Abstract
Objectives To describe the first experience with a free vastus lateralis muscle-only flap to be used to cover and protect the exposed skull base and carotid artery from radiotherapy-induced skull base osteoradionecrosis (ORN).
Design Retrospective review of a case series.
Setting Tertiary academic medical center.
Participants Post treatment nasopharyngeal carcinoma patients with skull base ORN.
Main Outcome Measures Coverage of the internal carotid artery (ICA).
Results Four patients underwent the procedure. Following the procedure, all patients were documented to have adequate viable soft tissue covering their ICA. Topical nasal steroids were prescribed to all patients as florid granulation tissue was noted to occur overlying the muscle flap in the early postoperative period. There were no flap failures. All patients noted an improvement in speech, cacosmia, and nasal crusting. No significant epistaxis occurred following surgery. Choanal stenosis was noted in three patients.
Conclusion For skull base ORN resulting from the treatment of nasopharyngeal carcinoma (NPC) with radiotherapy that fails conservative management, an open approach to the nasopharynx, that allows debridement then placement of a vastus lateralis muscle-only free flap for coverage, offers a unique and viable approach to the management of this challenging condition.
Keywords: skull base, nasopharyngeal carcinoma, osteoradionecrosis, vastus lateralis, free flap
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in Southern China and Hong Kong. 1 The incidence of NPC is the highest in patients between 40 and 59 years of age. 2 Treatment is radical radiotherapy with or without chemotherapy, depending on the disease stage.
Complications of the radiotherapy in this setting include otitis media with effusion, sensorineural hearing loss, cranial nerve palsies, chronic sinusitis, temporal lobe necrosis, dysphagia, and trismus. Osteoradionecrosis (ORN) of the skull base is another complication and one that is potentially serious. It typically arises one or more years after the treatment of NPC. 3 ORN of the skull base occurs with varying degrees of severity and a significant proportion; ∼10% of patients develop this complication following their treatment for NPC. 4 ORN of the skull base impacts patients and their families as they suffer from foul smelling nasal discharge, nasal crusting, hypernasal speech, recurrent epistaxis, and even central nervous system infections. The most feared complication is that of an exposed internal carotid artery (ICA) that subsequently ruptures. Devitalized bone becomes infected with bacteria that breach the irradiated mucosa, which then breaks down and ulcerates allowing further contamination of the necrotic bone. A laterally expanding ulcer may eventually lead to the exposure of the ICA. Already damaged by radiotherapy, the unsupported and unprotected artery is further weakened by contamination from nasopharyngeal bacteria and dry air from the crippled nasal cavity and mucosa. Protecting the exposed and necrotic bone from nasopharyngeal bacteria by debriding and covering it, together with the ICA if exposed, with healthy vascularized tissue halts the cycle of bone infection, necrosis, and mucosal ulceration and protects a vulnerable ICA. This drastically mitigates symptoms and risk of carotid rupture.
Here we describe the first experience with a free vastus lateralis muscle-only flap to be used to cover and to protect the exposed skull base and carotid artery from contamination by nasopharyngeal bacteria and dry air as treatment for radiotherapy-induced skull base ORN.
Materials and Methods
Patient Selection
Patients selected were those with severe ORN of the nasopharyngeal skull base following radiotherapy for nasopharyngeal carcinoma without or with an exposed ICA risking rupture and whose condition was unresponsive to other forms of treatment for skull base ORN.
Surgical Procedure
Prior to surgery, all patients had computed tomography (CT) of the nasopharynx with contrast for the intraoperative image guidance system. At the beginning of the procedure, a skull post was secured to the frontal bone with screws and the surgical navigation system calibrated to aid in the subsequent localization and prevention of inadvertent injury to the ICA. All surgical procedures were performed with two surgical teams working simultaneously. The nasopharynx was approached through a maxillary swing procedure performed with a Weber–Ferguson–Longmire facial incision in three patients who had the procedure confined to the nasopharynx, and through a transpalatal approach in one patient who had a composite resection of the mandible for a second primary oral cavity carcinoma at the same time. With adequate surgical access to the nasopharynx, necrotic bone, necrotic mucosa, and necrotic soft tissue were debrided with continual reference to the image guidance views to ascertain the proximity of the ICA to the operative field. Fig. 1 illustrates necrotic tissue within the nasopharynx exposed through a maxillary swing approach.
Fig. 1.
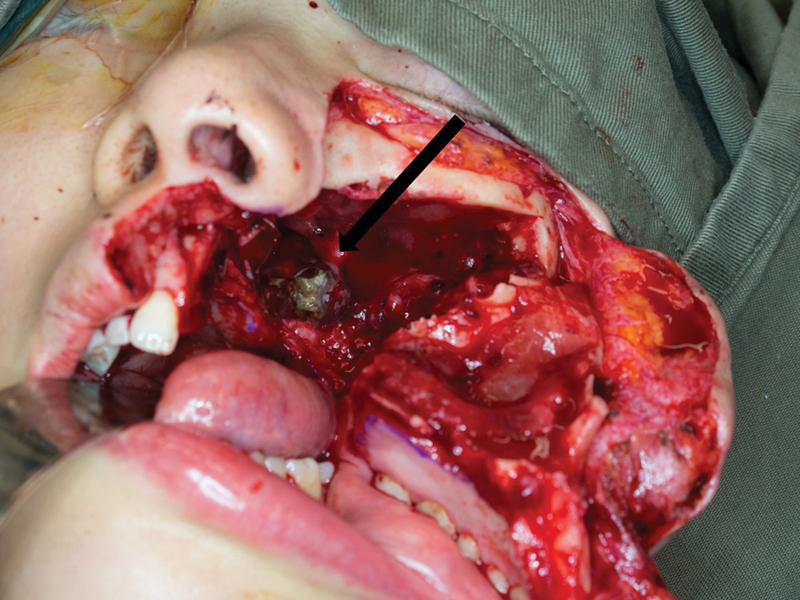
The image shows a nasopharynx exposed following a left maxillary swing. The black arrow points toward the necrotic bone in the left nasopharynx.
To harvest a muscle-only anterolateral thigh flap, a linear skin incision was made along a line from the anterior superior iliac spine to the lateral border of the patella. The dissection was then carried down through the fascia and the intermuscular septum identified between the rectus femoris and the vastus lateralis by retracting the rectus femoris medially. The muscular perforators entering the vastus lateralis, which arise from the descending branch of the lateral circumflex femoral artery, were identified, and a muscle flap of required size was harvested with one to two perforators.
The flap was then inset into the nasopharynx, and the flap muscle fascia was tacked to the mucosa of the nasopharynx using Vicryl sutures and the flap vessels anastomosed to the superior thyroid artery and internal jugular vein in all patients. The muscle flap had been then supported with a nasopharyngeal airway for 2 weeks. All patients had a nasogastric feeding tube placed and tracheostomy performed because dysphagia and risk of aspiration are high in this group of patients. 5 6
Results
Four patients with characteristics listed in Table 1 underwent the procedure. Following the procedure, all patients were documented to have adequate viable soft tissue covering their ICA, as demonstrated in Fig. 2 . Patient 3 who had a composite mandibular resection for a mandibular alveolar second primary cancer in addition to the transpalatal approach to the skull base ORN developed a long-term dysphagia and required a percutaneous an endoscopic gastrostomy tube for feeding. Topical nasal steroids were prescribed to all patients, as florid granulation tissue was noted to occur overlying the muscle flap in the early postoperative period. There were no flap failures. Post-surgery follow-up ranged from 4.9 months to 34.8 months for the four patients. All patients noted an improvement in speech, cacosmia, and nasal crusting. No significant epistaxis occurred following surgery. Unilateral complete choanal stenosis was noted in three patients. Fig. 3 shows a postoperative view of a left nasal cavity with a mucosalized vastus lateralis flap.
Table 1. Patient characteristics with skull base osteoradionecrosis following treatment for nasopharyngeal carcinoma.
| Patient number | Gender | Age at time of surgery | Approach to nasopharynx | Initial T stage |
Number of RT courses | Exposed ICA | Recurrent epistaxis | Postoperative complications | Postoperative improvement | Months following surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 42 | Maxillary swing | 2a | 2 | Yes | No | Choanal stenosis | ICA covered, speech and cacosmia | 34.8 |
| 2 | Female | 38 | Maxillary swing | 1 | 2 | No | No | Choanal stenosis | Speech and cacosmia | 18.2 |
| 3 | Male | 53 | Transpalatal | 4 | 1 | Yes | No | Re-exposure of ICA temporarily | ICA covered, speech and cacosmia | 13.9 |
| 4 | Male | 47 | Maxillary swing | 1 | 2 | No | Yes | Choanal stenosis | Speech and cacosmia | 4.9 |
Abbreviations: ICA, internal carotid artery; RT, radiotherapy; T, T classification based on the American Joint Committee on Cancer (AJCC) 6 th edition.
Fig. 2.
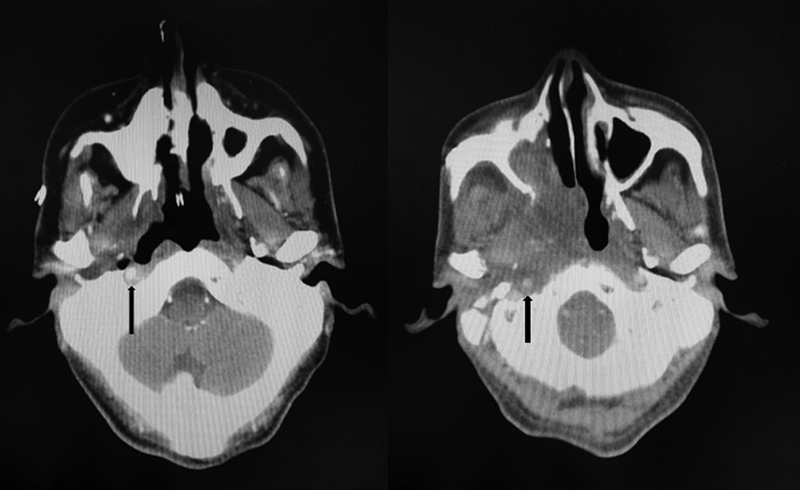
The left image shows an exposed internal carotid artery in the base of an extensive nasopharyngeal ulcer secondary to ORN on an axial CT scan with contrast (black arrow), while the right image shows the adequately covered nasopharynx and internal carotid artery (black arrow) after placement of a muscle-only vastus lateralis flap. CT, computerized tomography; ORN, osteoradionecrosis.
Fig. 3.
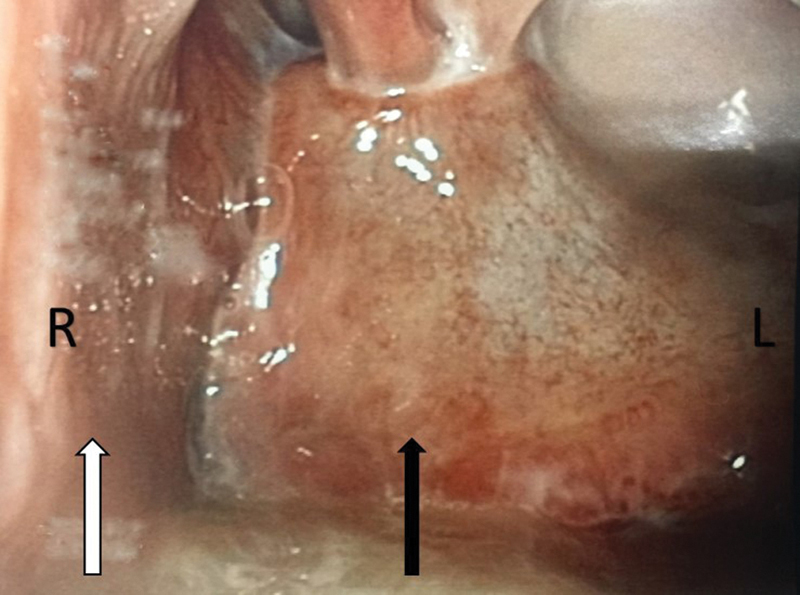
The image is an 8-week postoperative rigid endoscopy image of the left nasal cavity showing choanal stenosis and a completely mucosalized vastus lateralis muscle flap. The black arrow points to the mucosalized vastus lateralis muscle flap. The white arrow points to the septum. R = right, L = left.
Discussion
Long-term treatment-related toxicities from radiotherapy for NPC can be severely debilitating and are associated with major morbidity and mortality. 7 8 Besides the most feared complication of an ICA blowout, ORN can result in cacosmia, recurrent epistaxis, headaches, and intracranial infection; all of which have an impact on the patients and their families. 9 10
Initial treatment of skull base ORN involves conservative measures and hyperbaric oxygen (HBO). HBO does not cure ORN, but rather creates an environment conducive to tissue healing, which prevents infections. The sequestrum, however, remains as a nidus that ultimately needs to be surgically removed, if feasible, and in these cases, an endoscopic sequestrectomy has been advocated. 9 10 However, if there is great vessel exposure or a diffuse area of ORN of the skull base, endoscopic sequestrectomy neither addresses the ORN adequately nor can it safely prevent further complications. Utilizing the nasoseptal flap as described 11 is not always possible, given the extent of destruction by the ORN, loss of the choana, and sphenoid floor with resultant loss of the posterior septal branch of the sphenopalatine artery. Given the difficult location and important surrounding neurovascular structures, we utilized open access, including the maxillary swing and transpalatal approaches, with which we have a long and sound experience, to adequately expose the nasopharynx. To provide a rich blood supply to the debrided bone and soft tissues, an exposed ICA was covered when present, and to promote healing of irradiated bone and soft tissues, the vastus lateralis muscle-only free flap was used on the same principle that soft tissue only flaps are used in the management of mandibular ORN. 12 While a radial forearm free flap has been described to line the nasopharynx, 13 we chose to use the muscle-only free flap to provide bulk and an eventual mucosalized surface. The bulk ensures adequate protection of the great vessel (the ICA) and reduces the significant nasal regurgitation and hypernasal speech that patients treated for nasopharyngeal carcinoma experience. 14 Mucosalization of the surface of the free muscle flap is superior to that of a radial forearm free flap where the skin surface does not produce mucus resulting in crusting and cacosmia. Despite the endoscopic finding of choanal stenosis noted in three patients, they all noted and exhibited improved speech, swallowing, and cacosmia and were minimally concerned about their nasal obstruction from the stenosis, and no secondary procedures were required to correct the choanal stenosis. The reason for this improvement is the correction of the velopharyngeal insufficiency and hypernasal speech that affected these patients prior to surgery. Postoperative imaging in all patients confirmed adequate soft tissue cover of the previously exposed osteoradionecrotic bone.
Conclusion
For skull base ORN resulting from the treatment of NPC with radiotherapy that fails conservative management, an open approach to the nasopharynx that allows debridement then placement of a vastus lateralis muscle-only free flap for coverage of bone and vessels providing protection with well-vascularized tissue offers a unique and viable approach to the management of this challenging condition.
Conflict of Interest None.
Financial Disclosures
None.
References
- 1.Wei W I, Sham J S.Nasopharyngeal carcinoma Lancet 2005365(9476):2041–2054. [DOI] [PubMed] [Google Scholar]
- 2.Cao S M, Simons M J, Qian C N. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(02):114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam H C, Abdullah V J, Wormald P J, Van Hasselt C A. Internal carotid artery hemorrhage after irradiation and osteoradionecrosis of the skull base. Otolaryngol Head Neck Surg. 2001;125(05):522–527. doi: 10.1067/mhn.2001.118248. [DOI] [PubMed] [Google Scholar]
- 4.Lee C C, Ho C Y. Post-treatment late complications of nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2012;269(11):2401–2409. doi: 10.1007/s00405-011-1922-2. [DOI] [PubMed] [Google Scholar]
- 5.Ku P K, Vlantis A C, Leung S F et al. Laryngopharyngeal sensory deficits and impaired pharyngeal motor function predict aspiration in patients irradiated for nasopharyngeal carcinoma. Laryngoscope. 2010;120(02):223–228. doi: 10.1002/lary.20701. [DOI] [PubMed] [Google Scholar]
- 6.Ng L K, Lee K Y, Chiu S N, Ku P K, van Hasselt C A, Tong M C. Silent aspiration and swallowing physiology after radiotherapy in patients with nasopharyngeal carcinoma. Head Neck. 2011;33(09):1335–1339. doi: 10.1002/hed.21627. [DOI] [PubMed] [Google Scholar]
- 7.Lam J W, Chan J Y, Lui W M, Ho W K, Lee R, Tsang R K. Management of pseudoaneurysms of the internal carotid artery in postirradiated nasopharyngeal carcinoma patients. Laryngoscope. 2014;124(10):2292–2296. doi: 10.1002/lary.24721. [DOI] [PubMed] [Google Scholar]
- 8.Chen K C, Yen T T, Hsieh Y L et al. Postirradiated carotid blowout syndrome in patients with nasopharyngeal carcinoma: a case-control study. Head Neck. 2015;37(06):794–799. doi: 10.1002/hed.23671. [DOI] [PubMed] [Google Scholar]
- 9.Chang K P, Tsang N M, Chen C Y, Su J L, Hao S P. Endoscopic management of skull base osteoradionecrosis. Laryngoscope. 2000;110(07):1162–1165. doi: 10.1097/00005537-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Huang X M, Zheng Y Q, Zhang X M et al. Diagnosis and management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope. 2006;116(09):1626–1631. doi: 10.1097/01.mlg.0000230435.71328.b9. [DOI] [PubMed] [Google Scholar]
- 11.Adel M, Chang K P. Using a nasoseptal flap for the reconstruction of osteoradionecrosis in nasopharyngeal carcinoma: a case report. J Otolaryngol Head Neck Surg. 2016;45:27. doi: 10.1186/s40463-016-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang D W, Oh H K, Robb G L, Miller M J. Management of advanced mandibular osteoradionecrosis with free flap reconstruction. Head Neck. 2001;23(10):830–835. doi: 10.1002/hed.1121. [DOI] [PubMed] [Google Scholar]
- 13.Khoo M L, Soo K C, Gullane P J et al. Resurfacing of the nasopharynx after nasopharyngectomy using a free radial forearm flap. Head Neck. 2001;23(10):916–922. doi: 10.1002/hed.1132. [DOI] [PubMed] [Google Scholar]
- 14.Tong M C, Lee K Y, Yuen M T, Lo P S. Perceptions and experiences of post-irradiation swallowing difficulties in nasopharyngeal cancer survivors. Eur J Cancer Care (Engl) 2011;20(02):170–178. doi: 10.1111/j.1365-2354.2010.01183.x. [DOI] [PubMed] [Google Scholar]


