Abstract
Purpose
To evaluate and quantify visual function metrics to be used as predictors of AMD progression and visual acuity (VA) loss in patients with early and intermediate AMD.
Design
Baseline data of observational, cross-sectional, prospective study.
Methods
101 patients were enrolled at Duke Eye Center: 80 patients with AMD age-related eye disease study (AREDS) stage 2 (N=33) and stage 3 (N=47) and 21 age-matched, normal controls. A dilated retinal examination, macular pigment optical density measurements, and several functional assessments: best-corrected VA, mesopic microperimety with eye tracking (MAIA), dark adaptometry (AdaptDx), low luminance VA (LLVA) (standard using log 2.0 neutral density filter and computerized method) and cone contrast test (CCT) (Innova Systems Inc) were performed. Low luminance deficit (LLD) was defined as the difference in numbers of letters read at standard vs. low luminance. Group comparisons were performed to evaluate differences between the control and the AREDS 2 and AREDS 3 groups using two-sided significance tests.
Results
Functional measures that significantly distinguished between normal and AREDS3 were standard and computerized (0.5 cd/m2) LLVA, percent reduced threshold and average threshold on microperimetry, CCTs, and rod intercept on dark adaptation (p < 0.05). The AREDS 3 group demonstrated deficits in microperimetry reduced threshhold, computerized LLD2 and dark adaptation rod intercept (p < 0.05) relative to AREDS 2.
Conclusions and Relevance
Our study suggests that LLVA, MAIA microperimetry, CCT and dark adaptation may serve as functional measures of AMD progression.
Age-related macular degeneration (AMD) is a common and debilitating aging disease with significant mental health and quality of life implications. AMD is considered a “priority eye disease” by the World Health Organization, with global prevalence estimates reaching nearly 196 million by 20201. In patients with AMD, vision in dim lighting and at night is the most commonly reported visual defect. Consequently, patients often report difficulty driving, with mobility, peripheral vision in dim lighting and changing lighting conditions, symptoms which have been shown to lead to significant emotional distress in this patient population 2,3. Even in the early phases of disease when visual acuity is unaffected, these symptoms are present and associated with decreased sensitivity of the rod system responsible for scotopic vision and delayed dark adaptation 4,5.
Despite substantial improvements in treatment of wet AMD with the introduction of effective anti-VEGF therapy 6, there remains a significant clinical need to treat the pathology associated with dry AMD. Currently many new clinical trials have focused on developing therapies targeting oxidative stress 7 and stem cells 8, enhancers of retinal and choroidal blood flow 9, neuroprotective agents 10, and anti-complement factors11 for use in patients with geographic atrophy. To identify and develop treatments for early and intermediate stages of AMD before debilitating functional loss occurs in advanced disease, reliable functional endpoints are required. To meet this need, the objective of this study was to determine visual function measures that may be used to identify, evaluate, and quantify visual deficits in patients with early and intermediate AMD. Data collection was focused to improve the understanding of the natural history of early and intermediate AMD, to evaluate the functional characteristics of early and intermediate AMD using low luminance VA and low luminance deficit, dark adaptation, cone contrast function, and mesopic microperimetry, and to assess morphological characteristics of early and intermediate AMD on multi-modal retinal imaging. Herein, we evaluate the most comprehensive and extensive set of parameters tested in a large cohort of early-intermediate AMD patients measured in one study, which can be employed as robust clinical endpoints for future clinical trials aimed to test the efficacy of treatments for dry AMD.
Methods
Study Participants
The single center, prospective, exploratory observational study NCT01822873 at Duke University Medical Center was approved by the Duke University Health System Institutional Review Board (IRB) and was conducted in accordance with Good Clinical Practice (GCP) using the guidance documents and practices offered by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) or applicable international regulatory authority laws, regulations, and guidelines. Written informed consent was obtained from all study participants. Study subjects with AMD were identified from the existing population at Duke Eye Center or newly recruited individuals at the ophthalmology and optometry clinics at Duke Eye Center presenting for consultation. Spouses and friends of AMD subjects as well as Duke Optometry patients were recruited as controls participants. Inclusion criteria for participants with AMD were capacity and willingness to provide consent, age 50 to 90 years, Snellen visual acuity of 20/50 (logarithm of the minimum angle of resolution, 0.40) or better, pseodophakia or mild nuclear sclerotic (NS) cataract that is not visually significant (trace-1+ NS), diagnosis of early (Age-Related Eye Disease Study, AREDS category 2) or intermediate (AREDS category 3) AMD with the presence of drusen larger than 63 um and pigmentary anomalies. Drusenoid pigment epithelial detachments were allowed, but patients with geographic atrophy were excluded. Inclusion criteria for control subjects were identical for age and visual acuity, with no signs of AMD in either eye including reticular pseudodrusen, although fewer than 5 drusen <65um were allowed.
Individuals were excluded if they demonstrated inability to provide informed consent, phakic status with visually significant cataract in the study eye(s) (>1+ NS), any evidence of choroidal neovascularization or geographic atrophy in either eye, any ocular abnormality other than AMD or cataracts, in addition to not being able to perform any of the designated tests or complete the consent form for other health reasons. When both eyes met the inclusion criteria, the eye with better visual acuity was chosen as the study eye or the following algorithm was used if both had the same visual acuity: odd birth month–right eye was selected and even birth month – left eye was selected.
Functional Testing
The baseline clinic visit consisted of the psychophysical and physical tests: best corrected visual acuity (BCVA) (ETDRS), dark adaptometry, mesopic microperimetry, low luminance VA (LLVA) standard and computerized, cone contrast test (CCT), and macular pigment optical density (MPOD). Visual acuity and functional tests were performed before fundus imaging to prevent bleaching of the retina. Subjects wore their best correction for all tests. Best-corrected visual acuity was assessed by the ETDRS method and expressed in number of letters read 12,13. Standard low luminance visual acuity was performed by asking the subject to read the ETDRS chart through a 2.0 log neutral density filter that reduces luminance by 100 fold14. Low luminance deficit (LLD) was calculated by subtracting LLVA from BCVA in ETDRS letters. Computerized low luminance visual acuity and cone contrast test (CCT) were performed monocularly at 4 m using computerized tests developed at Innova Systems (Burr Ridge, IL). During the computerized LLVA test, subjects were presented a succession of lines composed of 5 Snellen letters of decreasing size on PC (Dell Optiplex 9010, Dell, Plano, TX) screen with the initial background luminance of 16 cd/m2 followed by a different set of Snellen lines with a background luminance of 1.3 cd/m2 (low luminance 1) or 0.5 cd/m2 (low luminance 2). The resulting computerized BCVA and LLVA in Snellen letters were recorded and converted to ETDRS letters.
Subjects then underwent CCT assessment, a computer-based method of quantifying color vision and deficits in cone color discrimination at the photoreceptor level (Innova Systems, Burr Ridge, IL) as previously described15. On CCT testing, 90–100% represents a normal score, 75–90% possible acquired color deficiency, and 0–75% color deficiency. Dark adaptometry and microperimetry testing were then administered after pupillary dilation with 1 drop of tropicamide 1% and phenylephrine 2.5% each. Dark adaptation was measured with the AdaptDx dark adaptometer (MacuLogix, Hummelstown, PA) on the study eye using a protocol modified for intermediate AMD subjects, while the fellow eye was occluded. The device and testing method are described in detail elsewhere16. Briefly, the subject’s eye was bleached by exposure to a 505nm photoflash equivalent to 76% bleaching level for rods. The stimulus light was a 505-nm, 2° circular test spot located at 5° on the inferior visual meridian16.
Retinal sensitivity assessment was performed using a microperimeter with eye tracking (Macular integrity assessment [MAIA] CenterVue, San Jose, CA), as previously described15 using the standard 37–10° MAIA grid. Two microperimetry measures were derived: average threshold and percent-reduced threshold (PRT). Average threshold is the average of all retinal sensitivities from all loci tested. Percent-reduced threshold is a derived functional index representing the percentage of abnormal retinal sensitivity thresholds below 25 dB.
Imaging
Retinal imaging included stereo color fundus photography (Zeiss FF 450 Plus IR; Carl Zeiss Meditec Inc., Dublin, CA), fundus autofluorescence (Spectralis 3 mode; Heidelberg Engineering GmbH, Heidelberg, Germany)15, spectral domain optical coherence tomography (SD-OCT; Spectralis 6-mode; Heidelberg Engineering US) and macular pigment optical density (MPOD) via dual-wavelength fundus autofluorescence (Heidelberg Spectralis HRA + OCT MultiColor17, Heidelberg Engineering GmbH, Heidelberg, Germany).
Autofluorescence images for MPOD analyses were obtained using the Spectralis acquisition software module version 5.6.2.2. The excitation spectra of two images, blue 488 nm and green 518 nm, were aligned and averaged by the Heidelberg Eye Explorer software (HEYEX, version 1.7.1.0) to create an MPOD map. The plateau or reference point was set to 7° and the average MPOD values were recorded at radii 0.25°, 0.5°, 1°, and 1.75°17.
Retinal specialists (EML, SC, LV) and a comprehensive ophthalmologist (AH) performed all clinical examinations. Color fundus photographs were graded by a medical retinal specialist (EML) by evaluating the extent of pigmentary changes and drusen size. Early AMD (AREDS category 2) was defined by the presence of many small drusen, few intermediate drusen (63–124 μm in diameter), and/or retinal pigment epithelium abnormalities, whereas intermediate AMD (ARED3 category 3) was defined by extensive intermediate drusen and at least 1 large drusen (>125 microns). Fundus autofluorescence and spectral optical coherence tomography images were used to exclude major divergences from the disease staging on color fundus images.
Data management
Data was collected from case report forms and double-entered into the Research Electronic Data Capture (RedCap) database by certified data entry analysts from Duke Office of Clinical Research. Additional data quality assurance and integrity checks were carried out using SAS software version 9.3 (SAS institute, Inc. Cary, NC).
Statistical analysis
Statistical comparisons were performed using two-sided significance tests. Data analysis was carried out using SAS software version 9.3 (SAS institute, Inc. Cary, NC). Baseline demographic and clinical variables were summarized for each AMD group. Comparisons of continuous baseline variables among AMD groups used analysis of variance (or Kruskal-Wallis tests) and between group comparisons used t-tests (or Wilcoxon rank sum tests). All variables with the exception of LLD, were normally distributed, thus non-parametric tests were performed. Comparisons of discrete baseline variables among AMD groups employed chi-square or Fisher’s exact tests. For each continuous variable, frequency and percentage of values greater than the mean plus two standard deviations were tabulated and compared among groups using Fisher’s exact test.
Results
A total of 101 participants were enrolled in the study (80 AMD subjects, and 21 healthy controls) and underwent all study assessments. Participant characteristics are listed in Table I. Of the subjects with AMD, 33 had diagnosed AREDS 2 (early AMD) and 47 had diagnosed AREDS 3 (intermediate AMD). There was no overall significant intergroup variation in age, sex, race, ethnicity or smoking history (p > 0.63 for all). There were also no observed differences in common comorbidities including history or treatment of hypertension and diabetes mellitus, or history of myocardial infarction, coronary artery disease, dyslipidemia or dementia (p > 0.32 for all). The presence of other ocular conditions was infrequent in this population, with a low number of patients carrying the diagnosis of glaucoma suspect (2), Fuch’s dystrophy (2) and dry eye (6 patients). The few cases of glaucoma suspect and Fuch’s dystrophy were found in intermediate AMD group. There was no significant intergroup variation between groups related to dry eye (p = 0.18).
Table I.
Demographics and Past Medical History
| Variable | Statistic | Early AMD | Intermediate AMD | Normal | P-Value Overall |
|---|---|---|---|---|---|
|
| |||||
| Age | N | 33 | 47 | 21 | 0.64 |
|
| |||||
| Mean (SD) | 71.8 (8.3) | 70.4 (6.9) | 71.7 (7.4) | ||
|
| |||||
| Min/Median/Max | 57, 71, 89 | 50, 71, 84 | 60, 75, 82 | ||
|
| |||||
| Sex | 0.63 | ||||
| Male | N (%) | 13 (39) | 14 (30) | 8 (38) | |
|
|
|||||
| Female | N (%) | 20 (61) | 33 (70) | 13 (62) | |
|
| |||||
| Race | 1.00 | ||||
| White (Caucasian) | N (%) | 33 (100) | 45 (96) | 21 (100) | |
|
|
|||||
| Black or African American | N (%) | 0 | 1 (2) | 0 | |
|
|
|||||
| Native Hawaiian or Pacific Islander | N (%) | 0 | 1 (2) | 0 | |
|
| |||||
| Ethnicity | 0.71 | ||||
| Not Hispanic or Latino | N (%) | 28 (88) | 44 (94) | 20 (95) | |
|
|
|||||
| Hispanic or Latino | N (%) | 1 (3) | 0 | 0 | |
|
|
|||||
| Unknown/Not reported | N (%) | 3 (9) | 3 (6) | 1 (5) | |
|
| |||||
| Smoking | 0.66 | ||||
| Never | N (%) | 14 (44) | 22 (47) | 10 (50) | |
|
|
|||||
| Former | N (%) | 13 (41) | 23 (49) | 9 (45) | |
|
|
|||||
| Current | N (%) | 3 (9) | 1 (2) | 0 | |
|
|
|||||
| Unknown/Not reported | N (%) | 2 (6) | 1 (2) | 1 (5) | |
|
| |||||
| Past Medical History | |||||
| Hypertension | N (%) | 19 ( 58) | 24 ( 51) | 14 ( 67) | 0.46 |
|
|
|||||
| Diabetes mellitus | N (%) | 2 (6) | 7 ( 15) | 3 ( 14) | 0.48 |
|
|
|||||
| Myocardial infarction | N (%) | 0 | 0 | 1 (5) | |
|
|
|||||
| CAD | N (%) | 1 ( 3) | 4 ( 9) | 3 ( 14) | 0.32 |
|
|
|||||
| Dyslipidemia | N (%) | 17 ( 52) | 19 ( 40) | 8 ( 38) | 0.51 |
|
|
|||||
| Dementia | N (%) | 0 | 0 | 0 | --- |
|
| |||||
| Past Ocular History | |||||
|
|
|||||
| Glaucoma Suspect | N (%) | 0 | 2 ( 4) | 0 | --- |
|
|
|||||
| Fuchs Dystrophy | N (%) | --- | |||
|
|
|||||
| Dry Eye | N (%) | 4 ( 12) | 1 ( 2) | 1 ( 5) | 0.18 |
Continuous among-group comparison based ANOVA
Categorical among-group comparisons based on Fisher’s exact test.
Evaluation of visual acuity measures (BCVA and LLVA) between groups
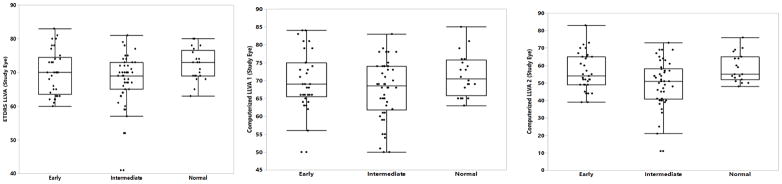
Various visual acuity measures are indicated in Table II (standard) and Table III (computerized). Standard best corrected visual acuity (BCVA) performance (p > 0.06 for all comparisons) and intraocular pressure (p = 0.11) (not shown) were similar between control and AMD groups. Unlike standard BCVA, however, computerized BCVA was significantly different between both early and intermediate AMD as compared to normal controls. Standard LLVA was significantly depressed in intermediate AMD patients relative to normal subjects (p = 0.03); however, this difference was absent when early AMD patients were compared to the normal group (p = 0.24) and did not distinguish intermediate from early disease (p = 0.43). Despite differences in standard LLVA, the mean standard LLD was not different between groups (p = 0.23). However, there were a significant number of patients with early and intermediate AMD with calculated LLD levels greater than two standard deviations above the mean of the normal group (Supplementary Table I, p = 0.04). The sensitivity of computerized LLVA to differentiate between patients in normal, early AMD, and intermediate AMD groups was dependent on the level of low luminance. Patients with intermediate AMD performed significantly worse than normal controls on computerized LLVA when background luminance was 0.5 cd/m2 (LLVA2, p = 0.01); this was not true for a luminance level of 1.3 cd/m2 (LLVA1, p = 0.09) (Figure 1). No differences were observed between normal and early AMD with either luminance level (LLVA1 p = 0.43, LLVA2 p = 0.37); however, the difference between intermediated and early AMD nearly reaches significance using the 0.5 cd/m2 (LLVA2, p = 0.06). Computerized LLD1 showed no differences between groups (p = 0.53); however, the lower luminance based computerized LLD2 did detect significant differences between early and intermediate AMD (p = 0.04).
Table II.
Visual acuity measures: Standard
| Measure | Statistic | Early | Intermediate | Normal | P- value overall | P-value Early vs Intermediate | P-value Early vs Normal | P-value Intermediate vs Normal |
|---|---|---|---|---|---|---|---|---|
| N | 33 | 47 | 21 | |||||
| BCVA | Mean (SD) | 81.03 (5.36) | 81.30 (5.67) | 83.81 (4.45) | 0.15 | 0.73 | 0.07 | 0.10 |
| Min, Median, Max | 69.0, 82.0, 90.0 | 64.0, 83.0, 92.0 | 73.0, 84.0, 90.0 | |||||
| LLVA | Mean (SD) | 70.06 (6.56) | 68.17 (7.21) | 72.24 (4.76) | 0.10 | 0.43 | 0.24 | 0.03 |
| Min, Median, Max | 60.0, 70.0, 83.0 | 41.0, 69.0, 81.0 | 63.0, 73.0, 80.0 | |||||
| LLD | Mean (SD) | 10.97 (4.52) | 13.13 (5.80) | 11.57 (3.19) | 0.23 | 0.11 | 0.49 | 0.36 |
| Min, Median, Max | 4.0, 11.0, 20.0 | 4.0, 12.0, 34.0 | 7.0, 11.0, 17.0 |
Overall p-value based on Kruskal-Wallis test of difference among medians
Pair-wise p-values based on Wilcoxon rank sum test of difference between medians.
Table III.
Visual acuity measures: Computerized
| Measure | Statistic | Early | Intermediate | Normal | P-value Overall | P-value Early vs Intermediate | P-value Early vs Normal | P-value Intermediate vs Normal |
|---|---|---|---|---|---|---|---|---|
| BCVA | N | 33 | 45 | 21 | 0.07 | 0.95 | 0.04 | 0.04 |
| Mean (SD) | 81.79 (6.03) | 81.09 (6.54) | 85.00 (5.50) | |||||
| Min, Median, Max | 70.0, 81.0, 98.0 | 60.0, 82.0, 90.0 | 73.0, 84.0, 95.0 | |||||
| LLVA1 | N | 33 | 46 | 20 | 0.21 | 0.29 | 0.43 | 0.09 |
| Mean (SD) | 70.00 (7.94) | 67.48 (8.20) | 71.55 (5.92) | |||||
| Min, Median, Max | 50.0, 69.0, 84.0 | 50.0, 68.5, 83.0 | 63.0, 70.5, 85.0 | |||||
| LLD1 | N | 33 | 45 | 20 | 0.53 | 0.40 | 0.29 | 0.73 |
| Mean | 11.79 (6.08) | 13.22 (5.92) | 13.60 (5.25) | |||||
| Min, Median, Max | 1.0, 13.0, 28.0 | 2.0, 13.0, 25.0 | 4.0, 13.0, 23.0 | |||||
| LLVA2 | N | 33 | 46 | 19 | 0.02 | 0.06 | 0.37 | 0.01 |
| Mean | 56.62 (10.69) | 50.15 (13.00) | 58.79 (8.13) | |||||
| Min, Median, Max | 39.0, 54.0, 83.0 | 11.0 51.0, 73.0 | 48.0, 55.0, 76.0 | |||||
| LLD2 | N | 33 | 45 | 19 | 0.08 | 0.04 | 0.71 | 0.13 |
| Mean | 25.27 (9.99) | 30.71 (10.86) | 26.21 (7.33) | |||||
| Min, Median, Max | 5.0, 27.0, 45.0 | 11.0, 31.0, 59.0 | 7.0, 26.0, 42.0 |
Overall p-value based on Kruskal-Wallis test of difference among medians
Pair-wise p-values based on Wilcoxon rank sum test of difference between medians.
Figure 1. Boxplot of Low Luminance Visual Acuity, standard and computerized.
Boxplot showing LLVA testing: (Left) standard and computerized at (Middle) 1.3 cd/m2 (LLVA1) or (Right)) 0.5 cd/m2 (LLVA2) for control and each AMD AREDS clinical group. LLVA measures are reported in ETDRS letters.
Evaluation of additional psychophysical measures between groups
The additional psychophysical measures included macular pigment optical density (MPOD; Table IV), cone contrast test (CCT) in Table V and mesopic microperimetry testing, and dark adaptation in Table VI.
Table IV.
Additional psychophysical measures: Macular Pigment Optical Density
| Variable | Statistic | Early | Intermediate | Normal | P-value Overall | P-value Early vs Intermediate | P-value Early vs Normal | P-value Intermediate vs Normal |
|---|---|---|---|---|---|---|---|---|
| Avg OD on radius 0.25 | N | 33 | 47 | 21 | 0.61 | 0.35 | 0.86 | 0.54 |
| Mean (SD) | 0.64 (0.22) | 0.68 (0.27) | 0.63 (0.19) | |||||
| Min, Median, Max | 0.3, 0.6, 1.2 | 0.2, 0.7, 1.2 | 0.3, 0.6, 1.0 | |||||
| Avg OD on radius 0.5 | N | 33 | 47 | 21 | 0.52 | 0.35 | 0.95 | 0.34 |
| Mean (SD) | 0.54 (0.18) | 0.60 (0.24) | 0.54 (0.18) | |||||
| Min, Median, Max | 0.2, 0.5, 0.9 | 0.1, 0.6, 1.1 | 0.2, 0.5, 0.9 | |||||
| Avg OD on radius 1 | N | 33 | 47 | 21 | 0.56 | 0.51 | 0.59 | 0.33 |
| Mean (SD) | 0.44 (0.16) | 0.45 (0.19) | 0.42 (0.17) | |||||
| Min, Median, Max | 0.2, 0.4, 0.7 | 0.0, 0.5, 0.8 | 0.1, 0.4, 0.8 | |||||
| Avg OD on radius 1.75 | N | 33 | 47 | 21 | 0.63 | 0.86 | 0.44 | 0.37 |
| Mean (SD) | 0.23 (0.10) | 0.23 (0.11) | 0.20 (0.09) | |||||
| Min, Median, Max | 0.1, 0.2, 0.4 | 0.0, 0.2, 0.4 | 0.1, 0.2, 0.5 |
Overall p-value based on Kruskal-Wallis test of difference among medians.
Pair-wise p-values based on Wilcoxon rank sum test of difference between medians.
Table V.
Additional psychophysical measures: Cone Contrast Test
| Measure | Statistic | Early | Intermediate | Normal | P-value Overall | P-value Early vs Intermediate | P-value Early vs Normal | P-value Intermediate vs Normal |
|---|---|---|---|---|---|---|---|---|
| CCT (%) | ||||||||
| Red | N | 33 | 47 | 21 | 0.06 | 0.13 | 0.24 | 0.03 |
| Mean (SD) | 65.30 (11.11) | 56.81 (19.88) | 66.90 (21.88) | |||||
| Min, Median, Max | 50.0, 70.0, 90.0 | 10.0, 60.0, 85.0 | 5.0, 70.0, 95.0 | |||||
| Green | N | 33 | 46 | 21 | 0.01 | 0.06 | 0.15 | 0.01 |
| Mean (SD) | 71.97 (11.99) | 60.11 (23.23) | 74.76 (18.13) | |||||
| Min, Median, Max | 30.0, 75.0, 90.0 | 0.0, 70.0, 90.0 | 10.0, 80.0, 95.0 | |||||
| Blue | N | 33 | 47 | 21 | 0.02 | 0.06 | 0.29 | 0.01 |
| Mean (SD) | 77.42 (16.35) | 67.34 (23.19) | 81.43 (17.19) | |||||
| Min, Median, Max | 45.0, 80.0, 100.0 | 0.0, 70.0, 100.0 | 40.0, 90.0, 100.0 |
Overall p-value based on Kruskal-Wallis test of difference among medians.
Pair-wise p-values based on Wilcoxon rank sum test of difference between medians.
Table VI.
Additional psychophysical measures: Microperimetry and Dark Adaptation
| Measure | Statistic | Early | Intermediate | Normal | P-value Overall | P-value Early vs Intermediate | P-value Early vs Normal | P-value Intermediate vs Normal |
|---|---|---|---|---|---|---|---|---|
| Microperimetry | ||||||||
| Average Threshold (dB) | N | 32 | 47 | 20 | 0.02 | 0.10 | 0.17 | 0.006 |
| Mean (SD) | 26.45 (2.76) | 25.20 (3.17) | 27.02 (3.63) | |||||
| Min, Median, Max | 18.7, 26.1, 30.6 | 17.9, 25.3, 30.6 | 14.4, 27.7, 31.3 | |||||
| Percent ReducedThreshold (%) | N | 31 | 47 | 20 | 0.001 | 0.03 | 0.09 | <0.001 |
| Mean (SD) | 24.92 (26.33) | 42.32 (35.11) | 15.40 (25.61) | |||||
| Min, Median Max | 0.0, 21.6, 100.0 | 0, 37.8, 100.0 | 0.0, 2.7, 94.6 | |||||
| Dark adaptation and rod intercept (min) | N | 30 | 38 | 21 | <0.001 | <0.001 | 0.09 | <0.001 |
| Mean (SD) | 7.71 (5.03) | 13.18 (6.40) | 6.20 (5.20) | |||||
| Min, Median, Max | 1.9, 6.6, 20.0 | 1.7, 13.3, 20.0 | 1.1, 4.2, 18.4 |
Overall p-value based on Kruskal-Wallis test of difference among medians.
Pair-wise p-values based on Wilcoxon rank sum test of difference between medians.
Analysis of MPOD was also performed but revealed no significant differences between groups. The average optical densities (OD) were found to be highest for the intermediate AMD group and lowest for the control group at the smallest 3 eccentricities tested; however, this trend did not reach statistical significance. This was true even after adjusting for age (data not shown; p >0.56 at all radii 0.25°, 0.5°, 1°, and 1.75°). Additionally, an inverse relationship between OD values and distance from the foveal center was observed, with average OD of 0.64 detected at 0.25°, 0.56 at 0.5°, 0.44 at 1°, 0.22 at 1.75° indicating an approximately 35% decrease between the closest and furthest distance tested.
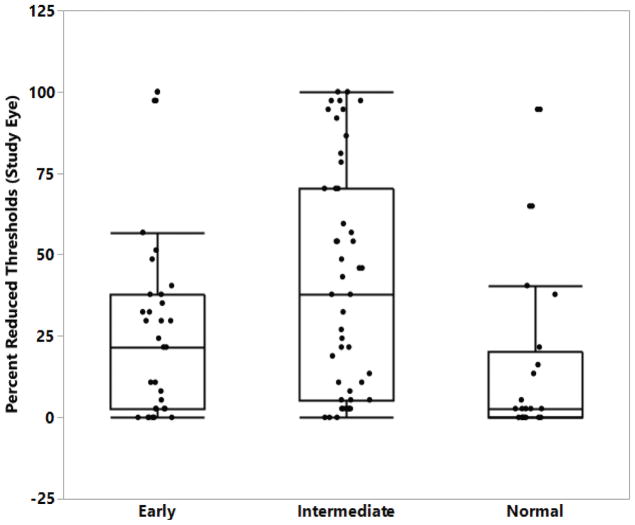
Representative images from CCT, Microperimetry and dark adaptation are indicated in Figure 2. Red, green, and blue CCT were all significantly reduced in intermediate AMD patients compared to normal subjects (p = 0.03, p = 0.01, p = 0.01), with green CCT showing the greatest degree of difference. Microperimetry percent reduced threshold served as a strong measure to distinguish between groups, as this metric was found to be significantly higher for patients with intermediate AMD as compared to normal (p = < 0.01) or early AMD patients (p = 0.03) (Figure 3). Microperimetry average threshold was significantly decreased in intermediate AMD patients versus controls (p < 0.01); however, no difference was observed when the two AMD groups were compared to each other, nor when early AMD was compared to the normal control group. The dark adaptation rod intercept was markedly greater in intermediate AMD patients than patients in either early AMD or normal control groups (p < 0.01 for both measures). In addition, the rod intercept of a significant number of patients with intermediate AMD (17) was greater than 2 SD above the mean measured from patients in the normal control group (Supplemental Table I, p < 0.01).
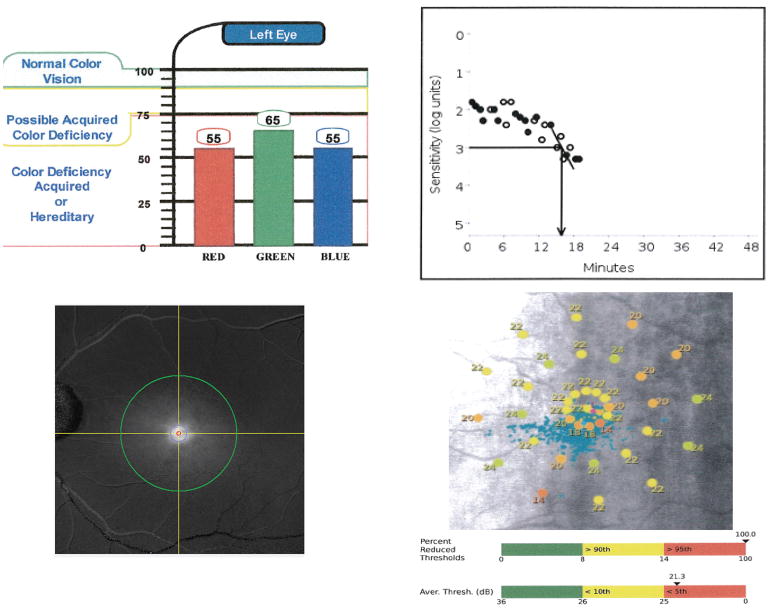
Figure 2. Examples of functional testing performed in a patient with intermediate AMD.
(Top Left) Report of the cone contrast test (CCT) showing a marked reduction in all cone tones; 90–100% represents a normal score. (Top Right) Dark adaptometry graph with rod intercept of 16 min (normal range < 7 min). (Bottom Left) Macular pigment optical density report screen: green circle represents plateau or reference point set at 7°, red circle at 0.5° and blue circle at 1° from foveal center. (Bottom Right) MAIA microperimetry SLO image showing decreased retinal sensitivities in multiple loci (normal retinal sensitivity > 25 dB), reported average threshold and percent reduced threshold values.
Figure 3. Boxplot of mesopic microperimetry percent reduced threshold.
Boxplot showing percent reduced threshold (PRT) on microperimetry testing for control and each AMD AREDS clinical group. PRT measured by percent.
Discussion
The paucity of treatment options for patients with dry age related macular degeneration is a significant clinical problem. This clinical unmet need promises only to worsen overtime, as a significant portion of the global population enters the sixth and seventh decade of life 18. Substantial barriers limiting the ability to effectively develop new treatments are a lack of markers of disease progression and of clinical trial endpoints that can reliably indicate disease progression.
To meet this need, several studies have been performed to understand the functional changes associated with dry AMD onset and progression 14,19,20. While providing important key insights into dry AMD pathology, this body of work has largely been limited by small sample sizes and by the number of psychophysical measures examined. Our study is second in size only to Owsley et al, which enrolled a larger cohort (253 AMD patients) but performed a smaller number of psychophysical tests21. A portion of the panel of psychophysical measures employed were previously described in our pilot study15, but improvements were made to the protocol and standard LLVA and we added a computerized method for BCVA and LLVA, dark adaptometry and MPOD.
As previously indicated, LLVA is a highly efficient and effective means of assessing central-cone mediated function under conditions of reduced luminance 15. The measure is further improved by computerization, allowing for standardization of operator and user-related factors, as well as facilitating ease of acquisition of multiple tests on the same interface. MAIA microperimetry serves as an robust measure of retinal sensitivity and indicator of foveal dysfunction19, while CCT serves as a sensitive index of color deficiencies that may indicate early stages of disease progression, even preceding the onset of visual acuity loss 22. Dark adaptation was added to the original panel to serve as a measure of visual cycle defects that appear early in AMD, as slowing of the recovery of light activity after exposing photopigment has previously been suggested to be linked to AMD onset and progression 16.
Consistent with previous work, we observed no differences in standard BCVA 14,20,23. Interestingly, however, computerized BCVA was significantly decreased in both early and intermediate AMD relative to controls but did not distinguish early and intermediate disease. This may be explained by the fact the mean BCVA score was greater, although not reaching statistical significance, in the control group using the computerized test as compared to the standard test, while the mean scores in early and intermediate AMD did not differ between standard and computerized. Given that we observed no differences between groups using standard BCVA, it is possible that the computerized method provides more sensitivity to differentiate between early-intermediate AMD and normal controls. Alternatively, this assessment may be too variable or the study is underpowered to detect subtle differences in BCVA. The data presented here is limited to the functional measures at baseline. Our continued longitudinal study with follow up visits every 6 months for 24 months will determine whether this measure is a sensitive or specific indicator of AMD onset or progression, given that photopic VA loss is not a common complaint associated with early stages of AMD24,25.
In the current study, we have suggested that LLVA (LLVA2: 0.5 cd/m2), MAIA microperimetry (percent reduced threshold and average threshold), CCT and dark adaptation (rod intercept) may be considered reliable markers for detecting intermediate AMD on a functional level. Several studies have previously indicated the value of LLVA as a clinical measure for detection of AMD. Puell et al was among the first to demonstrate that LLVA was an optimal measurement in participants with early stages of AMD, as they observed impairment in LLVA before changes in BCVA 23. Wu et al built upon these results by examining the extent to which LLVA is affected at differing clinical AMD severities: comparing measures across six clinical severity groups, a trend for LLVA to detect greater functional deficit than BCVA in eyes with increasingly poorer retinal sensitivity was revealed 19. Consistent with these findings, our study demonstrates that standard LLVA was significantly depressed between the more severe cases of AMD (AREDS 3) and normal controls but that this difference did not exist between the early cases (AREDS 2) and normal. This same trend was observed on computerized LLVA testing when background luminance was reduced to 0.5cd/m2. However this was not shown when background luminance was increased to 1.3cd/m2, indicating that the sensitivity of this test is dependent upon the level of background light provided.
Our study demonstrated variable results using LLD measures. Despite differences in standard LLVA, the mean standard LLD was not different between groups (p = 0.23). While computerized LLD1 also showed no differences between groups (p = 0.53); however, the lower luminance based computerized LLD2 did detect significant differences between early and intermediate AMD (p = 0.04). The findings in previous work are variable. Puell et al detected a significantly greater LLD in early stages of AMD when compared to control 23. In contrast, Wu et al detected LLD changes between AMD and control participants only when analyzing patients with non-foveal GA 19. Generally, the mechanism responsible for increased LLD in eyes with AMD is not clearly understood. Furthermore, the lack of consistency in the use of LLD to distinguish early AMD versus normal and intermediate disease versus normal subjects limits its potentially utility as a progression marker. As indicated by our data, the degree to which BCVA and LLVA differ is largely influenced by the amount of background luminance. Computerization influenced both BCVA and LLVA, indicating greater sensitivity in a more standardized setting. Given that only two luminance levels were tested in the current study, the possibility remains that more precise titration of luminance conditions in the setting of a computerized test will provide more robust and reproducible indications of differences in visual acuity measures at different levels of AMD severity. Furthermore, this also provides indication that the BCVA component of the measure may not be sensitive enough and therefore may influence the LLD measurements, an issue that may be addressed by increasing the study sample size.
In comparison to visual acuity measures, retinal sensitivity as measured by microperimetry has been suggested to be a topographic and more sensitive method for detecting functional deficits in patients at risk for AMD as compared to LLVA20. In our study, microperimetry percent reduced threshold served as the strongest measure to distinguish between all groups in our study: patients with intermediate AMD demonstrated significantly worse measures compared to normal (p = <0.01) or early AMD patients (p = 0.03). While differences were observed with microperimetry average threshold across groups, we found PRT to be superior to average threshold at differentiating between different degrees of severity of AMD between groups as well as relative to normal patients.
Cone contrast testing provides unique insight into visual defects related to color vision. Our results indicate dysfunction of S, M, L cones in intermediate AMD patients relative to normal subjects, as blue, red, and green CCT were all significantly different between these groups. These results controverted prior findings of greater S (blue) cone and rod pathway vulnerability in early disease over the L (red) and M (green) cone pathway26 and was not observed in early AMD patients in our study. LLVA, both standard and computerized, was positively correlated with both CCT blue and green, suggesting that the mesopic function attributed to LLVA testing is predominantly cone-mediated.
Dark adaptometry was added to the original panel that had been employed in our previous pilot study as a measure of visual cycle changes that may occur as eyes progress from normal to AMD. The dark adaptation rod intercept was markedly higher in intermediate AMD patients than in subjects with either early AMD or normal control groups. These data are in disagreement with previous studies that showed difference between early AMD and normal control participants on dark adaptation using a standard protocol (98% bleach at 12° on the inferior visual meridian)27 , however it is consistent with the more recent work that employed the modified protocol (76% bleach at 5° on the inferior visual meridian) as was used in our study 16 . In addition, the rod intercept of a significant number of patients with intermediate AMD was greater than 2 SD above the mean measured from patients in the normal control group, suggesting that intermediate patients, if affected, show a significant decline in this visual function. Rod intercept was negatively correlated with visual acuity measures (e.g. BCVA, LLVA), likely reflecting progressive rod impairment with increasing stages of AMD. Interestingly, there was no strong relationship of rod intercept with the other psychophysical measures.
Macular pigment (MP) has been shown to facilitate fine central and color vision 28 as well as demonstrate unique short wavelength light filtering properties 29. There is also evidence to suggest that MP may protect the macula from oxidative damage 30,31 . As such, elevated MPOD is predicted to prevent the occurrence and progression of AMD 32,33. Despite these indications, the results of previous studies investigating the relationship between MP and AMD have been mixed 34,35, 36. Collectively, literature results suggest that the sensitivity of MPOD to predict AMD may be limited to the most severe form of disease. Nevertheless, it is important to note that the lack of definitive conclusions relating MPOD to AMD across several studies may be confounded by differences in methodology and small sample sizes studied. As such, additional studies using the Heidelberg Spectralis in more advanced stages of AMD are required prior to conclusively determine the utility of MPOD measures for predicting outcomes related to AMD onset or progression.
In summary, our study supports the use of computerized LLVA, CCT, dark adaptometry, and MAIA microperimetry as functional endpoints in patients with early and intermediate dry AMD. Our previous pilot trial indicated that these tests were both well tolerated and had high test-retest reliability15. For LLVA, a computerized LLVA2 with a low luminance level of 0.5cd/m2 was the most sensitive for detecting differences between control and intermediate AMD. All CCTs were effective at distinguishing control and intermediate disease. Rod intercept as measured by dark adaptometry with a modified protocol also successfully differentiated intermediate AMD from normal controls. Finally, microperimetry average threshold as well as percentage reduced threshold served as the most promising and sensitive measures to distinguish groups: both early and intermediate from normal. Importantly, our data failed to provide many robust measures which differentiate early versus intermediate disease, with the exception of microperimetry. As such, this large, prospective study indicates that these functional endpoints may serve as good candidates for endpoints that may detect disease progression from normal to onset of early stage disease. Key data to confirm our current assumptions based on the cross-sectional data at baseline will be gained as to the utility of these tests to track disease progression as part of the longitudinal study that is currently underway. Collectively, the results from these two studies will provide much needed comprehensive insight into the natural history of AMD and into early endpoints for future clinical trials in this population.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institute of Health/National Eye Institute (K23EY026988), Research to Prevent Blindness and, through Duke University, and industry support from Hoffmann-La Roche.
-
Financial Disclosures:
Kimberly J. Cocce – No disclosures; Sandra S. Stinnett – No disclosures; U.F.O. Luhmann is employee of F.Hoffmann-La Roche. Ltd; Lejla Vajzovic – No disclosures ; Anupama Horne – No disclosures; Stefanie G. Shuman – No disclosures Cynthia A. Toth receives financial support from Genetech and holds a patent with Alcon Laboratories.
Scott W. Cousins has financial relationships with B&L (consulting and grant for a trial), Stealth BioTherapeutics (consulting and grant for trial), Alimera Sciences (consulting), PanOptica (consulting and equity), TheraKine (consulting/directorship), and Eyedesis (founder, equity and consulting). Eleonora M. Lad receives financial support from Hoffman La Roche.
Other Acknowledgements – none
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Owsley C, McGwin G, Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47(2):528–535. doi: 10.1167/iovs.05-1222. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 4.Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109(7):1235–1242. doi: 10.1016/s0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- 5.Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999;128(1):45–53. doi: 10.1016/s0002-9394(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 6.Comparison of Age-related Macular Degeneration Treatments Trials Research G. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe GJ, Schmitz-Valckenberg S, Boyer D, et al. Randomized Trial to Evaluate Tandospirone in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The GATE Study. Am J Ophthalmol. 2015;160(6):1226–1234. doi: 10.1016/j.ajo.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. 2013;36(7):385–395. doi: 10.1016/j.tins.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SY, Bourgeois H, Corbe C, et al. Randomized clinical trial France DMLA2: effect of trimetazidine on exudative and nonexudative age-relatedmacular degeneration. Retina. 2012;32(4):834–843. doi: 10.1097/IAE.0b013e31822058a3. [DOI] [PubMed] [Google Scholar]
- 10.Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012;53(12):7484–7491. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- 11.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 13.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 14.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(9):1480–1488. 1488e1481–1482. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandramohan A, Stinnett SS, Petrowski JT, et al. Visual Function Measures in Early and Intermediate Age-Related Macular Degeneration. Retina. 2016;36(5):1021–1031. doi: 10.1097/IAE.0000000000001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427–1431. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennison JL, Stack J, Beatty S, Nolan JM. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp Eye Res. 2013;116:190–198. doi: 10.1016/j.exer.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Colby SLaJMO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Population Estimates and Projections. 2015 https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf.
- 19.Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121(8):1612–1619. doi: 10.1016/j.ophtha.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015;133(4):442–448. doi: 10.1001/jamaophthalmol.2014.5963. [DOI] [PubMed] [Google Scholar]
- 21.Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G., Jr Comparison of Visual Function in Older Eyes in the Earliest Stages of Age-related Macular Degeneration to Those in Normal Macular Health. Curr Eye Res. 2016;41(2):266–272. doi: 10.3109/02713683.2015.1011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabin J, Gooch J, Ivan D. Rapid quantification of color vision: the cone contrast test. Invest Ophthalmol Vis Sci. 2011;52(2):816–820. doi: 10.1167/iovs.10-6283. [DOI] [PubMed] [Google Scholar]
- 23.Puell MC, Barrio AR, Palomo-Alvarez C, Gomez-Sanz FJ, Clement-Corral A, Perez-Carrasco MJ. Impaired mesopic visual acuity in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(11):7310–7314. doi: 10.1167/iovs.11-8649. [DOI] [PubMed] [Google Scholar]
- 24.Parisi V, Perillo L, Tedeschi M, et al. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina. 2007;27(7):879–890. doi: 10.1097/IAE.0b013e318042d6aa. [DOI] [PubMed] [Google Scholar]
- 25.Vujosevic S, Smolek MK, Lebow KA, Notaroberto N, Pallikaris A, Casciano M. Detection of macular function changes in early (AREDS 2) and intermediate (AREDS 3) age-related macular degeneration. Ophthalmologica. 2011;225(3):155–160. doi: 10.1159/000320340. [DOI] [PubMed] [Google Scholar]
- 26.Scholl HP, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45(2):574–583. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 27.Owsley C, McGwin G, Jr, Jackson GR, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114(9):1728–1735. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch J, Curcio CA. The spatial resolution capacity of human foveal retina. Vision Res. 1989;29(9):1095–1101. doi: 10.1016/0042-6989(89)90058-8. [DOI] [PubMed] [Google Scholar]
- 29.Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992;32(1):105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- 30.Wrona M, Rozanowska M, Sarna T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic Biol Med. 2004;36(9):1094–1101. doi: 10.1016/j.freeradbiomed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504(1):56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62(6 Suppl):1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 33.Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 34.Raman R, Biswas S, Gupta A, Kulothungan V, Sharma T. Association of macular pigment optical density with risk factors for wet age-related macular degeneration in the Indian population. Eye (Lond) 2012;26(7):950–957. doi: 10.1038/eye.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsika C, Tsilimbaris MK, Makridaki M, Kontadakis G, Plainis S, Moschandreas J. Assessment of macular pigment optical density (MPOD) in patients with unilateral wet age-related macular degeneration (AMD) Acta Ophthalmol. 2011;89(7):e573–578. doi: 10.1111/j.1755-3768.2011.02170.x. [DOI] [PubMed] [Google Scholar]
- 36.Ren XT, Gu H, Han X, et al. Measurement of macular pigment optical density among healthy Chinese people and patients with early-stage age-related macular degeneration. Int J Ophthalmol. 2015;8(6):1190–1195. doi: 10.3980/j.issn.2222-3959.2015.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.