Abstract
Objective The objective of this study was to examine the effect of cochlear dose on hearing preservation in stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (fSRT) for vestibular schwannoma (VS).
Design This is a retrospective case–control study.
Setting This study was completed at the Ronald Reagan UCLA Medical Center, a university-affiliated tertiary care center.
Participants Patients who underwent SRS (marginal dose of 12 Gy) or fSRT (marginal dose of 50.4 Gy) procedures for VS were included in the study.
Main Outcome Measures The main outcome measure was hearing preservation. Audiometric data, when available, were used to determine the level of hearing according to the Gardner Robertson scale.
Results A total of 38 patients (14 SRS and 24 fSRT) were analyzed. SRS patients with decreased hearing received a significantly higher minimum cochlear dose (7.41 vs. 4.24 Gy, p = 0.02) as compared with those with stable hearing. In fSRT patients, there were no significant differences in cochlear dose for patients with decreased hearing as compared with those with stable hearing. For SRS patients, who received a minimum cochlear dose above 6 Gy, there was a significant risk of decreased hearing preservation (odds ratio: 32, p = 0.02).
Conclusion Higher minimum cochlear dose was predictive of decreased hearing preservation following SRS. Though the study is low powered, the radiation dose to the cochlea should be a parameter that is considered when planning SRS or fSRT therapies for patients with VS.
Keywords: cochlear dose, hearing, stereotactic radiosurgery, fractionated stereotactic radiotherapy, vestibular schwannoma
Introduction
Vestibular schwannomas (VSs) are benign tumors that develop from myelin-forming Schwann cells that encompass the eighth cranial nerve. Stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (fSRT) have emerged as noninvasive alternatives to microsurgery for the treatment of VS, with high rates of tumor control and relatively fewer side effects documented in the literature. 1 2 3 Adverse effects associated with radiation-based therapies include tissue toxicity and damage to adjacent cranial nerves, but improved imaging modalities and lower marginal doses have minimized such morbidities. 4 5
A major complication of SRS and fSRT is hearing decline. Hearing deterioration immediately following radiation therapy is rare and is often caused by neural edema, demyelination, or inflammation at the site of the lesion. 6 7 However, the cause for delayed hearing loss remains unclear, with proposed mechanisms including gradual loss of microvessels, thrombosis of the internal auditory artery, and direct or immune-mediated injury to the vestibulocochlear nerve or cochlea hair cells. 6 8 The adverse effects of SRS and fSRT may be associated with radiation dose deliver to the cochlea.
Components of the cochlea are sensitive to radiation exposure and may be damaged as a result of radiation-based treatments. 9 Studies have shown that hearing loss following SRS was correlated with the treatment parameters of the intracanalicular part of the tumor and the radiation dose to the cochlea. 10 11 However, Paek et al measured the radiation dose delivered to the cochlea, vestibulocochlear nerve, and cochlear nucleus in the brain stem, and found that a maximum dose of ≥ 10 Gy to the cochlear nucleus was a significant predictor of hearing loss. 12 Although SRS and fSRT aim to control tumor progression, preserve hearing, and minimize complications, the effects of these treatments on cochlear dose and hearing outcomes are not well established. In this single institutional experience, we estimate the dose of radiation delivered to the cochlea during SRS and fSRT, and analyze its effect on hearing preservation.
Methods
Patient Selection
A retrospective review was conducted to identify all patients who received SRS or fSRT for VS at a single tertiary care hospital between years 2009 and 2014. Patients with complete hearing loss, those without pretreatment imaging to evaluate cochlea size, or those with clinical follow-up duration less than 12 months were excluded. Patients with bilateral tumors or other intracranial lesions were also excluded.
Treatment Parameters
Treatment imaging for radiation therapy was acquired with 1.5 mm slice thickness computed tomography (CT) and 1.5 or 3.0 T magnetic resonance imaging (MRI). CT to MRI fusion was performed to define the target tumor and normal structures for planning. iPlan RT Dose treatment planning software (BrainLAB, Munich, Germany) was used to generate beam portals and calculate radiation dose via a pencil beam algorithm for a 6-MV Novalis Tx linear accelerator (BrainLAB) using a HD120 multileaf collimator with 2.55 mm leaf width. The treatment plans were reviewed and approved in a collaborative manner by a neurosurgeon and a radiation oncologist.
Treatment was delivered via dynamic conformal arc or intensity modulated radiotherapy technique depending on the ability to improve sparing of normal tissue structures. Patients treated with SRS received a marginal dose of 12 Gy prescribed to the 90% isodose line. Patients treated with fSRT received a marginal dose of 50.4 Gy delivered through 28 fractions of 1.80 Gy prescribed to the 90% isodose line. All patients were immobilized in custom thermoplastic facemasks (BrainLAB) created at the time of the CT simulation scan. Final alignment was accomplished with ExacTrac image guidance system (BrainLAB) using stereoscopic X-ray for patient positioning and verification. For the SRS cohort, treatment setup tolerance was set to 1 mm, and verification imaging was performed prior to each beam or arc. For the fSRT cohort, treatment setup tolerance was set to 2 mm, and verification imaging was performed prior to the first beam or arc. Verification imaging was also repeated if the patient moved or if the treatment couch rotated.
Hearing Outcomes
Hearing was categorized as serviceable, poor, or no hearing. Serviceable hearing was defined as the capacity to use the phone unaided and to discriminate normal speech in the affected ear. Only patients with serviceable or poor hearing at initial evaluation were included in the study. Audiometric data were used to determine the level of hearing according to the Gardner Robertson (GR) scale. GR grades I to II were classified as serviceable hearing, GR grades III to IV as poor hearing, and GR grade V as no hearing. If pure tone average (PTA) and speech discrimination (SD) did not correlate to the same grade, the lower grade was used. For 15 patients, individual audiometric data were unavailable and hearing function was obtained from a combination of patient reports and physical examinations. Hearing outcome was dichotomized as either stable or decreased by comparing hearing at the last clinical follow-up with the initial pretreatment hearing.
Dosimetry Calculations
Cochlea volumes were determined from T1- and T2-weighted MRI scans and confirmed on bone windows of the planning CT scans ( Fig. 1 ). The minimum, mean, and maximum cochlear radiation doses were obtained from the treatment planning software. Integrated radiation doses delivered to the cochlea volumes were calculated from dose–volume histograms. Cochlea volumes receiving greater than 5.3 Gy with SRS, and 40.5 and 48.6 Gy with fSRT were recorded. The 5.3 Gy cutoff value was chosen based on studies by Brown et al and Kano et al, which reported significantly improved hearing outcomes when the cochlea received less than 5.3 Gy. 13 14 The 40.5 and 48.6 Gy cutoffs were chosen based on studies by Rasmussen et al and Thomas et al. The authors of those studies reported that patients with preserved hearing received 40.5 and 48.6 Gy to a smaller percentage of the cochlea volume than those with decreased hearing. 15 16 These volumes and measurements were completed retrospectively by the first author (L.K.C.) and verified by the second author (N.U.).
Fig. 1.

Images demonstrating the method of contouring the cochlea using ( A ) T2-weighted magnetic resonance images and confirmed with the ( B ) planning computed tomography scans.
Statistical Analysis
Statistical analyses were performed using SPSS v.22.0 (IBM Corporation, Armonk, New York, United States). Univariate analysis was completed using Mann–Whitney's U -test to compare clinical and dosimetric parameters between the stable and decreased hearing subgroups. Multivariate analysis was performed by testing for collinearity, and if present, only one offending variable (e.g., minimum dose OR mean dose OR maximum dose OR integrated dose) was included in the logistic regression at a time. The dependent variable was hearing outcome. Threshold cutoff values were then determined using either Fisher's exact test or Mann–Whitney's U -test. Kaplan–Meier's survival curves were used to evaluate hearing decline. All tests for significance were two tailed, with p -values < 0.05 considered statistically significant.
Results
A total of 67 patients received SRS or fSRT for VS between 2009 and 2014. Twelve patients were excluded for having no pretreatment hearing, 14 patients were excluded for insufficient clinical follow-up, 2 patients were excluded due to other intracranial lesions, and 1 patient was excluded due to incomplete treatment. The final study population included 38 patients treated with SRS ( n = 14) and fSRT ( n = 24) between May 2009 and November 2014. Patient characteristics for the SRS and fSRT groups are summarized in Table 1 .
Table 1. Summary of patient characteristics.
| Variable | Overall mean | SRS mean (range) |
fSRT mean (range) |
|---|---|---|---|
| Patients, n | 38 | 14 | 24 |
| Sex, n (%) | |||
| Female | 21 | 9 (64.3) | 12 (50.0) |
| Male | 17 | 5 (35.7) | 12 (50.0) |
| Age, y | 61.8 | 65.3 (40.2–79.4) | 59.7 (34.5–79.7) |
| Follow-up, mo | 42.6 | 38.3 (13.3–67.5) | 45.1 (12.7–84.8) |
| Tumor laterality, n (%) | |||
| Right | 17 | 1 (7.1) | 16 (66.7) |
| Left | 21 | 13 (92.9) | 8 (33.3) |
| Prior treatment, n (%) | 0 | 0 (0) | 0 (0) |
| Tumor volume, cm 3 | 2.13 | 1.44 (0.16–3.16) | 2.53 (0.10–11.54) |
| Local tumor control, n (%) | 34 | 13 (92.9) | 21 (87.5) |
| Cochlea volume, mm 3 | 80.3 | 73.8 (41–121) | 85.3 (47–131) |
| Pretreatment hearing, n (%) | |||
| Serviceable | 25 | 7 (50.0) | 18 (75.0) |
| Poor | 13 | 7 (50.0) | 6 (25.0) |
Abbreviations: fSRT, fractionated stereotactic radiotherapy; SRS, stereotactic radiosurgery.
Radiosurgery Group
The initial pretreatment evaluation included seven patients (50%) with serviceable hearing and seven patients (50%) with poor hearing. By the last clinical follow-up, 4 patients (29%) had decreased hearing, and 10 patients (71%) had stable hearing. Of the seven patients who initially presented with serviceable hearing, two patients (29%) had decreased hearing, while five patients (71%) had stable hearing at last follow-up ( Fig. 2 ). The hearing statuses of patients before and after SRS are summarized in Table 2 . Patients with decreased hearing had a mean of 11.6 months (range: 6.6–16.9) before hearing decline. The 5-year hearing preservation rate for all patients after SRS was 64%.
Fig. 2.
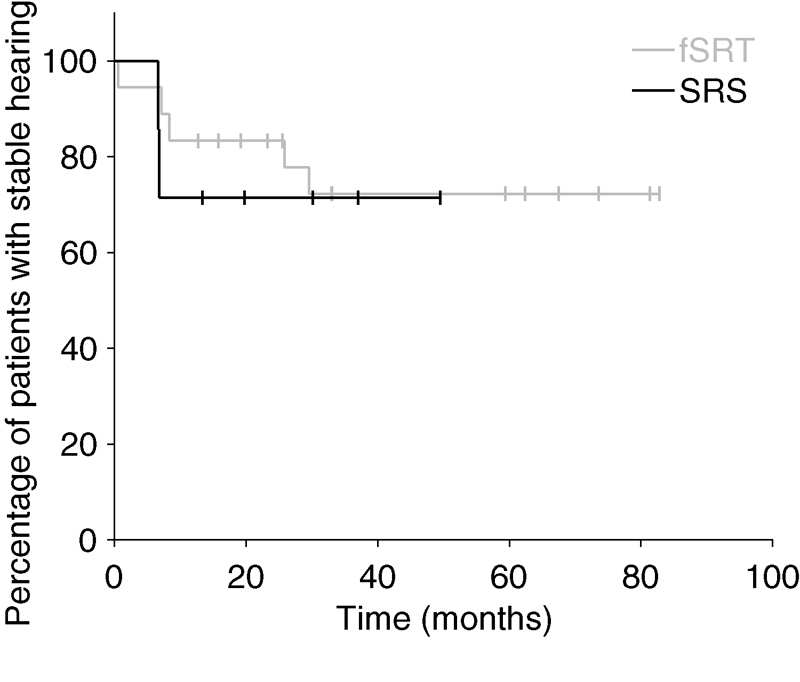
Hearing preservation of patients with serviceable hearing treated with SRS and fSRT. fSRT, fractionated stereotactic radiotherapy; SRS, stereotactic radiosurgery.
Table 2. Patient hearing status before and after SRS.
| Posttreatment hearing, n | Serviceable | Poor | No |
|---|---|---|---|
| Serviceable | 5 | 2 | 0 |
| Poor | 0 | 7 | 0 |
Abbreviation: SRS, stereotactic radiosurgery.
The mean cochlea volume was 73.8 mm 3 (range: 41–121). The minimum, mean, and maximum radiation doses delivered to the cochlea were 5.37 Gy (range: 2.5–10.1), 8.31 Gy (range: 4.1–11.9), and 10.8 Gy (range: 6.2–12.9), respectively. The mean integrated radiation dose delivered to the cochlea was 0.6 mJ (range: 0.3–1.1). Fig. 3 shows the averaged dose–volume histograms of the cochlea in patients with stable and decreased hearing after SRS ( p < 0.001).
Fig. 3.

Averaged dose–volume histograms of the cochlea for patients with stable and decreased hearing after SRS treatment. The cochlea volume received higher radiation dose in patients with decreased hearing than in patients with stable hearing ( p < 0.001). SRS, stereotactic radiosurgery.
Results of different clinical and dosimetric parameters in GR grades I to IV patients with stable and decreased hearing after SRS are summarized in Table 3 . When comparing the two groups, tumor volume ( p = 0.05) and minimum cochlear radiation dose ( p = 0.02) were significantly different. All other parameters were statistically insignificant. Cochlea volume, tumor volume, minimum radiation dose, and percentage of cochlea receiving greater than 5.3 Gy were then entered as covariates into multivariate logistic regression models. For SRS patients who received a minimum cochlear dose above 6 Gy, there was a significant risk of decreased hearing preservation (odds ratio: 32, p = 0.02).
Table 3. Comparison of hearing outcomes in GR grades I to IV patients after SRS.
| Variable | Hearing outcome after SRS | ||
|---|---|---|---|
| Stable mean ± SD |
Decreased mean ± SD |
p -Value | |
| Patients, n (%) | 9 (64.3) | 5 (35.7) | |
| Female, n (%) | 5 (55.6) | 4 (44.4) | 0.38 |
| Age, y | 65.0 ± 11.5 | 65.8 ± 6.8 | 0.89 |
| Follow-up, mo | 38.1 ± 20.6 | 38.8 ± 8.3 | 0.94 |
| Pretreatment hearing, n (%) | |||
| GR grade 1 | – | – | |
| GR grade 2 | 3 (33.3%) | 4 (80%) | 0.27 |
| GR grade 3 | 6 (66.7%) | 1 (20%) | 0.27 |
| GR grade 4 | – | – | |
| Cochlea volume, mm 3 | 87.4 ± 27.7 | 62.8 ± 15.9 | 0.10 |
| Tumor volume, cm 3 | 1.96 ± 1.2 | 0.51 ± 0.49 | 0.05 |
| Local tumor control, n (%) | 9 (100) | 4 (80) | 0.18 |
| Radiation delivered to cochlea | |||
| Minimum dose, Gy | 4.24 ± 1.89 | 7.41 ± 2.45 | .02 |
| Mean dose, Gy | 7.26 ± 2.74 | 10.21 ± 2.19 | .06 |
| Maximum dose, Gy | 10.12 ± 2.62 | 12.00 ± 1.28 | .13 |
| Integrated dose, mJ | 0.58 ± 0.28 | 0.62 ± 0.06 | .52 |
| Percentage of cochlea receiving, % | |||
| > 5.3 Gy | 76.3 ± 35.8 | 95.8 ± 9.34 | 0.26 |
Abbreviations: GR, Gardner Robertson; SD, standard deviation; SRS, stereotactic radiosurgery.
In determining cutoff values, a significant difference in the number of patients with preserved hearing was seen with a minimum cochlear radiation dose less than 5.4 Gy ( p = 0.02) or a mean cochlear radiation dose less than 8.8 Gy ( p = 0.02). There was also a significant difference in the percentage of cochlea volume receiving greater than 6.5 Gy between those with stable and decreased hearing ( p = 0.04).
Fractionated Radiotherapy Group
The initial pretreatment evaluation included 18 patients (75%) with serviceable hearing and 6 patients (25%) with poor hearing. By the last clinical follow-up, 9 patients (38%) had decreased hearing, and 15 patients (63%) had stable hearing. Of the 18 patients who initially presented with serviceable hearing, 5 patients (28%) had decreased hearing, while 13 patients (72%) had stable hearing at last follow-up ( Fig. 2 ). The hearing statuses of patients before and after fSRT are summarized in Table 4 . Patients with decreased hearing had a mean of 15.7 months (range: 0.6–38.0) before hearing decline. The 5-year hearing preservation rate for all patients after fSRT was 63%.
Table 4. Patient hearing status before and after fSRT.
| Pretreatment hearing, n | Serviceable | Poor | No |
|---|---|---|---|
| Serviceable | 14 | 4 | 0 |
| Poor | 0 | 5 | 1 |
Abbreviation: fSRT, fractionated stereotactic radiotherapy.
The mean cochlea volume was 85.3 mm 3 (range: 47–131). The minimum, mean, and maximum radiation doses delivered to the cochlea were 30.1 Gy (range: 12.1–47.6), 42.4 Gy (range: 30.2–50.7), and 50.3 Gy (range: 37.9–53.7), respectively. The mean integrated radiation dose delivered to the cochlea was 3.5 mJ (range: 1.9–6.0). Fig. 4 shows the averaged dose–volume histograms of the cochlea in patients with stable and decreased hearing after fSRT ( p = 0.42).
Fig. 4.

Averaged dose–volume histogram of the cochlea for patients with stable and decreased hearing after fSRT treatment. The cochlea volume did not receive higher radiation dose in patients with decreased hearing than in patients with stable hearing ( p = 0.42). fSRT, fractionated stereotactic radiotherapy.
Results of different clinical and dosimetric parameters in GR grades I to IV patients with stable and decreased hearing after fSRT are summarized in Table 5 . None of the 13 parameters was statistically different between the two groups. Cochlea volume and integrated dose were then entered as covariates into multivariate logistic regression models with no variables kept in the final model each time.
Table 5. Comparison of hearing outcomes in GR grades I to IV patients after fSRT.
| Variable | Hearing outcome after fSRT | ||
|---|---|---|---|
| Stable mean ± SD |
Decreased mean ± SD |
p -Value | |
| Patients, n (%) | 15 (62.5) | 9 (37.5) | |
| Female, n (%) | 7 (46.7) | 5 (55.6) | > 0.99 |
| Age, y | 58.4 ± 16.2 | 61.9 ± 12.2 | 0.86 |
| Follow-up, mo | 45.0 ± 25.6 | 45.1 ± 19.9 | 0.77 |
| Pretreatment hearing, n (%) | |||
| GR grade 1 | 5 (33.3) | 2 (22.2) | |
| GR grade 2 | 8 (53.3) | 3 (33.3) | |
| GR grade 3 | 2 (13.3) | 4 (44.4) | |
| GR grade 4 | – | – | |
| Cochlea volume, mm 3 | 77.7 ± 23.6 | 97.9 ± 27.9 | 0.13 |
| Tumor volume, cm 3 | 3.12 ± 3.27 | 1.56 ± 1.68 | 0.29 |
| Local tumor control, n (%) | 13 (86.7) | 8 (88.9) | > 0.99 |
| Radiation delivered to cochlea | |||
| Minimum dose, Gy | 31.29 ± 9.45 | 28.22 ± 9.57 | 0.45 |
| Mean dose, Gy | 42.26 ± 6.60 | 42.68 ± 6.32 | 0.86 |
| Maximum dose, Gy | 49.90 ± 3.79 | 50.93 ± 1.91 | 0.48 |
| Integrated dose, mJ | 3.36 ± 1.06 | 3.90 ± 1.01 | 0.22 |
| Percentage of cochlea receiving, % | |||
| > 40.5 Gy | 70.4 | 67.7 | 0.67 |
| > 48.6 Gy | 23.6 | 23.5 | 0.94 |
Abbreviations: fSRT, fractionated stereotactic radiotherapy; GR, Gardner Robertson; SD, standard deviation.
Discussion
In recent decades, radiation therapy has emerged as an effective treatment for VS. Although radiation based therapies originally saw high rates of cranial nerve neuropathies, better tumor targeting and decreased radiation dosing have resulted in reduced rates of facial and trigeminal neuropathies. 17 18 However, rates of hearing loss remain close to 50%. 19 20 21 Interest in prognostic factors associated with hearing outcomes in patients with VS has grown. Tumor size, patient age, and pretreatment hearing status have been reported to predict hearing outcomes following SRS. 13 22 23 Recently, cochlear dose and irradiation of inner ear structures have been associated with decreased hearing following radiation therapy. 10 13 24 25 26 27 28
In this study, the authors investigated the radiation delivered to cochlea and its effects on hearing preservation following SRS and fSRT for VS. To our knowledge, this is the third study to investigate the prognostic implication of cochlear dose after fSRT. 15 16 Our analyses suggest that cochlear radiation dose is better associated with hearing outcomes following SRS than with fSRT. However, given the higher prescription isodose line and larger tumor volumes for the fSRT method, a more gradual dose gradient is achieved than in SRS. As such, there is likely less variation in overall cochlear dose with fSRT than with SRS.
Hearing Outcome after SRS and fSRT
In our cohort of patients with VS and serviceable hearing, the rate of hearing preservation was 71% in the SRS group and 72% in the fSRT group. Various rates of hearing preservation have been previously reported for both SRS and fSRT. Studies using SRS with median doses of 12 to 13 Gy have reported hearing preservation rates of 22 to 77%. 20 29 30 31 Studies investigating conventional fSRT with median doses of 46.8 to 52.5 Gy delivered in 25 to 29 fractions have reported hearing preservation rates of 54 to 93%. 32 33 34 The discrepancy between reported values can be explained in part by disparities in the definition of hearing loss. Studies may report hearing loss as any decline in GR grade or a specific increase in PTA or SD. Furthermore, comparison between different studies is often confounded by difference treatment schemes and follow-up lengths. Regardless, our rates of hearing preservation appear consistent within the reported range. 35 However, the course of hearing loss following radiation therapy also requires further investigation.
In a study by Combs et al, hearing loss was found to be most significant within the first 6 to 10 months after treatment. 36 Conversely, Choy et al reported that hearing deterioration was mainly observed within the first 2 years after treatment. 19 Hearing loss has also been observed at longer intervals. Chopra et al reported hearing preservation of 74% at the 3-year follow-up which declined to 44% by the 10-year follow-up. 30 In another study, Hasegawa et al reported a drop from 43% at the 5-year follow-up to only 34% at the 8-year follow-up. 37 In our study, hearing loss occurred at a mean of 10 months after SRS and 15 months after fSRT. The exact course of hearing deterioration following SRS and fSRT may not be fully elucidated. Therefore, the need for long-term follow-up is required to accurately evaluate hearing preservation as auditory function appears to decline even years after treatment.
Cochlear Dose in SRS
Several previous studies have demonstrated the adverse effects of radiation on inner ear structures. 9 38 39 40 The first report of unexpectedly high doses of radiation being delivered to inner ear structures during VS treatment was in 2003 by Linskey et al. 41 Specifically, the authors retrospectively evaluated the radiation dose delivered to different temporal bone structures in 54 patients during Gamma Knife surgery with a mean tumor marginal dose of 14.2 Gy. The authors found that 14.8% of patients received cochlear doses greater than 12 Gy. This led the authors to hypothesize that radiation delivered to the inner ear contributed to decreased hearing following radiation-based therapies. Since then, numerous studies have validated the role of cochlear dose in decreased hearing following SRS. 10 11 13 16 24 25 26 27 28 Recent studies have attempted to better quantify this relationship by identifying a specific radiation threshold that avoids suboptimal outcomes.
Although there is a consensus that cochlear dose correlates with hearing outcomes, the specific radiation dose that the cochlea can tolerate requires further elucidation. Prior studies support avoiding a cochlear dose of 3 to 5 Gy during SRS. 14 24 25 Jacob et al reported that patients who received a mean dose less than 5 Gy to the cochlea were more likely to maintain serviceable hearing. 25 Another study demonstrated that a radiation dose less than 4.2 Gy to the central cochlea was predictive of preserved hearing in patients younger than 60 years. 14 Baschnagel et al evaluated the hearing outcomes of 40 patients with serviceable hearing after Gamma Knife surgery for VS and found that a mean cochlear dose less than 3 Gy had a 2-year hearing preservation rate of 91% compared with 59% in those who received greater than 3 Gy. 24 However, the authors reported that even low cochlear irradiation had a dose–response relationship on hearing loss with none of the patients receiving a mean cochlear dose less than 2 Gy losing serviceable hearing at last follow-up. Brown et al proposed that the cochlea volume irradiated above a certain threshold, rather than a specific point radiation dose, predicted hearing loss. 13 The authors suggested limiting the percentage of the cochlea volume exposed to greater than 5.3 Gy. Specifically, for every single percentage increase in cochlea volume receiving greater than 5.3 Gy, there was a 0.168 dB increase in PTA at last follow-up.
Compared with prior literature, the SRS patients in this study received a higher overall cochlear dose. We attributed this finding to a large proportion of SRS patients presenting with nonserviceable hearing, and thus treatment planning for these patients may not have specifically constrained the cochlea. Nevertheless, there was a significant difference in the minimum dose delivered to the cochlea volume between those with stable and decreased hearing on univariate analysis. Our findings suggest that limiting the minimum cochlear dose to less than 5.4 Gy and potentially restricting mean cochlear dose to less than 8.8 Gy results in more patients with preserved hearing. Furthermore, we found a significant relationship between the percentage of the cochlea volume receiving greater than 6 Gy and decreased hearing outcomes.
Cochlear Dose in fSRT
Few studies have directly investigated the effect of cochlear dose on hearing outcomes after fSRT for VS treatment. Thomas et al analyzed hearing outcomes of 34 patients treated with 50 Gy delivered in 25 fractions with a tumor dose of 45 Gy prescribed to 90% isodose. 16 The authors recorded the percentage of cochlea receiving 90, 80, and 50% of the prescription dose, as well as the minimum and maximum point doses to the cochlea. The authors found a significant difference in all five dosimetric variables in those with less than 15 dB increase in speech reception threshold and those with greater than 15 dB increase at last follow-up. Similarly, Rasmussen et al measured the percentage of the cochlea receiving a minimum of 90% of the total radiation dose in 15 patients who initially presented with serviceable hearing and underwent treatment with 54 Gy delivered in 27 to 30 fractions. 15 The authors reported a significant positive correlation between cochlear dose and an increased speech reception threshold, but not with SD loss. In a prospective study of head-and-neck cancers, Pan et al reported that a mean cochlear dose less than 45 Gy was favorable for hearing preservation. 42
Limitations
Several limitations were made apparent during data synthesis. First, compared with those patients treated with SRS, patients treated with fSRT had larger tumors and higher rates of serviceable hearing. This selection bias may reflect the current literature reporting higher hearing preservation rates with fSRT than with SRS. Second, a large number of patients who presented with complete hearing loss or those with insufficient follow-up duration were excluded. This decreased our sample size and thus, reduced our statistical power. Audiometry results were also incomplete, and therefore, PTA and SRT could not be analyzed as primary outcome measures. Third, a minimum follow-up period of 12 months may not adequately encompass the course of hearing deterioration following SRS or fSRT. Finally, possible inaccuracies in iPlan RT Dose for small field dosimetry have been previously reported. However, for field sizes larger than 10 mm the error is less than 4% and typically below 3%. Future investigations should include a randomized controlled trial comparing SRS and fSRT.
Conclusion
Our results reveal that higher minimum cochlear dose was predictive of decreased hearing preservation following SRS and should be considered in VS treatment. In addition, the percentage of the cochlea volume exposed to more than 6 Gy should be limited. Conversely, cochlear dose delivered in fSRT did not predict hearing loss. Consensus regarding the dose below which radiation poses no adverse effects to the cochlea has not be reached. The cochlear nerve is intrinsic to the target volume, and therefore, hearing loss may be an inevitable consequence regardless of dose.
Funding Statement
Funding Lawrance Chung was partially supported by the AMA Foundation Seed Grant and the AΩA Carolyn L. Kuckein Student Research Fellowship. Thien Nguyen and John P. Sheppard are recipients of the David Geffen Medical Scholarship. Carlito Lagman was partially supported by the Gurtin Skull Base Research Fellowship. Isaac Yang was partially supported by the UCLA Visionary Ball Fund Grant, the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, the Jason Dessel Memorial Seed Grant, the UCLA Honberger Endowment Brain Tumor Research Seed Grant, and the Stop Cancer! Research Career Development Award.
Footnotes
Conflict of Interest Dr Yang reports personal fees from BrainLAB, personal fees from Baxter, outside the submitted work.
References
- 1.Arthurs B J, Lamoreaux W T, Mackay A R et al. Gamma Knife radiosurgery for vestibular schwannomas: tumor control and functional preservation in 70 patients. Am J Clin Oncol. 2011;34(03):265–269. doi: 10.1097/COC.0b013e3181dbc2ab. [DOI] [PubMed] [Google Scholar]
- 2.Mulder J J, Kaanders J H, van Overbeeke J J, Cremers C W. Radiation therapy for vestibular schwannomas. Curr Opin Otolaryngol Head Neck Surg. 2012;20(05):367–371. doi: 10.1097/MOO.0b013e328357d337. [DOI] [PubMed] [Google Scholar]
- 3.Rykaczewski B, Zabek M. A meta-analysis of treatment of vestibular schwannoma using Gamma Knife radiosurgery. Contemp Oncol (Pozn) 2014;18(01):60–66. doi: 10.5114/wo.2014.39840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondziolka D, Lunsford L D, McLaughlin M R, Flickinger J C. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426–1433. doi: 10.1056/NEJM199811123392003. [DOI] [PubMed] [Google Scholar]
- 5.Miller R C, Foote R L, Coffey R J et al. Decrease in cranial nerve complications after radiosurgery for acoustic neuromas: a prospective study of dose and volume. Int J Radiat Oncol Biol Phys. 1999;43(02):305–311. doi: 10.1016/s0360-3016(98)00397-6. [DOI] [PubMed] [Google Scholar]
- 6.Farrell C J, Andrews D W. New York: Springer; 2008. Acoustic tumors: viewpoint—stereotactic radiotherapy. [Google Scholar]
- 7.Pensak M L. New York: Thieme; 2001. Controversies in Otolaryngology. [Google Scholar]
- 8.Delbrouck C, Hassid S, Massager N et al. Preservation of hearing in vestibular schwannomas treated by radiosurgery using Leksell Gamma Knife: preliminary report of a prospective Belgian clinical study. Acta Otorhinolaryngol Belg. 2003;57(03):197–204. [PubMed] [Google Scholar]
- 9.Linskey M E, Johnstone P A. Radiation tolerance of normal temporal bone structures: implications for Gamma Knife stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;57(01):196–200. doi: 10.1016/s0360-3016(03)00413-9. [DOI] [PubMed] [Google Scholar]
- 10.Massager N, Nissim O, Delbrouck Cet al. Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome J Neurosurg 2013119(Suppl):733–739. [DOI] [PubMed] [Google Scholar]
- 11.Massager N, Nissim O, Delbrouck C et al. Role of intracanalicular volumetric and dosimetric parameters on hearing preservation after vestibular schwannoma radiosurgery. Int J Radiat Oncol Biol Phys. 2006;64(05):1331–1340. doi: 10.1016/j.ijrobp.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Paek S H, Chung H T, Jeong S S et al. Hearing preservation after Gamma Knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104(03):580–590. doi: 10.1002/cncr.21190. [DOI] [PubMed] [Google Scholar]
- 13.Brown M, Ruckenstein M, Bigelow Det al. Predictors of hearing loss after Gamma Knife radiosurgery for vestibular schwannomas: age, cochlear dose, and tumor coverage Neurosurgery 20116903605–613., discussion 613–614 [DOI] [PubMed] [Google Scholar]
- 14.Kano H, Kondziolka D, Khan A, Flickinger J C, Lunsford L D. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg. 2009;111(04):863–873. doi: 10.3171/2008.12.JNS08611. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen R, Claesson M, Stangerup S E et al. Fractionated stereotactic radiotherapy of vestibular schwannomas accelerates hearing loss. Int J Radiat Oncol Biol Phys. 2012;83(05):e607–e611. doi: 10.1016/j.ijrobp.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Di Maio S, Ma R et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg. 2007;107(05):917–926. doi: 10.3171/JNS-07/11/0917. [DOI] [PubMed] [Google Scholar]
- 17.Foote K D, Friedman W A, Buatti J M, Meeks S L, Bova F J, Kubilis P S. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95(03):440–449. doi: 10.3171/jns.2001.95.3.0440. [DOI] [PubMed] [Google Scholar]
- 18.Yang I, Sughrue M E, Han S J et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009;93(01):41–48. doi: 10.1007/s11060-009-9842-3. [DOI] [PubMed] [Google Scholar]
- 19.Choy W, Spasic M, Pezeshkian P et al. Outcomes of stereotactic radiosurgery and stereotactic radiotherapy for the treatment of vestibular schwannoma. Neurosurgery. 2013;60 01:120–125. doi: 10.1227/01.neu.0000430307.78949.4e. [DOI] [PubMed] [Google Scholar]
- 20.Lunsford L D, Niranjan A, Flickinger J C, Maitz A, Kondziolka D.Radiosurgery of vestibular schwannomas: summary of experience in 829 cases J Neurosurg 2013119(Suppl):195–199. [PubMed] [Google Scholar]
- 21.Yang I, Sughrue M E, Han S J et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112(04):851–859. doi: 10.3171/2009.8.JNS0985. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T. Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg. 2011;115(06):1078–1086. doi: 10.3171/2011.7.JNS11749. [DOI] [PubMed] [Google Scholar]
- 23.Kano H, Kondziolka D, Khan A, Flickinger J C, Lunsford L D.Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma: clinical article J Neurosurg 2013119(Suppl):863–873. [DOI] [PubMed] [Google Scholar]
- 24.Baschnagel A M, Chen P Y, Bojrab D et al. Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg. 2013;118(03):571–578. doi: 10.3171/2012.10.JNS12880. [DOI] [PubMed] [Google Scholar]
- 25.Jacob J T, Carlson M L, Schiefer T K, Pollock B E, Driscoll C L, Link M J.Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions Neurosurgery 20147405466–474., discussion 474 [DOI] [PubMed] [Google Scholar]
- 26.Lasak J M, Klish D, Kryzer T C, Hearn C, Gorecki J P, Rine G P. Gamma Knife radiosurgery for vestibular schwannoma: early hearing outcomes and evaluation of the cochlear dose. Otol Neurotol. 2008;29(08):1179–1186. doi: 10.1097/MAO.0b013e31818b6639. [DOI] [PubMed] [Google Scholar]
- 27.Timmer F C, Hanssens P E, van Haren A E et al. Gamma Knife radiosurgery for vestibular schwannomas: results of hearing preservation in relation to the cochlear radiation dose. Laryngoscope. 2009;119(06):1076–1081. doi: 10.1002/lary.20245. [DOI] [PubMed] [Google Scholar]
- 28.Wackym P A, Runge-Samuelson C L, Nash J J et al. Gamma Knife surgery of vestibular schwannomas: volumetric dosimetry correlations to hearing loss suggest stria vascularis devascularization as the mechanism of early hearing loss. Otol Neurotol. 2010;31(09):1480–1487. doi: 10.1097/MAO.0b013e3181f7d7d4. [DOI] [PubMed] [Google Scholar]
- 29.Boari N, Bailo M, Gagliardi Fet al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients J Neurosurg 2014121(Suppl):123–142. [DOI] [PubMed] [Google Scholar]
- 30.Chopra R, Kondziolka D, Niranjan A, Lunsford L D, Flickinger J C. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68(03):845–851. doi: 10.1016/j.ijrobp.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Fukuoka S, Takanashi M, Hojyo A, Konishi M, Tanaka C, Nakamura H. Gamma Knife radiosurgery for vestibular schwannomas. Prog Neurol Surg. 2009;22:45–62. doi: 10.1159/000163382. [DOI] [PubMed] [Google Scholar]
- 32.Champ C E, Shen X, Shi W et al. Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery. 2013;73(03):489–496. doi: 10.1227/NEU.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 33.Litre F, Rousseaux P, Jovenin N et al. Fractionated stereotactic radiotherapy for acoustic neuromas: a prospective monocenter study of about 158 cases. Radiother Oncol. 2013;106(02):169–174. doi: 10.1016/j.radonc.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Woolf D K, Williams M, Goh C L et al. Fractionated stereotactic radiotherapy for acoustic neuromas: long-term outcomes. Clin Oncol (R Coll Radiol) 2013;25(12):734–738. doi: 10.1016/j.clon.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Fong B M, Pezeshkian P, Nagasawa D T, De Salles A, Gopen Q, Yang I. Hearing preservation after LINAC radiosurgery and LINAC radiotherapy for vestibular schwannoma. J Clin Neurosci. 2012;19(08):1065–1070. doi: 10.1016/j.jocn.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Combs S E, Welzel T, Schulz-Ertner D, Huber P E, Debus J. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2010;76(01):193–200. doi: 10.1016/j.ijrobp.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118(03):557–565. doi: 10.3171/2012.10.JNS12523. [DOI] [PubMed] [Google Scholar]
- 38.Li J J, Guo Y K, Tang Q L et al. Prospective study of sensorineural hearing loss following radiotherapy for nasopharyngeal carcinoma. J Laryngol Otol. 2010;124(01):32–36. doi: 10.1017/S0022215109991435. [DOI] [PubMed] [Google Scholar]
- 39.Low W K, Burgess R, Fong K W, Wang D Y. Effect of radiotherapy on retro-cochlear auditory pathways. Laryngoscope. 2005;115(10):1823–1826. doi: 10.1097/01.mlg.0000175061.59315.58. [DOI] [PubMed] [Google Scholar]
- 40.Tan P X, Du S S, Ren C, Yao Q W, Yuan Y W. Radiation-induced cochlea hair cell death: mechanisms and protection. Asian Pac J Cancer Prev. 2013;14(10):5631–5635. doi: 10.7314/apjcp.2013.14.10.5631. [DOI] [PubMed] [Google Scholar]
- 41.Linskey M E, Johnstone P A, O'Leary M, Goetsch S. Radiation exposure of normal temporal bone structures during stereotactically guided Gamma Knife surgery for vestibular schwannomas. J Neurosurg. 2003;98(04):800–806. doi: 10.3171/jns.2003.98.4.0800. [DOI] [PubMed] [Google Scholar]
- 42.Pan C C, Eisbruch A, Lee J S, Snorrason R M, Ten Haken R K, Kileny P R. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61(05):1393–1402. doi: 10.1016/j.ijrobp.2004.08.019. [DOI] [PubMed] [Google Scholar]


