Abstract
Objectives To review a surgical series of petroclival meningiomas and the factors considered in the choice of approach.
Design Retrospective review.
Setting The study was conducted in a university hospital in southern Brazil.
Participants Twenty-two patients with petroclival meningioma originating from the upper two-thirds of the clivus medial to the fifth cranial nerve.
Main Outcome Measures Gross-total resection, mortality, major morbidity, new cranial nerve deficits and tumor progression or recurrence.
Results Retrosigmoid approach was used in tumors <3 cm and in those at or below the internal auditory meatus. Posterior petrosectomy was performed for tumors extending into the middle fossa. Gross-total resection was performed in 11 patients (50%). The mean follow-up time was 32 months (6–75 months). There were four cases of tumor progression or recurrence, which were treated with radiosurgery.
Conclusions Resection of petroclival meningiomas remains challenging. In most cases, the retrosigmoid approach was sufficient, without affecting the degree of tumor resection. Petrosal approaches were reserved for patients with tumor extension into the middle fossa.
Keywords: brain tumor, clivus, meningioma, petroclival, skull base
Introduction
Petroclival meningiomas remain one of the most challenging surgical lesions of the skull base. Over the past three decades, advances in microsurgical techniques, new operating microscopes, ultrasonic aspirators, intraoperative neuromonitoring, and advances in intensive care and microsurgical anatomy have led to better outcomes than has the natural history of the disease, with acceptable morbidity. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Posterior fossa meningiomas can be located at different sites in relation to the clivus. Conceptually, petroclival meningiomas are located medial to the fifth cranial nerve (CN V). 1 15 16 Petrous, tentorial, cavernous sinus, and mid-clival meningiomas and meningiomas originating from the anterior border of the foramen magnum are not considered petroclival meningiomas, and the decision on which approach to use depends greatly on this factor. Petroclival meningiomas frequently displace the brainstem and basilar artery posteriorly and to the contralateral side. They may also displace or involve CN III, IV, and V, displace CN VII laterally and CN VI medially, and extend into the internal auditory meatus, jugular foramen, Meckel's cave, Dorello's canal, and the ipsilateral cavernous sinus. 13 15
Because of the rarity of these tumors and the different management philosophies in skull base approaches, there is currently no high-quality, high-level decision based on randomized controlled trials establishing the superiority of one surgical approach over another. The trans-temporal and fronto-orbito-zygomatic approaches can reduce the operative distance to the tumor and give a wider exposition of the tumor and its relationship with the CNs. However, the retrosigmoid approach results in fewer approach-related complications and is less time consuming. Some authors state that the main reason for subtotal resection is the lack of a dissection plane or infiltration into CNs, brainstem, or major vessels, 17 18 regardless of the approach.
The aim of this study was to review a series of petroclival meningiomas and assess the factors used to determine the choice of surgical approach.
Methods
Of a series of 53 patients treated for posterior fossa meningiomas by the first author (Gustavo Rassier Isolan) between 2007 and 2014, 32 had petroclival meningiomas. Of these, 22 met the criteria for “true” petroclival meningioma: those originating from the upper two-thirds of the clivus medial to the CN V. 15 Their medical records, imaging studies, and pathology reports were reviewed. Ten patients were not operated on: two refused surgery due to advanced age or comorbidities; four were asymptomatic with a predominantly cavernous pattern; and four showed no tumor growth on serial magnetic resonance imaging (MRI). Because these 10 patients had no clinical visual loss or extraocular muscle impairment, they were primarily referred for upfront radiosurgery or radiotherapy. Gross-total resection (GTR) was defined as Simpson grade I, II, and III resection 19 ( Table 1 ), confirmed by postoperative gadolinium-enhanced MRI. The study was approved by the Research Ethics Committee at Hospital de Clínicas de Porto Alegre.
Table 1. Simpson grading system for meningiomas according to the extent of resection.
| Grade | Definition |
|---|---|
| I | Macroscopically complete removal with excision of dural attachment and abnormal bone |
| II | Macroscopically complete removal with endothermy coagulation (Bovie or laser) of dural attachment |
| III | Macroscopically complete removal without resection or coagulation of extradural extensions |
| IV | Partial removal leaving intradural tumor in situ |
| V | Simple decompression with or without biopsy |
Results
Clinical Characteristics
Twenty-two patients underwent resection of petroclival meningiomas over a 7-year period; there were 18 women, and the mean age was 52.4 years (range 35–82 years). Headache was the most common presenting symptom, observed in 18 patients, followed by hearing loss ( n = 12), facial numbness ( n = 5), ataxia ( n = 3), progressive hemiparesis ( n = 2), and facial weakness ( n = 2). Trigeminal neuropathic pain refractory to conventional medical therapy was the main symptom in two patients. Difficulty swallowing due to lower CN deficits was present in three patients. Four patients had papilledema due to hydrocephalus. One older patient had severe visual loss, which justified the surgical approach. MRI findings are shown in Table 2 .
Table 2. Magnetic resonance imaging findings.
| Parameter | No. of cases |
|---|---|
| Brainstem compression | 11 |
| Tumor size (in cm) | |
| > 6 | 11 |
| 3–6 | 3 |
| < 3 | 8 |
| Extension | |
| Into CS | 9 |
| Into IAM | 7 |
| Into JF | 3 |
| BA displacement | 8 |
| BA encasement | 3 |
| Plane on T2-weighted images | 15 |
| Brainstem edema | 3 |
| Supratentorial ventricular dilatation with ICH signals | 4 |
Abbreviations: BA, basilar artery; CS, cavernous sinus; IAM, internal auditory meatus; ICH, intracranial hypertension; JF, jugular foramen.
Pathology
Except for one Grade 2 meningioma with brainstem edema and one Grade 3 meningioma, all other tumors in this series were classified as World Health Organization (WHO) Grade 1.
Approaches
Early in the series, lateral skull base approaches (petrosal approaches) were performed in patients, regardless of tumor size ( Fig. 1 ). At that time, posterior petrosectomy was performed in six patients ( Figs. 2 , 3 , and 4 ), and total petrosectomy—double petrosal—was used in two patients. After 2011, the retrosigmoid approach was used in tumors <3 cm and in those at or below the internal auditory meatus ( Fig. 5 ). The posterior petrosal approach was the choice in tumors extending into the middle fossa, as it allows simultaneous exposure of the middle and posterior fossa. In these cases, magnetic resonance angiography was performed to identify the insertion of the vein of Labbé. If the vein of Labbé drained into the superior petrosal sinus or the patient had a high jugular bulb, the lateral skull base approaches were not considered. 20 Table 3 shows the approaches used in this series and their results. The fronto-orbito-zygomatic approach was reserved for a specific case ( Fig. 4 ).
Fig. 1.
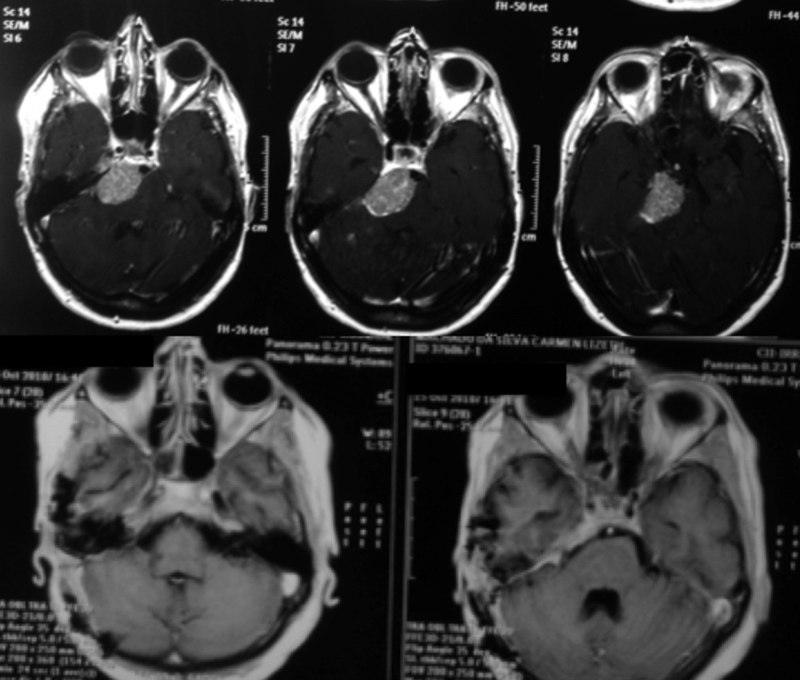
Petroclival meningioma of the middle third of the clivus in a patient with preserved hearing. A posterior petrosectomy was performed with complete tumor resection without postoperative deficits.
Fig. 2.
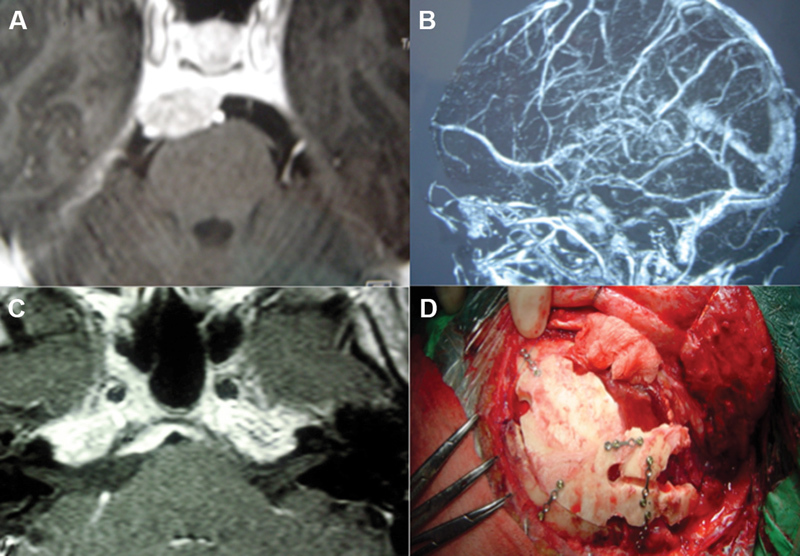
A 43-year-old patient with an asymptomatic petroclival meningioma involving the upper and middle clivus. A posterior petrosectomy was performed without labyrinthectomy due to preoperative hearing preservation. ( A ) Gadolinium-enhanced T1-weighted MRI showing the tumor. ( B ) Venous MR angiography showing posterior insertion of the vein of Labbé in the superior petrosal sinus, not contraindicating complete petrosal approach. ( C ) Gadolinium-enhanced T1-weighted MRI 3 months after surgery showing Simpson III tumor resection, as expected through intraoperative view. ( D ) Mastoid reconstruction. The patient had no postoperative deficits and showed no recurrence 6 years after surgery. MR, magnetic resonance; MRI, magnetic resonance imaging.
Fig. 3.
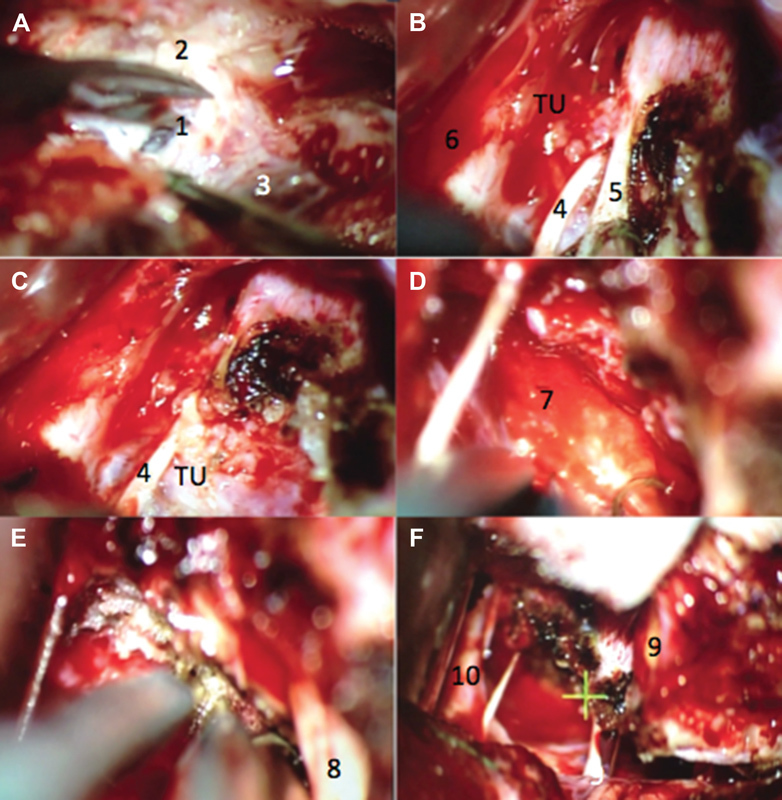
Previous case. ( A ) Presigmoid dural opening. ( B ) Coagulation of the tentorium and identification of the trochlear nerve before cutting the tentorium. ( C ) Tentorium free edge was released and reflected, noting the relationship of the tumor with the trochlear nerve. ( D ) Clivus insertion after tumor resection. ( E ) Clivus dural coagulation after tumor resection. ( F ) Cranial nerve preservation within the surgical field after tumor resection. 1. Presigmoid dura mater; 2. mastoid portion of the temporal bone; 3. sigmoid sinus; 4. trochlear nerve; 5. tentorial notch; 6. temporal lobe; 7. clivus; 8. trigeminal nerve; 9. superior petrosal vein; and 10. basilar artery. TU. Tumor.
Fig. 4.
Patient with prior posterior fossa tumor resection and adjuvant whole-brain radiotherapy 20 years earlier. Neurological examination showed paralysis of all cranial nerves related to the cavernous sinus and right amaurosis, ataxia. The new MRI revealed an enlargement of the tumor extending into the upper clivus, orbit, infratemporal, and pterygopalatine fossae ( A and B ). A combined approach was used (fronto-orbito-zygomatic craniotomy with previous petrosectomy), with complete tumor resection and wide reconstruction using fat and a temporalis muscle flap ( C ). The siphon and petrous segment of the internal carotid artery were skeletonized after resection of the petrous apex in the Mullan's triangle and Kawase's triangle to resect tumor extensions into the pterygopalatine and infratemporal fossae, respectively ( D ). The patient died due to a postoperative hemispheric venous infarction and pulmonary sepsis 3 weeks after surgery. The pathological study demonstrated anaplastic meningioma. MRI, magnetic resonance imaging.
Fig. 5.
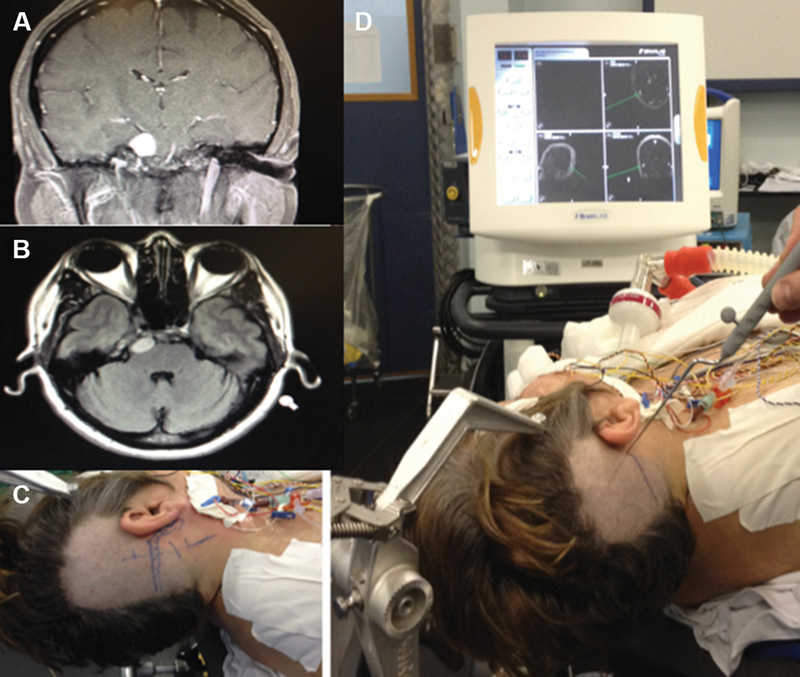
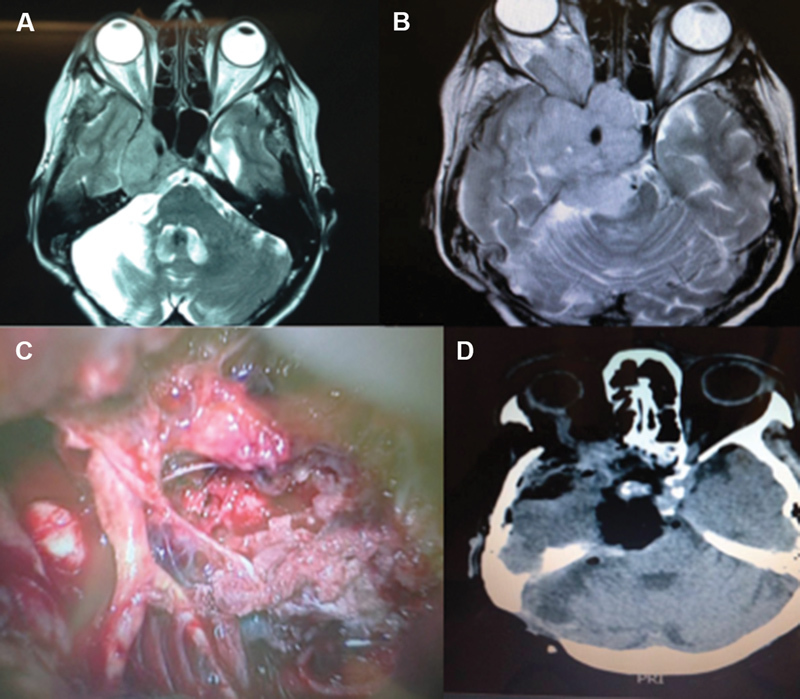
Patient with a small petroclival meningioma in the upper clivus that was resected by the retrosigmoid approach. Coronal ( A ) and axial ( B ) gadolinium-enhanced MRI scans. Incision site (C) and neuronavigation showing the junction position of the transverse and sigmoid sinuses (D).
Table 3. Results according to surgical approach.
| Surgical approach | n | Tumor size (>diameter – cm) |
GTR (%) | Mortality ( n ) | Major morbidity ( n ) | Permanent new CN deficits ( n ) | Tumor progression or recurrence ( n ) | Radiotherapy or RS ( n ) |
|---|---|---|---|---|---|---|---|---|
| Retrosigmoid | 8 | 4 to >6 2 to 3–6 2 to <3 |
87,5 | 0 | 0/8 | 2/8 | 1/8 | 1 |
| Posterior petrosectomy | 6 | 4 to 3–6 2 to >6 |
66,7 | 0 | 2/6 | 1/6 | 1/6 | − |
| Total petrosectomy | 2 | 1 to >6 1 to 3–6 |
50 | 1 | 2/2 | 2/2 | 0/2 | − |
| FOZ | 2 | 1 to 3–6 1 to >6 |
50 | 1 | 1/2 | 1/2 | 1/2 | 1 |
| Pretemporal | 1 | 1 to <3 | 0 | 0 | 0/1 | 0/1 | 1/1 | 1 |
| Combined (FOZ + anterior petrosectomy) | 1 | 1 to <3 | 0 | 0 | 1/1 | 1/1 | 0/1 | − |
| Endoscopic endonasal | 1 | 1 to 3–6 | 0 | 0 | 0/1 | 0/1 | 0/1 | 1 |
| VPS | 1 | 1 to >6 | 0 | 0 | 0/1 | 0/1 | 0/1 | 1 |
Abbreviations: CN, cranial nerve; FOZ, fronto-orbito-zygomatic; GTR, gross-total resection; RS, radiosurgery; VPS, ventriculoperitoneal shunt.
One patient had a small sphenopetroclival meningioma with invasion of the sphenoid sinus causing rhinorrhea. In this case, extended endoscopic endonasal approach was used for partial resection of the lesion inside the sphenoid sinus. The fistula was treated with a pedicled nasoseptal flap with postoperative resolution of the cerebrospinal fluid (CSF) leak, and the patient was later referred for radiosurgery. Over 1 year of follow-up, MRI showed no evidence of tumor growth. 21
GTR was achieved in 11 patients (50%). The reasons for incomplete resection were as follows: tumor adherence to the brainstem in two patients; adherence to major blood vessels in two patients; and venous infarction of the temporal lobe in two patients, which required interruption of the procedure. In three patients, some minor residual tumor was deliberately left in the region of the cavernous sinus. In two cases of sphenopetroclival meningioma, the goal was to perform a partial resection for optic nerve decompression. These five patients with subtotal resection were supplemented with radiosurgery. In addition, tumor progression was observed in two patients with residual tumor, and both underwent radiosurgery. The remaining patients with small residual tumor are currently being followed.
In this initial series, we have not changed our impression regarding GTR between intraoperative evaluation and postoperative MRI. It should be taken into account that we considered Simpson I, II, and even III as GTR, as in most other series reported in the literature. Using this concept, we already know intraoperatively whether GTR was achieved. There is a risk of failing to perceive part of the tumor in the middle fossa if a retrosigmoid approach is used. To avoid this, we performed a posterior petrosal approach that provided a full view of the middle fossa extension. In cases of cavernous sinus invasion, we also anticipated that part of the tumor had to be considered a “leave-me-alone lesion,” except in cases with visual impairment caused by this component.
Morbidity and Mortality
Tables 3 and 4 show surgically induced CN deficits, improvement in previous deficits, and the Simpson grade of resection. There were two perioperative deaths in this series: an older patient, who was operated on due to severe visual loss, died 3 weeks after surgery due to complications of pneumonia and sepsis; and another patient died due to extensive malignant cerebral venous infarction after complete resection of sphenopetroclival tumor 15 days after surgery.
Table 4. Surgically induced deficits and improved previous deficits and correlation with Simpson grade of resection.
| Simpson grade | New postoperative or worsened CN deficit | New deficit 6 months later | Improvement of previous deficit |
|---|---|---|---|
| I (0) | – | – | – |
| II (11) | 9 | 5 | 3 |
| III (7) | 2 | 1 | 0 |
| IV (4) | 1 | 0 | 1 |
| V (0) | – | – | – |
Abbreviation: CN, cranial nerve.
Early in the series, two patients had a stroke resulting in major neurological deficits: one left temporal lobe venous infarction and one brainstem infarction. The former case had a large petroclival meningioma, presenting tetraparesis and paralysis of right CN V to XII, and developed hemiplegia in the postoperative period and pneumonia. In the latter case, the venous infarction was probably due to brain retraction during anterior petrosectomy. This patient underwent emergency decompressive craniectomy 24 hours after surgery, but his aphasia and right hemiplegia persisted. Another patient developed posterior fossa hypertension after a suboccipital retrosigmoid approach, with persistent hemiparesis even after emergency decompressive surgery. This patient had pneumonia and critical illness polyneuromyopathy. There was no specific description between these ischemic events and tumor consistency as measured by T2 signal, presence of CSF cleft between tumor and brainstem on MRI, or MRI evidence of brainstem edema.
New postoperative cranial neuropathies occurred in seven patients. The CN dysfunctions observed after surgery were facial numbness ( n = 2), facial weakness ( n = 5), abducens nerve palsy ( n = 2), trochlear nerve palsy ( n = 3), and oculomotor nerve palsy ( n = 1); of these, four were transient. Three patients had permanent deficits 6 months after surgery. Cross-facial nerve grafting for facial nerve reanimation and muscle transposition were performed in three patients by the plastic surgery team. One patient had persistent CN VI palsy and was referred to a neuro-ophthalmologist. Patients with CN IV palsy reported diplopia; however, after 6 months, symptoms were mild, and none of the patients needed further treatment.
Four patients had hydrocephalus before surgery and underwent ventriculoperitoneal shunt placement. One patient developed hydrocephalus after surgery. CSF leaks (rhinorrhea) occurred in two patients after a petrosal approach and were treated with lumbar drainage. These two patients also required ventriculoperitoneal shunt placement. Three patients developed meningitis, which was successfully treated. Two patients with refractory trigeminal neuropathic pain improved their pain scores in the immediate postoperative period due to decompression of the trigeminal nerve ( Figs. 6 and 7 ). One patient with a large calcified petroclival meningioma and swallowing deficit underwent partial tumor resection, and his deficit improved postoperatively. He had been followed up for 5 years, and no new symptoms or tumor growth occurred. Another patient who also had a huge petroclival meningioma had progressive hemiparesis due to brainstem compression. This patient had facial and lower CN palsies due to two attempts of resection in another service. The patient's hemiparesis improved postoperatively.
Fig. 6.
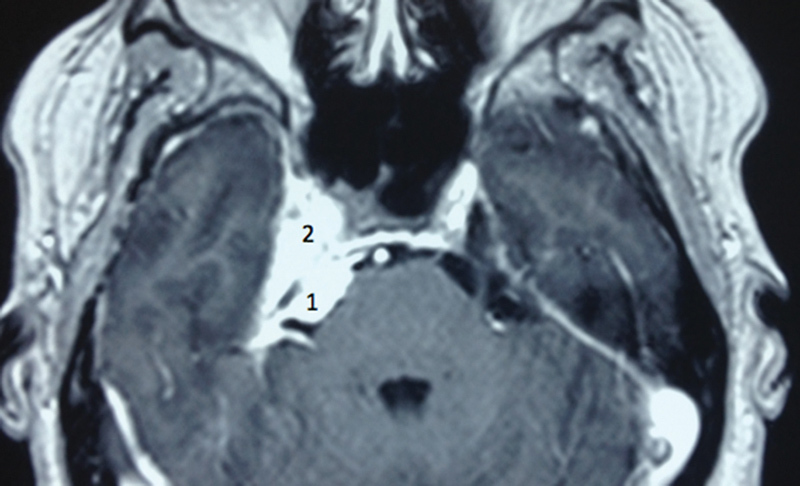
Axial gadolinium-enhanced T1-weighted MRI showing a sphenopetroclival meningioma. A 48-year-old patient presented refractory trigeminal neuropathic pain for 4 months. After partial resection of the petroclival portion of the tumor, her pain improved, and she remained with hypoesthesia. After 2 years, the tumor grew, and she was treated with radiosurgery, without development of trigeminal neuropathy. 1. Clival portion and 2. cavernous portion. MRI, magnetic resonance imaging.
Fig. 7.
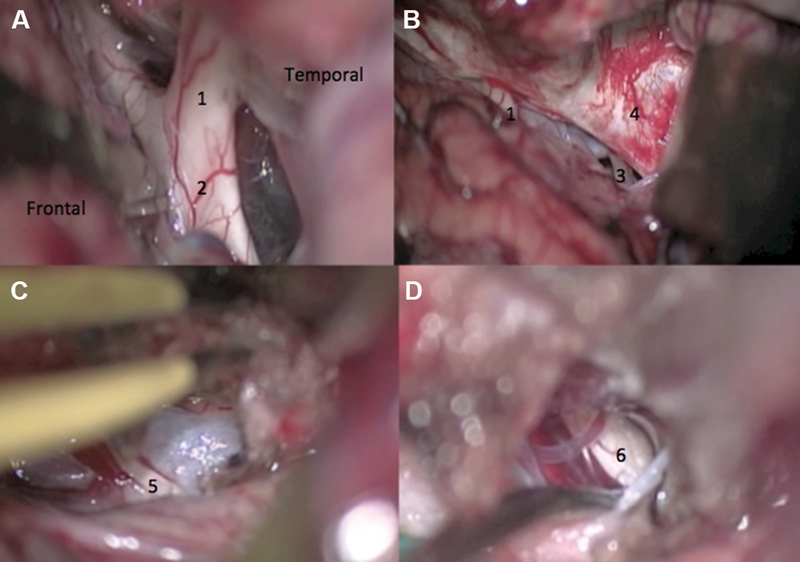
Intraoperative view of a pretemporal approach for sphenopetroclival meningioma resection causing neuropathic pain in the right trigeminal nerve. ( A ) Wide dissection of the Sylvian fissure. ( B ) Temporal lobe was retracted posteriorly to expose the crural and ambient cisterns. ( C ) Coagulation and incision of the free edge of the tentorium after trochlear nerve dissection. ( D ) Resection of the posterior fossa tumor component with decompression of the trigeminal nerve. 1. Optic nerve; 2. optic chiasm; 3. oculomotor nerve; 4. wall of the cavernous sinus; 5. trochlear nerve; and 6. trigeminal nerve.
Tumor Recurrence
Adequate radiographic follow-up (minimum of 6 months) was available for 14 patients, and recurrence rates were calculated based on the follow-up of these patients. The mean follow-up time was 32 months (range 6–75 months). In three of 14 patients followed up for >6 months, tumor progression or recurrence was observed. In two patients, in whom the cavernous sinus component of the sphenopetroclival meningioma was left intact, this component started to grow toward the incisural space as noted in postoperative MRIs 7 and 11 months after surgery, respectively. One patient was referred for radiosurgical treatment, and the other was being followed up due to relatively slow growth. Another patient presented a small tumor recurrence at the clivus. All three of these patients were asymptomatic.
Discussion
The slow-growing pattern of petroclival meningiomas is usually associated with the onset of symptoms only after reaching a large size. Van Havenberg et al 22 investigated 21 patients with petroclival meningioma treated conservatively, with a minimum follow-up of 4 years. They reported tumor growth in 76% of cases and clinical deterioration in 63%. Bricolo et al 2 reported that an average of 2.5 to 4.5 years elapse between the onset of symptoms and confirmation of diagnosis, which delays treatment. Jung et al 23 reported a series of 38 patients who had subtotal resection. Linear growth was 0.37 cm/year, and volume increased by 4.94 cm 3 /year. However, 60% of patients showed no signs of disease progression. Watchful waiting may be an option for patients who are poor candidates for surgery, elderly patients, patients with very small asymptomatic lesions, or when the patient is unwilling to undergo surgical treatment. In these cases, MRI may be repeated every 6 months or when new symptoms arise.
Most petroclival meningiomas are benign lesions. Complete resection is generally the only possible curative treatment, but it is often impossible due to invasion of the cavernous sinus, CNs, vessels, and pia mater. The size, consistency, and biological behavior of the tumor are other factors limiting the extent of resection. The best surgical outcomes are usually achieved with small tumors (up to 3 cm in diameter). 9 24 They may carry the greatest potential for cure, possibly with the least morbidity. 24 Nevertheless, these patients are also the best candidates for radiosurgery. 10
Subtotal resection with or without adjuvant therapy is usually performed when there is invasion of the cavernous sinus. Little et al 7 found that subtotal resection in patients with adherent or fibrous tumors significantly reduced the rate of postoperative neurological deficits without a significant increase in the rate of tumor recurrence. Nanda et al 13 , in a series of 50 patients with petroclival meningioma, achieved GTR in 28% of cases, with good functional outcome in 92%; their primary surgical goal was to achieve maximal tumor resection while maintaining or improving function. They suggested that residual or recurrent tumors might be treated with stereotactic radiosurgery (SRS). In the present series, involvement of the cavernous sinus in asymptomatic patients has led to the choice of follow-up and adjuvant treatment with radiosurgery in case of tumor recurrence.
Fractionated stereotactic radiotherapy and radiosurgery may be indicated as first-line or adjuvant therapy for skull base meningiomas and are considered to provide good outcomes in terms of tumor control and preservation of neurological function. 25 26 27 Nicolato et al 26 , in a series of posterior fossa meningiomas treated with gamma knife radiosurgery, reported that the only factor to influence the efficacy of radiosurgery to a significant extent was the biological nature of the meningioma (WHO grades 2 and 3). In 1998, Subach et al 28 reported good outcomes in 62 cases of petroclival meningioma, with CN deficits occurring in 8% of patients. Iwai et al 27 also reported good results in a series of seven patients with large petroclival and cavernous sinus meningiomas treated with gamma knife radiosurgery in a two-stage procedure. Xu et al 29 recommended that radiosurgery should be considered for petroclival meningiomas on a case-by-case basis, taking into account patient age, size, and location of residual tumor, and pathologic features. In 2010, Flannery et al 30 published their 21-year experience with gamma knife treatment of petroclival meningiomas. The authors were able to avoid initial or additional resection in 98% of patients with a low risk of radiation-related adverse effects and believed that radiosurgery should be considered a first-line option for patients with small, symptomatic petroclival meningiomas. Conversely, in their study, overall 5- and 10-year progression-free survival rates were 91% and 86%, respectively.
Long-term reports of radiosurgical results have shown actuarial progression-free survival rates in skull base meningiomas of 97.2% at 10 years. Kreil et al 25 reported 44 petroclival meningiomas from 200 patients with benign skull base meningiomas in 5 to 12 years of follow-up; 99 patients received gamma knife radiosurgery after microsurgical resection, and 101 patients underwent upfront SRS. The median tumor volume was 6.5 cm 3 . Five patients needed repeated microsurgical resection following SRS (2.5%). Hence, for particular cases of small sphenopetroclival meningiomas with cavernous sinus symptoms (CN III, IV, or VI paresis), we consider radiosurgery the initial treatment of choice. However, further studies reporting 20-year results or even longer post-radiosurgery observation are necessary. This is important not just for estimating the long-term outcomes of radiosurgical treatment, but also to evaluate surgical outcomes in patients in whom radiosurgery was unable to control tumor growth. Conversely, we believe that surgery should be the primary treatment for petroclival meningiomas in patients with good clinical condition. Besides that, in the presence of neuropathic pain refractory to medical therapy or progressive visual loss due to compression of the optic apparatus, we have performed surgical decompression prior to radiosurgery.
The choice of surgical approach is typically based on the location, extent of tumor involvement, and experience of the surgeon. The involvement of venous structures must be taken into account, such as the vein of Labbé, the superior petrosal and transverse sinuses, and the petrosal vein. This is important especially for the petrosal approaches. 20 For tumors extending into the middle fossa, Samii et al proposed the suprameatal approach, 18 which includes drilling of the temporal bone above the internal auditory meatus to reach the middle fossa. However, Chen et al 12 postulated that the exposure of this part of the tumor would be inadequate, because of the small angle for tumor resection, and the risk of postoperative neurological deficits would be more important than achieving GTR with tumor extension into the cavernous sinus. They considered that tumors invading the cavernous sinus could not be removed completely.
Almefty et al reported a series of 64 patients and found that complete resection (Grade I or II) of petroclival meningiomas was possible in 76.4% of cases. 15 The authors suggested that, when circumstances prevent complete resection, residual tumors can be managed by watchful waiting until there is evidence of progression, at which time a new intervention could be planned. Regarding approaches, we believe that the suboccipital retrosigmoid approach for tumors without extension into the middle fossa is usually sufficient. With the aid of long microsurgical instruments, ultrasonic aspirators and a surgical microscope with great mobilization capacity for changing the angle of view of the surgical field, the retrosigmoid approach provides satisfactory access to the tumor component, although from a different angle of view compared with petrosal approaches.
This does not mean that we consider the petrosal approach an aggressive approach, as did Bricolo et al by considering the retrosigmoid approach, subtemporal approach and their combination “less-aggressive” skull base approaches. 2 However, the latter are becoming more common in the surgical removal of petroclival meningiomas. 2 26 Bricolo et al 2 used the retrosigmoid approach alone in 65% of 110 consecutive patients. Bambakidis et al 9 reviewed 46 patients who underwent surgical treatment of petroclival meningiomas. The rate of GTR was 43%. Cases in which a retrosigmoid resection was performed showed no reduction in tumor progression and recurrence rates. They found that the mean length of stay for patients undergoing a less-aggressive approach was 1 week less than that for patients undergoing an aggressive approach (17 vs 23 days). Goel 6 also found no association between the surgical approaches and the extent of resection. Table 5 shows the comparative results with major surgical series of petroclival meningiomas. 4 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48
Table 5. Comparative results with major surgical series of petroclival meningiomas.
| Reference | N | Gross-total resection a (%) | Mortality (%) | Major morbidity (%) | New cranial nerve deficits (%) |
|---|---|---|---|---|---|
| Yasargil et al. 1 | 20 | 35 | 10 | 26 | 50 |
| Mayberg and Simon 31 | 35 | 26 | 9 | 34 | 54 |
| Nishimura et al. 32 | 24 | 63 | 8 | 33 | 91 |
| Tatagiba et al. 4 | 54 | 70 | 2 | 24 | 37 |
| Bricolo et al. 2 | 33 | 79 | 9 | 39 | 76 |
| Spetzler et al. 33 | 18 | 78 | 0 | 11 | 39 |
| Kawase et al. 3 | 42 | 76 | 0 | 12 | 36 |
| Coudwell et al. 5 | 109 | 69 | 3.7 | 15 | 33 |
| Zentner et al. 34 | 19 | 68 | 5 | 11 | 34 |
| Goel 6 | 24 | 67 | 0 | 29 | 29 |
| Abdel Aziz et al. 35 | 35 | 37 | 0 | 9 | 31 |
| Little et al. 7 | 137 | 40 | 0.7 | 26 | 22 |
| Park et al. 36 | 49 | 20 | 2 | 28.6 | 28.6 |
| Mathiesen et al. 37 | 29 | 48 | 0 | 7 | 21 |
| Natarajan et al. 8 | 150 | 32 | 0 | 22 | 20.3 |
| Bambakidis et al. 9 | 46 | 43 | 0 | 41 | 30 |
| Ramina et al. 10 | 67 | 55 | 3 | 12 | 33 |
| Tahara et al. 38 | 15 | 50 | 13 | 20 | 50 |
| Seifert 11 | 148 | 37 | 0 | 31 | 22 |
| Li et al. 39 | 57 | 58 | 2 | 42 | 67 |
| Yang et al. 40 | 41 | 61 | 0 | 66 | 8 |
| Yamakami et al. 41 | 32 | 59 | 6 | 28 | 22 |
| Watanabe et al. 42 | 26 | 42 | 0 | 15 | 15 |
| Shi et al. 43 | 14 | 86 | 0 | 43 | 43 |
| Chen et al. 12 | 82 | 56 | 5 | 44 | 39 |
| Nanda et al. 13 | 50 | 28 | 6 | 44 | 32 |
| Kusumi et al. 44 | 23 | 47 | 0 | 22 | 43 |
| Matsui 45 | 15 | 67 | 0 | 27 | 27 |
| Li et al. 14 | 259 | 52.5 | 1.2 | 54 | 54 |
| Almefty et al. 15 | 64 | 64 | 8 | 25 | 39 |
| Morisako et al. 46 | 60 (24/36) b | EOR 96.1/92.7 c | 1.7 | 25 | 46.7 |
| da Silva & de Freitas 47 | 8/16 | 87.5 | 0 | 37.5 | 37.5 |
| Tatagiba et al. 4 | 29/87 | 66 | 0 | 24 | 34 |
| Zhou et al. 48 | 24 | 33.3 | 0 | 20.8 | 37.5 |
| Isolan et al., 2015 | 22 | 50 | 4.5 | 27.3 | 13.6 |
Data in bold correspond to the current study.
Simpson grades I, II, and III.
24 cases in the early group (1990–1999) and 36 cases in the late group (2000–2009).
Extent of resection (EOR) was calculated as follows: EOR(%) = (preoperative tumor volume − postoperative tumor volume)/preoperative tumor volume × 100.
Tumors with extension into the middle fossa, more precisely extending above the tentorium notch, were approached through an access to the lateral skull base. Once the tentorium is completely transected, petrosal approaches provide a better view of the entire tumor extension in a single procedure in cases of real midline retroclival tumors, with supratentorial and middle fossa invasion.
Although endoscopic endonasal approaches are becoming increasingly applied, they were used in only one case in which the patient had rhinorrhea due to erosion of the sphenoid sinus by a small sphenopetroclival tumor, which was later referred for radiosurgery. The endoscopic approach for petroclival endonasal meningiomas would theoretically have the advantage of dealing with the CNs at a distance from the surgeon, i.e., the rear surface of the tumor. Moreover, except for midclival meningiomas, the lateral location and displacement of both abducens nerves to the same side would likely increase the risk of CN VI palsy. In addition, the deep surgical field and increased risk of CSF fistula are also disadvantages compared with the approaches commonly used for resection of this type of tumor.
Tumor progression or recurrence was observed in three of 14 patients (21.4%) followed for >6 months, and in four (18%) of the total 22 patients included in the study; all of them had undergone subtotal resection. The small number of patients and relatively short follow-up in our series prevented us from performing additional analyses. Nevertheless, none of the patients with GTR had recurrence.
Conclusions
Resection of petroclival meningiomas remains challenging. Early in the series, petrosal approaches were used primarily, but, over the course of the learning curve, the retrosigmoid approach was considered generally sufficient for most patients, without affecting the degree of tumor resection. In asymptomatic cases extending into the cavernous sinus, follow-up was performed, and radiosurgery in case of tumor growth. The petrosal approaches were reserved for patients with tumor extension into the middle fossa.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Conflicts of Interest None.
References
- 1.Yasargil M G, Mortara R W, Curcic M.Meningiomas of basal posterior cranial fossa Adv Tech Stand Neurosurg 19803–115.. doi: 10.1007/978-3-7091-7051-9_1 [Google Scholar]
- 2.Bricolo A P, Turazzi S, Talacchi A, Cristofori L.Microsurgical removal of petroclival meningiomas: a report of 33 patients Neurosurgery 19923105813–828., discussion 828 [DOI] [PubMed] [Google Scholar]
- 3.Kawase T, Shiobara R, Toya S.Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality Acta Neurochir (Wien) 1994129(3-4):113–120. [DOI] [PubMed] [Google Scholar]
- 4.Tatagiba M, Samii M, Matthies C, Vorkapic P. Management of petroclival meningiomas: a critical analysis of surgical treatment. Acta Neurochir Suppl (Wien) 1996;65:92–94. doi: 10.1007/978-3-7091-9450-8_25. [DOI] [PubMed] [Google Scholar]
- 5.Couldwell W T, Fukushima T, Giannotta S L, Weiss M H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84(01):20–28. doi: 10.3171/jns.1996.84.1.0020. [DOI] [PubMed] [Google Scholar]
- 6.Goel A. Extended lateral subtemporal approach for petroclival meningiomas: report of experience with 24 cases. Br J Neurosurg. 1999;13(03):270–275. doi: 10.1080/02688699943673. [DOI] [PubMed] [Google Scholar]
- 7.Little K M, Friedman A H, Sampson J H, Wanibuchi M, Fukushima T.Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients Neurosurgery 20055603546–559., discussion 546–559 [DOI] [PubMed] [Google Scholar]
- 8.Natarajan S K, Sekhar L N, Schessel D, Morita A.Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up Neurosurgery 20076006965–979., discussion 979–981 [DOI] [PubMed] [Google Scholar]
- 9.Bambakidis N C, Kakarla U K, Kim L Jet al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review Neurosurgery 2007610502202–209., discussion 209–211 [DOI] [PubMed] [Google Scholar]
- 10.Ramina R, Fernandes Y B, Coelho Neto M. Berlin: Springer Berlin Heidelberg; 2008. Petroclival meningiomas: diagnosis, treatment, and results; pp. 121–135. [Google Scholar]
- 11.Seifert V. Clinical management of petroclival meningiomas and the eternal quest for preservation of quality of life: personal experiences over a period of 20 years. Acta Neurochir (Wien) 2010;152(07):1099–1116. doi: 10.1007/s00701-010-0633-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen L F, Yu X G, Bu B, Xu B N, Zhou D B. The retrosigmoid approach to petroclival meningioma surgery. J Clin Neurosci. 2011;18(12):1656–1661. doi: 10.1016/j.jocn.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Nanda A, Javalkar V, Banerjee A D. Petroclival meningiomas: study on outcomes, complications and recurrence rates. J Neurosurg. 2011;114(05):1268–1277. doi: 10.3171/2010.11.JNS10326. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Hao S Y, Wang L et al. Surgical management and outcomes of petroclival meningiomas: a single-center case series of 259 patients. Acta Neurochir (Wien) 2013;155(08):1367–1383. doi: 10.1007/s00701-013-1795-9. [DOI] [PubMed] [Google Scholar]
- 15.Almefty R, Dunn I F, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: results of surgical management. J Neurosurg. 2014;120(01):40–51. doi: 10.3171/2013.8.JNS13535. [DOI] [PubMed] [Google Scholar]
- 16.Castellano F, Ruggiero G. Meningiomas of the posterior fossa. Acta Radiol Suppl. 1953;104:1–177. [PubMed] [Google Scholar]
- 17.Samii M, Tatagiba M.Experience with 36 surgical cases of petroclival meningiomas Acta Neurochir (Wien) 1992118(1-2):27–32. [DOI] [PubMed] [Google Scholar]
- 18.Samii M, Tatagiba M, Carvalho G A. Retrosigmoid intradural suprameatal approach to Meckel's cave and the middle fossa: surgical technique and outcome. J Neurosurg. 2000;92(02):235–241. doi: 10.3171/jns.2000.92.2.0235. [DOI] [PubMed] [Google Scholar]
- 19.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(01):22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafez A, Nader R, Al-Mefty O. Preservation of the superior petrosal sinus during the petrosal approach. J Neurosurg. 2011;114(05):1294–1298. doi: 10.3171/2010.6.JNS091461. [DOI] [PubMed] [Google Scholar]
- 21.Wayhs S Y, Lepski G A, Frighetto L, Isolan G R. Petroclival meningiomas: Remaining controversies in light of minimally invasive approaches. Clin Neurol Neurosurg. 2017;152:68–75. doi: 10.1016/j.clineuro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M.Natural history of petroclival meningiomas Neurosurgery 2003520155–62., discussion 62–64 [DOI] [PubMed] [Google Scholar]
- 23.Jung H W, Yoo H, Paek S H, Choi K S.Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases Neurosurgery 20004603567–574., discussion 574–575 [DOI] [PubMed] [Google Scholar]
- 24.Ramina R, Neto M C, Fernandes Y B, Silva E B, Mattei T A, Aguiar P H.Surgical removal of small petroclival meningiomas Acta Neurochir (Wien) 200815005431–438., discussion 438–439 [DOI] [PubMed] [Google Scholar]
- 25.Kreil W, Luggin J, Fuchs I, Weigl V, Eustacchio S, Papaefthymiou G. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76(10):1425–1430. doi: 10.1136/jnnp.2004.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolato A, Foroni R, Pellegrino M et al. Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg. 2001;44(04):211–217. doi: 10.1055/s-2001-19934. [DOI] [PubMed] [Google Scholar]
- 27.Iwai Y, Yamanaka K, Nakajima H. Two-staged gamma knife radiosurgery for the treatment of large petroclival and cavernous sinus meningiomas. Surg Neurol. 2001;56(05):308–314. doi: 10.1016/s0090-3019(01)00622-x. [DOI] [PubMed] [Google Scholar]
- 28.Subach B R, Lunsford L D, Kondziolka D, Maitz A H, Flickinger J C.Management of petroclival meningiomas by stereotactic radiosurgery Neurosurgery 19984203437–443., discussion 443–445 [DOI] [PubMed] [Google Scholar]
- 29.Xu F, Karampelas I, Megerian C A, Selman W R, Bambakidis N C. Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus. 2013;35(06):E11. doi: 10.3171/2013.9.FOCUS13319. [DOI] [PubMed] [Google Scholar]
- 30.Flannery T J, Kano H, Lunsford L D et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. 2010;112(05):957–964. doi: 10.3171/2009.8.JNS09695. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg M R, Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg. 1986;65(02):160–167. doi: 10.3171/jns.1986.65.2.0160. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura S, Hakuba A, Jang B J, Inoue Y. Clivus and apicopetroclivus meningiomas--report of 24 cases. Neurol Med Chir (Tokyo) 1989;29(11):1004–1011. doi: 10.2176/nmc.29.1004. [DOI] [PubMed] [Google Scholar]
- 33.Spetzler R F, Daspit C P, Pappas C T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76(04):588–599. doi: 10.3171/jns.1992.76.4.0588. [DOI] [PubMed] [Google Scholar]
- 34.Zentner J, Meyer B, Vieweg U, Herberhold C, Schramm J. Petroclival meningiomas: is radical resection always the best option? J Neurol Neurosurg Psychiatry. 1997;62(04):341–345. doi: 10.1136/jnnp.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdel Aziz K M, Sanan A, van Loveren H R, Tew J M, Jr, Keller J T, Pensak M L.Petroclival meningiomas: predictive parameters for transpetrosal approaches Neurosurgery 20004701139–150., discussion 150–152 [PubMed] [Google Scholar]
- 36.Park C K, Jung H W, Kim J E, Paek S H, Kim D G.The selection of the optimal therapeutic strategy for petroclival meningiomas Surg Neurol 20066602160–165., discussion 165–166 [DOI] [PubMed] [Google Scholar]
- 37.Mathiesen T, Gerlich A, Kihlström L, Svensson M, Bagger-Sjöbäck D.Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas Neurosurgery 20076006982–991., discussion 991–992 [DOI] [PubMed] [Google Scholar]
- 38.Tahara A, de Santana P A, Jr, Calfat Maldaun M V et al. Petroclival meningiomas: surgical management and common complications. J Clin Neurosci. 2009;16(05):655–659. doi: 10.1016/j.jocn.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Li P L, Mao Y, Zhu W, Zhao N Q, Zhao Y, Chen L. Surgical strategies for petroclival meningioma in 57 patients. Chin Med J (Engl) 2010;123(20):2865–2873. [PubMed] [Google Scholar]
- 40.Yang J, Fang T, Ma S et al. Large and giant petroclival meningiomas: therapeutic strategy and the choice of microsurgical approaches - report of the experience with 41 cases. Br J Neurosurg. 2011;25(01):78–85. doi: 10.3109/02688697.2010.539716. [DOI] [PubMed] [Google Scholar]
- 41.Yamakami I, Higuchi Y, Horiguchi K, Saeki N.Treatment policy for petroclival meningioma based on tumor size: aiming radical removal in small tumors for obtaining cure without morbidity Neurosurg Rev 20113403327–334., discussion 334–335 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Katayama Y, Fukushima T, Kawamata T. Lateral supracerebellar transtentorial approach for petroclival meningiomas: operative technique and outcome. J Neurosurg. 2011;115(01):49–54. doi: 10.3171/2011.2.JNS101759. [DOI] [PubMed] [Google Scholar]
- 43.Shi W, Shi J L, Xu Q W, Che X M, Ju S Q, Chen J. Temporal base intradural transpetrosal approach to the petoclival region: an appraisal of anatomy, operative technique and clinical experience. Br J Neurosurg. 2011;25(06):714–722. doi: 10.3109/02688697.2011.562991. [DOI] [PubMed] [Google Scholar]
- 44.Kusumi M, Fukushima T, Mehta A I et al. Tentorial detachment technique in the combined petrosal approach for petroclival meningiomas. J Neurosurg. 2012;116(03):566–573. doi: 10.3171/2011.11.JNS11985. [DOI] [PubMed] [Google Scholar]
- 45.Matsui T. Therapeutic strategy and long-term outcome of meningiomas located in the posterior cranial fossa. Neurol Med Chir (Tokyo) 2012;52(10):704–713. doi: 10.2176/nmc.52.704. [DOI] [PubMed] [Google Scholar]
- 46.Morisako H, Goto T, Ohata K. Petroclival meningiomas resected via a combined transpetrosal approach: surgical outcomes in 60 cases and a new scoring system for clinical evaluation. J Neurosurg. 2015;122(02):373–380. doi: 10.3171/2014.8.JNS132406. [DOI] [PubMed] [Google Scholar]
- 47.da Silva C E, de Freitas P E. Large and giant skull base meningiomas: the role of radical surgical removal. Surg Neurol Int. 2015;6:113. doi: 10.4103/2152-7806.159489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q J, Liu B, Geng D J et al. Microsurgery with or without neuroendoscopy in petroclival meningiomas. Turk Neurosurg. 2015;25(02):231–238. doi: 10.5137/1019-5149.JTN.8670-13.1. [DOI] [PubMed] [Google Scholar]


