Summary
According to learning-based models of behavior, food cue reactivity and craving are conditioned responses that lead to increased eating and subsequent weight gain. However, evidence supporting this relationship has been mixed. We conducted a quantitative meta-analysis to assess the predictive effects of food cue reactivity and craving on eating and weight-related outcomes. Across 69 reported statistics from 45 published reports representing 3,292 participants, we found an overall medium effect of food cue reactivity and craving on outcomes (r = 0.33, p <0.001; approximately 11% of variance), suggesting that cue exposure and the experience of craving significantly influence and contribute to eating behavior and weight gain. Follow-up tests revealed a medium effect size for the effect of both tonic and cue-induced craving on eating behavior (r = 0.33). We did not find significant differences in effect sizes based on body mass index, age, or dietary restraint. However, we did find that visual food cues (e.g. pictures and videos) were associated with a similar effect size to real food exposure and a stronger effect size than olfactory cues. Overall, the present findings suggest that food cue reactivity, cue-induced craving and tonic craving systematically and prospectively predict food-related outcomes. These results have theoretical, methodological, public health and clinical implications.
Keywords: Craving, cue reactivity, eating behavior, food, weight gain
For most people, the mere sight or smell of warm chocolate chip cookies initiates a strong desire to eat, also known as craving. Such craving is a form of food cue reactivity: a conditioned response to food that is frequently accompanied by increased salivation, physiological arousal and neural activity in regions such as the ventral striatum (VS) (1–7). It has been proposed that as conditioned responses, craving and other forms of food cue reactivity should lead to increased eating behavior and subsequent weight gain (8,9); however, the evidence for this relationship has been inconsistent. We undertook this systematic review and meta-analysis to (i) summarize the findings in this area, (ii) quantitatively assess whether cue reactivity is prospectively associated with eating and weight gain, (iii) quantitatively assess whether craving specifically is prospectively associated with eating and weight gain and (iv) estimate the magnitude of these predictive relationships if they are found. We further aimed to investigate whether specific types of cue reactivity (e.g. craving versus neural activity) account for more variance in eating and weight gain and to assess the role of potential moderators such as body mass index (BMI), age, dietary restraint and gender. Assessing contributors to eating and weight gain is especially important given rising rates of obesity in the USA, in what has been described as a ‘toxic food environment’ filled with an abundance of food cues (e.g. in popular advertising) that may induce craving and cue overeating and weight gain.
Food cue reactivity and craving are conditioned responses
In learning-based models of behavior, food is considered an unconditioned stimulus, and food effects (e.g. salivation and digestive processes) are unconditioned responses. After repeated exposure, food-related cues (e.g. the sight of cookies) that are present at the time of eating acquire the ability to predict eating, becoming conditioned stimuli that evoke conditioned responses (including those that prepare the body to digest the food (10)). Consistently, animal work has demonstrated that cues experimentally paired with food (e.g. the sound of a bell) evoke strong conditioned responses, including salivation in dogs (11), ghrelin secretion in sheep (12) and dopamine neuron firing in monkeys and rats (13,14).
For humans in the natural ecology, food cues that become conditioned are typically environmental, such as the sight and smell of food, but can also include interoceptive cues such as stress, negative affect, hormonal fluctuation and food-related cognitions (e.g. (15)). Such conditioned cues consistently induce conditioned physiological responses including increased salivation, heart rate, gastric activity and neural activity in the VS (1–7). According to the ‘cued overeating’ model (8,9), these physiologically conditioned responses are consciously experienced as craving and are commonly termed cue-induced craving (Table 1). Indeed, exposure to food cues strongly and reliably produces the conscious experience of craving, defined here as ‘an intense desire or urge to eat’ (8,16–20). Importantly, recent experimental work has shown that conditioned cue craving associations are learned quickly and robustly in the laboratory (21–24) so that even previously neutral stimuli paired with chocolate elicit cue-induced responses, including self-reported craving, after a single-session classical conditioning procedure (25).
Table 1.
Definitions of the most frequently used terms in this manuscript
| Useful definitions |
|---|
| Cue reactivity: conditioned responses to cues, including physiological reactivity and craving |
| Craving: a strong, conscious desire |
| Cue-induced craving: self-reported craving in response to cues |
| Tonic craving: self-reported craving in the absence of external cues |
Cue reactivity and craving may increase eating and weight
The ‘cued overeating’ model (8,9) predicts that cue reactivity, and its consciously experienced form of craving, increase the likelihood and amount of food intake. Consistent with this prediction, both cue exposure and responses to cues are associated with subsequent food seeking and eating in animals (14,26–29). Interestingly, exposure to cues previously associated with high-calorie foods not only increases consumption of those high-calorie foods but also increases consumption of other foods (29). In humans, food cue exposure has been shown to increase eating in adults (16,22,30–41) and children (1,42–49). Similarly, cue-induced physiological responses and neural activity have been prospectively associated with eating (1–4,50–52) and weight gain (53–55), including in children (55). The experience of cue-induced craving specifically has also been linked to subsequent eating behavior (3,16–19). Another form of craving, tonic craving, arises independently of external cues (56) and is typically measured using multi-item craving scales that ask about experiences of craving, such as “thinking about my favorite foods makes my mouth water” or “if I am craving something, thoughts of eating it consume me” (57). Like cue-induced craving, tonic craving has been associated with increased eating (58–62) and long-term weight gain (63–66).
However, for both cue reactivity and craving, some studies have failed to show associations with increased eating and weight (22,34,35,40,41,52,67–71), while other studies found this association with physiological cue reactivity but not craving (53–55,72). These inconsistencies suggest that either (i) all forms of cue reactivity, including craving, increase eating and weight gain, but that some studies were not able to capture this relationship, (ii) cue reactivity leads to increased eating and weight gain, but this does not depend on the conscious experience of craving or (iii) cue reactivity of any form does not reliably increase eating or weight gain. The present meta-analysis was designed to resolve among these accounts by quantitatively summarizing across all studies, including those that report no association between cue reactivity or craving and eating or weight outcomes. This investigation is important because these processes may influence important health-related outcomes, such as obesity (73).
Potential impact of cue reactivity and craving on obesity
Today, two-thirds of the USA is overweight or obese, whereas only 15% were 30 years ago (74). This is concerning because obesity is currently the second leading cause of preventable disease and death in the USA (75), with elevated BMI accounting for about 2.8 million deaths each year worldwide (76). Population-level shifts in rates of obesity involve broad environmental changes as well as individual-level processes (77–85). On a population level, environmental factors include ubiquitous advertising for appetizing, high-calorie foods (86,87), which some have described as part of a ‘toxic food environment’ (84,88). Accordingly, individuals who live in a food environment that includes advertised, high-caloric foods (e.g. fast food, such as McDonalds) eat those foods more frequently and have higher BMI (adults: (89–91); children: (92)).
Concurrently, processes such as cue reactivity and craving could explain how such environmental factors influence eating behavior and weight gain on the individual level. Although weight gain involves complex interactions between environmental and physiological systems, these conditioned, learning-based processes may contribute to rising rates of obesity (8,9,84,88,93,94) and low efficacy in weight loss interventions (95–100). For instance, pervasive exposure to food advertisements may strengthen the salience of food cues and their effects on physiological cue reactivity and craving, leading to more frequent food consumption (86). If these processes systematically lead to increased eating and weight, they can serve as important targets for public health and clinical intervention and can facilitate the development of new treatments to prevent and combat rising rates of obesity.
Parallels with drug cue reactivity and drug craving
Over the past 25 years, cue reactivity and craving and their ability to predict clinical outcomes have been most thoroughly investigated in the context of drug addiction. Although some comparisons between drug addictions and eating behaviors remain controversial (101–104), many parallels are evident in work on drug cues and food cues, and the craving responses they elicit. For example, a wealth of animal studies has shown that cues paired with drug taking (i.e. ‘drug cues’) elicit a variety of conditioned responses and become conditioned predictors of drug taking (105,106). In humans, such drug cues may include the sight of drugs, drug paraphernalia, images of drug taking or of people previously associated with drug cues (107). Several meta-analyses have demonstrated that, in response to such drug cues, drug users show increased physiological reactivity (e.g. heart rate and skin conductance) (108), exhibit increased neural activity in the VS (109–112) and report drug craving (108). In turn, both drug cue exposure and self-reported craving for drugs increase the likelihood of subsequent use across drugs of abuse, including nicotine, alcohol, cocaine, methamphet-amine and opioids (for review, see (113)). Much of this work supported the recent inclusion of self-reported craving for drugs as one of the DSM-5 diagnostic criteria for substance use disorders (20). However, although drug and food cue reactivity involve similar learning-based processes, unlike for addictions, food craving is not a standard clinical or diagnostic measure for obesity or eating disorders. Nevertheless, for food as with drugs, cue reactivity, and particularly the conscious experience of craving, may be important predictors of outcomes related to eating behavior, obesity and eating-related psychopathology.
The present investigation
Cue reactivity and craving for food are conditioned responses that have been associated with increased food consumption and weight gain in some studies; however, findings have been inconsistent, and the validity and strength of these effects are an ongoing question. To address this, we conducted a systematic review and quantitative meta-analysis investigating the prospective effect of (i) food cue reactivity, (ii) cue-induced craving and (iii) tonic craving on food-related outcomes, in order to determine how strongly these factors predict eating behavior and weight. We hypothesized that we would find significant prospective relationships between these three factors and food-related outcomes (Hypotheses 1–3). We also hypothesized that BMI, age, dietary restraint and gender would moderate cue reactivity effects (Hypothesis 4) and that exposure to visual food cues would be as powerful as in vivo exposure to ‘real food’ (Hypothesis 5). Overall, we expected medium-sized effects, with cue reactivity and craving explaining a significant amount of variance in subsequent outcomes.
Methods
Literature search and study selection
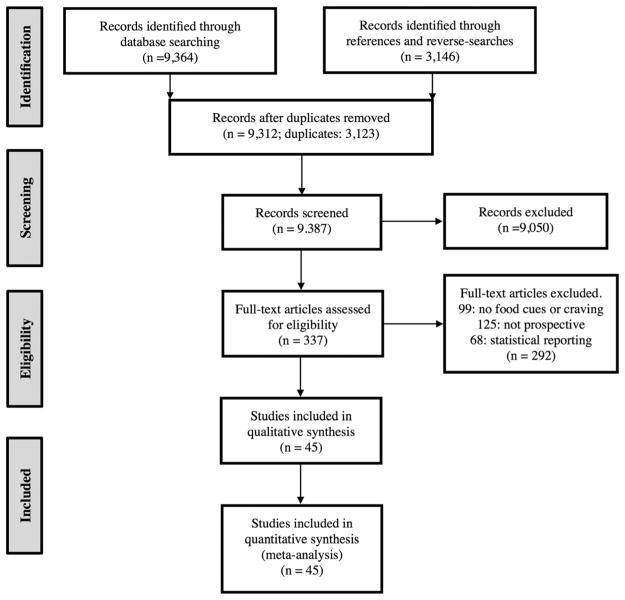
We conducted literature searches using PubMed and Google Scholar. Searches included the keywords ‘food’ or ‘eat*’ or ‘weight’ or ‘BMI’, in combination with ‘crave’ or ‘craving’ or ‘urge’ or ‘desire’ or ‘cue reactivity’ or ‘cue’. We conducted additional searches using the previous search terms and population-specific terms, such as ‘dieter’ or ‘restrained eater’ or ‘bariatric’ or ‘overweight’ or ‘eating disorder’. Further, to ensure that we included as many relevant studies as possible and to identify additional studies that fit inclusion criteria, we searched reference sections of included papers and of relevant literature reviews and performed reverse searches on included papers using Google Scholar. These searches yielded a total of 9,387 independent entries for papers published before October 2014, which were screened by three independent researchers and narrowed to 337 papers (see Fig. 1 for schematic depiction, using the preferred reporting items for systematic reviews and meta-analyses (114)).
Figure 1.
Study selection and exclusion Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) diagram depicting study selection and exclusion.
We ultimately included studies that met the following criteria: (1a) participants were exposed to a food cue and/or (1b) completed a self-report craving measure;(2) at least one unhealthy food consumption or weight outcome measure was reported; (3) cue exposure and/or craving was measured before the eating or weight outcome measure (i.e. prospectively, not retrospectively or cross-sectionally); and (4) at least one reported analysis assessed the relationship between self-reported craving, cue exposure condition or any measure of cue reactivity and outcome. Measures of cue reactivity included (i) cue-induced peripheral physiological reactivity (e.g. heart rate and salivation), (ii) cue-induced fMRI activation and (ii) cue-induced craving. We limited fMRI results to the VS because the VS is necessary for Pavlovian conditioning (115) and has been consistently associated with cue reactivity for both drugs (109,110) and food (5), and with self-reported craving for both drugs and food (116). Final inclusion/exclusion was determined independently by the two authors, yielding 45 studies included in analyses. Any conflicts were discussed between the authors until full agreement was reached.
Data extraction and reduction
From each included study, we extracted N (number of included participants), outcome type (food consumption, weight or BMI), timing of outcome measure (immediately following to two years later), means and standard deviations for cue-conditions and the reported statistics (see Table 2 for additional details). We further coded each study as belonging to one of several study types: (i) ‘cue-condition’ included studies that reported outcomes after exposing participants to food-related cues (such as visual cues, olfactory cues or real food) compared with a control condition, (ii) ‘cue reactivity’ included studies that reported statistical relationships between peripheral physiological reactivity (e.g. heart rate, gastric activity and salivation) or neural activity in response to food cues and outcomes, (iii) ‘cue-induced craving’ included studies that reported relationships between self-reported craving in response to food cues and outcomes and (4) ‘tonic craving’ included studies that measured craving in the absence of external cues and reported its relationship with outcomes.
Table 2.
Characteristics of included studies
| Author | Year | Measures | N | Study type | Cue type | Clinical | BMI | Restraint | Gender | Age | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batra† | 2013 | Behavioral | 95 | TC | Real food | No | OW/OB | — | Mixed | Adult | Weight |
| Birch* | 1989 | Behavioral | 29 | CC | Other | No | — | — | Mixed | Children | Eating |
| Boutelle | 2014 | Behavioral | 15 | CC | Other | No | OW | — | Mixed | Children | Eating |
| Buckland* | 2013a | Behavioral | 21 | CC | Olfactory | No | Lean/OW | Both | Female | Adult | Eating |
| Buckland* | 2013b | Behavioral | 13 | TC | Real food | No | Lean/OW | Both | Female | Adult | Eating |
| Buijzen† | 2008 | Behavioral | 234 | CC | Visual | No | — | — | Mixed | Children | Eating |
| Coehlo, Ilder | 2011 | Behavioral | 58 | CC | Olfactory | No | Lean/OW | Both | Female | Adult | Eating |
| Coehlo, Jansen* | 2009 | Behavioral | 48 | CC | Real food | No | Lean/OW | Both | Female | Adult | Eating |
| Coehlo, Polivy* | 2009 | Behavioral | 104 | CC | Olfactory | No | Lean/OW | Both | Female | Adult | Eating |
| Cornell* | 1989 | Behavioral | 52 | CC/CIC | Real food | No | Lean/OW | Yes | Mixed | Adult | Eating |
| Cushing | 2014 | Behavioral | 16 | TC | — | Bariatric | OW/OB | — | Mixed | Children | Weight |
| Demos | 2012 | fMRI | 48 | CR | Visual | No | Lean/OW | — | Female | Adult | Weight |
| Fedoroff* | 1997 | Behavioral | 91 | CIC | Olfactory | No | — | Both | Female | Adult | Eating |
| Fedoroff† | 2003 | Behavioral | 132 | CIC | Olfactory | No | — | Both | Female | Adult | Eating |
| Ferriday | 2008 | Behavioral | 50 | CC | Real food | No | Lean/OW | Both | Female | Adult | Eating |
| Folkvord | 2013 | Behavioral | 270 | CC | Visual | No | Lean/OW | — | Mixed | Children | Eating |
| Folkvord | 2014 | Behavioral | 261 | CC | Visual | No | Lean | — | Mixed | Children | Eating |
| Gilhooly† | 2007 | Behavioral | 32 | TC | — | No | OW | Both | Female | Adult | Weight |
| Halford, Boyland* | 2007 | Behavioral | 93 | CC | Real food | No | Lean/OW/OB | — | Mixed | Adult | Eating |
| Halford, Gillespie* | 2004 | Behavioral | 42 | CC/CR | Visual | No | Lean/OW/OB | — | Mixed | Children | Eating |
| Harris | 2012 | Behavioral | 152 | CC | Visual | No | — | — | Mixed | Children | Eating |
| Jakubowicz† | 2012 | Behavioral | 193 | TC | Real food | No | OW/OB | — | Mixed | Children | Weight |
| Jansen, Nederkoorn, Roefs* | 2011 | Behavioral | 58 | CC | Real food | No | — | Yes | Mixed | Adult | Eating |
| Jansen, Nederkoorn, van Baak* | 2009 | Behavioral | 12 | CC | Real food | No | Lean/OW | Yes | Female | Adult | Eating |
| Jansen, Theunissen* | 2003 | Behavioral | 16 | CR | Real food | No | Lean/OW | — | Mixed | Children | Eating |
| Jansen and van den Hout* | 1991 | Behavioral | 19 | CC | Real food | No | — | Both | Female | Adult | Eating |
| Jansen, Vanreyten* | 2008 | Behavioral | 91 | CC | Real food | No | Lean/OW | — | Female | Adult | Eating |
| Lambert* | 1991 | Behavioral | 23 | CC | Visual/Real | No | — | No | Mixed | Adult | Eating |
| Larsen | 2012 | Behavioral | 109 | CC | Olfactory | No | Lean | Both | Female | Adult | Eating |
| Lawrence† | 2012 | fMRI | 25 | CR | Visual | No | Lean/OW | — | Female | Adult | Eating |
| Lopez* | 2014 | fMRI | 31 | CR/CIC | Visual | No | — | Both | Female | Adult | Eating |
| Martin† | 2008 | Behavioral | 91 | TC | Real food | No | OW/OB | — | Mixed | Adult | Eating |
| Mehta† | 2012 | fMRI | 23 | CR | Visual | No | Lean/OW/OB | — | Mixed | Adult | Eating |
| Murdaugh | 2012 | fMRI | 25 | CR | Visual | No | OW/OB | — | Mixed | Adult | Weight |
| Nederkoorn | 2002 | Behavioral | 44 | CR | Real food | Both | Lean | Both | Female | Adult | Eating |
| Nederkoorn, Jansen | 2000 | Behavioral | 24 | CR/CIC | Real food | No | Lean/OW | No | Female | Adult | Eating |
| Nederkoorn, Smulders* | 2004 | Behavioral | 72 | CR/CIC | Real food | Both | Lean | — | Female | Adult | Eating |
| Ng* | 2013 | Behavioral | 50 | CIC | Real food | Both | OW/OB | — | Female | Adult | Eating |
| Overduin | 1997 | Behavioral | 20 | CC | Other | No | Lean | — | Mixed | Adult | Eating |
| Rogers* | 1989 | Behavioral | 12 | CR | Visual | No | Lean | Both | Female | Adult | Eating |
| van den Akker | 2013 | Behavioral | 70 | CC | Other | No | Lean | No | Female | Adult | Eating |
| Vogele and Florin* | 1996 | Behavioral | 60 | CR/CIC | Real food | Both | Lean/OW | Both | Mixed | Adult | Eating |
| Wonderlich-Tierney | 2013 | Behavioral | 83 | CC | Visual | No | Lean/OW | — | Mixed | Adult | Eating |
| Yokum | 2014 | fMRI | 24 | CR | Visual | No | Lean/OW/OB | — | Mixed | Children | Weight |
| Zoon | 2014 | Behavioral | 50 | CC | Olfactory | No | Lean/OW | Both | Female | Adult | Eating |
studies that contributed multiple independent, nested effect sizes;
studies that contributed multiple effect sizes and were averaged to adjust for non-independence.
CC, cue condition studies (reported outcomes after exposing participants to food-related cues compared with a control condition); CR, cue reactivity studies (reported statistical relationships between physiological reactivity (e.g. heart rate, gastric activity and salivation) or neural activity in response to food cues and outcomes); CIC, cue-induced craving studies (reported relationships between self-reported craving in response to food cues and outcomes); TC , tonic craving studies (measured craving in the absence of cues and reported its relationship with outcomes); OW, overweight; OB, obese.
Each piece of information was extracted and coded by one of the two authors and then checked by the other. Any inconsistencies were resolved in discussion until perfect agreement was reached. Extracted statistics fell into several categories:
Between-group comparisons between cue-exposure and no-cue exposure groups on food consumption (F or t statistics);
Within-group comparisons between cue-exposure and no-cue exposure conditions (F or t statistics);3. Standardized beta weights (β) from multiple regression models predicting the effect of cue-exposure, cue-reactivity or self-reported craving on eating or weight outcome;
Pearson correlation coefficient (r) as an index of effect size of the relationship between cue-response and subsequent eating or weight outcome. Cue-responses were either (a) cue-induced physiological reactivity (e.g. heart rate, salivation), (b) cue-induced neural response in VS, or (c) cue-induced craving;
Pearson correlation coefficient (r) as an index of effect size of the relationship between tonic measures of craving and subsequent eating or weight outcome.
We also extracted information about potential moderators, including participant pre-study BMI (lean/overweight, measured prior to cue exposure/craving measurements), participant age (children/adults), restrained eating status (restrained eaters/non-restrained eaters), gender (male/female/ mixed) and type of food cue (real food/visual/olfactory/ other). We included any reported measure of BMI and restrained eating status. Further, we defined a sample as representing ‘children’ when the mean age was <18 years. This set of studies (NSTUDIES = 11) included children and adolescents ages 3–17 years. Examining these moderators in this meta-analysis could identify differences in the strength of cue reactivity effects and explain variability in findings and/or effect sizes across different studies (see Supporting Information for additional details).
Statistical analyses
All analyses were conducted using the Comprehensive Meta-Analysis Version 3.0 software program (Biostat; Englewood, NJ, 2015) (117), following previously published meta-analyses (118–121).
Calculation of effect sizes
For between-group and within-group comparisons, means, standard deviations and/or test statistics were used to calculate Cohen’s d, and then d was converted to Pearson’s r (122,123). For beta weights, we used imputation to convert β to r (124). The inclusion of beta weights in meta-analyses is somewhat controversial, because beta coefficients reflect the influence of all predictor variables in a regression equation. Nevertheless, we decided to include them for several reasons: (i) such studies directly test for a predictive relationship between cue reactivity measures and food outcome and are therefore relevant to this meta-analysis, (ii) such beta coefficients are actually more conservative than r (123), (iii) omitting them would increase the sampling error (123,124), (iv) empirical work has found that r can be reliably imputed from β values (124) and (v) such methods have been successfully used in published meta-analyses (e.g. (125,126)). Pearson’s correlation coefficients (r) (those extracted from included studies as well as those converted from other statistics) were converted to a standard normal z-score metric using Fisher’s r–z transformation (127). After all effect sizes were converted to z-scores, each effect size was weighted based on sample size and used to create an average effect size r, as in previously published meta-analyses (e.g. (119,120,128,129)). We used Cohen’s criteria for small (r = 0.10), medium (r = 0.30) and large (r = 0.50) effect sizes (130).
Adjusting for dependencies in effect size
Several studies reported multiple non-independent statistics using the same study population (e.g. multiple correlation coefficients between craving and consumption of several food types; see Table 2 for details). In these cases, we aggregated non-independent effect sizes by averaging reported statistics. In cases in which studies reported several independent statistics (e.g. in independent participant groups within a study), we reported these as ‘nested’ statistics within these studies. For instance, we included two correlation coefficients from a single study when they independently represented overweight and lean individuals (1,47,48,131) and independently restrained and unrestrained eaters (17,18,31,33,60). We also included multiple statistics when they independently represented different cue conditions (e.g. olfactory versus control and visual versus control (4,17)) or independent measures of craving (e.g. self-report by scale and cue-induced craving (3,48,50,132,133)). This approach is analogous to methods used in previously published meta-analyses (118,120).
Meta-analytic random effects models
We applied random effects models to the main analyses because we expected differences in sample and methodology across studies and wanted to allow for population inferences (122,127). Such models are more conservative and have lower Type I error rates than fixed effects models, and allow for generalizability of findings across new samples. We also calculated a Q statistic to measure heterogeneity in effect sizes across studies. For moderator-based analyses, a significant Q would suggest that compared groups (e.g. high versus low BMI) exhibit different relationships between cue reactivity or craving and outcome. Because we included a relatively small number of studies in our moderator analyses (see previous discussion), we evaluated moderators even in the absence of a significant Q, as suggested by Rosenthal and DiMatteo (123). Further, we used mixed models to test the effects of categorical moderators (e.g. gender, dietary restraint and fixed) on study effect sizes (random).
Publication bias
Because studies with non-significant findings may not be published, and therefore cannot be included in meta-analyses (i.e. ‘the file drawer problem’), we conducted the most conservative analyses of publication bias to assess the effect of missing studies on meta-analytic results using two approaches (134). First, we calculated Rosenthal’s fail-safe N to determine the number of studies with a null effect that would be necessary to render the meta-analytic results non-significant (p >0.05) (123). Using a second approach, we created a funnel plot depicting standard error by Fisher’s z (135), assessed for publication bias (136–138) and conducted a trim-and-fill analysis (139,140) to estimate the number of studies with negative findings that are potentially missing from the literature.
Results
Included studies and outcome variables
Of the 12,510 articles initially identified through online, reverse and reference searches, 3,123 were duplicates, and 9,387 were screened for eligibility. Nine thousand fifty were initially excluded (primarily for studying animals, not including an outcome variable or not measuring food cue reactivity/craving), and 337 studies were fully assessed for eligibility. Of these, 99 studies were excluded for not containing food-related cues or not measuring cue reactivity, 125 for not prospectively testing the effect of cue reactivity on outcomes and 68 for not reporting statistics of interest. Ultimately, 45 studies were included in analyses, with 69 reported statistics, representing 3,292 participants (Fig. 1). Of the statistics included, 35 were ‘cue-condition’ statistics (NPARTICIPANTS = 1,834), 15 were ‘cue-reactivity’ statistics (NPARTICIPANTS = 458), 12 were ‘cue-induced craving’ statistics (NPARTICIPANTS = 546) and 7 were ‘tonic craving’ statistics (NPARTICIPANTS = 454). We included 53 statistics from behavioral studies (NPARTICIPANTS = 2,520), 8 from fMRI (NPARTICIPANTS = 248) and 8 from other psychophysiological measures (e.g. salivation/heart rate; NPARTICIPANTS = 524; Table 2).
In addition, 35 statistics included overweight or obese individuals (NPARTICIPANTS = 1,649) and 18 included restrained eaters (NPARTICIPANTS = 919). Forty-two statistics were female-only samples (NPARTICIPANTS = 1,749), 26 statistics were from mixed male/female samples (NPARTICIPANTS = 1,523) and only one statistic represented males only (NPARTICIPANTS = 20). Nineteen statistics included a mean age under the age of 18 years (‘children’; NPARTICIPANTS = 953).
Thirty-two statistics measured responses to real food cues, whereas 13 measured responses to olfactory cues, 17 to visual cues (pictures and videos) and 2 used ‘other’ cue types (e.g. auditory and environmental). NSTUDIES = 39 included exposure to cues, of which NSTUDIES = 34 reported the length of exposure to food cues (RangeMINUTES: 0.5–40, MeanMINUTES = 9.73 and SDMINUTES = 7.97) and NSTUDIES = 5 did not report length of exposure to cues.
The outcome variable from 54 statistics was food amount consumed immediately following a laboratory manipulation (measured in grammes or calories; NPARTICIPANTS = 2,457). Eight statistics reported food amount consumed over 1–2 weeks after the study period (NPARTICIPANTS = 391) and 7 statistics reported change in weight or BMI over time (4 months–2 years; NPARTICIPANTS = 444; Table 2). Sample size of individual studies ranged from NPARTICIPANTS = 3 to 234, with a mean sample size of 66.51 (SD = 53.06).
Overall effect on outcomes
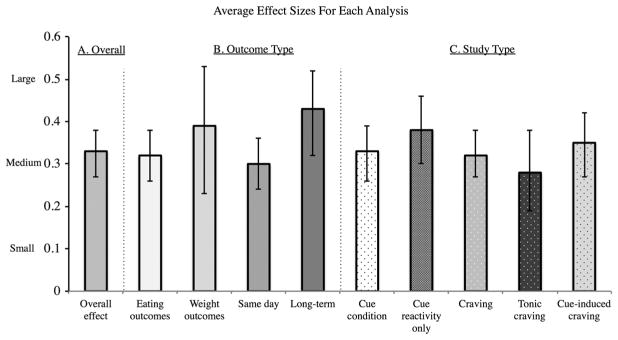
We first performed an omnibus analysis across all the included studies (NSTUDIES = 45, NPARTICIPANTS = 2,948) and found a significant overall combined effect size of r = 0.33 (CI: 0.27–0.38, z = 10.69, p <0.001), suggesting a medium prospective effect of cue reactivity and craving on eating and weight-related outcomes. Overall, cue reactivity and craving measures explained approximately 11% of variance in subsequent eating and weight-related outcomes.
Next, we compared effect sizes for food and weight-related outcomes separately. The type of outcome measure did not influence effect size (Q(1) = 0.68, p = 0.41). Tested separately, cues and craving had medium effects on both eating (r = 0.32, CI: 0.26–0.38, z = 9.53, p <0.001) and weight outcomes (r = 0.39, CI: 0.23–0.53, z = 4.59, p <0.001). Finally, long-term outcome measures were associated with larger effect sizes than short-term outcomes (Q(1) = 4.30, p = 0.04; same day; r = 0.30, CI: 0.24–0.36, z = 8.93, p <0.001; >1 week; r = 0.43, CI: 0.32–0.52, z = 7.19, p <0.001; Figs. 2 and 3).
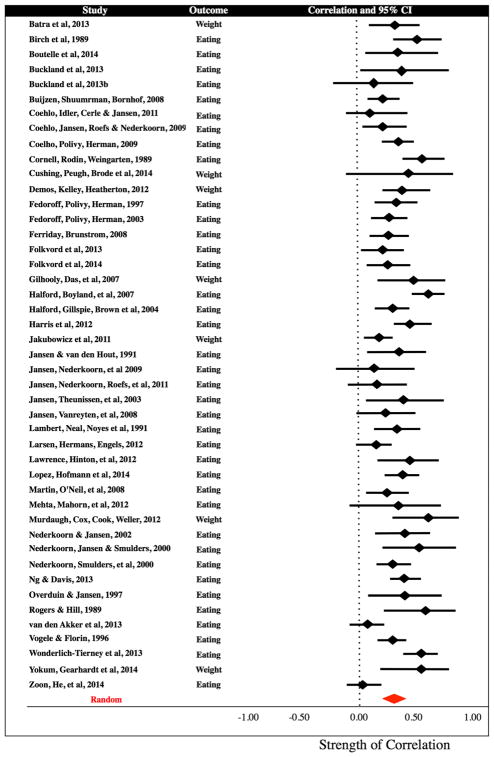
Figure 2.
Forrest plot of effect sizes. Statistics were converted to a standardized r and are plotted with 95% confidence intervals. The x-axis indicates the strength of the correlation coefficient, and shows that the overall effect favours a positive relationship between variables.
Figure 3.
Average effect sizes for Hypotheses 1–3. Average effect sizes and confidence interval: (A) overall, across all study types; (B) separately by outcome type; and (C) separately by study type. All effect sizes are medium or medium-to-large following Cohen’s d convention. ‘Cue condition’ includes all studies that measured responses to cue exposure, including cue-condition, cue reactivity (physiological and neural) and cue-induced craving. ‘Craving’ includes both tonic and cue-induced craving. The effect size in males represents NSTATISTICS = 1.
Hypothesis 1: Effect of Food Cue Reactivity on Outcomes
To test the hypothesis that food cue reactivity predicts outcomes, we calculated the average effect size across all cue condition and cue reactivity studies (including cue-induced craving, neural activity and peripheral physiology, but excluding tonic craving). We found a significant overall combined effect size of r = 0.33 (CI: 0.27–0.40, z = 9.73, p <0.001; Fig. 3), suggesting a medium effect of cue reactivity on eating and weight outcomes, accounting for 11% of the variance. Type of outcome measure did not influence effect size (Q(1) = 2.04, p = 0.15). Cue-condition and cue reactivity paradigms had medium-to-large effects on both eating (r = 0.32, CI: 0.26–0.39, z = 9.08, p <0.001) and weight outcomes (r = 0.51, CI: 0.26–0.69, z = 3.69, p <0.001). In addition, we found that cue reactivity had a larger effect on longer-term outcomes (Q(1) = 7.20, p <0.01; same day: r = 0.29, CI: 0.23–0.36, z = 8.56, p <0.001; >1 week: r = 0.48, CI: 0.36–0.59, z = 6.98, p <0.001).
To examine whether type of food cue reactivity exhibited different relationships with outcome, we compared across types of cue condition and cue reactivity studies. We did not find a significant difference, with all study types averaging a medium effect size (Q(1) = 3.73, p = 0.16; cue-induced craving: r = 0.40, CI: 0.27–0.52, z = 5.74, p <0.001; cue-condition: r = 0.29, CI: 0.22–0.36, z = 7.58, p <0.001; cue reactivity: r = 0.41, CI: 0.28–0.52, z = 5.83, p <0.001). Within cue reactivity studies, we did not find a significant difference between peripheral physiological and neutral activity measures (Q(1) = 0.74, p = 0.39; physiology: r = 0.35, CI: 0.23–0.46, z = 5.27, p <0.001; neural: r = 0.42, CI: 0.30–0.53, z = 6.15, p <0.001), such that both were significantly related to outcomes.
Hypotheses 2 and 3: Effect of Cue-induced Craving and Tonic Craving on Outcomes
Next, to test the hypothesis that craving predicts outcomes, we averaged effect sizes for studies that included cue-induced craving or tonic craving measures. We found that craving had a medium effect size on outcomes (r = 0.33, CI: 0.27–0.40, z = 9.40, p <0.001), which did not vary based on short versus long-term outcomes (Q(1) = 1.55, p = 0.21; Fig. 3). Then, we compared the effect sizes of cue-induced and tonic craving measures. We found no difference in effect sizes (Q(1) = 3.41, p = 0.10), such that cue-induced craving and tonic craving each contributed significantly to outcomes (cue-induced: r = 0.38, CI: 0.30–0.46, z = 8.36, p <0.001; tonic: r = 0.27, CI: 0.18–0.36, z = 5.77, p <0.001). All cue-induced craving studies reported eating outcomes. Tonic craving predicted both eating (r = 0.26, CI: 0.08–0.42, z = 2.75, p <0.001) and weight outcomes (r = 0.27, CI: 0.17–0.37, z = 5.07, p = 0.001) to the same degree (Q(1) = 0.02, p = 0.89).
Hypotheses 4 and 5: Effects of Moderator Variables
Body mass index
To test whether pre-study BMI moderated the effects of cue reactivity and craving on outcome, and was therefore associated with variability in effect sizes, we compared statistics with lean participants (BMI ≤ 24.9) to those with overweight or obese participants (BMI ≤ 25). Studies that did not explicitly report participant BMI or included mixed samples were excluded from this analysis (NSTUDIES = 26). We found no effect of BMI group (Q(1) = 0.89, p = 0.35). Samples with lean participants (r = 0.30, CI: 0.17 – 0.43, z = 4.28, p <0.001) and overweight participants (r = 0.40, CI: 0.25 – 0.53, z = 4.85, p <0.001) both exhibited significant, medium-sized relationships between cue-reactivity/ craving and outcomes (Figure S1).
Age
We tested whether studies with samples of children (mean age <18) or adults showed stronger effects. We did not find a significant difference in effect sizes between adults and children (Q(1) = 2.88, p = 0.09). Studies with adults and children both exhibited medium effect sizes of cues/craving on eating and weight outcomes (children: r = 0.40, CI: 0.30–0.50, z = 7.14, p <0.001; adults: r = 0.30, CI: 0.24–0.36, z = 8.84, p <0.001; Figure S1).
Restrained eating
To test whether restrained eating is associated with a larger effect of cue reactivity and craving on eating and weight, we compared statistics with restrained eaters to those with non-restrained eaters. We included all reported measures of dietary restraint. Studies that did not explicitly assess dietary restraint or that only reported statistics from mixed samples were excluded from this analysis (NSTUDIES = 29). We did not find a significant difference based on dietary restraint (Q(1) = 2.90, p = 0.09). The effect sizes for restrained eaters was medium-sized (r = 0.31, CI: 0.20 – 0.42, z = 5.12, p <0.001) and for non-restrained eaters was small-to-medium (r = 0.19, CI: 0.10–0.28, z = 4.05, p <0.001; Figure S1).
Gender
A single study reported separate statistics for male participants. Therefore, to examine whether gender influences the magnitude of association, we compared 42 statistics with women only (NPARTICIPANTS = 1,749), 26 mixed gender (NPARTICIPANTS = 1,523) and one with male-only participants (NPARTICIPANTS = 20). We found a significant effect of gender (Q(2) = 9.61, p = 0.01), such that the effect size for mixed-gender samples was larger than for females. Despite this, the effect size for females and mixed-gender samples were both medium (female: r = 0.28, CI: 0.21–0.34, z = 8.01, p <0.001; mixed: r = 0.39, CI: 0.32–0.46, z = 9.73, p <0.001). The one effect size in males was large, with a sizable confidence interval, although this single effect size should be interpreted with caution (r = 0.73, CI: 0.35–0.90, z = 3.26, p = 0.001; Figure S1). Differences between mixed and female samples remained significant when excluding the single statistic in a male sample. No included studies reported gender-related differences in outcomes.
Food cue type
We tested whether food cue type influenced the magnitude of observed effects and found a significant difference (Q(2) = 6.28, p = 0.04). Both real food cues and visual cues (pictures and videos) were associated with medium-sized effects on eating and weight (real food: r = 0.36, CI: 0.28–0.43, z = 9.04, p <0.001; visual: r = 0.37, CI: 0.27–0.45, z = 7.43, p <0.001). In contrast, olfactory cues were associated with a small-to-medium effect size (r = 0.21, CI: 0.09–0.32, z = 3.42, p = 0.001; Fig. 4).
Figure 4.
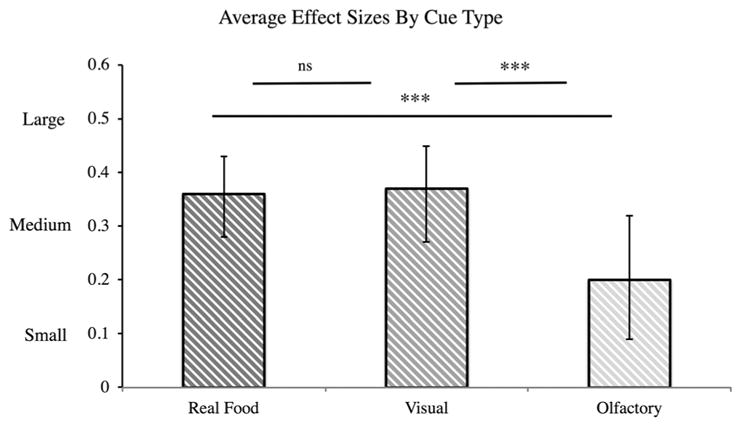
Average effect sizes by cue type (Hypothesis 5). Effect sizes for visual and real food cues were not significantly different; both were significantly bigger than for olfactory cues. ‘***’ indicates that p < 0.001.
Publication bias
First, we calculated Rosenthal’s fail-safe N to determine the number of studies with a null effect that would be necessary to render the meta-analytic results non-significant. Overall, the fail-safe N for the omnibus analysis was NSTUDIES = 4,186. In other words, 4,186 studies with null results would be required to suggest that cue reactivity and craving are not prospectively associated with eating and weight outcomes. Testing separately by type of outcome, we found fail-safe NSTUDIES = 3,015 for studies reporting eating outcomes and fail-safe NSTUDIES = 89 for weight outcomes. The fail-safe N across cue condition, cue reactivity and cue-induced craving studies was NSTUDIES = 3,295 (Hypothesis 1) and fail-safe N for cue-induced craving and tonic craving studies was NSTUDIES = 367 (Hypotheses 2 and 3; cue-induced NSTUDIES = 141; tonic NSTUDIES = 48). For moderator comparisons, the fail-safe Ns were still quite high, with smaller fail-safe Ns corresponding with smaller number of included studies:lean fail-safe NSTUDIES = 306, overweight NSTUDIES = 234, children NSTUDIES = 491, adult NSTUDIES = 1,785, restrained NSTUDIES = 46 and non-restrained NSTUDIES = 49 (Hypothesis 4). Similarly, for cue-type analyses, the fail-safe Ns for real food cues, visual cues and olfactory cues were 5 to 50 times the observed study sample (fail-safe NSTUDIES = 962, NSTUDIES = 498 and NSTUDIES = 39, respectively; Hypothesis 5). Overall, fail-safe N analyses indicated that a very large number of studies reporting null results would be needed to render the reported meta-analytic results non-significant.
Using a second approach, we calculated Kendall’s tau and Egger’s regression intercept and conducted a trim-and-fill analysis. Kendall’s tau (τ = 0.36, zτ = 3.27, p <0.01) indicated some risk of publication bias; however, Egger’s regression intercept did not detect bias (t(33) = 0.84, p = 0.27). The trim-and-fill analysis filled in 15 studies left of the mean and zero studies right of the mean, adjusting to an unbiased effect size r = 0.24 (CI: 0.21–0.27; Fig. 5), which is still substantial. Overall, these analyses also suggest that the present meta-analytic results are resilient to publication bias and that even adjusting for publication bias, food cue reactivity and craving still predict eating and weight outcome with a medium effect size.
Figure 5.
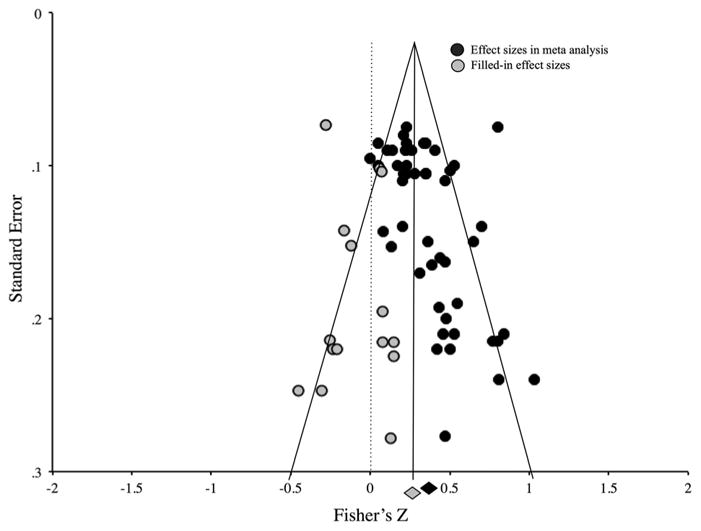
Funnel plot depicting standard error of effect sizes. Funnel plot shows minimal publication bias in the overall model using the calculated Fisher’s z-value for each study in a trim-and-fill analysis. Black circles represent studies included in the analysis, and grey circles represent filled-in studies. The black diamond is the observed effect, and the grey diamond is the adjusted effect after adding the filled-in studies, which is still significant.
Discussion
The present meta-analysis is the first quantitative examination of the effect of food cue reactivity and craving on subsequent eating behavior and weight gain. Across 45 published studies and 69 reported statistics representing 3,292 participants, we found a significant medium effect (r = 0.33) of food cue reactivity and craving on eating and weight, measured prospectively with outcomes in the short-term and long-term. Our results demonstrate a robust prospective and predictive relationship between measures of food cue reactivity, including the conscious experience of craving and subsequent food-related outcomes. Further, we found consistent, medium effect sizes across several specific analyses, in confirmation of our hypotheses. In support of our first hypothesis that cue reactivity increases eating, we found that (i) exposure to food cues increases eating (‘cue condition’ studies) and (ii) the magnitude of physiological and neural cue reactivity to food cues relates prospectively to eating and weight gain over time (‘cue reactivity’ studies). In support of our second hypothesis, we found that magnitude of cue-induced craving relates prospectively to eating and weight gain. In support of our third hypothesis, we found that magnitude of tonic craving relates to eating and weight gain prospectively. We also found that real food and visual cues were both more strongly related to outcomes than olfactory cues and that BMI, age, dietary restraint and gender did not modify the strength of the findings. Overall, our results suggest that food cue reactivity and craving explain a substantial amount (7–26%) of the variance in food-related outcomes. In turn, this suggests a causal effect of cue exposure and craving on eating behavior and weight. Our results have important theoretical and methodological implications for the use and application of food cue reactivity paradigms, as well as implications for our understanding of obesity prevention and treatment.
Theoretical implications
Our work provides strong empirical support for the idea that exposure and reactivity to food cues, including craving, increase eating and weight, and thus may contribute to rising and persistent rates of obesity. Indeed, our findings are consistent with both conditioned, learning-based models (21–24) and their theoretical applications to obesity (80–84,93,94,99,141). For instance, Jansen (8,9) proposed that eating triggered by increased availability and advertising of food can lead to steady weight gain on a population level and interfere with the efficacy of obesity treatment. Although the increasing presence of food and food cues has been described as a ‘toxic food environment’ that causes a population-wide increase in BMI (e.g. (84,88)), it has been difficult to determine causal relationships between the environment and obesity from cross-sectional studies (142). Importantly, our meta-analytic work demonstrates that responses to food cues systematically lead to subsequent eating and weight gain across both experimental and prospective studies, providing evidence of a consistent, predictive relationship on an individual level. Furthermore, these effects generalize across individual differences in BMI, age, dietary restraint and gender. Thus, our work supports the theory that, on a large scale, the abundance of food and food cues in the modern ‘toxic food environment’ may function as conditioned stimuli that serve as triggers for increased food consumption and can lead to weight gain on a population level (80–85,93,94,99,141).
Implications for obesity prevention and policy
By establishing the link between food cues and eating, our work can help build a framework to investigate how individual behavior is influenced by environment–level interventions. Because we find that exposure to food cues reliably leads to eating and weight gain, our work is consistent with the suggestion that exposure to food advertisements and increased access to unhealthy foods can have deleterious effects. In that, the findings support obesity prevention policies that reduce unhealthy food cue exposure, including limiting food advertisements or access to high-caloric, nutrient-poor foods. In one analysis, we specifically found that visual exposure to pictures or videos of food (such as in food advertising and commercials) increases eating behavior and weight, including several studies in the present meta-analysis that used real-life food advertisements as food cues in experimental settings (47,48,143). Accordingly, this could support policy initiatives limiting television and print advertisements for energy dense products (144) as has been previously implemented for cigarettes (145,146). Our findings support the removal of cues to and availability of unhealthy foods in schools, an intervention that has already demonstrated some effectiveness in preventing unhealthy weight gain in children (147). Because we found that cue-related effects are similar in children and adults, these interventions may also be applicable for adults. Indeed, it has been shown that reducing the visibility of food cues in an office environment reduces consumption of unhealthy foods in adults (148). In sum, our work highlights a mechanism involved in food consumption that explains why a reduction in exposure to food cues can be an important target for obesity prevention policies.
Methodological implications
Our work has methodological implications for the use and application of cue reactivity and craving paradigms. Learning-based models suggest that olfactory cues should be better predictors of food consumption than visual cues because olfactory cues are always present during food consumption and the conditioned stimulus that predicts the unconditioned stimulus best should elicit the strongest conditioned responses (8). Consistently, the use of visual stimuli has been criticized for failing to represent real-world food consumption, although it mirrors advertising for food (e.g. (149)). Nevertheless, we found that reactivity to visual food cues (e.g. pictures and videos) is as strongly predictive of eating behavior and weight gain as reactivity to real food (and that both are more strongly predictive than olfactory cues). That visual food cue exposure was as strongly associated with eating behavior as real food exposure suggests that cue-based learning processes are powerful for food. This finding is especially informative for neuroimaging studies that frequently use images to provoke craving (5,53,55,150) and for studies that use images to investigate the regulation of craving (151,152). Overall, our findings support the ecological validity of a variety of food cues (including visual food cues) to elicit cue reactivity and craving and suggest that this directly relates to real-life behavior, because their effect on subsequent food-related outcomes is comparable with real food exposure.
Parallels with drug cue reactivity and addictions
As reviewed earlier, cue reactivity and craving for food and drugs share many similarities. In fact, it has been proposed that drugs ‘hijack’ a hedonic, dopamine-modulated reward system that originally evolved for food and that subserves processes including cue reactivity and craving for both food and drugs (5,101,153). It is this system that is altered in addiction and may be altered in obesity and eating disorders (154–156). Here, we report that food cue reactivity and craving predict eating and weight, which further parallels findings in addiction linking drug cue reactivity and craving to drug taking and clinical outcomes for addictive disorders (113,121,157). However, despite such similarities, cue reactivity and craving are central aspects of the diagnosis and treatment of addictive disorders, whereas they are under-utilized in the context of eating behavior.
Some previous proposals to investigate similarities between food and addiction have focused on the concept of ‘food addiction’ and have been met with controversy (101–104). We propose that continued construct-focused and mechanism-focused investigation into similarities between drug taking and eating behavior may be useful for illuminating shared processes across addictions and eating-related psychopathology (as proposed by the Research Domain Criteria framework (158)). Our work specifically suggests that cue reactivity and craving may be cross-diagnostic and clinically relevant. As such, they could be used to more accurately describe and measure eating behavior, as they have been in addictive disorders. This insight may further encourage the adoption of other paradigms from the addictions field to measure-related processes, such as regulation of craving, impulsivity and cognitive control. Such processes could explain additional variance in food-related outcomes and facilitate comparisons between food and drug-seeking behavior and related psychopathology.
In addition, our work is relevant to an ongoing debate in the addictions literature about the influence of cue-induced versus tonic craving on outcomes (159–163). We found that both tonic and cue-induced craving were associated with subsequent eating and weight-related outcomes, suggesting that both of these forms of craving can serve a predictive function and may be useful to measure in clinical and treatment settings. Finally, these parallels suggest that treatment approaches for obesity and related disorders can draw from insights and findings already established for addiction, as elaborated in the succeeding discussion.
Treatment implications for obesity and related disorders
Our findings are consistent with and suggest explanatory mechanisms for the efficacy of pharmacological, behavioral and psychological treatments for obesity, eating disorders and addictions that both reduce craving and improve outcomes (73,164–166). For example, craving-targeting medications increase weight loss in overweight individuals, paralleling findings in addiction that demonstrate reductions in drug use with pharmacotherapy (167,168). Specifically, pharmacotherapies that reduce craving, such as bupropion and naltrexone, are associated with reduced self-reported craving and reduced BMI in weight loss trials (95,169–172). In addition, stimulants such as methylphenidate also reduce craving for and consumption of food (173). Similarly, in drug addiction, such pharmacotherapies reduce craving for and consumption of alcohol (174,175), methamphetamine (176) and nicotine (177).
Several psychological treatments also target the associations between cue exposure, craving and food consumption, including cue exposure and response prevention treatments (CERP), cognitive behavioral therapy (CBT) and mindfulness-based therapies (MBTs). CERP attempts to extinguish associations between a cue, conditioned responses and a behavior by preventing the behavior from occurring in a cued context, consistent with learning models (8,9). Such exposures occur over prolonged periods (e.g. 60 min, multiple times per week), in contrast to the relatively short exposures used by studies included in this meta-analysis (e.g. ~10 min) (178–180). Accordingly, prolonged exposure to chocolate cues (181), as well as smoking (182) and alcohol cues (183), reduces physiological reactivity in response to these cues. As a treatment, CERP also reduces self-reported craving (178) and binge eating in individuals with bulimia nervosa (178,180,184,185). However, extinction of cue-response pairings can be context-specific and may therefore still be reinstated in new environments (100,186–189) or through incubation of craving (190), even after extinction, limiting the potential application of CERP. Consistently, clinical trials for CERP have been mixed for other eating disorders (191), obesity (192) and addictions (193,194).
In contrast, other psychological treatment approaches assume that the association between food cues, craving and food consumption may not be perfectly extinguished and provide skills to manage diverse food-related contexts. For instance, CBT employs cognitive and behavioral strategies to reduce the influence of food cues and craving on eating behavior and weight. Cognitive strategies include the regulation of craving through cognitive reappraisal, which reduces neural reactivity and self-reported craving for high-calorie foods (116,151,152,195), cigarettes (116,151), alcohol (196), cocaine (197) and methamphetamine (198). Behavioral strategies to reduce the influence of food cues and craving in CBT include the following: determining the antecedents of eating behavior (e.g. food cues and craving), intervening to prevent consequences (i.e. food consumption), stimulus control (e.g. reduction of food cues in the personal environment), regular meal planning (to reduce vulnerability to food cues and craving) and exposure-based exercises (to reduce the salience of ‘trigger’ foods and contexts) (199). CBT is effective in the treatment of a wide range of conditions (200), including eating disorders (164,201), obesity (202,203) and drug addictions (204). Further, regulation of food craving specifically has been examined as part of obesity interventions for children (205) and adults (206), although this work is in its early stages. Finally, MBTs that teach individuals to notice and accept the experience of craving have demonstrated effectiveness at reducing craving for food and weight in both lean and obese adults (207–210) as well as reducing episodes of binge eating in individuals with binge eating disorder (211), bulimia nervosa (212,213) or following bariatric surgery (214). In drug addiction, MBTs reduce craving and use of drugs (215–217), and reductions in craving reportedly mediate reductions in drug use (215,217).
Overall, much evidence suggests that treatments that target cue-outcome associations and/or craving-outcome associations can reduce eating, improve weight outcomes and reduce drug use in addictions. We propose that the findings reported in this meta-analysis provide explanatory mechanisms for the efficacy of such treatments, although this should be investigated directly using mediation models in future clinical work (e.g. (215)). Such continued investigation into craving-targeted pharmacotherapy, behavioral treatment and psychotherapy for obesity and related disorders may increase the efficacy of existing treatment approaches.
Methodological issues and future work
One limitation of this meta-analysis is that it could not include all studies in the field; rather, it was inherently limited to studies that reported appropriate statistics. Unfortunately, approximately 20% of the studies that met our initial inclusion criteria were excluded because of limited or incomplete statistical reporting. This highlights the importance of reporting of sample size, means, standard deviations, test statistics and effect sizes in all future work, in order to increase accurate and transparent data reporting and to contribute to future meta-analyses and the overall development of the field.
Relatedly, we may not have been able to detect significant effects in some of our moderator analyses because of underreporting of group-based statistics or because of a small or imbalanced number of studies included in such analyses. Future work should continue to assess whether individual differences moderate the relationship between cue reactivity, craving and eating behavior as the number of published studies increases. This is especially important because some cross-sectional studies have demonstrated heightened cue reactivity in overweight individuals and restrained eaters (overweight: (1,41,218–220); restrained: (17,18,30,221)) as well as increased craving (19,64,222). Further, failure to detect differences based on dietary restraint measurements may be due to inconsistent and varied measurement of dietary restraint across studies (223–225) (Supporting Information). If cross-sectional differences in BMI and dietary restraint extend to predictive relationships with eating and weight gain, then food cue reactivity and craving could serve as markers of increased risk for restrained eating or becoming overweight.
In addition, some of the effects we detected may be due to small or imbalanced numbers of studies included in moderator-based analyses. For instance, we detected a stronger effect in mixed gender than female-only samples, contrary to the suggestion that females are more cue reactive (e.g. (226); Supporting Information). However, this comparison included almost double the number of mixed gender (NSTATISTICS = 42) compared with female-only statistics (NSTATISTICS = 26) and only one statistic in males. We hope that future studies will include more male-only and mixed-gender samples to further test for gender differences and will also test for any menstrual-cycle variations in females that may influence cue reactivity and craving. Similarly, we found that cue reactivity and craving were more strongly associated with long-term outcomes than short-term outcomes, which may also be attributable to an imbalanced number of studies (short-term: NSTUDIES = 35; long-term: NSTUDIES = 10). Future work could examine how far into the future the assessment of food cue responses and craving can predict outcomes and whether this is related to underlying trait-level individual differences.
Further, although a substantial literature suggests that appetitive conditioning increases, and depends upon, expectancy of reward (e.g. (227–231)), expectancy to eat did not affect the meta-analytic result (Supporting Information). Nevertheless, expectancy of food may be an important avenue for future work despite reported null effects on physiological reactivity to food cues (232), food craving (233) and food consumption (232,233).
Finally, given the parallels drawn herein between food and drug cue reactivity and craving, it may be useful to statistically compare these effects in future work. Notably, one prior meta-analysis of craving and cigarette smoking found similarly sized predictive effects (rs = 0.20–0.34) (121). Future meta-analyses should directly compare the predictive effects of cue reactivity and craving on cigarettes-related, drug-related and food-related outcomes.
Conclusions
Most people have an intuitive sense that wanting to eat a cookie increases one’s chance of eating cookies. The present meta-analysis addressed this systematically by quantitatively examining the effects of food cue reactivity and craving on eating and weight-related outcomes. We found a medium-sized effect for all measures of food cue reactivity and craving on food outcomes, consistent with the ‘cued overeating’ model (8,9). We also found that reactivity to visual cues (pictures and videos) was as strongly related to outcome as reactivity to real food, which has important methodological implications. Our results parallel findings in the drug literature and suggest that these phenomena are cross-diagnostic and may have important clinical utility. This supports targeting food cue exposure and craving as part of obesity-related public health interventions as well as clinical treatments for obesity, weight loss and eating disorders.
Supplementary Material
Acknowledgments
This work was funded by K12 DA00167 and P50 DA09241. We are grateful to Jessica Hallam and Payal Marathe for their help and to Darby Henry, Avram Holmes, Jutta Joormann, Maggie Mae Mell, Matthew Schafer and Wendy Sun for their comments on previous versions of this manuscript.
Footnotes
Conflict of interest statement
The authors report no conflicts of interest.
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Jansen A, Theunissen N, Slechten K, et al. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 2.Nederkoorn C, Jansen A. Cue reactivity and regulation of food intake. Eat Behav. 2002;3:61–72. doi: 10.1016/s1471-0153(01)00045-9. [DOI] [PubMed] [Google Scholar]
- 3.Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35:45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- 4.Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav. 1989;14:387–397. doi: 10.1016/0306-4603(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 5.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Nederkoorn C, Smulders F, Havermans R, Jansen A. Exposure to binge food in bulimia nervosa: finger pulse amplitude as a potential measure of urge to eat and predictor of food intake. Appetite. 2004;42:125–130. doi: 10.1016/j.appet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 9.Jansen A, Havermans RC, Nederkoorn C. Cued overeating. In: Preedy VR, Watson RR, Martin CR, editors. Handbook of Behavior, Food and Nutrition. Springer; New York: 2011. pp. 1431–1443. [Google Scholar]
- 10.Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: neural bases and implications for psychopathology. Neurosci Biobehav Rev. 2007;31:426–440. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlov IP. Conditioned Reflexes. Dover Publications; New York: 1927. [Google Scholar]
- 12.Sugino T, Hasegawa Y, Kurose Y, Kojima M, Kangawa K, Terashima Y. Effects of ghrelin on food intake and neuroendocrine function in sheep. Anim Reprod Sci. 2004;82–83:183–194. doi: 10.1016/j.anireprosci.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 14.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Ponteza MN. Neural correlates of stress- and food-cue induced food craving in obesity. Diabetes Care. 2012;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornell CE, Rodin J, Weingarten HP. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 17.Fedoroff I, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- 18.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters’ responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 19.Ng L, Davis C. Cravings and food consumption in binge eating disorder. Eat Behav. 2013;14:472–475. doi: 10.1016/j.eatbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.APA. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 21.Bongers P, van den Akker K, Havermans R, Jansen A. Emotional eating and Pavlovian learning: does negative mood facilitate appetitive conditioning? Appetite. 2015;89:226–236. doi: 10.1016/j.appet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 22.van den Akker K, Jansen A, Frentz F, Havermans RC. Impulsivity makes more susceptible to overeating after contextual appetitive conditioning. Appetite. 2013;70:73–80. doi: 10.1016/j.appet.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 23.Papachristou H, Nederkoorn C, Beunen S, Jansen A. Dissection of appetitive conditioning. Does impulsivity play a role? Appetite. 2013;69:46–53. doi: 10.1016/j.appet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Van Gucht D, Vansteenwegen D, Beckers T, Hermans D, Baeyens F, Van den Bergh O. Repeated cue exposure effects on subjective and physiological indices of chocolate craving. Appetite. 2008;50:19–24. doi: 10.1016/j.appet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Van Gucht D, Vansteenwegen D, Van den Bergh O, Beckers T. Conditioned craving cues elicit an automatic approach tendency. Behav Res Ther. 2008;46:1160–1169. doi: 10.1016/j.brat.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 27.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012;59:437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes (Lond) 2009;33:693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coelho JS, Polivy J, Herman CP, Pliner P. Wake up and smell the cookies. Effects of olfactory food-cue exposure in restrained and unrestrained eaters. Appetite. 2009;52:517–520. doi: 10.1016/j.appet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Buckland NJ, Finlayson G, Hetherington MM. Pre-exposure to diet-congruent food reduces energy intake in restrained dieting women. Eat Behav. 2013;14:249–254. doi: 10.1016/j.eatbeh.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? Br J Nutr. 2008;100:1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- 33.Jansen A, van den Hout M. On being led into temptation: “counterregulation” of dieters after smelling a “preload”. Addict Behav. 1991;16:247–253. doi: 10.1016/0306-4603(91)90017-c. [DOI] [PubMed] [Google Scholar]
- 34.Jansen A, Nederkoorn C, van Baak L, Keirse C, Guerrieri R, Havermans R. High-restrained eaters only overeat when they are also impulsive. Behav Res Ther. 2009;47:105–110. doi: 10.1016/j.brat.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Jansen A, Nederkoorn C, Roefs A, Bongers P, Teugels T, Havermans R. The proof of the pudding is in the eating: is the DEBQ-external eating scale a valid measure of external eating? Int J Eat Disord. 2011;44:164–168. doi: 10.1002/eat.20799. [DOI] [PubMed] [Google Scholar]
- 36.Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51:556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Overduin J, Jansen A. Conditioned insulin and blood sugar responses in humans in relation to binge eating. Physiol Behav. 1997;61:569–575. doi: 10.1016/s0031-9384(96)00504-5. [DOI] [PubMed] [Google Scholar]
- 38.Wonderlich-Tierney AL, Wenzel KR, Vander Wal JS, Wang-Hall J. Food-related advertisements and food intake among adult men and women. Appetite. 2013;71:57–62. doi: 10.1016/j.appet.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Lambert KG, Neal T, Noyes J, Parker C, Worrel P. Food-related stimuli increase desire to eat in hungry and satiated human subjects. Curr Psychol Res Rev. 1991;10:297–303. [Google Scholar]
- 40.Larsen JK, Hermans RC, Engels RC. Food intake in response to food-cue exposure. Examining the influence of duration of the cue exposure and trait impulsivity. Appetite. 2012;58:907–913. doi: 10.1016/j.appet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Zoon HF, He W, de Wijk RA, de Graaf C, Boesveldt S. Food preference and intake in response to ambient odours in overweight and normal-weight females. Physiol Behav. 2014;133:190–196. doi: 10.1016/j.physbeh.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- 43.Boutelle KN, Kuckertz JM, Carlson J, Amir N. A pilot study evaluating a one-session attention modification training to decrease overeating in obese children. Appetite. 2014;76:180–185. doi: 10.1016/j.appet.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buijzen M, Schuurman J, Bomhof E. Associations between children’s television advertising exposure and their food consumption patterns: a household diary-survey study. Appetite. 2008;50:231–239. doi: 10.1016/j.appet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Folkvord F, Anschutz DJ, Buijzen M, Valkenburg PM. The effect of playing advergames that promote energy-dense snacks or fruit on actual food intake among children. Am J Clin Nutr. 2013;97:239–245. doi: 10.3945/ajcn.112.047126. [DOI] [PubMed] [Google Scholar]
- 46.Folkvord F, Anschutz DJ, Nederkoorn C, Westerik H, Buijzen M. Impulsivity, “advergames,” and food intake. Pediatrics. 2014;133:1007–1012. doi: 10.1542/peds.2013-3384. [DOI] [PubMed] [Google Scholar]
- 47.Halford JC, Boyland EJ, Hughes G, Oliveira LP, Dovey TM. Beyond-brand effect of television (TV) food advertisements/ commercials on caloric intake and food choice of 5–7-year-old children. Appetite. 2007;49:263–267. doi: 10.1016/j.appet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM. Effect of television advertisements for foods on food consumption in children. Appetite. 2004;42:221–225. doi: 10.1016/j.appet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Harris JL, Speers SE, Schwartz MB, Brownell KD. US Food Company Branded Advergames on the Internet: children’s exposure and effects on snack consumption. J Child Media. 2012;6:51–68. [Google Scholar]
- 50.Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014;25:1337–1344. doi: 10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta S, Melhorn SJ, Smeraglio A, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 53.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murdaugh DL, Cox JE, Cook EW, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunbar MS, Shiffman S, Kirchner TR, Tindle HA, Scholl SM. Nicotine dependence, “background” and cue-induced craving and smoking in the laboratory. Drug Alcohol Depend. 2014;142:197–203. doi: 10.1016/j.drugalcdep.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the state and trait food cravings questionnaire. Behav Ther. 2000;31:151–173. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 58.Martin CK, O’Neil PM, Tollefson G, Greenway FL, White MA. The association between food cravings and consumption of specific foods in a laboratory taste test. Appetite. 2008;51:324–326. doi: 10.1016/j.appet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill AJW, Weaver CFL, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187–197. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- 60.Buckland NJ, Finlayson G, Hetherington MM. Slimming starters. Intake of a diet-congruent food reduces meal intake in active dieters. Appetite. 2013;71:430–437. doi: 10.1016/j.appet.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 61.Moreno-Dominguez S, Rodriguez-Ruiz S, Martin M, Warren CS. Experimental effects of chocolate deprivation on cravings, mood, and consumption in high and low chocolate-cravers. Appetite. 2012;58:111–116. doi: 10.1016/j.appet.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Cleobury L, Tapper K. Reasons for eating ‘unhealthy’ snacks in overweight and obese males and females. J Hum Nutr Diet. 2014;27:333–341. doi: 10.1111/jhn.12169. [DOI] [PubMed] [Google Scholar]
- 63.Batra P, Das SK, Salinardi T, et al. Relationship of cravings with weight loss and hunger. Results from a 6 month worksite weight loss intervention. Appetite. 2013;69:1–7. doi: 10.1016/j.appet.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Crowley N, Madan A, Wedin S, et al. Food cravings among bariatric surgery candidates. Eat Weight Disord. 2014;19:371–376. doi: 10.1007/s40519-013-0095-y. [DOI] [PubMed] [Google Scholar]
- 65.Gilhooly CH, Das SK, Golden JK, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes (Lond) 2007;31:1849–1858. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- 66.Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids. 2012;77:323–331. doi: 10.1016/j.steroids.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Leahey TM, Bond DS, Raynor H, et al. Effects of bariatric surgery on food cravings: do food cravings and the consumption of craved foods “normalize” after surgery? Surg Obes Relat Dis. 2012;8:84–91. doi: 10.1016/j.soard.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cushing CC, Benoit SC, Peugh JL, Reiter-Purtill J, Inge TH, Zeller MH. Longitudinal trends in hedonic hunger after Roux-en-Y gastric bypass in adolescents. Surg Obes Relat Dis. 2014;10:125–130. doi: 10.1016/j.soard.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morewedge CK, Huh YE, Vosgerau J. Thought for food: imagined consumption reduces actual consumption. Science. 2010;330:1530–1533. doi: 10.1126/science.1195701. [DOI] [PubMed] [Google Scholar]
- 70.Missbach B, Florack A, Weissmann L, Konig J. Mental imagery interventions reduce subsequent food intake only when self-regulatory resources are available. Front Psychol. 2014;5:1391. doi: 10.3389/fpsyg.2014.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coelho JS, Idler A, Werle COC, Jansen A. Sweet temptation: effects of exposure to chocolate-scented lotion on food intake. Food Quality and Preference. 2011;22:780–784. [Google Scholar]
- 72.Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A. Neural predictors of chocolate intake following chocolate exposure. Appetite. 2015;87:98–107. doi: 10.1016/j.appet.2014.12.204. [DOI] [PubMed] [Google Scholar]
- 73.Potenza MN, Grilo CM. How relevant is food craving to obesity and its treatment? Front Psychiatry. 2014;5:164. doi: 10.3389/fpsyt.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States. U S Department of Health and Human Services; 2012. [Google Scholar]
- 75.Center for Disease Control. Obesity Statistics. 2013 [Google Scholar]
- 76.World Health Organization. Global status report on non-communicable diseases. 2010. [Google Scholar]
- 77.Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- 78.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 79.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Nestle M. Why Calories Count: From Science to Politics. Univeristy of California Press; Berkeley, CA: 2012. [PubMed] [Google Scholar]
- 81.Popkin BM. The World is Fat: The Fads, Trends, Policies, and Products that are Fattening the Human Race. Avery; New York: 2009. [Google Scholar]
- 82.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 83.Kessler DA. The End of Overeating: Taking Control of the Insatiable American Appetite. Emmaus, PA: Rodale; 2009. [Google Scholar]
- 84.Brownell KD, Horgen KB. Food Fight: The Inside Story of the Food Industry, America’s Obesity Crisis, and What We Can Do About It. McGraw Hill, Contemporary Books; New York: 2004. [Google Scholar]
- 85.Polivy J, Herman CP. Eating in response to exernal cues. In: Gill T, editor. Managing and Preventing Obesity: Behavioral Factors and Dietary Interventions. Elsevier; Cambridge, UK: 2014. [Google Scholar]
- 86.Harris JL, Pomeranz JL, Lobstein T, Brownell KD. A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Annu Rev Public Health. 2009;30:211–225. doi: 10.1146/annurev.publhealth.031308.100304. [DOI] [PubMed] [Google Scholar]
- 87.Gearhardt AN, Bragg MA, Pearl RL, Schvey NA, Roberto CA, Brownell KD. Obesity and public policy. Annu Rev Clin Psychol. 2012;8:405–430. doi: 10.1146/annurev-clinpsy-032511-143129. [DOI] [PubMed] [Google Scholar]
- 88.Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol. 2002;70:510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- 89.Prentice AMJ, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4:187–194. doi: 10.1046/j.1467-789x.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 90.Jeffery RW, Baxter J, McGuire M, Linde J. Are fast food restaurants an environmental risk factor for obesity? Int J Behav Nutr Phys Act. 2006;3:2. doi: 10.1186/1479-5868-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenheck R. Fast food consumption and increased caloric intake: a systematic review of a trajectory towards weight gain and obesity risk. Obes Rev. 2008;9:535–547. doi: 10.1111/j.1467-789X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 92.McClure AC, Tanski SE, Gilbert-Diamond D, et al. Receptivity to television fast-food restaurant marketing and obesity among U.S. youth. Am J Prev Med. 2013;45:560–568. doi: 10.1016/j.amepre.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang GJ, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Byrne S, Cooper Z, Fairburn CG. Psychological predictors of weight regain in obesity. Behav Res Ther. 2004;42:1341–1356. doi: 10.1016/j.brat.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Weiss EC, Galuska DA, Khan LK, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 98.Sitton SC. Role of Craving for Carbohydrates upon Completion of a Protein-sparing Fast. Psychol Rep. 1991;69:683–686. doi: 10.2466/pr0.1991.69.2.683. [DOI] [PubMed] [Google Scholar]
- 99.Martin CK, McClernon FJ, Chellino A, Correa JB. Handbook of Behavior, Food, and Nutrition. In: Preedy VR, Watson RR, Martin CR, editors. Food Cravings: A Central Construct in Food Intake Behavior, Weight Loss, and the Neurobiology of Appetitive Behavior. Springer; New York, USA: 2011. pp. 741–755. [Google Scholar]
- 100.van den Akker K, Havermans RC, Bouton ME, Jansen A. How partial reinforcement of food cues affects the extinction and reacquisition of appetitive responses. A new model for dieting success? Appetite. 2014;81:242–252. doi: 10.1016/j.appet.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 101.Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci. 2012;13:514. doi: 10.1038/nrn3212-c1. author reply 514. [DOI] [PubMed] [Google Scholar]
- 103.Ziauddeen H, Farooqi IS, Fletcher PC. Food addiction: is there a baby in the bathwater? Nat Rev Neurosci. 2012;13:514–514. [Google Scholar]
- 104.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- 105.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 106.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 109.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Engelmann JM, Versace F, Robinson JD, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 112.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2012;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kober H, Mell MM. Neural mechanisms underlying craving and the regulation of craving. In: Wilson SJ, editor. Handbook on the Cognitive Neuroscience of Addiction. Wiley-Blackwell; Oxford, UK: 2015. pp. 195–218. [Google Scholar]
- 114.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:1–28. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 116.Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis: Statistics in Practice. Wiley; New York, NY: 2009. [Google Scholar]
- 118.Thomas JJ, Vartanian LR, Brownell KD. The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: meta-analysis and implications for DSM. Psychol Bull. 2009;135:407–433. doi: 10.1037/a0015326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uttal DH, Meadow NG, Tipton E, et al. The malleability of spatial skills: a meta-analysis of training studies. Psychol Bull. 2013;139:352–402. doi: 10.1037/a0028446. [DOI] [PubMed] [Google Scholar]
- 120.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 121.Gass JC, Motschman CA, Tiffany ST. The relationship between craving and tobacco use behavior in laboratory studies: a meta-analysis. Psychol Addict Behav. 2014;28:1162–1176. doi: 10.1037/a0036879. [DOI] [PubMed] [Google Scholar]
- 122.Rosenthal R. Meta-analytic Procedures for Social Research. Sage; Newbury Park, CA: 1991. [Google Scholar]
- 123.Rosenthal R, DiMatteo M. Meta analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- 124.Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31:2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prati G, Pietrantoni L. Optimism, social support, and coping strategies as factors contributing to posttraumatic growth: a meta-analysis. J Loss Trauma. 2009;14:364–388. [Google Scholar]
- 127.Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Bull. 2001;6:161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- 128.Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 129.Freund PA, Kasten N. How smart do you think you are? A meta-analysis on the validity of self-estimates of cognitive ability. Psychol Bull. 2012;138:296–321. doi: 10.1037/a0026556. [DOI] [PubMed] [Google Scholar]
- 130.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum; Hillside, N.J: 1988. [Google Scholar]
- 131.Carpenter CL, Wong AM, Li Z, Noble EP, Heber D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity (Silver Spring) 2013;21:E467–E473. doi: 10.1002/oby.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodin JM, Mancuso J, Granger J, Nelbach E. Food cravings in relation to body mass index, restraint and estradiol levels: a repeated measures study in healthy women. Appetite. 1991;17:177–185. doi: 10.1016/0195-6663(91)90020-s. [DOI] [PubMed] [Google Scholar]
- 133.Vogele C, Florin I. Psychophysiological responses to food exposure: an experimental study in binge eaters. Int J Eat Disord. 1997;21:147–157. doi: 10.1002/(sici)1098-108x(199703)21:2<147::aid-eat5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 134.Sutton AJ, Duval SJ, Tweedie R, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Egger M, Davey Smith G, Altman D. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Begg CB. Publication bias. In: Cooper HM, Hedges LV, editors. The Handbook of Research Synthesis. Russel Sage Foundation; New York: 1994. [Google Scholar]
- 137.Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J Roy Stat Soc. 1988:419–463. Series A. [Google Scholar]
- 138.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. In: Egger M, Davey Smith G, editors. Systematic Reviews in Health Care: Meta-analysis in Context. BMJ Books; London: 2000. pp. 347–369. [DOI] [PubMed] [Google Scholar]
- 139.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 140.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 141.Lee RE, McAlexander KM, Banda JA. Reversing the Obesogenic Environment. Human Kinetics; Champaign, IL: 2011. [Google Scholar]
- 142.Kirk SF, Penney TL, McHugh TL. Characterizing the obesogenic environment: the state of the evidence with directions for future research. Obes Rev. 2010;11:109–117. doi: 10.1111/j.1467-789X.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- 143.Boulos R, Vikre EK, Oppenheimer S, Chang H, Kanarek RB. ObesiTV: how television is influencing the obesity epidemic. Physiol Behav. 2012;107:146–153. doi: 10.1016/j.physbeh.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 144.Veerman JL, Van Beeck EF, Barendregt JJ, Mackenbach JP. By how much would limiting TV food advertising reduce childhood obesity? Eur J Public Health. 2009;19:365–369. doi: 10.1093/eurpub/ckp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brownell KD, Warner KE. The perils of ignoring history: big tobacco played dirty and millions died. How similar is big food? Milbank Q. 2009;87:259–294. doi: 10.1111/j.1468-0009.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bayer R, Kelly M. Tobacco control and free speech – an American dilemma. New Engl J Med. 2010;362:281–283. doi: 10.1056/NEJMp0909626. [DOI] [PubMed] [Google Scholar]
- 147.Driessen CE, Cameron AJ, Thornton LE, Lai SK, Barnett LM. Effect of changes to the school food environment on eating behaviors and/or body weight in children: a systematic review. Obes Rev. 2014;15:968–982. doi: 10.1111/obr.12224. [DOI] [PubMed] [Google Scholar]
- 148.Wansink B, Painter JE, Lee YK. The office candy dish: proximity’s influence on estimated and actual consumption. Int J Obes (Lond) 2006;30:871–875. doi: 10.1038/sj.ijo.0803217. [DOI] [PubMed] [Google Scholar]
- 149.Ledoux T, Nguyen AS, Bakos-Block C, Bordnick P. Using virtual reality to study food cravings. Appetite. 2013;71:396–402. doi: 10.1016/j.appet.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 150.Wagner DD, Boswell RG, Kelley WM, Heatherton TF. Inducing negative affect increases the reward value of appetizing foods in dieters. J Cogn Neurosci. 2012;24:1625–1633. doi: 10.1162/jocn_a_00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010;106:52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Giuliani NR, Calcott RD, Berkman ET. Piece of cake. Cognitive reappraisal of food craving. Appetite. 2013;64:56–61. doi: 10.1016/j.appet.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia-Garcia I, Horstmann A, Jurado MA, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. 2014;15:853–869. doi: 10.1111/obr.12221. [DOI] [PubMed] [Google Scholar]
- 154.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010;18:341–351. doi: 10.1002/erv.1048. [DOI] [PubMed] [Google Scholar]
- 156.Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15:1167–1182. doi: 10.1093/ntr/nts268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 159.Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 160.Tiffany ST. Response to Perkins (2009): the continuing conundrum of craving. Addiction. 2009;104:1617–1622. doi: 10.1111/j.1360-0443.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- 161.Sayette MA, Tiffany ST. Peak provoked craving: an alternative to smoking cue-reactivity. Addiction. 2013;108:1019–1025. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shiffman S. Commentary on Perkins (2009): responses to smoking cues are relevant to smoking and relapse. Addiction. 2009;104:1617–1622. doi: 10.1111/j.1360-0443.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 163.Shiffman S, Dunbar M, Kirchner T, et al. Smoker reactivity to cues: effects on craving and on smoking behavior. J Abnorm Psychol. 2013;122:264–280. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005–2012. Int J Eat Disord. 2013;46:462–469. doi: 10.1002/eat.22103. [DOI] [PubMed] [Google Scholar]
- 165.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carroll KM. Cognitive-behavioral therapies. In: Galanter M, Kleber HD, editors. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. American Psychiatric Publishing; Arlington, VA: 2008. pp. 349–360. [Google Scholar]
- 167.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1414. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 168.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 169.Anderson JW, Greenway FL, Fujoka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind placebo-controlled trial. Obes Res. 2002;10:633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 170.Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 171.Greenway FL, Fujioka F, Pladowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 172.Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davis C, Levitan RD, Kaplan AS, Kennedy JL, Carter JC. Food cravings, appetite, and snack-food consumption in response to a psychomotor stimulant drug: the moderating effect of “food-addiction”. Front Psychol. 2014;5:403. doi: 10.3389/fpsyg.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 175.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 176.Newton TF, Roache JD, De La Garza R, 2nd, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 177.Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- 178.Jansen A, Broekmate J, Heymans M. Cue-exposure vs. self-control in the treatment of binge eating: a pilot study. Behav Res Ther. 1992;30:235–241. doi: 10.1016/0005-7967(92)90069-s. [DOI] [PubMed] [Google Scholar]
- 179.Bulik CMS, Sullivan PF, Carter FA, McIntosh VV, Joyce PR. The role of exposure with response prevention in the cognitive-behavioral therapy for bulimia nervosa. Psychol Med. 1998;28:611–623. doi: 10.1017/s0033291798006618. [DOI] [PubMed] [Google Scholar]
- 180.Martinez-Mallen E, Castro-Fornieles J, Lazaro L, et al. Cue exposure in the treatment of resistant adolescent bulimia nervosa. Int J Eat Disord. 2007;40:596–601. doi: 10.1002/eat.20423. [DOI] [PubMed] [Google Scholar]
- 181.Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A. The craving stops before you feel it: neural correlates of chocolate craving during cue exposure with response prevention. Cereb Cortex. 2014;24:1589–1600. doi: 10.1093/cercor/bht016. [DOI] [PubMed] [Google Scholar]
- 182.Choi JS, Park S, Lee JY, et al. The effect of repeated virtual nicotine cue exposure therapy on the psychophysiological responses: a preliminary study. Psychiatry Investig. 2011;8:155–160. doi: 10.4306/pi.2011.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vollstadt-Klein S, Loeber S, Kirsch M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 184.McIntosh VV, Carter FA, Bulik CM, Frampton CM, Joyce PR. Five-year outcome of cognitive behavioral therapy and exposure with response prevention for bulimia nervosa. Psychol Med. 2011;41:1061–1071. doi: 10.1017/S0033291710001583. [DOI] [PubMed] [Google Scholar]
- 185.Toro J, Cervera M, Feliu MH, et al. Cue exposure in the treatment of resistant bulimia nervosa. Int J Eat Disord. 2003;34:227–234. doi: 10.1002/eat.10186. [DOI] [PubMed] [Google Scholar]
- 186.Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Processes. 2012;90:130–141. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van den Akker K, Havermans RC, Jansen A. Effects of occasional reinforced trials during extinction on the reacquisition of conditioned responses to food cues. J Behav Ther Exp Psychiatry. 2015;48:50–58. doi: 10.1016/j.jbtep.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 188.Van Gucht D, Vansteenwegen D, Beckers T, Van den Bergh O. Return of experimentally induced chocolate craving after extinction in a different context: divergence between craving for and expecting to eat chocolate. Behav Res Ther. 2008;46:375–391. doi: 10.1016/j.brat.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 189.Bouton ME. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103:51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 190.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koskina A, Campbell IC, Schmidt U. Exposure therapy in eating disorders revisited. Neurosci Biobehav Rev. 2013;37:193–208. doi: 10.1016/j.neubiorev.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 192.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: a randomized controlled trial. J Consult Clin Psychol. 2011;79:759–771. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 194.Havermans RC, Jansen ATM. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- 195.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60:213–220. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 196.Naqvi N, Kober H, Ochner CN, Morgenstern J. Cognitive regulation of cue-induced craving in alcohol-dependent and social drinkers. Drug Alcohol Depend. 2014;140(39):343–349. doi: 10.1111/acer.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Volkow ND, Fowler JS, Wang GJ, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lopez R, Onyemekwu C, Hart CL, Ochsner KN, Kober H. Boundary conditions of methamphetamine craving. Clinical & Experimental Psychopharmacology. 2015 doi: 10.1037/pha0000049. in press. [DOI] [PMC free article] [PubMed]
- 199.Brownell KD. The LEARN Program for Weight Management. American Health Publishing; Dallas, TX: 2000. [Google Scholar]
- 200.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wilson GT, Fairburn CG. Treatments for eating disorders. In: Nathan PE, Gorman JM, editors. A Guide to Treatments that Work. Oxford University Press; New York: 2007. [Google Scholar]
- 202.Cooper Z, Doll HA, Hawker DM, et al. Testing a new cognitive behavioral treatment for obesity: a randomized controlled trial with three-year follow-up. Behav Res Ther. 2010;48:706–713. doi: 10.1016/j.brat.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cooper Z, Fairburn CG. Cognitive behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford Press; New York: 2002. [Google Scholar]
- 204.Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Boutelle KN, Zucker N, Peterson CB, Rydell S, Carlson J, Harnack LJ. An intervention based on Schachter’s externality theory for overweight children: the regulation of cues pilot. J Pediatr Psychol. 2014;39:405–417. doi: 10.1093/jpepsy/jst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol Behav. 2015;138:124–132. doi: 10.1016/j.physbeh.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alberts HJ, Mulkens S, Smeets M, Thewissen R. Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite. 2010;55:160–163. doi: 10.1016/j.appet.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 208.Dalen J, Smith BW, Shelley BM, Sloan AL, Leahigh L, Begay D. Pilot study: Mindful Eating and Living (MEAL): weight, eating behavior, and psychological outcomes associated with a mindfulness-based intervention for people with obesity. Complement Ther Med. 2010;18:260–264. doi: 10.1016/j.ctim.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 209.O’Reilly GA, Cook L, Spruijt-Metz D, Black DS. Mindfulness-based interventions for obesity-related eating behaviors: a literature review. Obes Rev. 2014;15:453–461. doi: 10.1111/obr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Alberts HJ, Thewissen R, Raes L. Dealing with problematic eating behavior. The effects of a mindfulness-based intervention on eating behavior, food cravings, dichotomous thinking and body image concern. Appetite. 2012;58:847–851. doi: 10.1016/j.appet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 211.Kristeller J, Wolever RQ, Sheets V. Mindfulness-based eating awareness training (MB-EAT) for binge eating: a randomized clinical trial. Mindfulness. 2014;5:282–297. [Google Scholar]
- 212.Katterman SN, Kleinman BM, Hood MM, Nackers LM, Corsica JA. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. 2014;15:197–204. doi: 10.1016/j.eatbeh.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 213.Hill DM, Craighead LW, Safer DL. Appetite-focused dialectical behavior therapy for the treatment of binge eating with purging: a preliminary trial. Int J Eat Disord. 2011;44:249–261. doi: 10.1002/eat.20812. [DOI] [PubMed] [Google Scholar]
- 214.Leahey TM, Crowther JH, Irwin SR. A cognitive-behavioral mindfulness group therapy intervention for the treatment of binge eating in bariatric surgery patients. Cognitive and Behavioral Practice. 2008;15:364–375. [Google Scholar]
- 215.Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addict Behav. 2013;38:1563–1571. doi: 10.1016/j.addbeh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119:72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Elwafi HM, Witkiewitz K, Mallik S, Brewer JA. Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use. Drug Alcohol Depend. 2013;130:222–229. doi: 10.1016/j.drugalcdep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes (Lond) 2011;35:142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- 219.Hendrikse JJ, Cachia RL, Kothe EJ, McPhie S, Skouteris H, Hayden MJ. Attentional biases for food cues in overweight and individuals with obesity: a systematic review of the literature. Obes Rev. 2015;16:424–432. doi: 10.1111/obr.12265. [DOI] [PubMed] [Google Scholar]
- 220.Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52:614–620. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 221.Meule A, Vogele C, Kubler A. Restrained eating is related to accelerated reaction to high caloric foods and cardiac autonomic dysregulation. Appetite. 2012;58:638–644. doi: 10.1016/j.appet.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 222.Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 223.Stice E, Cooper JA, Schoeller DA, Tappe K, Lowe MR. Are dietary restraint scales valid measures of moderate- to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychol Assess. 2007;19:449–458. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- 224.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 225.Stice E, Sysko R, Roberto CA, Allison S. Are dietary restraint scales valid measures of dietary restriction? Additional objective behavioral and biological data suggest not. Appetite. 2010;54:331–339. doi: 10.1016/j.appet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang GJ, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bolles RC. Reinforcement, expectancy, and learning. Psychol Rev. 1972;79:394–409. [Google Scholar]
- 228.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 229.Field M, Duka T. Smoking expectancy mediates the conditioned responses to arbitrary smoking cues. Behav Pharmacol. 2001;12:183–194. doi: 10.1097/00008877-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 230.Jones A, Hogarth L, Christiansen P, Rose AK, Martinovic J, Field M. Reward expectancy promotes generalized increases in attentional bias for rewarding stimuli. Q J Exp Psychol (Hove) 2012;65:2333–2342. doi: 10.1080/17470218.2012.686513. [DOI] [PubMed] [Google Scholar]
- 231.Hogarth L, Duka T. Human nicotine conditioning requires explicit contingency knowledge: is addictive behavior cognitively mediated? Psychopharmacology (Berl) 2006;184:553–566. doi: 10.1007/s00213-005-0150-0. [DOI] [PubMed] [Google Scholar]
- 232.Hardman CA, Scott J, Field M, Jones A. To eat or not to eat. The effects of expectancy on reactivity to food cues. Appetite. 2014;76:153–160. doi: 10.1016/j.appet.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 233.Werthmann J, Roefs A, Nederkoorn C, Jansen A. Desire lies in the eyes: attention bias for chocolate is related to craving and self-endorsed eating permission. Appetite. 2013;70:81–89. doi: 10.1016/j.appet.2013.06.087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.