Abstract
OBJECTIVES
A common presumption in sleep research is that “normal” human sleep should show high night-to-night consistency. Yet, intra-individual sleep variation in small-scale subsistence societies has never been studied to test this idea. In this study, we assessed the degree of nightly variation in sleep patterns among Tsimane forager-horticulturalists in Bolivia, and explored possible drivers of the intra-individual variability.
METHODS
We actigraphically recorded sleep among 120 Tsimane adults (67 female), aged 18-91, for an average of 4.9 nights per person using the Actigraph GT3X and Philips Respironics Actiwatch 2. We assessed intra-individual variation using intra-class correlations and average deviation from each individual’s average sleep duration, onset, and offset times ( ).
RESULTS
Only 31% of total variation in sleep duration was due to differences among different individuals, with the remaining 69% due to nightly differences within the same individuals. We found no statistically significant differences in Tsimane sleep duration by day-of-the-week. Nightly variation in sleep duration was driven by highly variable sleep onset, especially for men. Nighttime activities associated with later sleep onset included hunting, fishing, housework, and watching TV.
CONCLUSIONS
In contrast to nightly sleep variation in the United States being driven primarily by “sleeping-in” on weekends, Tsimane sleep variation, while comparable to that observed in the US, was driven by changing “bedtimes”, independent of day-of-the-week. We propose that this variation may reflect adaptive responses to changing opportunity costs to sleep/nighttime activity.
Keywords: Sleep, Actigraphy, Tsimane, Opportunity costs, traditional society
Subfield: Human biology [living humans; behavior, ecology, physiology, anatomy], Theory
INTRODUCTION
There is currently no consensus definition of normal human sleep. A long-held presumption in sleep research is that artificial light and electronic devices in contemporary urban, industrialized populations have disrupted natural sleep rhythms and dramatically reduced average sleep duration at the cost of impaired health (Bonnet, 2000; National Heart Lung and Blood Institute, 2003; National Center on Sleep Disorders Research, 2011; Yetish et al., 2015). The implication is that sleep duration from non-industrial, non-electric populations would represent a healthier, species-typical sleep pattern. Yet recent sleep research conducted among Hadza and San hunter-gatherers and Tsimane horticulturalists cast doubt on this supposition, finding that their average nightly sleep duration is largely comparable to that of people living in the United States, despite lacking electricity and maintaining a subsistence lifestyle (Yetish et al., 2015; Samson et al., 2017b). Building on these findings, this study addresses another common supposition in sleep research: that “normal” sleep duration is generally consistent from night-to-night for a given individual (Sharpley et al., 1990; Zheng et al., 2012; Gaines et al., 2015). We assess the night-to-night, within-individual variation in sleep patterns among Tsimane hunter-horticulturalists in lowland Bolivia. No study to date has yet reported the extent of within-individual variation in sleep patterns in a subsistence society, nor explored sources of that variability.
Few studies of sleep conducted in North America, Europe, or Asia have focused on short-term within-individual variation in sleep duration (see Bin et al., 2012 for a list of countries and associated summary sleep measures). When reported, nightly sleep consistency is often represented by the intra-class correlation (ICC), which ranges from 0 (no nightly consistency) to 1 (same sleep duration every night). Based on 3-night actigraphic sleep recordings, people in the United States have been found to exhibit highly variable sleep duration from night-to-night, with ICC estimates ranging from 0.15-0.50 (Yang et al., 2001; Tworoger et al., 2005; Zheng et al., 2012; Gaines et al., 2015). This effect has been driven primarily by distinct sleep schedules during weekdays versus weekends, whereby the average adult in the United States sleeps 1.1 fewer hours per night on weekdays, due to 1.23 hours earlier sleep onset and 2.25 hours earlier sleep offset, according to self-reported sleep patterns (Soehner et al., 2011). A similar pattern of shorter sleep on weekdays has also been observed in several other countries, and not only among adults (Bin et al., 2012). Indeed, less sleep during the school-week has been observed among school children, adolescents, and college students, using both reported and objective measures of sleep (Yang et al., 2001; Tworoger et al., 2005; Chang et al., 2009; Miller et al., 2010; Crowley et al., 2014). The predominant interpretation is that the weekday/weekend sleep dichotomy is a product of the standardized work schedules and social constraints typical of modern post-industrial economies (Wittmann et al., 2006; Roenneberg et al., 2012).
In this study, we used actigraphic sleep recordings of free-living Tsimane adults to investigate nightly variation in sleep duration and timing. Since Tsimane are a modern horticultural population having experienced increasing access to market goods and Bolivian institutions over the past four decades, we first tested for any association between day of the week and sleep duration. Though Tsimane still engage in many traditional subsistence activities, town visits to procure healthcare or market goods often occur on weekends, and any religious services typically occur on Sundays. Next, we assessed the degree of intra-individual variation in sleep duration and analyze whether such variation was driven primarily by variability in sleep onset or sleep offset times. To contextualize this variation, we used reports of nightly (after dusk) activities recorded during morning-after interviews to investigate which activities were associated with particularly early and late sleep onset times. Given prior field experience with Tsimane, we expect variability in sleep onset and offset to be greater among men due to opportunistic nighttime hunting and fishing, although variability due to late night socializing was predicted to be similar for men and women.
METHODS
Study population
The Tsimane population is an indigenous Amazonian group, living in villages of 50-500 near the market town of San Borja, El Beni, Bolivia (see Gurven et al., 2017). The terrain is dense neo-tropical rainforest (largely secondary forest), and is very difficult to traverse by road or river. As a result, Tsimane villages remain relatively isolated, with most villages lacking both running water and electricity. Much of the Tsimane economy is based on non-market subsistence, as most adults produce food for themselves and their children by hand-cultivating small garden plots, fishing, hunting wild game, and gathering forest products (Gurven et al., 2017). As such, their way of life is distinctly separated from the five-day workweek standard in the United States and other post-industrial economies, making them a valuable study population for investigating intra-individual variation in sleep.
Study design
This study was conducted from June-December 2013 under the umbrella of the Tsimane Health and Life History Project (THLHP), which provided all of the estimates of study participant ages as well as logistical support (see Gurven et al., 2017). During this period, the THLHP mobile clinic traveled among 13 villages, collecting anthropological and biomedical information, while providing free medical examinations and treatment to village residents, regardless of their research participation. Since the inception of the THLHP in 2002, research participation in the Tsimane population has exceeded 85% (Gurven et al., 2017). We attempted to solicit all Tsimane adults over 40 years of age for participation in this study, in concordance with the THLHP mission of studying aging and senescence (29 men, 30 women). To complement this sample, we randomly sampled an approximately equal number of men and women between the ages of 18 and 40 (24 men, 37 women). Only two adults declined the invitation, citing a desire to avoid the inconvenience of disrupting their normal daily routines. In total, 120 adults were sampled (67 female), with a mean±SD age of 40.0±15.8 (range 18-91).
The only exclusion criteria applied during the process of random sampling was the continued presence in the same village where the mobile clinic was located for the duration of the intended observation period. More than 90% of potential subjects who agreed to participate qualified for participation. The majority of those who did not were younger adults, especially men, who traveled to work on short-term wage labor projects for non-Tsimane employers.
Adults who agreed to participate were issued wrist-worn sleep monitors to wear for 24-hours per day, with the exception of during bathing. Subjects with watches were then free to return to their normal activities. Devices were retrieved either 3 or 7 nights later, determined by the logistic considerations of battery life and specific devices used (explained further in next section). In many cases subjects could not be found or their travel plans changed, which resulted in extended sleep recordings for some subjects and truncated recordings for others. Recording length durations were not systematically associated with individual participant characteristics like age, sex, or village.
Built around this baseline protocol, we also conducted brief sleep interviews, in-home, for three consecutive nights of sleep recording. This interview was used to capture reports of dinner times, post-dinner nighttime activities, and hunger level at the time of sleep initiation. To avoid interfering with participants’ daily activity, participants were instructed to follow their normal schedules, even if it meant leaving home before GY and a Tsimane researcher arrived at their house to conduct this interview. As a result, the total number of interviews conducted on this sample of 120 people was only 283 out of a planned 360 interviews.
Sunrise and sunset times during the study period, used as controls in some analyses, came from the open-access data provided by the NOAA Solar Calculator (http://www.esrl.noaa.gov/gmd/grad/solcalc/).
Wrist-worn accelerometry for sleep monitoring
Accelerometry has been extensively validated against polysomnography, the “gold-standard” for measuring sleep (de Souza et al., 2003). Accelerometry tends to overestimate sleep by about 20 minutes per night (Cole et al., 1992), but is easily administered in a field setting with limited infrastructure. Polysomnography is cumbersome and invasive, typically requiring subjects to sleep in a laboratory setting. Another advantage of accelerometry is that it appears to be immune to the “first-night effect” that affects polysomnographic data (van Hilten et al., 1993).
This study began with 4 Actilife GT3X devices, provided by the THLHP, to conduct sleep recordings. After the first two months of data collection, 10 Philips Respironics Actiwatch 2 devices were provided by the Center for Sleep Research at UCLA (Director Jerome Siegel). Using both allowed us to collect substantially more data per unit time, and consequently double the sample size. Both devices have been validated numerous times, including against one another (de Souza et al., 2003; Cellini et al., 2013; Zinkhan et al., 2014; Lee and Suen, 2017). In addition, we conducted an independent validation, whereby a subset of 9 Tsimane participants were asked to wear both sleep monitors on the same wrist at the same time for 3 nights per person. After data transformation (see supplementary materials), the Cole sleep-scoring algorithm was applied to both datasets to produce a binary score of sleep versus wake for every 1-minute epoch recorded (Cole et al., 1992). Comparing the measurements of sleep duration for the 9 participants wearing both devices at the same time, we found that over the course of a night, from 7PM to approximately 9AM (840 minutes), sleep evaluations agreed on an average of 91.9% of 1-minute epochs, with an average deviation in measurements of total sleep duration of only 4.8 ± 45.7 minutes per night.
Using the total dataset for the current analyses (n=120), we tested for any statistically significant association between the device used and each of the key sleep measures of interest. We found that the Actigraph measurements of sleep offset time were, on average, 28 minutes later (p<.001, df=118). However, we did not find any statistically significant differences in mean sleep duration or sleep onset measurements (note: total sleep duration is almost never the sum total of all time between sleep onset and offset; see supplementary materials section on sleep efficiency). There were no statistically significant differences in any of the measures of intra-personal variation in sleep duration, onset, or offset for each subject. The six regression models used to test for the effects of sleep recording device used are provided in Table S1. In all regression analyses, sleep monitoring device used was included as a control variable, referred to in-text as “device used”.
Including all Tsimane subjects whose average sleep was presented in Yetish et al., 2015 (47 Tsimane adults, recorded for 6-8 nights each), we sampled a total of 120 adults (67 female), for a mean±SD of 5.0±2.1 (range 3-10) nights of sleep per person. The number of nights recorded per participant was unsurprisingly correlated with device used (r=0.721, p<.001), as Actigraphs can only record for 3 nights maximum on a single battery charge (with a 60 hz sampling rate). Table S2 shows the sample size as a function of number of nights recorded. To assess the effect of recording duration on our results, we modeled how the number of nights recorded for each person affected measurements of sleep duration and timing. Controlling for sunset time (and any associated seasonal effects) and sleep monitoring device used, the number of nights of observation was associated with more variable sleep onset time (b=6.6 minutes per night, p=.014, df=95; Figure 1) and marginally more variable sleep duration (b=3.3 minutes per night, p=.099, df=95). It was not statistically significantly associated with any other measure of sleep (see Figure S1 for composite graph showing each measure of sleep as a function of number of nights recorded). There were no statistically significant interaction effects between device used and duration of sleep recording in predicting mean and intra-personal variation in sleep onset, offset, and duration. We include “number of nights recorded” as a control variable in all analyses of intra-individual variation in sleep timing or duration.
Figure 1.
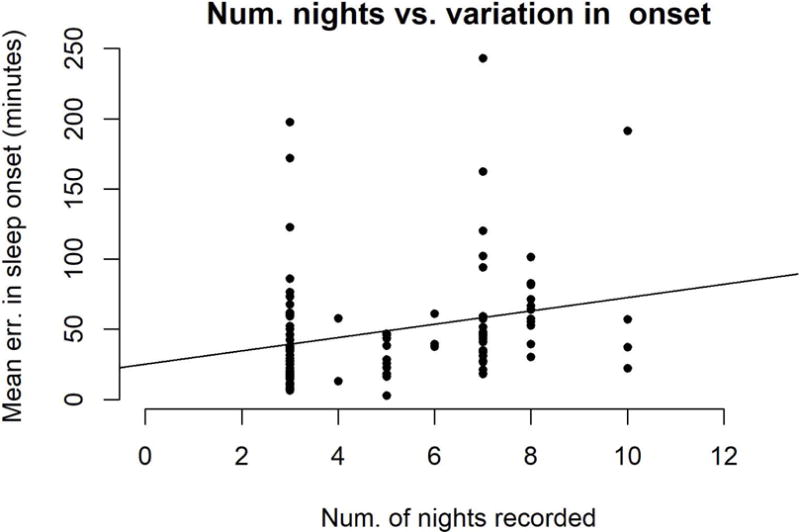
Variation in sleep onset is shown as a function of the number of nights of sleep recorded for each participant (best fit line y = 25.2 + 4.7*x). The profile of sleep recording lengths is bi-modally distributed around 3 nights and 7 nights, which is insufficient to test for a non-linear relationship. However, since a significant linear relationship was found, we include “number of nights recorded” as a control variable.
Data coding process and statistical analysis
An individual’s particular sleep pattern can be represented by the following equation:
where Yij is a single observation for subject i on night j of the specific sleep measure of interest (sleep duration, onset, or offset), μi is the average value for subject i, and εij is the “error”, or deviation between subject i’s sleep on night j and subject i’s average sleep over the entire sleep recording. While most previous studies have focused on individual means and inter-individual variability (σμ), this study investigates how εij varies in the Tsimane population.
εij is the “intra-individual, night-to-night variation” in the specified sleep parameter, whereas , the average of εij over all j nights for each subject i. is calculated by following equation:
We use t-tests and Bartlett’s tests to assess differences in means and variances, respectively. Linear regressions were used for analyses of individual level differences (μ and ε values) while adjusting for duration of sleep recordings.
We use linear mixed-effects regression models to predict daily sleep behavior, and we include random intercepts for individual ID to adjust for repeated observations on the same individuals and the variable length of sleep recordings among study participants. This statistical model can parcel variance out into the μi and εij terms, and it generates intra-class correlations (ICC), i.e. proportion of the overall correlation between values of Yij due to within-individual differences:
ICC facilitates distinguishing how much of the total variance in a recorded sleep measure exists at the inter-individual level versus intra-individual level; if inter-individual level differences (σμ) account for a relatively large proportion of the variance, ICC is high, whereas if inter-individual level differences are near 0, then ICC is also near 0. Conditional R2 is also used in conjunction with the mixed-effects regression, as it represents the sum of all variance explained by both fixed and random effects (Nakagawa et al., 2017). Using ICC and conditional R2 together allows for comparing regression models that include an overlapping set of predictors to assess whether additional independent variables explain variation at the intra-individual level or inter-individual level (see supplementary materials). Details on coding issues and R packages used are provided in the supplementary materials.
Survey responses on nighttime activities were categorized based on the potential impact different activities may have on sleep. Impact was based mostly on whether the participant left the house or employed certain types of modern technology. The 7 categories include: listening to the radio, watching TV, hunting/fishing, relaxation, “sleep after dinner”, housework (e.g. washing clothes, needlework, weaving, tool repair), and social visitation (i.e. either visiting a neighbor’s house or receiving a guest). “Relaxation” is a rough translation of the Tsimane word “ji’jutyiti”, which refers to resting seated around a low fire, often while chatting. If these activities occurred at a neighbor’s house, or if other visitors were present, we coded this as “social visitation”. Similarly, if participants reported listening to the radio while “relaxing”, that was coded as listening to the radio rather than relaxation.
We asked about nighttime hunger prior to sleep using a verbal Likert scale: “very hungry”, “somewhat hungry”, “somewhat full”, or “very full”. To complement descriptive analyses, this was coded into numerical values from 1 to 4 to use compare average hunger score on nights of hunting and fishing against those of any other activity.
RESULTS
Tsimane sleep exhibits no day-of-the-week effect
Controlling for sunset time and device used, neither Tsimane sleep duration (Figure 2) nor sleep offset time varied by day of the week to a statistically significant degree (see Tables S3a–c for full regression results). Sleep onset time was later on Thursday night than on Sunday, Monday, Tuesday, or Friday nights, but no other day-of-the-week comparisons were statistically significant.
Figure 2.
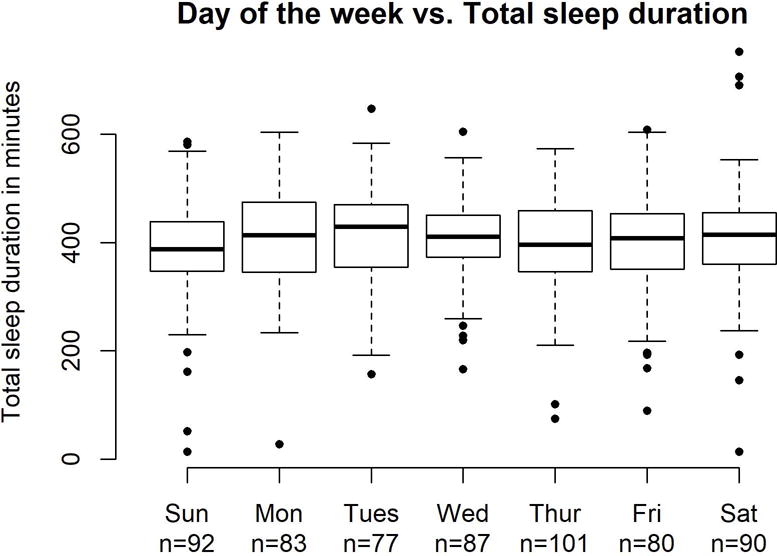
Sleep duration (in minutes) by day of the week. Boxplots show 25th percentile, median (black line), and 75th percentile. Day of the week refers to the day the person woke up, not the day they went to bed (i.e. Saturday night through Friday night).
Assessing the degree of intra-personal variation in sleep
To assess intra-individual variation in sleep patterns, we calculated values for each subject (the mean deviation between sleep observed on a given night and that same subject’s mean sleep observed). The mean values for men and women were 56.0 and 42.6 minutes, respectively. On average, men and women went to sleep at 9:44PM and 9:03PM, respectively, and awoke the next morning at 5:42AM and 5:37AM, respectively (Table 1).
Table 1.
Descriptive summary of sleep variables among 120 Tsimane adults. These include average sleep duration, onset, and offset (μi), and the mean intra-personal variation ( ) in these parameters. Presented values are mean ± standard deviation.
| Men | Women | |
|---|---|---|
| Num. of subjects (n) | 53 | 67 |
| Mean num. of nights recorded per person | 5.1 nights ± 2.3 | 4.9 nights ± 2.1 |
| Age | 42.3 ± 15.9 | 38.3 ± 15.7 |
| Mean total sleep (μi) | 380.1 min. ± 68.8 | 415.5 min. ± 57.2 |
| Mean intra-personal deviation in total sleep ( i) | 56.0 min. ± 38.1 | 42.6 min. ± 22.0 |
| Mean sleep onset (μi) | 9:44PM ± 91.3 | 9:03PM ± 51.8 |
| Mean intra-personal deviation in sleep onset ( i) | 62.9 min. ± 55.4 | 39.0 min. ± 21.1 |
| Mean sleep offset (μi) | 5:42AM ± 49.4 | 5:37AM ± 44.8 |
| Mean intra-personal deviation in sleep offset ( i) | 29.7 min. ± 20.4 | 23.9 min. ± 14.6 |
| Mean circadian phase measure (CPM) (μi) | 1:43AM ± 61.3 | 1:20AM ± 39.7 |
| Mean intra-personal deviation in CPM ( i) | 51.7 min. ± 40.2 | 29.5 min. ± 16.3 |
In a linear mixed-effects regression model predicting sleep duration with no covariates but including the individual ID random effect, we find that 31.1% of the variation was explained by the model (i.e., ICC = conditional R2 = .311); thus, 68.9% of the total variation in sleep duration is due to differences within individuals over time (Table S4). After adding age, sex, device used, and sunset time into the model as control variables, ICC dropped to 0.268 and conditional R2 increased to 0.317, indicating that the majority of variability in sleep duration captured by the control variables exists at the inter-individual level, already captured by the random effect of individual ID (see supplementary materials for further discussion of ICC versus conditional R2). Moreover, it suggests that up to 68.3% of the variation in sleep duration exists at the within-individual level and cannot be explained by changes in sunset times.
Nightly variation in sleep duration varied notably throughout the population. There was a broader distribution of nightly sleep consistency scores for men than women (variance ratio of 2.99, Bartlett’s test p<.001, df=43). The same was true for sleep onset (variance ratio of 6.90, p<.001, df=43) and sleep offset (variance ratio of 1.96, p=.020, df=43; Figure 3).
Figure 3.
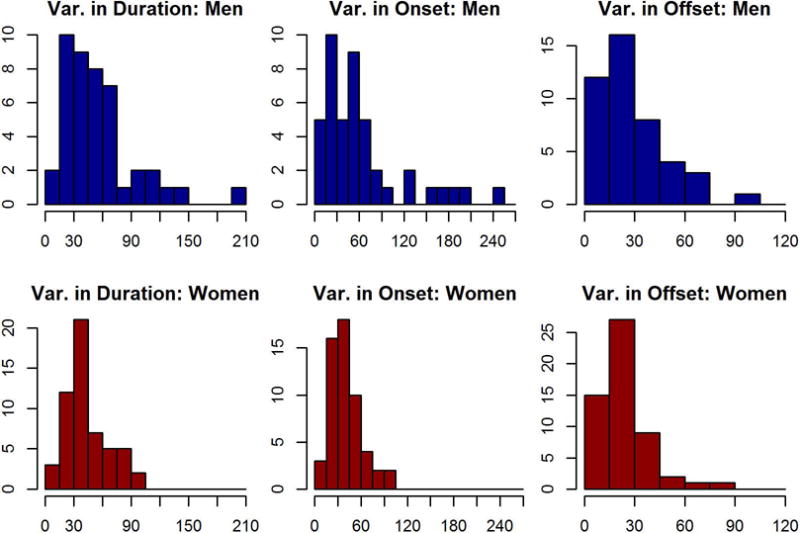
The intra-personal variation in sleep duration, onset, and offset for men and women. Men’s values are plotted on the top row, in blue, and women’s on the bottom, in red. 15 minute bins were used for all three plots.
Men had more nightly-variation in sleep duration than women (b=12.6 minutes, p=.043, df=94), adjusting for device used, length of sleep recording, and average sunset time over the period of observation. Sleep consistency did not vary significantly with age for either men (b=−0.54 min/year, p=.123, df=41) or women (b=−0.30 min/year, p=.111, df=52), with the same controls (Figure 4).
Figure 4.
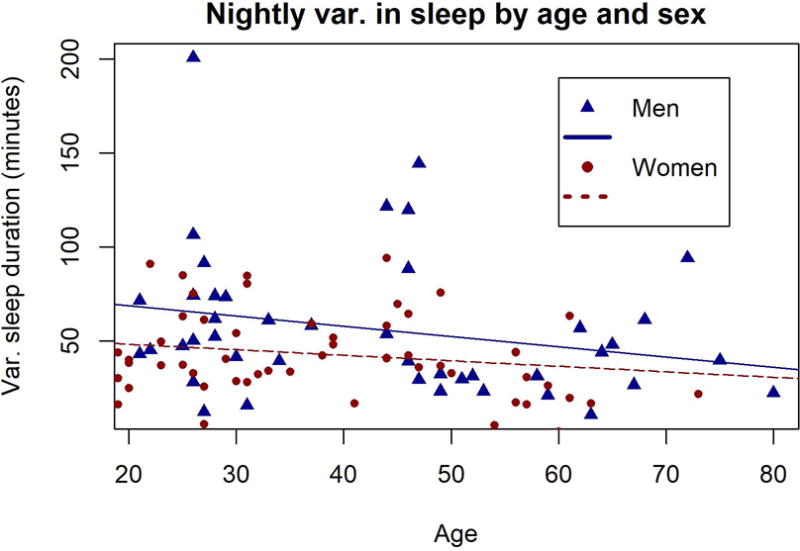
Night-to-night variation in sleep duration over the adult life course for men (blue triangles) and women (red circles). Each data point reflects an individual’s average deviation from their personal average sleep duration ( ).
Comparing the variability of sleep onset versus sleep offset
Over 610 total person-nights of observation, the correlation coefficients between sleep onset and total sleep duration was −0.729, and between sleep offset and sleep duration was 0.228. However, after adjusting for individual, device used, and sunset time in a linear mixed-effects regression model, the effects of sleep onset and sleep offset on total sleep duration were of relatively similar magnitude: b=−0.812 for sleep onset, p<.001; b=0.802 for sleep offset, p<.001; df=187.
Nightly variation ( ) in sleep onset was greater than in sleep offset, for both men (62.9 versus 29.7 minutes, t-test p<.001, df=54) and women (38.9 versus 23.9 minutes, p<.001, df=95; Table 1). Controlling for device used and length of sleep recording, longer average sleep duration was associated with lower intra-personal deviation in sleep onset time for men (b=−0.46, p<.001, df=40), but not for women (b=−0.04, p=.482, df=51). Similarly, with the same controls, longer average sleep duration was associated with lower intra-personal deviation in sleep offset time for men (b=−0.16, p<.001, df=40), but not for women (b=0.03, p=.434, df=51).
Figure 5 illustrates sleep onset and sleep offset clock-times for all study participants. Subjects are sorted vertically by descending average sleep duration (μi) with 95% prediction intervals. This visual display of individual sleep patterns corroborates the analysis above where both earlier sleep onset time and later sleep offset time are associated with greater total sleep duration in both men and women. There is also greater variability in sleep onset and offset times among men than women.
Figure 5.
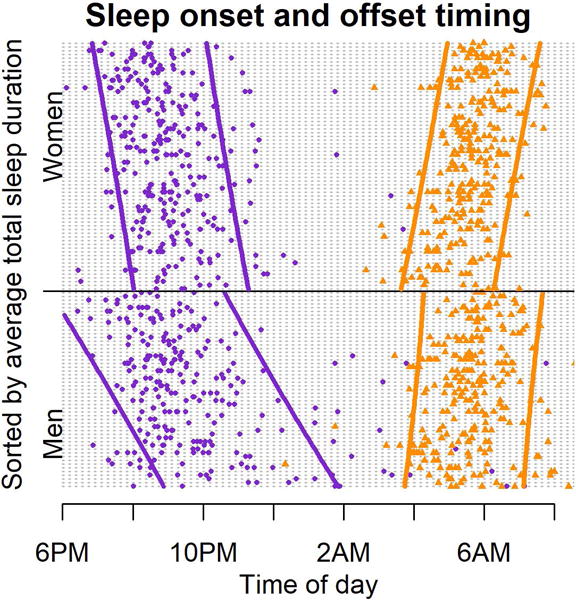
Sleep onset (purple circles) and sleep offset times (orange triangles), for women (top) and men (bottom). Each horizontal entry represents a different individual, vertically sorted by descending nightly sleep duration. 95% prediction intervals, calculated using individual average sleep onset and sleep offset times, are shown with solid lines.
Exploring after-dusk activities and their association with sleep
A typical Tsimane day ends with bathing at 5:49PM for women and 6:09PM for men (t-test p=.127, df=66), followed shortly thereafter by eating dinner at 6:31PM for women and 7:00PM for men (t-test p=.008, df=239). Given that Tsimane do not use clocks or watches, these times based on self-reports should be considered rough estimates. Controlling for sex, reported average dinner time did not change significantly from June to December (b=0.3 minutes, p=.930, df=242). Over the duration of the study, sunset time changed from the earliest time of 6:04PM in June to the latest time of 6:48PM in December (note that there is no daylight savings time clock adjustment in Bolivia). Sunrise changed from the earliest time of 5:47AM to the latest time of 6:53AM, and was correlated with sunset time with Pearson’s r = −0.837.
For each sex, we analyzed activity composition by tertiles of sleep onset time: early (before 8:42PM for men; before 8:24PM for women), middle (8:42PM-9:48PM for men; 8:24PM-9:12PM for women), and late (after 9:48PM for men; after 9:12PM for women) (Tables 2–3). Overall, relaxation is the most commonly reported activity (89 out of 278 person-nights), followed by listening to the radio (72), sleep (42), and domestic chores (22).
Table 2.
Men’s nightly activities from self-report. Activities ranked from most to least commonly reported by tertile of sleep onset time. Activity profiles for quantiles 1 and 2 show minimal differences, but night hunting/fishing and watching TV are listed at later sleep onset times. Note that the unit of measure for this analysis is a single evening, and a single participant could potentially contribute data points to all three quantiles.
| Quantile 1 | Quantile 2 | Quantile 3 | |
|---|---|---|---|
| Onset | 6:24PM-8:42PM | 8:42PM-9:48PM | 9:48PM-8:54AM |
| Mean sleep duration | 445.7 minutes | 397.6 minutes | 293.5 minutes |
| Mean sleep offset | 5:24AM | 5:26AM | 6:04AM |
| Activity 1 | Relax (12) | Relax (20) | Hunt/fish (12) |
| Activity 2 | Listen to Radio (12) | Listen to Radio (12) | Just sleep (9) |
| Activity 3 | Just sleep (7) | Just sleep (8) | Relax (8) |
| Activity 4 | Other (2) | Social visit (2) | Listen to Radio (7) |
| Activity 5 | Work around the house (2) | Other (1) | Social visit (3) |
| Activity 6 | Watch TV (1) | – | Watch TV (3) |
| Activity 7 | – | – | Other (1) |
| Activity 8 | – | – | Work around the house (1) |
| Total observations | 36 | 43 | 44 |
Table 3.
Women’s nightly activities from self-report. Activities ranked from most to least commonly reported by tertiles of sleep onset time. Post-dinner activity profiles are more similar across tertiles among women than among men. Household chores and watching TV were associated with later sleep onset time. Note that the unit of measure for this analysis is a single evening, and a single participant could potentially contribute data points to all three quantiles.
| Quantile 1 | Quantile 2 | Quantile 3 | |
|---|---|---|---|
| Onset | 6:06PM-8:24PM | 8:24PM-9:12PM | 9:12PM-3:42AM |
| Mean sleep duration | 463.9 minutes | 424.2 minutes | 372.7 minutes |
| Mean sleep offset | 5:23AM | 5:31AM | 5:50AM |
| Activity 1 | Relax (16) | Relax (18) | Listen to Radio (17) |
| Activity 2 | Listen to Radio (10) | Listen to Radio (13) | Relax (15) |
| Activity 3 | Watch TV (3) | Just sleep (6) | Work around the house (12) |
| Activity 4 | Just sleep (2) | Social visit (5) | Just sleep (10) |
| Activity 5 | Social visit (2) | Work around the house (5) | Watch TV (6) |
| Activity 6 | Work around the house (2) | Watch TV (4) | Social visit (4) |
| Activity 7 | Hunt/fish (1) | – | Hunt/fish (1) |
| Activity 8 | Other (1) | – | Other (1) |
| Total observations | 37 | 51 | 66 |
We more formally tested how activities affect sleep onset, duration, and offset using mixed-effects regressions that adjust for sunset time, age, sex, device used, and individual random effects. Sunset time does not account for any of the variation in sleep onset, duration, or offset, despite being a statistically significant predictor of each of the three in their respective null models (see Tables S5a–c for comparison of ICC and conditional R2 values across four models predicting sleep onset, duration, and offset, respectively). When including nighttime activities in the model, sunset time remains a statistically significant predictor of sleep duration and offset, but drops out of the model as a significant predictor of sleep onset (Table 4: Model 1). Relaxation, the most commonly reported activity, was used as a baseline (intercept) for statistical comparison (setting sunset equal to its mean of 6:27PM). The associated average sleep onset, duration, and offset were 9:45PM, 414 minutes, and 6:09AM, respectively (Table 4). Nighttime activity explains approximately 21%, 14%, and 7% of the variation in sleep onset, duration, and offset (Tables S5a–c). Those engaging in nighttime hunting or fishing had a sleep onset 208 minutes (p<.001) later than those relaxing (baseline; Table 4: Model 1). Similarly, later sleep onset times were found for those engaging in social visitation (b=65 mins, p=0.012) and watching TV (b=41mins, p=.041; Table 4: Model 1). Only nighttime hunting and fishing were associated with a statistically significant difference in sleep offset (b=30 minutes later, p=.037; Table 4: Model 3). Thus, taken together, fishing and hunting reduce total sleep duration by 139 minutes (p<.001), social visitation by 78 minutes (p=.001) and TV watching by 55 minutes (p=.005; Table 4: Model 2).
Table 4.
Assessing the effect of nighttime activity on sleep onset (Model 1), duration (Model 2), and offset (Model 3), controlling for age, sex, device used, and sunset time, we see that hunting and fishing are associated with the largest significant effects on sleep onset, duration, and offset. Watching TV and working around the house are also associated with later sleep onset and shorter sleep duration, but not any significant changes in sleep offset.
| Model 1 (Onset) | Model 2 (Duration) | Model 3 (Offset) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Est | Std Err | p | Est | Std Err | p | Est | Std Err | p | |
| Intercept/Relaxing | 9:45PM | 741.078 | 0.015 | 413.728 | 684.374 | 0 | 6:09AM | 423.501 | 0 |
| Age | −1.236 | 0.409 | 0.003 | 0.114 | 0.378 | 0.764 | −0.579 | 0.234 | 0.015 |
| Male | 36.986 | 13.251 | 0.006 | −39.503 | 12.227 | 0.002 | 0.89 | 7.576 | 0.907 |
| Device used | −7.435 | 13.858 | 0.593 | 18.567 | 12.864 | 0.152 | −12.063 | 7.899 | 0.13 |
| Sunset time (minutes) | −28.93 | 40.307 | 0.474 | −126.817 | 37.224 | 0.001 | −160.93 | 23.033 | 0 |
| Hunting/fishing | 207.952 | 24.837 | 0 | −138.618 | 23.575 | 0 | 29.603 | 13.996 | 0.037 |
| ‘Just sleep’ | 15.554 | 17.821 | 0.385 | −7.556 | 16.9 | 0.656 | −1.889 | 10.048 | 0.851 |
| Listen to radio | 3.991 | 13.739 | 0.772 | 3.409 | 13.035 | 0.794 | −2.05 | 7.744 | 0.792 |
| Other | −11.57 | 35.195 | 0.743 | 25.706 | 33.439 | 0.444 | −12.216 | 19.826 | 0.539 |
| ‘Social visit’ | 29.062 | 21.938 | 0.188 | −22.588 | 20.931 | 0.283 | 5.737 | 12.334 | 0.643 |
| Watch TV | 64.51 | 25.242 | 0.012 | −78.287 | 23.615 | 0.001 | −14.91 | 14.329 | 0.3 |
| Housework | 41.81 | 20.255 | 0.041 | −54.705 | 19.234 | 0.005 | −15.453 | 11.413 | 0.179 |
| Model DF | 109 | 108 | 109 | ||||||
| ICC for ID | 0.318 | 0.279 | 0.337 | ||||||
| Cond. R2 | 0.520 | 0.459 | 0.548 | ||||||
Given the central importance of food acquisition for altering sleep patterns, we queried participants about their motivations for hunting and fishing specifically at night. 28% of participants reported eating no dinner on the nights they went hunting/fishing compared to 10% who reported any other nighttime activity (t-test p=.166, df=13). On a 1-4 scale representing “very full” to “very hungry”, the average hunger level reported by those who went hunting/fishing was 2.8 versus 2.3 for those reporting any other activities (t-test p=.104, df=14). Upon further inquiry of 14 men who went night hunting, the most common response for why they went at night was that “they really needed to on that particular night” (n=7 reports). Other reasons included “I only do it at night (n=3), “I was busy during the day” (n=3), or “it’s easier at night” (n=1). Similarly, of 11 adults who reported engaging in household chores at night did so because they were “busy during the day” (n=5), “it could not wait” (n=3), “it was easier at night” (n=1), “I wasn’t tired” (n=1), or it was a rare exception (n=1). Those who watched TV reported doing so specifically at night because they were “busy during the day” (n=4), they “only watch at night and it is easier to watch at night” (n=1), or no particular reason (n=1).
DISCUSSION
In this paper, we used wrist-worn actigraphic sleep monitors to investigate the degree of variation in sleep duration and timing among Tsimane adults. Unlike in the United States and other post-industrial economies, we found that sleep duration had little to no variation as a function of day-of-the-week. Nevertheless, sleep duration varied substantially from night-to-night. This variation was primarily driven by variation in sleep onset (bedtime), particularly among Tsimane men. We found that subsistence activities of hunting and fishing, visiting or hosting neighbors, and watching TV were the main activities affecting later sleep initiation and shorter total duration.
With an ICC of 0.31 for sleep duration, up to 69% of variation in Tsimane sleep exists at the intra-individual level, suggesting that a person’s sleep duration on a given night may be more likely to resemble the sleep duration of another person than that same person’s sleep on the following night. This degree of variation is comparable to that previously reported in the United States, where recorded ICC values range from 0.15-0.50 (Tworoger et al., 2005; Zheng et al., 2012; Gaines et al., 2015); Tsimane within-individual standard deviation was 65 minutes, compared to 76 minutes observed in the United States (see supplemental materials; Knutson et al., 2007). The nightly variation in sleep duration in the United States is driven primarily by the difference in sleep patterns during weekdays versus weekends, especially for sleep offset times (Tworoger et al., 2005; Soehner et al., 2011; Crowley et al., 2014). Given the lack of rigid scheduling of work-related activities, we found no significant changes in sleep duration over different days of the week, and instead found that nightly variation in Tsimane sleep duration was driven primarily by substantial fluctuation in sleep onset times. To our knowledge, this is the first examination of intra-individual variation in sleep patterns in a subsistence-level society.
Towards an explanation for nightly variation in sleep patterns
In the United States and other post-industrial economies, the variability driven by the weekday/weeknight sleep dichotomy is typically regarded as an unhealthy pattern, particularly because of the associated desynchrony with solar light cycles and central role of electric lighting (Bonnet, 2000; Wittmann et al., 2006; Roenneberg et al., 2012; Czeisler, 2013). While Tsimane sleep exhibits considerable nightly variability, there is no associated desynchrony with sunrise and sunset times; sleep offset, typically occurring at approximately the same time as dawn (see Yetish et al., 2015), remained consistent from night-to-night, and sleep onset, while highly variable, still occurred after sunset. Given the limited presence of electric lighting, modern technology, and market economics in the Tsimane environment, there is currently little established theory to interpret nightly variability in Tsimane sleep.
We explored the relationship between sleep and nighttime activity, finding that 21% of the variability in Tsimane sleep onset time and 14% of sleep duration variability could be explained by reported nighttime activity. Television is a novel and uncommon feature of the Tsimane environment, but was nonetheless associated with a significant delay in sleep onset and shortening of sleep duration, similar to the effect seen in the United States (Basner and Dinges, 2009). Television, like many electric lights, emits blue light radiation, which can inhibit melatonin production and sleep initiation (Chellappa et al., 2013; Komada et al., 2015; Moderie et al., 2017). We did not record duration of TV watching nor melatonin levels, so we cannot assess whether the delay of sleep onset is due to blue light exposure or the direct loss of sleep from watching TV during hours normally spent sleeping.
Other nighttime activities were less dependent on modern technology yet still predicted later sleep onset and shorter sleep duration. Hunting and fishing had a larger effect on sleep than any other activities reported, including watching TV: sleep onset was delayed by an average of 208 minutes, offset was delayed by 30 minutes, and duration was shortened by 139 minutes. We found that Tsimane engage in nighttime hunting and fishing more when there was food scarcity or complaints of hunger. This may be an analogous phenomenon to one observed in the United States, where “[o]verwhelmingly, [analyses from The American Time Use Survey] point to work as the dominant waking activity that is performed instead of sleep in short sleepers (1.55 [hours more spent working] on weekdays and 1.86 [hours more spent working] on weekends/holidays compared to normal sleepers)” (Basner et al., 2014). The loss of sleep attributable to the need to spend more hours working each day is particularly pronounced among employed, lower income adults in the United States (Lauderdale et al., 2006). This type of sleep loss due to resource acquisition is typically interpreted as a negative consequence of poverty, under the presumption that people would prefer to sleep longer but are prevented from doing so by economic pressure. Rarely, if ever, is the loss of sleep interpreted as making the best of a bad situation.
Explanations highlighting the benefits of lost sleep are relatively uncommon and somewhat contentious in sleep research, but the benefits of sufficient sleep might trade-off against short-term payoffs from engaging in other activities. A reasonable hypothesis is that variability in the sleep/nighttime activity tradeoff may have adaptive value to accommodate availability of fitness-related opportunities. Across the animal kingdom, sleep appears to be homeostatically regulated, whereby “sleep deprivation” on one night is followed by a longer than usual “recovery” sleep the next night. Typically, sleep recovery is sufficient enough to counter many of the negative symptoms associated with sleep deprivation (Siegel, 2009). For animals, this is often thought to facilitate the avoidance of predators or other threats, as sleep is a state of high vulnerability. For humans, this homeostatic property of sleep is interpreted as a capacity to recover from undesired sleep loss imposed by external factors, as in the case where recovery sleep on the weekends helps counter some of the accumulated sleep debt from the work week (Wittmann et al., 2006; Chang et al., 2009; Roepke and Duffy, 2010). Building on recent studies recognizing the potential adaptive value of reducing sleep duration (Horne, 2011; Samson and Nunn, 2015), we hypothesize an alternative interpretation for the frequent cycles of sleep loss and rebound: individuals sleep less when opportunity costs are high, and make up that sleep at a later time, when the opportunity costs are lower. Though losing sleep may have harmful consequences, doing so may be adaptive under certain conditions. Given that short-term payoffs of activities leading to sleep loss may enhance fitness more than the longer-term costs detract from it, individuals may have difficulty recovering fully from accumulated sleep debt. Focusing on the productivity of nighttime work and perceived value of other nighttime activities among people in free-living populations may provide insightful tests of this hypothesis in future research.
Limitations and future directions
Many observational studies face a tradeoff between extending the period of observation and including a larger number of study participants. This study attempted to balance the two, using GT3X sleep monitors to record sleep for 3 nights, the maximum allowable with that device’s battery life, and Actiwatch 2 sleep monitors to perform longer recordings of 6-10 nights per participant. Since there is no consensus recommendation in the sleep literature for the requisite duration of actigraphic sleep recordings, we took advantage of this property of our database to assess the relationship between the length of participant sleep recordings and measurements of intra-personal variation in sleep patterns. We found that each additional night observed was associated with an increase of 3.3 minutes in the measure of intra-personal sleep duration, over the domain of 3-10 nights. This association with a key outcome variable of interest is a limitation of the current study. Given that longer recordings were associated with more variable sleep patterns, it is possible that the presented assessments of intra-individual variation are underestimated. There may be some asymptote to measurements of nightly sleep consistency, but that asymptote may not be approximated with recordings of 10 nights or fewer, so it is unknown how much our estimates of sleep variability would change with longer recordings. Nevertheless, it should be noted that even though the recordings for this study were relatively short, they are still longer than the 3-night recordings commonly found in other contemporary sleep studies. Future research investigating intra-individual variation may benefit from establishing a standard sleep recording length of 7 nights or longer, especially for comparing findings from different populations.
This investigation focused on sleep in a single population, which limits the applicability of conclusions. We can make no global claims about how much nightly variability in sleep is “normal” or healthy. However, our findings do refute the common supposition that nightly variability in sleep is primarily the product of artificial lighting or the 5-day workweek. Beyond this, it is difficult to compare variability in sleep among people living in the United States to that observed among Tsimane, as environments and lifestyles differ starkly. Moreover, different sub-populations within the United States likely exhibit different sleep patterns, likely as a function of vocational field. We focused on nighttime activity to explain some of the nightly variability in Tsimane sleep, but the advent of electric lighting and other modern technologies likely complicate the relationship between nighttime activities and sleep in the United States and other post-industrial or urban populations. More focused studies on intra-individual variation in sleep among people living in the United States may address this issue, especially by comparing patterns in different subpopulations with different vocations, work schedules, and usage patterns of personal electronics/TV. Moreover, we used a relatively coarse measure of nighttime activity to capture broad categories of reported activities. Using more nuanced and precise measures of nighttime activity may improve the amount of sleep variation explained in predictive models in the United States, Tsimane population, or elsewhere.
Few sleep studies have been conducted in non-electrified communities, and even fewer among small-scale subsistence populations. The significance of sleep in evolutionary and ecological contexts has generated increased attention recently, however, and thus more observational sleep studies in field populations have been conducted in the past few years than ever before (Knutson, 2013; de la Iglesia et al., 2015; Moreno et al., 2015; Yetish et al., 2015; Beale et al., 2017; Samson et al., 2017a; b; c; d). While these studies have contributed novel insights, they focused on addressing variation in sleep among individuals or groups. Future field research among subsistence-level populations can provide the necessary context to expand on our preliminary study of intra-individual sleep variation. As many populations are currently experiencing rapid socioeconomic change, there is ripe opportunity to assess the effects of changing work schedules, food security, and social environment on sleep ecology.
Supplementary Material
Acknowledgments
We thank Alberto Maito Nosa and Basilo Vie Tayo for their guidance and assistance during data collection, and THLHP personnel who provided assistance and support. We also thank Jerome Siegel for his contribution of research equipment and advice during manuscript preparation. Melissa Emery-Thompson, Jane Lancaster, and Joe Alcock all provided invaluable input on earlier versions of the paper. The authors would also like to thank the anonymous reviewers, whose comments helped improve the paper. Most of all, we thank all study participants for their participation and hospitality.
Contributor Information
Gandhi Yetish, University of California, Los Angeles, Psychiatry and Biobehavioral Sciences.
Hillard Kaplan, Chapman University, Economic Science Institute.
Michael Gurven, UCSB, Anthropology.
References
- Basner M, Dinges DF. Dubious bargain: trading sleep for Leno and Letterman. Sleep. 2009;32:747–752. doi: 10.1093/sleep/32.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Spaeth AM, Dinges DF. Sociodemographic Characteristics and Waking Activities and their Role in the Timing and Duration of Sleep. Sleep. 2014;37:1889–1906. doi: 10.5665/sleep.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale AD, Pedrazzoli M, Gonçalves B da SB, Beijamini F, Duarte NE, Egan KJ, Knutson KL, von Schantz M, Roden LC. Comparison between an African town and a neighbouring village shows delayed, but not decreased, sleep during the early stages of urbanisation. Sci Rep. 2017;7:5697. doi: 10.1038/s41598-017-05712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: A systematic review. Sleep Med Rev. 2012;16:223–230. doi: 10.1016/j.smrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Sleep Deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd. Philadelphia: W.B. Saunders Co; 2000. pp. 53–71. [Google Scholar]
- Cellini N, Buman MP, McDevitt EA, Ricker AA, Mednick SC. Direct comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiol Int. 2013;30:691–698. doi: 10.3109/07420528.2013.782312. [DOI] [PubMed] [Google Scholar]
- Chang A-M, Reid KJ, Gourineni R, Zee PC. Sleep Timing and Circadian Phase in Delayed Sleep Phase Syndrome. J Biol Rhythms. 2009;24:313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, Cajochen C. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22:573–580. doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, Barker DH, Carskadon MA. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA. Perspective: Casting light on sleep deficiency. Nature. 2013;497:S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- Gaines J, Vgontzas AN, Fernandez-Mendoza J, Basta M, Pejovic S, He F, Bixler EO. Short- and Long-Term Sleep Stability in Insomniacs and Healthy Controls. Sleep. 2015;38:1727–1734. doi: 10.5665/sleep.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, Hooper P, Kaplan H. The Tsimane Health and Life History Project: Integrating anthropology and biomedicine. Evol Anthropol. 2017;26:54–73. doi: 10.1002/evan.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hilten JJ, Braat EA, van der Velde EA, Middelkoop HA, Kerkhof GA, Kamphuisen HA. Ambulatory activity monitoring during sleep: an evaluation of internight and intrasubject variability in healthy persons aged 50–98 years. Sleep. 1993;16:146–50. doi: 10.1093/sleep/16.2.146. [DOI] [PubMed] [Google Scholar]
- Horne J. The end of sleep: “Sleep debt” versus biological adaptation of human sleep to waking needs. Biol Psychol. 2011;87:1–14. doi: 10.1016/j.biopsycho.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Knutson KL. Sleep duration, quality, and timing and their associations with age in a community without electricity in haiti. Am J Hum Biol. 2013;86:80–86. doi: 10.1002/ajhb.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada Y, Aoki K, Gohshi S, Ichioka H, Shibata S. Effects of television luminance and wavelength at habitual bedtime on melatonin and cortisol secretion in humans. Sleep Biol Rhythms. 2015;13:316–322. [Google Scholar]
- de la Iglesia HO, Fernández-Duque E, Golombek DA, Lanza N, Duffy JF, Czeisler CA, Valeggia CR. Access to Electric Light Is Associated with Shorter Sleep Duration in a Traditionally Hunter-Gatherer Community. J Biol Rhythms. 2015;30:342–350. doi: 10.1177/0748730415590702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- Lee PH, Suen LKP. The convergent validity of Actiwatch 2 and ActiGraph Link accelerometers in measuring total sleeping period, wake after sleep onset, and sleep efficiency in free-living condition. Sleep Breath. 2017;21:209–215. doi: 10.1007/s11325-016-1406-0. [DOI] [PubMed] [Google Scholar]
- Miller NL, Shattuck LG, Matsangas P. Longitudinal Study of Sleep Patterns of United States Military Academy Cadets. Sleep. 2010;33:1623–1631. doi: 10.1093/sleep/33.12.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderie C, Maren S, Van Der Dumont M. Circadian phase, dynamics of subjective sleepiness and sensitivity to blue light in young adults complaining of a delayed sleep schedule. Sleep Med. 2017;34:148–155. doi: 10.1016/j.sleep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Moreno CRC, Vasconcelos S, Marqueze EC, Lowden A, Middleton B, Fischer FM, Louzada FM, Skene DJ. Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci Rep. 2015;5:14074. doi: 10.1038/srep14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H, Johnson PCD, Schielzeth H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2017.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep Research Coordinating Committee T-N, editor. National Center on Sleep Disorders Research. National Institutes of Health Sleep Disorders Research Plan. 2011. [Google Scholar]
- National Heart Lung and Blood Institute. National sleep disorders research plan 2003 [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Roepke SE, Duffy JF. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat Sci Sleep. 2010;2:213–220. doi: 10.2147/NSS.S12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP. The evolution of human sleep: Technological and cultural innovation associated with sleep-wake regulation among Hadza hunter-gatherers. J Hum Evol. 2017a;113:91–102. doi: 10.1016/j.jhevol.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL. Hadza sleep biology: Evidence for flexible sleep-wake patterns in hunter-gatherers. Am J Phys Anthropol. 2017b;162:573–582. doi: 10.1002/ajpa.23160. [DOI] [PubMed] [Google Scholar]
- Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL. Chronotype variation drives night-time sentinel-like behaviour in hunter – gatherers. Proc R Soc B. 2017c;284 doi: 10.1098/rspb.2017.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson DR, Manus MB, Krystal AD, Fakir E, Yu JJ, Nunn CL. Segmented sleep in a nonelectric, small-scale agricultural society in Madagascar. Am J Hum Biol. 2017d:1–13. doi: 10.1002/ajhb.22979. [DOI] [PubMed] [Google Scholar]
- Samson DR, Nunn CL. Sleep intensity and the evolution of human cognition. Evol Anthropol. 2015;24:225–237. doi: 10.1002/evan.21464. [DOI] [PubMed] [Google Scholar]
- Sharpley AL, Solomon RA, Cowen PJ. Sleep stability with home sleep recording and automatic sleep stage analysis. Sleep. 1990;13:538–540. doi: 10.1093/sleep/13.6.538. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009 doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehner AM, Kennedy KS, Monk TH. Circadian Preference and Sleep-Wake Regularity: Associations With Self-Report Sleep Parameters in Daytime-Working Adults. Chronobiol Int. 2011;28:802–809. doi: 10.3109/07420528.2011.613137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–19. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Yang CM, Spielman AJ, D’Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–81. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]
- Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PRPR, Wilson C, McGregor R, Siegel JMJM. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25:2862–2868. doi: 10.1016/j.cub.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Sowers MF, Buysse DJ, Consens F, Kravitz HM, Matthews KA, Owens JF, Gold EB, Hall M. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN sleep study. J Clin Sleep Med. 2012;8:87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkhan M, Berger K, Hense S, Nagel M, Obst A, Koch B, Penzel T, Fietze I, Ahrens W, Young P, Happe S, Kantelhardt JW, Kluttig A, Schmidt-Pokrzywniak A, Pillmann F, Stang A. Agreement of different methods for assessing sleep characteristics: A comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Med. 2014;15:1107–1114. doi: 10.1016/j.sleep.2014.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


