Abstract
Objective
To use meta-analytic techniques to synthesize the findings of the current body of published literature regarding the risk of hypospadias resulting from parental exposure to pesticides.
Materials and methods
A search of Pub Med for original research published in English from January 1966 through March 2008 identified 552 studies, 90 of which were reviewed in detail. Nine studies met all study inclusion criteria. Two reviewers independently abstracted data from each included study. Any disagreements were resolved by consensus. Pooled risk ratios (PRRs) and confidence intervals (CIs) were calculated using both random and fixed effects models, along with statistical tests of homogeneity.
Results
Elevated but marginally significant risks of hypospadias were associated with maternal occupational exposure (PRR of 1.36, CI = 1.04–1.77), and paternal occupational exposure (PRR of 1.19, CI = 1.00–1.41). Subgroup analyses provided insights into needed designs for future studies. Notably, exposure assessment using a job-exposure matrix resulted in slightly higher estimated risk than agricultural occupation in fathers; but this effect was reversed in mothers, suggesting the importance of indirect and residential pesticide exposures in this group.
Conclusions
Despite potential exposure misclassification, which would tend to diminish observed associations, the previous literature indicates a modestly increased risk of hypospadias associated with pesticide exposure.
Keywords: Congenital abnormalities, Hypospadias, Meta-analysis, Pesticides, Pregnancy
Introduction
Hypospadias is estimated to affect 0.3–1% of live births and is characterized by an abnormal positioning of the meatus, the opening of the urethra, in males [1–3]. This malformation is most common among non-Hispanic whites and is least common among Hispanics [4], although recent data suggest that birth prevalence is increasing among nonwhites [3]. There is substantial unexplained variation in hypospadias rates both within and between countries [2,7]. Data from the US and several European countries from the 1970s to 1990s showed increases in overall rates which were unlikely to be due to changes in case ascertainment [5], though more recent data seem to indicate that this trend is leveling off or at least not as widespread as was once thought [6].
Because sex hormones play a strong role in fetal genitourinary development [3,7], it has been hypothesized that in utero exposure to endocrine-disrupting chemicals could contribute to hypospadias [8,9]. Such chemicals might have an estrogenic or androgen-antagonist effect [8]. In utero exposure to the synthetic estrogen diethylstilbestrol is a known risk factor for hypospadias [10—12]. Exposure to other synthetic estrogens and progestins, such as those used in oral contraceptives or assisted reproductive techniques, has also been associated with an increased risk of hypospadias in some [4,13—15], but not all, studies [16,17].
Several classes of pesticides have been shown to have endocrine-disrupting potential [18]. Approximately 60% of the herbicides applied in the US, by weight, have demonstrated endocrine-disrupting or reproductive effects in vitro or in animal studies [19], including commercial chlorphenoxy herbicides and glyphosate. The herbicide linuron, which binds weakly to the androgen receptor, was shown to increase rates of hypospadias in rats, as were the dicarboximide fungicides chlozolinate, iprodione, procy-midone, vinclozolin and dichlorodiphenyldichloroethylene (DDE) [20—22].
Despite laboratory evidence of endocrine-disruption by several pesticide classes, few recommendations have been issued by authoritative expert panels or advisory committees. The National Research Council’s comprehensive report on pesticides in the diets of infants and children specifically excluded exposure prior to the third trimester of pregnancy, remarking that ‘‘the origins of this broader concern with peri- and postnatal toxicology are inextricably rooted in experimental teratology’’ [23]. Experimental results in animals, they however note, may not be fully applicable to humans.
Animal models may not accurately reflect the typical human experience of pesticide exposure or metabolism of pesticides [24]. Measurement issues, however, have also plagued many human studies. Pesticide exposure might occur in many settings (occupationally, in the home or environmentally) and be mediated by personal behaviors and the use of protective equipment [25—27]. Even when pesticide exposure has been well measured, the low frequency of hypospadias has resulted in several studies that were underpowered to adequately detect a clinically significant increase in risk [28—32]. Further, the potential adverse effects of adjuvants used in commercial products—which typically make up 50—60% of the total product weight—have rarely been considered [18].
This meta-analysis was conducted to systematically review the available evidence of an association between pesticide exposure and hypospadias, to provide a quantitative summary of the estimated risk, and to identify areas where further study might be needed. Although a metaanalysis cannot overcome variations in measurement, it might be able to overcome a lack of precision and present a composite estimate of the association between pesticide exposure and hypospadias [33].
Methods
Relevant published studies from January 1966 through March 2008 were identified using Pub Med searches and reviewing references from selected citations. Search terms were included as both keywords and medical subject headings. Exposure terms used were pesticides, fungicides, fumigants, insecticides, herbicides, agriculture, agricultural chemicals, occupation, maternal occupation, paternal occupation, parental occupation, and hypospadias risk factors. Outcome terms used were hypospadias, genitourinary defects, birth defect, birth malformation, congenital malformation, congenital anomaly, and birth anomaly. The latter broad terms were selected to identify articles that examined multiple birth defects and might have reported results for hypospadias. Publicationswere restricted tothoseon humans.
Identified articles were reviewed for suitability for inclusion in the meta-analysis first by title, followed by abstract review, and then by full text review (Fig. 1). Among the excluded studies were two that solely examined the effects of serum DDT/DDE [34,35]; with widespread bans on use of DDT beginning in the 1970s, these studies have decreasing relevance and potential for intervention. Two other studies that used other congenital defects as a control group were also excluded [36,37]. Associations have been suggested between pesticides and several types of birth defects [38]; inclusion of these defects in the control group might significantly bias results towards the null. Because meta-analytic methods require that all risk estimates included in calculation of the pooled risk ratio (PRR) be independent from one another, two additional studies were excluded due to overlap between their study populations and another study population [38,39]. In each instance, the study with the larger sample size or more extensive adjustment of covariates was used in calculating the final PRRs. To assess bias introduced by excluding these two studies, the PRR was recalculated using the excluded studies while removing the alternative studies from the overlapping population.
Figure 1.
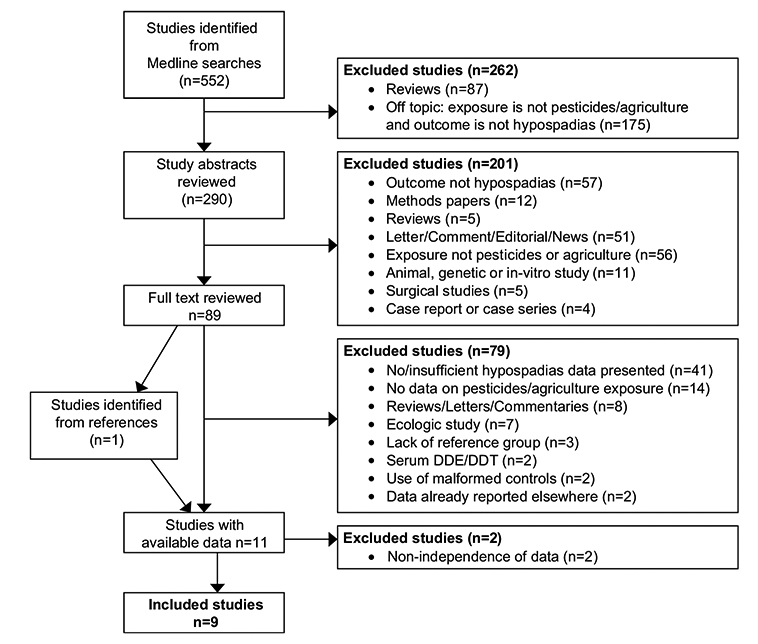
Flow scheme of process used to select studies for inclusion in the meta-analysis.
Reported risk ratios (either odds ratios or relative risks), confidence intervals (CIs), raw percentages and raw data were abstracted, where available, by two independent reviewers (CMR and PAR). Discrepancies in abstracted data were resolved by consensus. Inclusion criteria were presentation of either a reported risk ratio estimate and CI, or sufficient raw data to calculate a risk ratio between some measure of individual pesticide exposure and risk of hypospadias. Abstracted data from studies identified as suitable for inclusion were used to conduct a meta-analysis for reported type (maternal or paternal) of occupational exposure. Where data permitted, PRRs were calculated for study design and exposure period.
The natural log of the risk estimate and its variance was required for each study, and was either calculated from the reported risk ratios and CIs (n = 7) where available or from the raw (crude) data (n = 2). The most highly analytically-adjusted risk ratios available were used for calculation of the PRRs in the final model. Most exposure data were presented as a dichotomous variable (exposed/unexposed); multi-level exposure categories were similarly dichotomized to reduce the potential overuse of the highest exposure category.
PRRs were calculated using both fixed effects and random effects models [40,41]. Data were stratified into subgroups according to study design, exposure period and exposure assessment. The consistency of associations across studies was assessed using statistical tests of homogeneity [40]. Where multiple risk ratios were available from a single publication, the PRRs and CIs were recalculated using each available risk ratio estimate, regardless of inclusion in the final model, in order to detect a large change in the calculated PRRs and CIs associated with which risk ratio was selected from the multiple ratios presented in a particular study.
Results
Among the nine studies pooled in this meta-analysis, six of the studies evaluated both maternal and paternal exposure, two evaluated paternal exposure only, and one examined maternal exposure only (Table 1). Overall, cases and controls were most often identified from hospital or health care records, and the number of subjects enrolled was reported for most studies. Five studies provided risk ratios adjusted for potential confounders. Only one study attempted to stratify by severity or location of hypospadias, by evaluating all hypospadias and then restricting cases to hypospadias receiving surgical repair. The authors’ assumption was that only more severe (e.g. distal or proximal) hypospadias would receive repair; this assumption may not be appropriate in other settings. Outcome ascertainment might have been variable between the remaining studies, depending on how cases were identified. First-degree (glanular) hypospadias may not be immediately apparent at birth or prior to circumcision; therefore these cases may not be identified based on birth records.
Table 1.
Characteristics of study methods for the association between parental pesticide exposure and hypospadias
| Lead author and year | Study population and period | Subject selection (case:control) | Exposure assessment | Covariate adjustment* | Maternal exposure |
Paternal exposure |
||
|---|---|---|---|---|---|---|---|---|
| Exposure levels | Exposure period | Exposure levels | Exposure period | |||||
| Schwartz 1986 [49] | California, USA 1975–1978 | Hospital (12:2451) | Medical chart review | NO | Agricultural occupation vs non-agricultural occupation | Prenatal | Agricultural occupation vs non-agricultural occupatior | Prenatal |
| Schnitzer 1995 [43] | Georgia, USA 1968–1990 | Birth register (463:2388) | Parental interview | NO | Farm manager or worker (yes/no) | During 2 years before birth | ||
| Olshan 1991 [44] | British Columbia 1952–1973 | Birth defects registry (838:28,830) | Occupation recorded on birth record | YES | Occupation “farm managers and workers’’ (yes/no) | Birth | ||
| Garcia 1999 [47] | Spain 1993–1994 | Hospitals (18:18) | Parental interview | YES | Agricultural work ever (yes/no) Agricultural work during acute risk periods (yes/no) |
Ever/1 month prior through first trimester | Pesticide application ever (yes/no) Pesticide application in critical period (yes/no) |
Ever/3 months prior through first trimester |
| Carbone 2006 [50] | Ragusa, Italy 1998–2002 | Pediatric services (43:203) | Parental interview and JEM | YES | Work in agriculture (yes/no) Exposure to pesticidesa |
Before/during pregnancy | Work in agricultureb | Before/during pregnancy |
| Pierik 2004 [46] | The Netherlands 1991–2001 | Child health care centers (56:313) | Parental interview and JEM | NO | Probable pesticide exposure (yes/no) | First trimester | Exposure to pesticidesc Probable pesticide exposure (yes/no) | Periconception |
| Weidner 1998 [45] | Denmark 1983–1992 | Birth cohort (1345:23,273) | Maternal interview | YES | Farmer (yes/no) Gardener (yes/no) | Occupation during year of conception | Farmer (yes/no) Gardener (yes/no) | Occupation during year of conception |
| Zhu 2006 [30] | Denmark 1997–2003 | Birth cohort (172: 62,432) | Maternal interview | NO | Farmer (yes/no) Gardener (yes/no) | Occupation in pregnancy and 3 months prior | ||
| Kristensen 1997 [48] | Norway 1967–1991 | Birth register (270:253,498) | Birth record linked to agricultural census | YES | Farming parents (yes/no) Use of tractor spraying equipment, tractor spraying + grain cultivation |
Occupation at time of pregnancy and birth | Farming parents (yes/no) Use of tractor spraying equipment, tractor spraying + grain cultivation |
Occupation at time of pregnancy and birth |
Olshan et al. adjusted for parental ages, race, and outcome of previous pregnancies. Garcia et al. matched cases and controls according to hospital and approximate date of birth. Carbone et al. adjusted for birth weight, parity, mother’s age, mother’s education, TTP, condom use, mother’s gynecological diseases, father’s urogenital diseases, use of antiabortion drugs, mother’s alcohol use during pregnancy, and other parent’s exposure to pesticides. Weidner et al. adjusted for year of birth and birth weight. Kristensen et al. adjusted for year of birth, maternal age, geographical region, and parental consanguinity.
Exposure to pesticides was classified as: improbable or probable.
Agricultural work was classified as: no, in related occupations, work in the field, or work in a greenhouse.
Exposure to pesticides was classified as: improbable, probable or highly probable.
Pesticide exposure was most commonly assessed as selfreported agricultural occupation, though occupation recorded on the birth record or in the medical chart was also used. Two studies used a job-exposure matrix (JEM) to assess the probability of occupational exposure to pesticides, and one of these compared self-reported agricultural work to JEM-assigned exposure. The relevant period of exposure varied across all studies, with exposure at any time during the pregnancy ± pre-pregnancy period most often reported.
PRRs stratified by maternal and paternal exposure, study design, and exposure period are presented in Table 2. The studies reported homogenous PRR estimates (P > 0.40), thus fixed and random effects were similar or identical. Only results using random effects models are shown to provide the most conservative estimates. Total maternal occupation in agriculture or other pesticide-exposed occupations was associated with an excess risk of hypospadias (PRR of 1.36; CI = 1.04—1.77), and total paternal occupation in agriculture or other pesticide-exposed occupations was also associated with a small excess risk (PRR of 1.19; CI = 1.00—1.41) using all studies. Restriction of analyses to those studies with covariate adjustment produced small elevations in the PRR estimates. Self-reported maternal exposure produced similar results, but when maternal exposure was assigned by JEM the PRR dropped below unity. Results of a cohort analysis could not be calculated for paternal exposure; but the PRR for the two cohort studies of maternal exposure was elevated (PRR of 1.51, CI = 1.06—2.16). Whether using a JEM or self-reported exposure, paternal pesticide exposure produced PRRs greater than unity but non-significant. Data for evaluating the exposure period were only available for paternal studies, but assessment of exposure in the periconceptional and spermatogenesis ± periconceptional periods produced PRRs that were non-significant and less than unity.
Table 2.
Results from exposure-specific meta-analyses using a random effects model
| Category | All studies |
Studies with covariate adjustmenta |
||||||
|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | Homogeneity P-value |
n | OR | 95% CI | Homogeneity P-value |
|
| Maternal occupationb | 7 | 1.36 | 1.04–1.77 | 0.77 | 4 | 1.40 | 1.06–1.84 | 0.63 |
| Study design | ||||||||
| Case-control | 5 | 1.19 | 0.80–1.76 | 0.68 | 3 | 1.25 | 0.82–1.91 | 0.52 |
| Cohort | 2 | 1.51 | 1.06–2.16 | 0.94 | 1 | NCc | NCc | NCc |
| Exposure period | ||||||||
| Pregnancy ± pre-pregnancy | 7 | 1.36 | 1.04–1.77 | 0.77 | 4 | 1.40 | 1.06–1.84 | 0.63 |
| Exposure assessmentd,e | ||||||||
| Agricultural occupation | 5 | 1.36 | 1.01–1.82 | 0.53 | 4 | 1.42 | 1.05–1.92 | 0.60 |
| JEM-assessed pesticide exposure | 2 | 0.93 | 0.24–3.65 | 0.34 | 1 | NCc | NCc | NCc |
| Paternal occupationf | 8 | 1.19 | 1.00–1.41 | 0.69 | 5 | 1.23 | 1.03–1.47 | 0.74 |
| Study design | ||||||||
| Case-control | 7 | 1.11 | 0.92–1.34 | 0.86 | 4 | 1.16 | 0.95–1.41 | 0.95 |
| Cohortc | 1 | NCc | NCc | NCc | 1 | NCc | NCc | NCc |
| Exposure period | ||||||||
| Pregnancy ± pre-pregnancy | 8 | 1.19 | 1.00–1.41 | 0.69 | 5 | 1.23 | 1.03–1.47 | 0.74 |
| Periconceptiveg | 2 | 0.85 | 0.30–2.44 | 0.85 | 1 | NCc | NCc | NCc |
| Spermatogenesis ± periconceptionh | 2 | 0.84 | 0.41–1.73 | 0.44 | 1 | NCc | NCc | NCc |
| Exposure assessmente | ||||||||
| Agricultural occupation | 7 | 1.20 | 0.99–1.46 | 0.52 | 5 | 1.25 | 1.02–1.53 | 0.60 |
| JEM-assessed pesticide exposure | 2 | 1.28 | 0.50–3.27 | 0.29 | 1 | NCc | NCc | NCc |
OR = odds ratio; CI = confidence interval; NC = not calculated.
Refs. [30,43,46,49] excluded from studies with covariate adjustment due to lack of sufficient data.
Insufficient number of studies to calculate a pooled risk estimate.
Ref. [30] assessed exposure as occupation in “gardening” and was excluded.
Ref. [50] assessed exposure based on self-reported occupation in agriculture, and also used a JEM to assess pesticide exposure. Data from each evaluation were available, and therefore used in each of the exposure-assessment categories.
Ref. [30] excluded from paternal occupation analyses due to lack of data on paternal exposures.
Study includes any part of the 3 months prior to conception plus early pregnancy.
Exposure period assessed included the approximate time period of spermatogenesis, roughly 4 months prior to conception.
Discussion
This meta-analysis showed that maternal occupational exposure to pesticides or agricultural work was associated with a 36% increased risk of hypospadias overall, and paternal occupational exposure to pesticides or agricultural work was associated with a 19% increased risk of hypospadias. Though modest, these elevated risks may be clinically relevant given the enormous psychological and economic impact of hypospadias on families. The elevated risk observed in this meta-analysis may be an underestimate. Challenges in exposure assessment created the potential for misclassification in the pooled studies; this could have biased the risk ratio estimates towards the null. Given the spectrum of severity of hypospadias, there was also the potential for incomplete case ascertainment in some previous studies; this also may have diluted the observed overall effect of pesticide exposure.
The relationship between pesticide exposure and birth defects might be difficult to examine due to the potential critical period, which is typically defined as between weeks 8 and 14 but which may also involve latent effects [3,4,10,12]. Assessment of exposure only during weeks 8—14 might be misleading when subjects are continuously employed in a given occupation, creating the illusion of a ‘critical period’ when the actual critical exposure might have occurred outside this time frame.
Although maternal exposure during early gestation could alter the normal fetal environment and disrupt embryogenesis [42], high levels of pesticides have also been measured in seminal fluids [7]. Consequently, both parents’ exposures might be relevant. Of the nine studies included in this analysis, three did not assess both parents’ occupations [30,43,44], three assessed maternal and paternal exposures separately [45—47], two assigned exposure to both parents based on exposure by either parent, citing the familial nature of farm work [48,49], and only one considered each parent’s exposure separately while adjusting for the other parent’s exposure [50].
Residential pesticide exposure could not be evaluated in this meta-analysis, because only one included study evaluated probable exposure to pesticides at either work or at home [50]. Non-occupationally exposed individuals have been demonstrated to carry substantial levels of nonpersistent pesticide metabolites in biological samples [51]. Of the studies identified for this meta-analysis, two used a JEM to assign probable exposure, one asked for selfreports of pesticide exposure, and the remainder relied on either documented or self-reported work in agriculture. Expert assessment of a detailed occupational history by industrial hygienists has been demonstrated to be superior to self-assessed exposure [52], and JEMs are based on many assumptions that might lead to misclassification [53]. Any exposures might be modified by practices such as the use of personal protective equipment and the frequency of handwashing [25,54—56], though these were not assessed in any of the included studies. None of the studies were able to assess risks associated with specific pesticide brands or classes. The exposure misclassification in these studies would likely be random and therefore most likely bias results towards the null.
Rather than subjectively assigning a ‘quality’ score to the exposure assessment used by the included studies, we examined methodologic subcategories to elucidate potential sources of misclassification that may have caused underestimation of the true risk. Limiting analysis to studies with covariate adjustment yielded slight increases in the PRRs for all analyses, suggesting that cruder assessment might tend to bias the observed associations towards the null. The PRR obtained from cohort studies was also increased over that calculated from case-control studies; this might be due to better exposure assessment available in the cohort studies.
Publication bias, in which positive studies are more likely to be published than null studies, might have artificially inflated the calculated PRRs [33]. It is worth noting that only one of the included studies contributed a statistically significant result [48], suggesting that publication bias is unlikely for this disease-exposure relationship. Bias might also be introduced in a meta-analysis when authors make exclusion decisions. To assess potential bias introduced by authors’ inclusion decisions, analyses were conducted in which the PRR was recalculated after adding back each excluded study, both singly and in combination, while removing any overlapping study. These analyses found very little (<10%) change in either the magnitude or significance of the PRRs.
The results of this meta-analysis are further strengthened by their consistency with animal studies demonstrating the teratogenic potential of pesticides. The anti-androgenic effects of selected pesticides are well-defined from in vivo studies [57], and are important in light of the androgen-dependent normal urethral development in the fetus [2,9,28,58]. It is also possible that estrogenic or other endocrine-disrupting effects of pesticides play a role [8,59—61].
In conclusion, this meta-analysis of nine published studies demonstrated an elevated risk of hypospadias associated with maternal occupational exposure to pesticides or agricultural work, with a suggested increased risk for paternal exposure that was stronger when analysis was restricted to those studies with covariate adjustment. Potential misclassification of exposure, which would likely attenuate the relative risk estimates, posed a large threat in all publications used in this meta-analysis. This misclas-sification could mask a stronger relationship between specific types of pesticides and hypospadias, since pesticides and their adjuvants comprise a chemically diverse class. Despite this potential, the 19—51% increase in risk of hypospadias defects observed here may be clinically relevant given the economic, social and psychological burden of this malformation. Results of this meta-analysis are consistent with evidence from animal and developmental models. Future studies of pesticide exposure and hypospadias should attempt to describe pesticide exposure in terms of both quantity and type of pesticide.
Acknowledgements
This work was funded by a grant sponsored by the Centers for Disease Control and Prevention (U50/CCU 713238). Ms. Rocheleau also received fellowship support from a National Institute for Occupational Safety and Health, Occupational Epidemiology Training Grant (T42 OH008491). Study sponsors were not involved in the study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest statement
The authors of this manuscript do not have any competing financial or personal interests.
References
- [1].Stokowski L Hypospadias in the neonate. Adv Neonatal Care 2004;4:206—15. [DOI] [PubMed] [Google Scholar]
- [2].Manson JM, Carr MC. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res A Clin Mol Teratol 2003;67:825—36. [DOI] [PubMed] [Google Scholar]
- [3].Kurzrock EA, Karpmann E. Hypospadias: pathophysiology and etiologic theories. Pediatr Endocrinol Rev 2004;1:288—95. [PubMed] [Google Scholar]
- [4].Leung AKC, Robson WLM. Hypospadias: an update. Asian J Androl 2007;9:16—22. [DOI] [PubMed] [Google Scholar]
- [5].Toppari J, Kaleva M, Virtanen HE. Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Hum Reprod Update 2001;7: 282—6. [DOI] [PubMed] [Google Scholar]
- [6].Porter MP, Faizan MK, Grady RW, Mueller BA. Hypospadias in Washington State: maternal risk factors and prevalence trends. Pediatrics 2005;115:e495—9. [DOI] [PubMed] [Google Scholar]
- [7].Garcia AM. Occupational exposure to pesticides and congenital malformations: a review of mechanisms, methods, and results. Am J Ind Med 1998;33:232—40. [PubMed] [Google Scholar]
- [8].Sharpe RM, Skakkebaek NE. Are oestrogens involved n falling sperm counts and disorders of the male reproductive tract? Lancet 1993;341:1392—5. [DOI] [PubMed] [Google Scholar]
- [9].Rittler M, Castilla EE. Endocrine disruptors and congenital anomalies. Cad Saude Publica 2002;18:421—8. [DOI] [PubMed] [Google Scholar]
- [10].Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod 2006;21:666—9. [DOI] [PubMed] [Google Scholar]
- [11].Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 2002;359: 1102–7. [DOI] [PubMed] [Google Scholar]
- [12].Palmer J, Wise L, Robboy S, Titus-Ernstoff L, Noller K, Herbst A, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology 2005;16:583–6. [DOI] [PubMed] [Google Scholar]
- [13].Silver RI, Rodriguez R, Chang TS, Gearhart JP. In vitro fertilization is associated with an increased risk of hypospadias. J Urol 1999;161:1954–7. [PubMed] [Google Scholar]
- [14].Monteleone Neto R, Castilla EE, Paz JE. Hypospadias: an epidemiological study in Latin America. Am J Med Genet 1981; 10:5–19. [DOI] [PubMed] [Google Scholar]
- [15].Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005;159:957–62. [DOI] [PubMed] [Google Scholar]
- [16].Ericson A,Kallen B. Congenital malformations in infants born after IVF:a population-based study. Hum Reprod 2001; 16: 504–9. [DOI] [PubMed] [Google Scholar]
- [17].Kallen B Case control study of hypospadias, based on registry information. Teratology 1988;38:45–50. [DOI] [PubMed] [Google Scholar]
- [18].Lin N, Garry VF. In vitro studies of cellular and molecular developmental toxicity of adjuvants, herbicides, and fungicides commonly used in Red River Valley, Minnesota. J Toxicol Environ Health A 2000;60:423–39. [DOI] [PubMed] [Google Scholar]
- [19].Colborn T, Short P. Pesticide use in the U.S. and policy implications: a focus on herbicides. Toxicol Ind Health 1999; 15:241–76. [DOI] [PubMed] [Google Scholar]
- [20].Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylexyl pthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 1999; 15:94–118. [DOI] [PubMed] [Google Scholar]
- [21].Gray LE Jr, Wolf C, Lambright C, Mann P, Price M, Cooper R, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 1999; 15:94–118. [DOI] [PubMed] [Google Scholar]
- [22].Ostby J, Monosson E, Kelce WR, Gray LE. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 1999;15:48–64. [DOI] [PubMed] [Google Scholar]
- [23].National Research Council. Pesticides in the diets of infants and children. Washington: USA: National Academy of Science; 1993. [Google Scholar]
- [24].Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE, et al. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect 2000;108:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Coble J, Arbuckle T, Lee W, Alavanje M, Dosemeci M. The validation of a pesticide exposure algorithm using biological monitoring results. J Occup Environ Hyg 2005;2:194–201. [DOI] [PubMed] [Google Scholar]
- [26].Arcury TA, Quandt SA, Barr DB, Hoppin JA, McCauley L, Grzywacz JG, et al. Farmworker exposure to pesticides: methodologic issues for the collection of comparable data. Environ Health Perspect 2006;114:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nurminen T The epidemiologic study of birth defects and pesticides. Epidemiology 2001;12:145–6. [DOI] [PubMed] [Google Scholar]
- [28].Restrepo M, Munoz N, Day N, Parra J, Hernandez C, Blettner M, et al. Birth defects among children born to a population occupationally exposed to pesticides in Colombia. Scand J Work Environ Health 1990;16:239–46. [DOI] [PubMed] [Google Scholar]
- [29].Smith AH, Fisher DO, Pearce N, Chapman CJ. Congenital defects and miscarriages among New Zealand 2, 4, 5-T sprayers. Arch Environ Health 1982;37:197–200. [DOI] [PubMed] [Google Scholar]
- [30].Zhu JL, Hjollund NH, Andersen AM, Olsen J. Occupational exposure to pesticides and pregnancy outcomes in gardeners and farmers: a study within the Danish National Birth Cohort. J Occup Environ Med 2006;48:347–52. [DOI] [PubMed] [Google Scholar]
- [31].Lawson CC, Schnorr TM, Whelan EA, Deddens JA, Dankovic DA, Piacitelli LA, et al. Paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and birth outcomes of offspring: birth weight, preterm delivery, and birth defects. Environ Health Perspect 2004;112:1403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pacque M, Munoz B, Poetschke G, Foose J, Greene BM, Taylor HR. Pregnancy outcome after inadvertent ivermectin treatment during community-based distribution. Lancet 1990; 336:1486–9. [DOI] [PubMed] [Google Scholar]
- [33].Khoshdel A, Attia J, Carney SL. Basic concepts in meta-analysis: a primer for clinicians. Int J Clin Pract 2006;60:1287–94. [DOI] [PubMed] [Google Scholar]
- [34].Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol 2002;155:313–22. [DOI] [PubMed] [Google Scholar]
- [35].Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect 2005;113:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bianca S, Li Volti G, Caruso-Nicoletti M, Ettore G, Barone P, Lupo L, et al. Elevated incidence of hypospadias in two sicilian towns where exposure to industrial and agricultural pollutants is high. Reprod Toxicol 2003;17:539–45. [DOI] [PubMed] [Google Scholar]
- [37].Vrijheid M, Armstrong B, Dolk H, van Tongeren M, Botting B. Risk of hypospadias in relation to maternal occupational exposure to potential endocrine disrupting chemicals. Occup Environ Med 2003;60:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, De Gier RP, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr 2007; 166:671–8. [DOI] [PubMed] [Google Scholar]
- [39].Irgens A, Kruger K, Skorve AH, Irgens LM. Birth defects and paternal occupational exposure. Hypotheses tested in a record linkage based dataset. Acta Obstet Gynecol Scand 2000;79:465–70. [PubMed] [Google Scholar]
- [40].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [41].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [42].Nurminen T Maternal pesticide exposure and pregnancy outcome. J Occup Environ Med 1995;37:935–40. [DOI] [PubMed] [Google Scholar]
- [43].Schnitzer PG, Olshan AF, Erickson JD. Paternal occupation and risk of birth defects in offspring. Epidemiology 1995;6: 577–83. [DOI] [PubMed] [Google Scholar]
- [44].Olshan AF, Teschke K, Baird PA. Paternal occupation and congenital anomalies in offspring. Am J Ind Med 1991;20: 447–75. [DOI] [PubMed] [Google Scholar]
- [45].Weidner IS, Moller H, Jensen TK, Skakkebaek NE. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Perspect 1998;106:793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect 2004;112:1570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garcia AM, Fletcher T, Benavides FG, Orts E. Parental agricultural work and selected congenital malformations. Am J Epidemiol 1999;149:64–74. [DOI] [PubMed] [Google Scholar]
- [48].Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology 1997;8:537–44. [DOI] [PubMed] [Google Scholar]
- [49].Schwartz DA, Newsum LA, Heifetz RM. Parental occupation and birth outcome in an agricultural community. Scand J Work Environ Health 1986;12:51–4. [DOI] [PubMed] [Google Scholar]
- [50].Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. Cryptorchidism and hypospadias in the Sicilian district of Ragusa and the use of pesticides. Reprod Toxicol 2006;22:8–12. [DOI] [PubMed] [Google Scholar]
- [51].Bouvier G, Seta N, Vigouroux-Villard A, Blanchard O, Momas I. Insecticide urinary metabolites in nonoccupationally exposed populations. J Toxicol Environ Health B 2005;8:485–512. [DOI] [PubMed] [Google Scholar]
- [52].Fritschi L, Nadon L, Benke G, Lakhani R, Latreille B, Parent M, et al. Validation of expert assessment of occupational exposures. Am J Ind Med 2003;43:519–22. [DOI] [PubMed] [Google Scholar]
- [53].Tielemans E, Heederik D, Burdorf A, Vermeulen R, Veulemans H, Kromhout H, et al. Assessment of occupational exposures in a general population: comparison of different methods. Occup Environ Med 1999;56:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Curwin B, Sanderson W, Reynolds S, Hein M, Alavanja M. Pesticide use and practices in an Iowa farm family pesticide exposure study. J Agric Saf Health 2002;8:423–33. [DOI] [PubMed] [Google Scholar]
- [55].Dosemeci M, Alavanja M, Rowland AS, Mage D, Zahm S, Rothman N, et al. A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann Occup Hyg 2002;46:245–60. [DOI] [PubMed] [Google Scholar]
- [56].Arbuckle T, Burnett R, Cole D, Teschke K, Dosemeci M, Bancej C, et al. Predictors of herbicide exposure in farm applicators. Int Arch Occup Environ Health 2002;75: 406–14. [DOI] [PubMed] [Google Scholar]
- [57].Kang IH, Kim HS, Shin JH, Kim TS, Moon HJ, Kim IY, et al. Comparison of anti-androgenic activity of flutamide, vinclo-zolin, procymidone, linuron, and p,p′-DDE in rodent 10-day Hershberger assay. Toxicology 2004;199:145–59. [DOI] [PubMed] [Google Scholar]
- [58].Steinhardt G Endocrine disruption and hypospadias. Adv Exp Med Biol 2004;545:203–15. [DOI] [PubMed] [Google Scholar]
- [59].Hanke W, Jurewicz J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: an overview of current epidemiological evidence. Int J Occup Med Environ Health 2004; 17:223–43. [PubMed] [Google Scholar]
- [60].Chia SE, Shi LM. Review of recent epidemiological studies on paternal occupations and birth defects. Occup Environ Med 2002;59:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gray L, Ostby J, Furr J, Wolf C, Lambright C, Wilson V, et al. Toxicant-induced hypospadias in the male rat. Adv Exp Med Biol 2004;545:217–41. [DOI] [PubMed] [Google Scholar]


