138:1015–1020. [DOI] [PubMed] [Google Scholar]
- 9.Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res Rev 2008; 57:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandy S, Almeida OP, Fonte J, Lim D, Waterrus A, Spry N, et al. Chemical andropause and amyloid-beta peptide. JAMA 2001; 285:2195–2196. [DOI] [PubMed] [Google Scholar]
- 11.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology 2004; 29:1071–1081. [DOI] [PubMed] [Google Scholar]
- 12.Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis 2003; 5:267–269. [DOI] [PubMed] [Google Scholar]
- 13.Yao M, Nguyen TV, Rosario ER, Ramsden M, Pike CJ. Androgens regulate neprilysin expression: role in reducing beta-amyloid levels. J Neurochem 2008; 105:2477–2488. [DOI] [PubMed] [Google Scholar]
- 14.Amyloid Hardy J., the presenilins and Alzheimer’s disease. Trends Neurosci 1997; 20:154–159. [DOI] [PubMed] [Google Scholar]
- 15.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, et al. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem 2003; 87:1052–1055. [DOI] [PubMed] [Google Scholar]
- 16.Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci 2006; 26:13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Hikim AS, Ferrini M, Bonavera JJ, Vernet D, Leung A, et al. Male reproductive ageing: using the Brown Norway rat as a model for man. Novartis Found Symp 2002; 242:82–95; discussion 95–97. [PubMed] [Google Scholar]
- 18.Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakamura RM, Coulson AH, Korelitz JJ. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy In: Lepow IH, Crozier R, editors. Vasectomy: immunologic and pathophysiologic effects in animals and man. New York: Academic Press; 1979. p. 165. [Google Scholar]
- 19.Serafini P, Ablan F, Lobo RA. 5 alpha-reductase activity in the genital skin of hirsute women. J Clin Endocrinol Metab 1985; 60:349–355. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MP, Hickman LJ, Eckman CB, Uljon SN, Wang R, Golde TE. Gamma-secretase, evidence for multiple proteolytic activities and influence of membrane positioning of substrate on generation of amyloid beta peptides of varying length. J Biol Chem 1999; 274:11914–11923. [DOI] [PubMed] [Google Scholar]
- 21.Taylor G, Bardgett M, Farr S, Humphrey W, Womack S, Weiss J Aging of the brain-testicular axis: reproductive systems of healthy old male rats with or without endocrine stimulation. Proc Soc Exp Biol Med (New York, NY) 1996; 211:69–75. [DOI] [PubMed] [Google Scholar]
- 22.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 2007; 19:743–751. [DOI] [PubMed] [Google Scholar]
- 23.Roselli CE, Kaler LW, Resko JA. Hypothalamic aromatase activity in young and old male rats. Neurobiol Aging 1986; 7:121–125. [DOI] [PubMed] [Google Scholar]
- 24.Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol 1994; 49:B42–B50. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol 2009; 512:688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roselli CE, Resko JA. Effects of gonadectomy and androgen treatment on aromatase activity in the fetal monkey brain. Biol Reprod 1986; 35:106–112. [DOI] [PubMed] [Google Scholar]


