Abstract
Although the major white adipose depots evolved primarily to store energy, secrete hormones and thermo-insulate the body, multiple secondary depots developed additional specialized and unconventional functions. Unlike any other fat tissue, dermal white adipose tissue (dWAT) evolved a large repertoire of novel features that are central to skin physiology, which we discuss in this Review. dWAT exists in close proximity to hair follicles, the principal appendages of the skin that periodically grow new hairs. Responding to multiple hair-derived signals, dWAT becomes closely connected to cycling hair follicles and periodically cycles itself. At the onset of new hair growth, hair follicles secrete activators of adipogenesis, while at the end of hair growth, a reduction in the secretion of activators or potentially, an increase in the secretion of inhibitors of adipogenesis, results in fat lipolysis. Hair-driven cycles of dWAT remodelling are uncoupled from size changes in other adipose depots that are controlled instead by systemic metabolic demands. Rich in growth factors, dWAT reciprocally signals to hair follicles, altering the activation state of their stem cells and modulating the pace of hair regrowth. dWAT cells also facilitate skin repair following injury and infection. In response to wounding, adipose progenitors secrete repair-inducing activators, while bacteria-sensing adipocytes produce antimicrobial peptides, thus aiding innate immune responses in the skin.
Main
White adipose tissue (WAT) is widely distributed throughout the body in the form of depots that can both store nutrient-derived lipids and mobilize them upon demand1,2. Many features of WAT anatomy are well adapted for periodic bouts of expansion and emptying. WAT depots commonly reside in body sites, such as the abdominal cavity or subcutaneous space, that enable rapid change in volume without physical hindrance on vital organs. At the microscopic level, fat consists of adipocyte clusters surrounded by a web-like extracellular matrix that efficiently remodels — through the aid of many matrix-modifying enzymes3–5 — to accommodate for the rapid growth of WAT upon lipid accumulation6–10.
At the cell lineage level, WAT growth depends on adipose progenitors, including adipose stem cells (ASCs), that typically reside on small blood vessels11–14. Adipocyte maturation is driven by many signalling activities, including Bmp15,16, Fgf17,18, Hedgehog19,20, and Wnt pathways21–24. Therefore, in addition to adipokines, which are hormones derived from adipose tissues 1, WAT secretes multiple paracrine signalling and matrix-modifying factors. Naturally, in places where WAT contacts other dynamic tissues, including skin, mammary glands and bone marrow, signalling symbiosis becomes possible. Perhaps, like nowhere else in the body, this relationship evolved in the skin between dermal WAT (dWAT)25 and neighbouring cell populations, both during physiological hair growth-coupled remodelling and in repair processes, such as after infection and wounding.
Like other major WAT depots, dWAT performs several systemic functions (also known as metabolic functions). dWAT efficiently stores and releases lipids following changes in nutrient availability26, secretes several adipokines27–29, and adaptively thermo-insulates the body in response to external temperature changes30. Within the last decade, researchers have begun to unravel the tissue-specific, or the non-metabolic functions of dWAT in skin. The purpose of this Review is to summarize the novel developments in the dWAT field. We start by defining the place of dWAT in the context of skin anatomy. We then discuss the effects of dWAT on periodic hair growth, and the reciprocal effects of hair follicles on non-metabolic dWAT cycling. We discuss progenitor sources for cyclic dWAT remodelling, and how unique aspects of dWAT anatomy and physiology enable high-precision experimental inquiries into adipose development and signalling regulation. We conclude by addressing the emerging functions of dWAT in skin protection, including its role in antimicrobial defence, wound healing, and regeneration.
Anatomy of skin
Location of skin fat
Skin is comprised of several distinct layers and contains an array of ectodermal appendages. Epidermis forms the outermost layer and gives skin its barrier function. It rests on top of a layer of dermis, which is rich in fibroblasts and collagens. The latter sub-divides into thin, upper papillary dermis and thicker, lower reticular dermis1,2. Underlying and partially integrating into the reticular dermis is the dWAT3–6. In laboratory rodents, including mice and rats, dWAT forms a continuous layer that is 2 to 15 cells thick 7 demarcated from deeper subcutaneous fat by a sheet-like panniculus carnosus skeletal muscle layer5 (Fig. 1a). In other species, however, the microanatomy of dWAT can differ substantially. For instance, in the skin of naked mole-rats, dermal adipocytes exist only in isolated groups 8. In rabbit skin, dermal adipocytes are arranged into discontinuous clusters exclusively around hair follicles9 (Fig. 1b). In human skin, dWAT exists in a histologically distinct layer; however, because panniculus carnosus muscle is rudimental in humans, dWAT is in contact with subcutaneous fat3,10 (Fig. 1c). Furthermore, dWAT can substantially differ even in the same species between distinct body sites11, and this becomes accentuated in species with highly specialized skin. For instance, in bats, dermal adipocytes are prominent in dorsal and ventral skin, but are largely unidentifiable in the web-like wing skin12.
Figure 1. Distribution of dermal white adipose tissue (dWAT) in the skin.
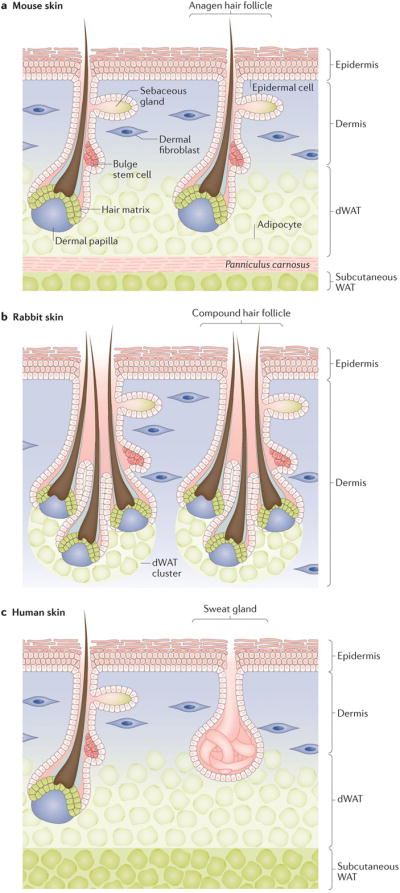
(a) In mice, dermal adipocytes form a continuous dWAT layer separated from subcutaneous WAT by sheet-like skeletal muscle (pink)5. Growing hair follicles are in close contact with dermal, but not subcutaneous, adipocytes. The key skin compartments (epidermis, dermis, dWAT, panniculus carnosus and subcutaneous WAT) are labelled. (b) In rabbit skin, multiple hair follicles form bundle-like compound units. Rabbit dWAT is discontinuous and clusters exclusively around hair follicle units9. (c) Human skin has a prominent dWAT layer and lacks a clear separation from subcutaneous WAT due to rudimental skeletal muscle3,10. Anagen hair follicles closely associate with dermal adipocytes. Sweat glands, common in humans throughout the skin, reside in dermis, and can contact the interface of dWAT32.
Hair follicles
Traversing through the skin are its ectodermal appendages – hair follicles and sweat glands. Hair follicles are stem cell-rich mini-organs that regenerate new hairs repetitively in a process known as the hair growth cycle. This regenerative cycle consists of three phases: active hair growth (anagen), regression (catagen), and rest (telogen)13,14. The hair follicle attains its largest size during anagen, when its proximal end, the hair bulb, extends deep into dWAT.
The hair bulb contains actively dividing epithelial matrix progenitors and specialized dermal papilla fibroblasts, which serve as the key signalling centre of the hair follicle15. Hair growth is sustained by proliferation and differentiation activities taking place in the hair matrix. Distally, above the dWAT, the hair follicle houses its stem cells16–19, including the so-called bulge stem cells — the principal hair-fated, long-lasting progenitors18,20. Above the bulge, the hair follicle contains an oil-producing sebaceous gland. Connecting the bulge with the bulb is the outer root sheath, which has a cylindrical shape.
Hair growth termination during catagen is mediated by events of terminal differentiation, apoptosis, and phagocytosis21–23. Dermal papilla fibroblasts and some epithelial outer root sheath cells survive catagen involution, move upwards toward the bulge, and constitute the lower portion of the resting telogen hair follicle. Surviving outer root sheath cells form the secondary hair germ compartment. At the onset of new anagen, secondary hair germ progenitors respond to activating signals from dermal papilla, divide, and fuel the rapid growth of the hair follicle24,25. Bulge progenitors divide with a delay and contribute progenies toward mature hair follicles in anagen 18,24.
The cellular dynamics described above occur in cycles, allowing each hair follicle to grow multiple rounds of hairs. Furthermore, in many species, thousands of neighbouring hair follicles regenerate collectively as dynamic hair growth waves9,11,26–29, therefore, their interactions with dWAT occur at the collective level. In addition to hair follicles, skin contains sweat glands consisting of a straight duct and secretory coil nested in the dermis30,31 and, sometimes, abuts dWAT32. While in humans sweat glands are distributed widely throughout the skin, in many other species, including mice, they are restricted to the paws 33,34.
Dermal adipocytes and hair follicles
One of the most distinguishing features of dWAT is its ability to periodically remodel in coordination with the hair cycle. This process, which has been described in the classic literature35–40, involves prominent dWAT thickening around anagen hair follicles and the subsequent thinning when hair follicles transition via catagen to telogen6,41.
The early stages of hair follicle and skin fat development seem to proceed independently from one another. In mouse skin, adipose precursors first appear on embryonic day (E) 16.542; at this stage only 3% of all hair follicles are partially matured43 (Fig. 2a). Uncoupling between early hair follicle development and adipose tissue development is supported by the fact that adipose progenitors form in the skin of mutant mice that have suppressed hair follicle morphogenesis41. However, adipose progenitors become coupled with and dependent on hair follicle signalling during the maturation phase. The first multilocular adipocytes appear between E17.5–18.5 around early anagen hair follicles, and their number and size quickly increase 4,41,44,45 (Fig. 2b) until around postnatal day (P) 12, when hair follicles are in full anagen4 (Fig. 2c). This process becomes largely disrupted when hair follicles are genetically converted into abnormal cysts41 or when sonic hedgehog (Shh), an important signalling factor for dWAT development, is deleted from hair follicles45.
Figure 2. Development and cyclic remodelling of dWAT.
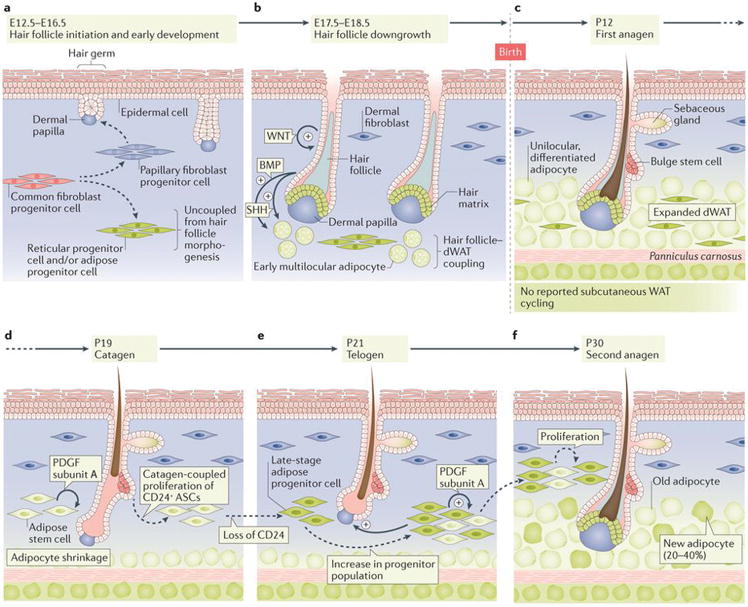
(a) In mice, the initial development of hair follicles and dWAT, which occurs between embryonic days (E) 12.5 to E16.5, are largely independent from one another41,42. Reticular progenitor cells and adipose progenitor cells arise from common fibroblast progenitors. Hair follicles develop at the interaction sites between embryonic epidermis and papillary fibroblast progenitor cells. (b) During E17.5 to E18.5, development of hair follicles and dWAT are coupled4,41,44,45,110. Enlarged hair follicles activate several pro-adipogenic signalling pathways, including Hedgehog45 and Bmp48. In response to hair follicle-derived signals, adipose progenitors rapidly differentiate. Small multilocular adipocytes appear first and then mature into large unilocular cells over several days. (c) dWAT peaks in thickness by postnatal day (P) 12, which is when anagen hair follicles fully develop4. dWAT contains differentiated adipocytes and adipocyte progenitor cells. (d, e) Between P19 to 21, hair follicles undergo regression into telogen stage. Concomitantly, dWAT collapses, partially through cell lipolysis to less than half of its anagen size4,41. At the same time, adipose stem cells (ASCs) expand via proliferation and give rise to late stage adipose progenitor cells (green)6. Autocrine Pdgfα mediates the expansion of adipose progenitor cells46. (f) During second anagen, up to 40% of adipocytes in enlarged dWAT are new, derived from expanded progenitors45.
When fully developed at P12, dWAT has an intricate microanatomy with adipocytes distributing under and around hair follicles in anagen phase so that they appear to be floating in an adipose cushion. Intriguingly, on P17, dWAT starts to collapse as hair follicles regress, and by P19–23, when hair follicles first enter telogen, it reduces to less than half the size it is during anagen4,41 (Fig. 2d, 2e). First telogen marks both the completion of hair follicle morphogenesis and the onset of its cyclic regeneration. A new nagen phase starts around P22–24, and this event is coupled with an increase in dWAT volume41,45,46 (Fig. 2f). Cyclic fluctuations in the volume of dWAT continue into adulthood, but skinny periods, which coincide with telogen of the hair cycle lengthen in duration as telogen phase itself in adult mice lengthens – often lasting several months29,47.
Cyclic remodelling of dWAT
The cyclic remodelling of dWAT raises several intriguing questions, answers to which could enrich our understanding of general adipose tissue biology. First, what are the cellular and signalling bases for cyclic dWAT remodelling? Does catagen-coupled reduction in adipose tissue volume involve emptying of mature adipocytes, their elimination by apoptosis, or a combination of both? On the other hand, is anagen-coupled dWAT expansion primarily achieved via hypertrophy of pre-existing preadipocytes or hyperplasia from early-stage progenitors? In terms of signalling, what are the hair follicle-derived regulators of dWAT remodelling, both during anagen and catagen? Second, is dWAT heterogeneous, with only some adipocytes being sensitive to hair cycle changes? For example, lipid-filled adipocytes persist in telogen skin, even in old mice, when the telogen phase becomes extended, although the number of adipocytes that do persist is low 28,47. Third, what are the reciprocal signalling effects of dWAT on hair cycle, and is hair follicle or dWAT the dominant driver of their coupled remodelling events? Below we review our current understanding of the above questions regarding cyclic dWAT remodelling and outline knowledge gaps.
Cellular and signalling bases
The formation of the dermal adipose lineage seems to rely on the same key transcriptional regulators that operate in other WAT depots. Adipogenic lineage specification in skin, at least in part, depends on Zfp423 activity48,49. Both Cebpα44 and Pparγ41 are expressed during dWAT development, which Pparγ regulates. The deletion of Pparγ under adipose-specific Adipoq-Cre driver results in the systemic lipoatrophy, including in dermal fat50. Temporally, adipogenic specification of skin fibroblast progenitors begins at E16.542. Committed progenitors then mature into Cebpα-positive preadipocytes44 and further mature into lipid-laden adipocytes. These processes continue until ~ P12, when dWAT thickness peaks4,41,44,45. Next, as hair follicles enter telogen, dWAT rapidly reduces in size by ~ 50%. Intriguingly, this decrease is not associated with substantial apoptosis4, but rather with cell size reduction6, suggesting that partial lipolysis is the underlying mechanism (Fig. 2d). As such, lipolysis during the early postnatal period, at the time of rapid body growth, is not coupled to systemic nutrient shortage and is a unique feature of dWAT. Instead, lipolysis is coupled with the catagen phase, indicating that either regressing hair follicles secrete factors that trigger lipolysis, or that they cease to secrete anti-lipolysis factors, which are abundant during anagen4. One such anti-lipolysis candidate factor is Shh, whose expression shuts off in catagen hair follicles, while in anagen it is abundant and signals to activate Pparγ45 (Fig. 3).
Figure 3. Signalling crosstalk between dWAT and hair follicles in mice.
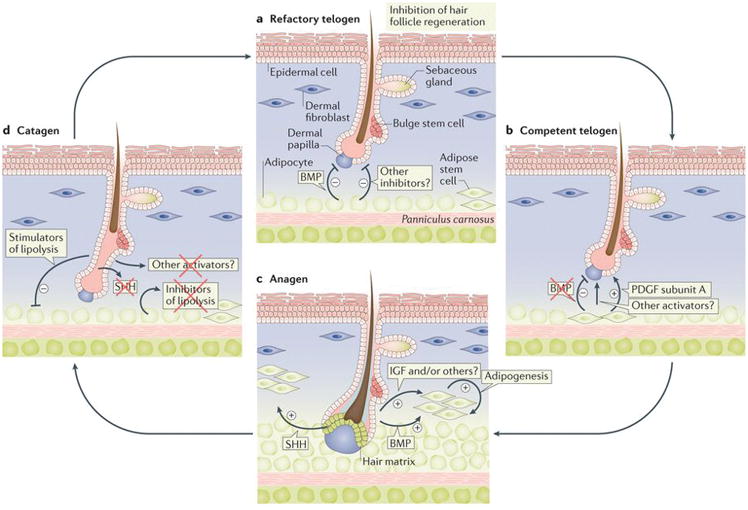
(a) During early, refractory telogen, dermal adipocytes signal to inhibit anagen entry by hair follicles. This inhibitory effect is in part mediated by dermal adipocyte-derived Bmp ligands28. (b) During late, competent telogen, dermal adipocytes reduce Bmp ligand production. Reduction in hair cycle inhibitors enables hair follicles to easily respond to other growth-activating stimuli; hence they become competent11,26,28. At the same time, adipose progenitors secrete Pdgfα, which has hair cycle-activating effects6. (c) During anagen, enlarged hair follicles secrete ligands for multiple pro-adipogenic signalling pathways, including Bmp48 and Hedgehog45. These ligands act on dermal preadipocytes to stimulate their differentiation into lipid-laden adipocytes. Other putative signals from hair follicles, not indicated here, could also induce adipogenesis. (d) During catagen, dWAT reduces in size, mediated, in part, by lipolysis. The signalling mechanism of dWAT reduction is not fully understood, but it probably involves the loss of HF-derived adipogenic activators and/or production of additional anti-adipogenic factors.
The subsequent expansion of dWAT during a new hair cycle is complex, and involves both hypertrophy45 and hyperplasia6,46. The first wave of hyperplasia occurs before anagen onset, during the preceding catagen, when Cd24+ ASCs transiently proliferate6. The fact that hyperplasia occurs before the onset of anagen, suggests that the triggers for ASC hyperplasia are secreted either by the regressing hair follicles or by the surrounding mature adipocytes that undergo lipolysis. Once triggered, hyperplasia is driven by Pdgfα, which is secreted by Cd24+ ASCs and their progenies, Cd24-negative preadipocytes46. The outcome of ASC hyperplasia is the enrichment of dWAT for differentiation-ready preadipocytes ahead of the new hair cycle. Interestingly, cellular dynamics in dWAT parallel these in the hair follicles, where a new population of ‘primed’ secondary hair germ progenitors is also set aside for a new hair cycle during the preceding catagen phase24,25. The primed progenitors in dWAT and hair follicles might serve to sensitize skin to various regeneration stimuli.
The expansion of dWAT is tightly coupled to and follows the enlargement of anagen hair follicles45. When early anagen hair follicles grow into the dWAT layer, new lipid-laden adipocytes start to rapidly appear around them45,46. Driving this process are the progenitor cells, either actively forming via proliferation45 or those that were set aside during the preceding catagen46. By the time dWAT has fully expanded in mid-anagen, it comprises 20–40% of newly formed adipocytes, with the rest of cells being the pre-existing adipocytes45 (Fig. 2f). Furthermore, contributing to dWAT expansion is hypertrophy of newly formed and pre-existing adipocytes.
It remains unclear which hair follicle-derived signals induce the rapid expansion of dWAT. Anagen hair follicles secrete ligands and antagonists for multiple signalling pathways with known roles in adipogenesis, including Wnt51–53, Bmp54–57, Tgfβ58–60, Fgf61,62, Igf63,64, Pdgf65,66, and Hedgehog67,68. Considering this, hair follicle to dWAT communication can occur via multiple channels; Hedgehog signalling is one such channel (Fig. 3c). Anagen hair follicles secrete Shh ligands that drive the proliferation of adipose precursors and their terminal differentiation, in part via Pparγ activation45. dWAT expansion largely stops when Shh is genetically deleted from the hair follicle epithelium, or when adipose precursors are made insensitive to Shh following the precursor-specific deletion of Smoothened45 Moreover, skin-specific overexpression of Shh causes excessive dermal fat expansion to result in dWAT of nearly double the normal size45. Interestingly, the pro-adipogenic effect of Shh in dWAT contrasts with its inhibitory effect in other, non-skin adipose depots69–72.
Bmp signalling is another hair follicle to fat communication channel and anagen hair follicles secrete multiple Bmp ligands57,65. In non-skin fat, Bmps commit mesenchymal cells to the adipose lineage and promote their terminal differentiation via activation of the transcriptional regulator Zfp42349,73,74. In parallel, Bmp-Zfp423 signalling is necessary for adipogenesis in skin, both under normal conditions and following wounding (Fig. 4). Genetic ablation of Zfp423 or Bmp responsiveness in wound fibroblasts in mice in vivo prevents adipocyte regeneration around hair follicles, while Zfp423 deletion in normal skin causes noticeably thinner dWAT and smaller adipocytes48.
Figure 4. Role of dWAT in skin defence and regeneration in mice.
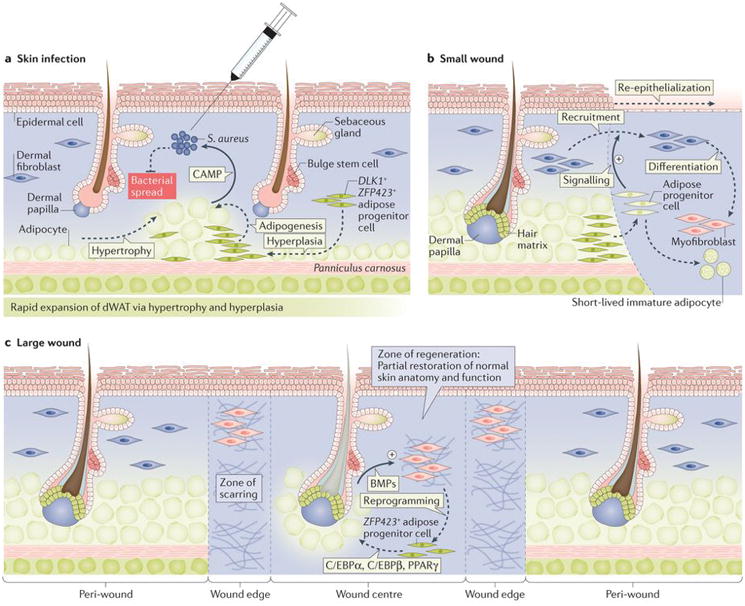
(a) Upon skin infection with Staphylococcus aureus, dWAT expands via hypertrophy and hyperplasia, irrespective of hair cycle stage. Responding to bacterial presence, expanded adipocytes secrete cathelicidin antimicrobial peptide (Camp), which has direct bacterial killing activity95. (b) Upon small excisional wounding, dWAT progenitors migrate from the wound edge into the wound bed and stimulate recruitment of fibroblasts, which are tasked with normal scar tissue formation89. (c) In large excisional wounds, new adipocytes regenerate de novo from non-adipogenic myofibroblasts. This process is driven by Bmp signals secreted by neogenic hair follicles in the wound centre. Bmps induce adipogenic lineage commitment of myofibroblasts, a process that depends on Zfp423 activation48.
Canonical Wnt signalling is another important regulator of dermal adipogenesis. Anagen hair follicles both respond to52,75 and modulate Wnt signalling via secreted ligands and antagonists51–53. The direct effect of canonical Wnt signalling is largely inhibitory in non-skin WAT76–79, and this holds true for dermal fat48,80. Dramatic dWAT loss is observed in mice whose dermal cells overexpress constitutive stabilized β-catenin80. Consistently, adipocytes fail to regenerate around anagen hair follicles in wounds of mice overexpressing canonical Wnt7a ligand48. Curiously, dWAT expands during anagen, when Wnt ligand secretion and activity peak in hair follicles. This suggests that anagen hair follicles can either balance their Wnt signalling outputs into the surrounding skin via secreted antagonists and/or override inhibitory Wnt activities via other signalling channels. Considering that the diffusion range for Wnt antagonists is longer than that for ligands, at least during embryonic skin development81, it is plausible that adipocytes are primarily exposed to hair follicle-secreted Wnt antagonists. Furthermore, canonical Wnt signalling exerts indirect pro-adipogenic effects via epithelial skin cells41,82.
The genetic overexpression of stabilized β-catenin in embryonic epidermis results in dramatically increased dermal adipogenesis even though hair follicle development is blocked. This indirect effect is mediated by several downstream factors, including Igf and Bmp ligands, that are secreted by Wnt-active epidermal cells41. The pro-adipogenic effect of Bmp signalling is further evident from skin wound healing study. Regeneration of adipocytes is largely prevented around anagen hair follicles in wounds of mice that overexpress the soluble Bmp antagonist Noggin48.
Importantly, systemic factors can override hair cycle-driven dWAT remodelling. For example, dWAT notably expands in size in obese db/db mice, even during telogen83. Moreover, dWAT becomes 80% thinner in mice housed under thermo-neutral conditions (31°C) compared with mice housed in typical laboratory conditions (21°C), which causes mild thermal stress7.
Heterogeneity of dermal adipocytes
Emerging evidence suggests that diversity exists between dermal adipocytes on several levels. First, there is chronologic heterogeneity. Once dWAT is mature, which occurs during the postnatal hair cycle, it is composed of two subsets of adipocytes — one subset of these adipocytes are those that are formed during skin morphogenesis and the other subset are adipocytes generated during anagen phase of hair cycle in adult mice 45,46. Second, heterogeneity exists between adipocytes with regards to their sensitivity to catagen. Although the total number of adipocytes substantially declines between anagen and telogen, mature adipocytes persist throughout the hair cycle4,6,45. The fact that some mature adipocytes remain throughout the hair cycle implies that these adipocytes are protected from catagen-driven lipolysis. A third type of adipocyte heterogeneity occurs at the spatial level. For instance, adipocytes are distributed along the length of the hair follicle; therefore, adipocytes located near to the hair bulb have a distinct spatial location compared with adipocytes located closer to the bulge stem cells. This difference in location means that adipocytes might be exposed to distinct signalling ‘experiences’ depending on the follicular compartment that they contact. For example, Shh production is restricted to the base of anagen hair follicle, where matrix cells reside45. Therefore, only the lowermost adipocytes will experience high levels of Shh. Finally, thickness of dWAT is heterogeneous between the skin regions. dWAT is thicker in the ventral skin than in the dorsal and very thin and discontinuous in the ear skin11.
Signalling effects of dWAT
WAT and hair follicles share a number of common pathways, therefore paracrine factors produced by dWAT can modulate activities linked to hair follicle growth. Mouse hair follicles cycle collectively, which requires signalling coordination between neighbouring hair follicles and non-hair cell populations26,27,29. Messages between hair follicles are commonly activating. Early anagen hair follicles signal to adjacent telogen hair follicles and stimulate them into growth (anagen). In turn, newly activated anagen hair follicles signal to their respective neighbouring telogen hair follicles and this wave-like activation spreads across the skin. This common activation process is, in part, mediated by Wnt signals9. However, if a wave reaches early telogen hair follicles that have recently completed their previous growth cycle, it stops and a sharp anagen–telogen boundary forms instead. This property of early telogen hair follicles is called telogen refractivity and it is, in part, mediated by Bmp2 produced by neighbouring adipocytes28. Bmp signalling maintains hair follicle stem cells in a quiescence state. Large regions of dWAT express Bmp2, therefore, many thousands of hair follicles can be made refractory all at the same time (Fig. 3a). By preventing early telogen hair follicles from re-entering anagen, this mechanism prevents hair overproduction on the skin.
While mature adipocytes signal to inhibit the hair cycle, adipose progenitors signal to stimulate it5. Hair follicle entry to anagen stalls when dWAT progenitors are depleted, either genetically in Ebf1 mutant mice, lacking a transcriptional factor that regulates normal WAT development84, or pharmacologically with Pparγ antagonists6. Transplantation of adipose progenitors into the skin induces precocious anagen in normal mice and rescues hair cycle defect in Ebf1 mutants6. Pdgfα, which is strongly expressed by adipose progenitors, might mediate their activating effect on resting hair follicles (Fig. 3b). Indeed, Pdgf signalling is necessary for normal hair growth66,85 and Pdgfα-coated beads alone can reactivate anagen in Ebf1 mutant skin6. The paracrine effect of dWAT on the surrounding stem cell niches parallels observations in the bone marrow, where adipocytes signal to haematopoietic progenitors to inhibit their regenerative activities86–88.
Taken together, the following principles underlie signalling symbiosis between hair follicles and dWAT. First, signalling crosstalk is possible because both tissues rely on the same pathways for their respective cellular activities5. Second, growing hair follicles enrich the signalling milieu of dWAT both because they form secretory hotspots and because adipocytes and their progenitors reside within the diffusion range for many soluble ligands and antagonists26,41,45,48. Conversely, dWAT enriches a signalling background in which hair follicles function, affecting their hair cycle decision-making6,28. Third, the growth-promoting signalling activities in anagen hair follicles also promote growth activities in dWAT, positively stimulating both hyperplasia and hypertrophy. In turn, changes in signalling activities in catagen hair follicles are such that they also drive dWAT regression, in part by stimulating lipolysis. On the other hand, due to the location of dWAT, dWAT signalling activities preferentially modulate growth versus quiescence decision making by telogen hair follicles. Working together, periodic signalling activities during hair cycle result in entanglement between growth and regression bouts of hair follicles and dWAT. Multiple pathways, beyond Bmp, Wnt, Pdgf, and Hedgehog might contribute to this signalling symbiosis; novel mutual regulations of hair follicle and dWAT cycling could to be identified in the near future.
Studying adipose lineage development
The close spatiotemporal coupling between hair follicles and dWAT during development, the fine microanatomy of dermal fat and a multitude of skin-specific genetic tools make dermal fat a useful model system for studying adipose lineage development. Morphologically and on gene expression, dermal adipocytes can be characterized as stereotypical white adipocytes. Indeed, when fully differentiated they have unilocular appearance4,45,46 and activate characteristic adipokine reporters for Adiponectin45,46,48 and Resistin48. However, in contrast to other WAT depots, dWAT morphogenesis is tightly controlled — in mice, adipose progenitors specify on E16.542 and, within days, they progress toward early adipocytes4,41,45, which then fully mature during the first two weeks after birth4. This tight control enables researchers to study the distinct cellular and signalling events in dermal adipose lineage with high temporal resolution. The fine microarchitecture of dWAT further enables investigators to precisely measure and detect the subtlest phenotypes presented as changes in layer thickness6,41,45,46,80,82. The level of phenotypic precision that dWAT affords researchers is not possible in other fat depots.
Several skin specific markers further aid experimental inquiries into dWAT lineage. They enable lineage tracing42,45,80,82 and the sorting of adipose progenitors6,45,46,80,82,89. During embryonic period, early mouse adipose precursors can be labelled with Dlk1–CreER42, while in adult mouse skin, a large portion of adipose progenitors can be marked using Pdgfrα–CreER45. A marker set, Linneg/Cd34+/Cd29+/Sca1+/Cd24+, originally developed for visceral and subcutaneous ASCs90,91, can also be used to sort dermal adipose progenitors for quantification and gene expression profiling6,46. Similar to visceral fat92, Cd24+ skin progenitors represent early ASCs necessary for long-term maintenance of dWAT46.
The close reliance of dWAT on signalling inputs from hair follicles for hyperplasia, hypertrophy and regression, and an array of hair-specific genetic tools provides a unique opportunity to study the role of regulatory pathways in fat biology. Keratin gene-based promoter drivers and Cre lines can be used to either delete or overexpress various ligands and antagonists in hair follicles and examine their effects on dWAT. Examples of mouse models with prominent dWAT phenotypes include epithelial-specific deletion of Shh under K15–CrePGR45, overexpression of stabilized β-catenin under K14–Cre41 and overexpression of Wnt7a and Bmp antagonist Noggin under K14 promoter48.
Another important tool for studying dermal adipogenesis is the model for wound-induced skin neogenesis93. In this model, large skin wounds in adult mice heal by regenerating new hair follicles in their center94 followed by new adipocytes surrounding such new hair follicles 48. Fat regeneration in wounds follows a precise timeline, and it depends on hair-derived signals. Adipose progenitors in the wound originate from contractile myofibroblasts, which can be labelled and targeted for gene deletion using contractile-specific Cre lines, such as Sma–CreER. Additionally, because all regenerated hair follicles and adipocytes in the wound can be quantified upon whole mount evaluation, adipose regeneration phenotypes can be sensitively calculated as adipocyte-to-hair follicle ratio 48. These features of the wound-induced neogenesis model enable study of the entire process of adipose lineage formation in adult animals with the resolution level that parallels that during embryonic skin development.
Defence functions of dermal fat
Residing near the interface with the outside environment, dWAT serves additional functions, such as aiding in skin defence after injury and infection89,95. Fllowing skin wounding, resident adipose progenitors activate near the wound edges and transiently migrate into the wound bed, where they differentiate. Once in the wound, they signal to facilitate efficient recruitment of fibroblasts, the building blocks of scar tissue89 (Fig. 4). Consistent with these findings, in Azip mice, which lack mature adipocytes, or in mice after treatment with Pparγ inhibitor, scars become weak and wounds can spontaneously reopen89. Dermal adipose progenitors can also directly contribute to scarring in mice, at least in the model of bleomycin (a DNA damaging agent) induced fibrosis. Following treatment with bleomycin, excessive skin scarring is accompanied by the loss of adipocytes and conversion of adipose progenitors into myofibroblasts96. In analogy with these observations, researchers have proposed that dWAT might contribute to skin fibrosis via the so-called adipocyte-to-myofibroblast transition mechanism during skin aging, including UV-induced aging97 and male pattern baldness98.
Bacterial skin infection also results in the activation of adipose progenitors and a rapid expansion of dWAT, which is mediated by hypertrophy and hyperplasia. Expanding adipocytes have high expression levels of cathelicidin antimicrobial peptide (Camp), which has direct bacterial killing activity95 (Fig. 4a). Adipocytes from Camp deficient mice lack antimicrobial activity and infection-fighting properties of the skin become largely compromised in Zfp423 mutants with deficient adipogenesis or after treatment with a Pparγ inhibitor95. Indeed, unlike control animals, the same dose of Staphylococcus aureus results in systemic bacteraemia in Zfp423 mutant mice95. Given the newly recognized importance of adipocytes in innate immune skin defence, the ability of large skin wounds to regenerate new fat48 is particularly important, as fat cells can endow scars with an extra layer of protection (Fig. 4c).
Future directions
Recognition of the non-metabolic functions of skin fat is rapidly growing99–102. Indeed, dWAT has emerged as an important signalling regulator of skin regeneration, both during the normal hair cycle6,28 and in response to wounding89. With similar function already noted in the bone marrow86–88, adipocytes and their progenitors could also be important modulators of regeneration in other organs. Fat-derived signals have also been implicated in abnormal tissue growth, such as cancer. Indeed, these so-called ‘cancer-associated adipocytes’ were shown to exert pro-oncogenic effects by supporting tumour metabolism, and creating favourable signalling and extracellular niche microenvironment103,104. The hair follicle-dWAT symbiosis paradigm provides a fruitful model for studying the mechanisms of adipose tissue interaction with other tissues types.
Cellular plasticity of adipose lineage following injury, disease, and ageing is another area of research that is undergoing rapid growth. Depending on the type of injury, adipose progenitors can either convert into scar-forming cells96 or regenerate anew from non-adipogenic scar myofibroblasts48. Although a better understanding of cellular lineages at the sites of skin injury is acutely needed105, these observations are important as they demonstrate that both adipogenic and non-adipogenic skin cells can be modulated to reduce and even reverse scarring. Anti-scarring strategies learned from the skin system will ultimately be applicable in other organs.
A unique aspect of hair follicle and dWAT ageing is its cyclic nature. Each bout of regeneration is associated with the use of a stem cell reserve, both in the hair follicles106 and dWAT46. Indeed, dWAT size and its Cd24+ ASCs become depleted with hair cycles and this ageing process accelerates when supernumerary hair cycles are induced by hair plucking46. Advanced stages of skin ageing are associated with prominent slowing of hair cycling47, permanent loss of at least some hair follicles107, and changes in the expression of hair cycle regulators by dWAT108. Future studies on skin ageing will probably yield new rejuvenation approaches centred around dermal adipose.
Lastly, the antimicrobial activities of dermal adipocytes95 point toward novel approaches to skin infection management. Clinically, the incidence of wound infections is high, and in certain instances, such as diabetic foot ulcers, they can be life threatening. Enhancing adipose regeneration either from endogenous progenitors or with the aid of autologous fat grafts can represent a novel approach in wound infection management. Intriguingly, animal species with highly developed blubber, such as dolphins, display profoundly enhanced infection fighting and wound healing abilities109. Exploring the innate immune biology of adipose tissue, including that in aquatic mammals, might lead to novel breakthroughs in fat-centred antibacterial strategies.
Key Points.
Dermal white adipose tissue (dWAT) is a specialized adipose depot of the skin with activities that are closely linked with those of hair follicles.
The development and remodelling of dWAT relies largely on paracrine signals generated by growing hair follicles.
Dermal adipocytes cyclically grow and shrink their lipid droplets in response to hair-derived signals and systemic factors, such as cold stress, can override this cycle.
Dermal adipocytes and their progenitors actively signal to neighbouring skin cell types, including hair follicle cells, and become important modulators of their growth activities.
Dermal adipogenesis is activated in response to bacterial skin infection, activates antimicrobial peptide secretion and serves an innate immune defence function.
Review criteria.
This review cites references identified using the following keywords: “adipose tissue”, “skin”, “hair follicle”, “wound”, “regeneration”, “infection”, “stem cells”, “signalling factor”. Additional relevant references were identified via forward citation analysis. The authors also used their own knowledge of the literature on skin fat, hair follicles and wound healing.
Acknowledgments
MVP is supported by the NIH NIAMS grants R01-AR067273, R01-AR069653, and Pew Charitable Trust grant. CFGJ is supported by NSF-GRFP (DGE-1321846), MBRS-IMSD training grant (GM055246) and Howard A. Schneiderman Graduate Fellowship Award.
Glossary terms
- Panniculus carnosus
a layer of striated muscle below dermal adipose tissue
Biographies
Christian F. Guerrero-Juarez obtained a dual B.S./B.A. degree in Biology and Biochemistry, respectively, from California State University, San Bernardino, California, USA. He is currently a graduate student at the University of California, Irvine in Irvine, California, USA. His work focuses on the mechanism of dermal fat regeneration in skin wounds.
Maksim V. Plikus obtained his Ph.D. in Pathology from the University of Southern California in Los Angeles, California, USA in the laboratory of Dr. Cheng-Ming Chuong, where he studied regulation of hair follicle regeneration. He trained as a postdoctoral fellow with Dr. George Cotsarelis at the University of Pennsylvania in Philadelphia, Pennsylvania, USA, where he studied regeneration of dermal adipocytes after wounding. He is currently an assistant professor in the Department of Developmental and Cell Biology at the University of California, Irvine in Irvine, California, USA. His laboratory investigates mechanisms of hair follicle and dermal adipose development and regeneration.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author contributions
C. F. G-J. and M. V. P. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the article before submission.
References
- 1.Harper RA, Grove G. Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science. 1979;204:526–527. doi: 10.1126/science.432659. [DOI] [PubMed] [Google Scholar]
- 2.Woodley DT. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol Clin. 2017;35:95–100. doi: 10.1016/j.det.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CA. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One. 2013;8:e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt B, Horsley V. Unravelling hair follicle-adipocyte communication. Exp Dermatol. 2012;21:827–830. doi: 10.1111/exd.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasza I, et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10:e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly TJ, Buffenstein R. Skin morphology and its role in thermoregulation in mole-rats, Heterocephalus glaber and Cryptomys hottentotus. J Anat. 1998;193( Pt 4):495–502. doi: 10.1046/j.1469-7580.1998.19340495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plikus MV, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker GE, et al. Subcutaneous abdominal adipose tissue subcompartments: potential role in rosiglitazone effects. Obesity (Silver Spring) 2008;16:1983–1991. doi: 10.1038/oby.2008.326. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, et al. A multi-scale model for hair follicles reveals heterogeneous domains driving rapid spatiotemporal hair growth patterning. Elife. 2017;6 doi: 10.7554/eLife.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madej JP, et al. Skin structure and hair morphology of different body parts in the Common Pipistrelle (Pipistrellus pipistrellus) Acta Zoologica. 2013;94:478–489. doi: 10.1111/j.1463-6395.2012.00578.x. [DOI] [Google Scholar]
- 13.Oh JW, et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J Invest Dermatol. 2016;136:34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller-Rover S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. The Journal of investigative dermatology. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4:a015180. doi: 10.1101/cshperspect.a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen KB, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 19.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 20.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 21.Mesa KR, et al. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foitzik K, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 23.Lindner G, et al. Analysis of apoptosis during hair follicle regression (catagen) Am J Pathol. 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- 26.Plikus MV, Chuong CM. Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harb Perspect Med. 2014;4:a015198. doi: 10.1101/cshperspect.a015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–868. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu CP, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobitz WC, Jr, Dobson RL. Dermatology: the eccrine sweat glands. Annu Rev Med. 1961;12:289–298. doi: 10.1146/annurev.me.12.020161.001445. [DOI] [PubMed] [Google Scholar]
- 32.Kimani JK. Some histological aspects of the palmar digital pads in the vervet monkey. Folia Primatol (Basel) 1983;41:147–155. doi: 10.1159/000156125. [DOI] [PubMed] [Google Scholar]
- 33.Lu CP, Polak L, Keyes BE, Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354 doi: 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montagna W. Some particularities of human skin and the skin of nonhuman primates. G Ital Dermatol Venereol. 1984;119:1–4. [PubMed] [Google Scholar]
- 35.Butcher EO. The hair cycles in the albino rat. The Anatomical Record. 1934;61:5–19. doi: 10.1002/ar.1090610103. [DOI] [Google Scholar]
- 36.Hansen LS, Coggle JE, Wells J, Charles MW. The influence of the hair cycle on the thickness of mouse skin. Anat Rec. 1984;210:569–573. doi: 10.1002/ar.1092100404. [DOI] [PubMed] [Google Scholar]
- 37.Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116:75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- 38.Moffat GH. The growth of hair follicles and its relation to the adjacent dermal structures. J Anat. 1968;102:527–540. [PMC free article] [PubMed] [Google Scholar]
- 39.Borodach GN, Montagna W. Fat in skin of the mouse during cycles of hair growth. J Invest Dermatol. 1956;26:229–232. doi: 10.1038/jid.1956.32. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs HF. A study of the post-natal development of the skin and hair of the mouse. The Anatomical Record. 1941;80:61–81. doi: 10.1002/ar.1090800108. [DOI] [Google Scholar]
- 41.Donati G, et al. Epidermal Wnt/beta-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1501–1509. doi: 10.1073/pnas.1312880111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 44.Wojciechowicz K, Markiewicz E, Jahoda CA. C/EBPalpha identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol. 2008;17:675–680. doi: 10.1111/j.1600-0625.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B, et al. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes & development. 2016;30:2325–2338. doi: 10.1101/gad.285429.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera-Gonzalez GC, et al. Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Cell Stem Cell. 2016;19:738–751. doi: 10.1016/j.stem.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CC, et al. Regenerative hair waves in aging mice and extra-follicular modulators follistatin, dkk1, and sfrp4. J Invest Dermatol. 2014;134:2086–2096. doi: 10.1038/jid.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plikus MV, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta RK, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandyba E, Kobielak K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells. 2014;32:886–901. doi: 10.1002/stem.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy S, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 54.Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- 57.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paus R, Foitzik K, Welker P, Bulfone-Paus S, Eichmuller S. Transforming growth factor-beta receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol. 1997;109:518–526. doi: 10.1111/1523-1747.ep12336635. [DOI] [PubMed] [Google Scholar]
- 59.Welker P, Foitzik K, Bulfone-Paus S, Henz BM, Paus R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor beta 1 and beta 3 in murine skin. Arch Dermatol Res. 1997;289:554–557. doi: 10.1007/s004030050239. [DOI] [PubMed] [Google Scholar]
- 60.Wollina U, Lange D, Funa K, Paus R. Expression of transforming growth factor beta isoforms and their receptors during hair growth phases in mice. Histol Histopathol. 1996;11:431–436. [PubMed] [Google Scholar]
- 61.Kawano M, et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124:877–885. doi: 10.1111/j.0022-202X.2005.23693.x. [DOI] [PubMed] [Google Scholar]
- 62.Rosenquist TA, Martin GR. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 63.Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- 64.Rudman SM, Philpott MP, Thomas GA, Kealey T. The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol. 1997;109:770–777. doi: 10.1111/1523-1747.ep12340934. [DOI] [PubMed] [Google Scholar]
- 65.Rezza A, et al. Signaling Networks among Stem Cell Precursors, Transit-Amplifying Progenitors, and their Niche in Developing Hair Follicles. Cell Rep. 2016;14:3001–3018. doi: 10.1016/j.celrep.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomita Y, Akiyama M, Shimizu H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J Dermatol Sci. 2006;43:105–115. doi: 10.1016/j.jdermsci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 68.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleury A, et al. Hedgehog associated to microparticles inhibits adipocyte differentiation via a non-canonical pathway. Sci Rep. 2016;6:23479. doi: 10.1038/srep23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh JM, et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3:25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Spinella-Jaegle S, et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 72.Kopinke D, Roberson EC, Reiter JF. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell. 2017;170:340–351 e312. doi: 10.1016/j.cell.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shao M, et al. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol Metab. 2017;6:111–124. doi: 10.1016/j.molmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao M, et al. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016;23:1167–1184. doi: 10.1016/j.cmet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi YS, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell stem cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loh NY, et al. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015;21:262–272. doi: 10.1016/j.cmet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villanueva CJ, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 2011;13:413–427. doi: 10.1016/j.cmet.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Jin Q, Lee JE, Su IH, Ge K. Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proc Natl Acad Sci U S A. 2010;107:7317–7322. doi: 10.1073/pnas.1000031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 80.Mastrogiannaki M, et al. beta-Catenin Stabilization in Skin Fibroblasts Causes Fibrotic Lesions by Preventing Adipocyte Differentiation of the Reticular Dermis. J Invest Dermatol. 2016;136:1130–1142. doi: 10.1016/j.jid.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 82.Lichtenberger BM, Mastrogiannaki M, Watt FM. Epidermal beta-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun. 2016;7:10537. doi: 10.1038/ncomms10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sumikawa Y, Inui S, Nakajima T, Itami S. Hair cycle control by leptin as a new anagen inducer. Exp Dermatol. 2014;23:27–32. doi: 10.1111/exd.12286. [DOI] [PubMed] [Google Scholar]
- 84.Hesslein DG, et al. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone. 2009;44:537–546. doi: 10.1016/j.bone.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–2621. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 86.Ambrosi TH, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017;20:771–784 e776. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab. 2016;27:392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 92.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, et al. Principles and mechanisms of regeneration in the mouse model for wound-induced hair follicle neogenesis. Regeneration (Oxf) 2015;2:169–181. doi: 10.1002/reg2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 95.Zhang LJ, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marangoni RG, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kruglikov IL, Scherer PE. Skin aging: are adipocytes the next target? Aging (Albany NY) 2016;8:1457–1469. doi: 10.18632/aging.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kruglikov IL, Scherer PE. Adipocyte-myofibroblast transition as a possible pathophysiological step in androgenetic alopecia. Exp Dermatol. 2017;26:522–523. doi: 10.1111/exd.13379. [DOI] [PubMed] [Google Scholar]
- 99.Kruglikov IL, Scherer PE. Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends Endocrinol Metab. 2016;27:1–10. doi: 10.1016/j.tem.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alexander CM, et al. Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shook B, et al. The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches. Annu Rev Cell Dev Biol. 2016;32:609–631. doi: 10.1146/annurev-cellbio-111315-125426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kruglikov IL, Scherer PE. Dermal adipocytes and hair cycling: is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp Dermatol. 2016;25:258–262. doi: 10.1111/exd.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horsley V, Watt F. Repeal and Replace: Adipocyte Regeneration in Wound Repair. Cell Stem Cell. 2017;20:424–426. doi: 10.1016/j.stem.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 106.Keyes BE, et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci U S A. 2013;110:E4950–4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsumura H, et al. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351:aad4395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 108.Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zasloff M. Observations on the remarkable (and mysterious) wound-healing process of the bottlenose dolphin. J Invest Dermatol. 2011;131:2503–2505. doi: 10.1038/jid.2011.220. [DOI] [PubMed] [Google Scholar]
- 110.Sardella C, et al. Delayed Hair Follicle Morphogenesis and Hair Follicle Dystrophy in a Lipoatrophy Mouse Model of Pparg Total Deletion. J Invest Dermatol. 2017 doi: 10.1016/j.jid.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


