Abstract
REV1 is an evolutionarily conserved translesion synthesis (TLS) DNA polymerase and an assembly factor key for the recruitment of other TLS polymerases to DNA damage sites. REV1-mediated recognition of ubiquitin in the proliferative cell nuclear antigen is thought to be the trigger for TLS activation. Here we report the solution NMR structure of a 108-residue fragment of human REV1 encompassing the two putative ubiquitin-binding motifs UBM1 and UBM2 in complex with ubiquitin. While in mammals UBM1 and UBM2 are both required for optimal association of REV1 with replication factories after DNA damage, we show that only REV1 UBM2 binds ubiquitin. Structure-guided mutagenesis in Saccharomyces cerevisiae further highlights the importance of UBM2 for REV1-mediated mutagenesis and DNA damage tolerance.
Keywords: translesion synthesis, human REV1, ubiquitin, NMR spectroscopy
Introduction
Cells have evolved a DNA damage response that includes DNA damage tolerance mechanisms contributing to cell survival and to mutagenesis [1-3]. Translesion synthesis (TLS) is an evolutionarily conserved DNA damage tolerance pathway operating with specialized DNA polymerases that bypass bulky adducts or abasic sites in damaged DNA [2,4-6]. Unlike high-fidelity polymerases that stall at DNA lesions, TLS polymerases can add nucleotides to replicating strands opposite lesions, albeit with low fidelity, which leads to mutagenesis. How DNA replication switches to TLS at damaged sites is poorly understood. In eukaryotes, mono-ubiquitylation of the replication clamp proliferating cell nuclear antigen (PCNA) on Lys164 by the DNA damage-triggered RAD18-RAD6 ubiquitin ligases controls the TLS switch[7-9]. Mono-ubiquitylation of PCNA is thought to activate REV1, a scaffold factor critical for the recruitment and assembly of other TLS polymerases at DNA damage sites, which thereby permits bypass of a variety of lesions [10-25] and of replication disturbances in the absence of DNA damage [26]. Because of its function in TLS and associated mutagenesis, REV1 has been referred to as a mutasome assembly factor [27]. In addition to being a general activator of TLS, REV1 is a TLS polymerase that incorporates dCMP opposite dGMP or apurinic/apyrimidinic sites in DNA [28,29]. The enzymology of yeast and human REV1 is well understood from kinetic characterization and from the 3D structures of the catalytic domain of REV1 bound to lesion-bearing DNA [30-35]. REV1 also likely plays a role in post-replication DNA repair [36].
Activation of REV1 and TLS by ubiquitylation of PCNA likely involves direct recognition of ubiquitin in the context of ubiquitylated PCNA (PCNAub) [13, 37-39]. Indeed, REV1 harbors two putative C-terminal ubiquitin-binding motifs (UBMs) [37,40]. Similar UBMs were also identified in TLS polymerase ι (Polι)[39,41,42]. To better understand the mechanism of ubiquitin recognition by REV1 and of TLS activation, we used NMR spectroscopy to probe the interaction of ubiquitin with a 108-residue segment of human REV1 (residues 932–1039) encompassing the predicted UBM1 and UBM2 domains.
Results and Discussion
The 108-residue apo REV1 fragment (hereafter called REV1) was mostly disordered based on NMR chemical shift values [43], with the exception of predicted helical conformations that matched the UBM1 and UBM2 regions (Fig. 1a). Marked changes in the 1H–15N heteronuclear single quantum coherence (HSQC) spectrum of REV1 after titration with ubiquitin were only detected for the UBM2 region indicating that UBM2, but not UBM1, contacted ubiquitin (Fig. 1b, c). Similarly, in the case of Saccharomyces cerevisiae REV1, only UBM2 bound ubiquitin [39]. The exchange between free and ubiquitin-bound human REV1 was fast to intermediate on the timescale of NMR chemical shifts allowing the dissociation constant (Kd) to be estimated. The NMR spectroscopy-derived Kd is ~27 μM (Fig. 2a), which is of the same order of magnitude as the isothermal titration calorimetry (ITC)-derived Kd of ~49 μM (Fig. 2b). The ITC stoichiometry number n is close to unity (n = ~0.9), consistent with only one ubiquitin molecule interacting with REV1 (Fig. 2b).
Fig. 1.
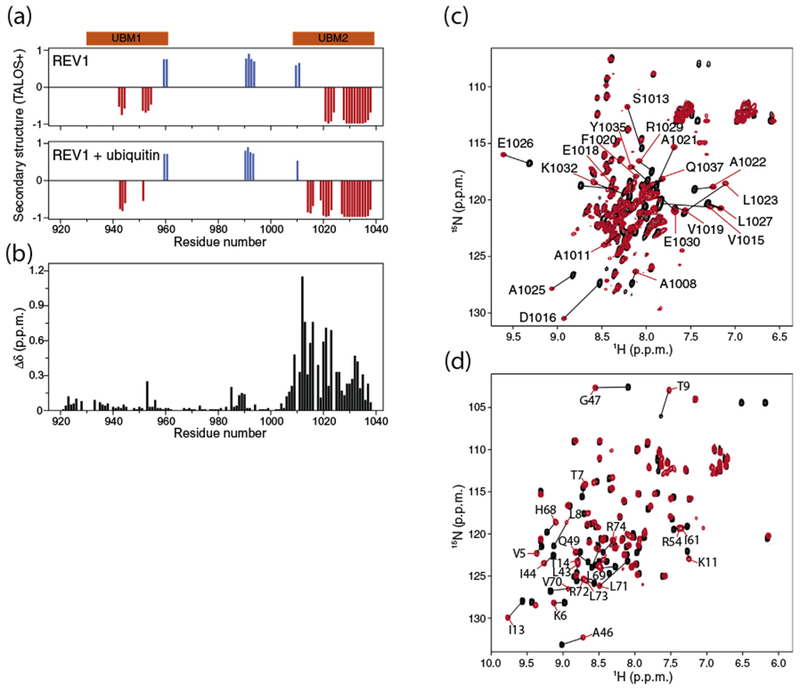
Interaction of human REV1 (932–1039) with ubiquitin. (a) Prediction of α-helical (red) and β-sheet (blue) regions in apo REV1 (top) and in REV1 titrated with threefold molar excess ubiquitin (bottom) based on TALOS+ analysis of Cα, Cβ, C′, HN and N NMR chemical shifts [43]. The UBM1 and UBM2 regions are shown in orange. (b) Chemical shift perturbations in the 1H–15N HSQC spectrum of 15N-labeled REV1 (1 mM concentration) caused by addition of threefold molar excess of unlabeled ubiquitin. The normalized chemical shift changes Δδ in parts per million (ppm) for the 1H–15N correlation signals are defined as . (c) Overlay of the 1H–15N HSQC spectra of 15N-labeled REV1 at 0.5 mM concentration before and after addition of threefold molar excess of unlabeled ubiquitin. (d) Overlay of the 1H–15N HSQC spectra of 15N-labeled ubiquitin at 0.3 mM concentration before and after addition of 5-fold molar excess of unlabeled REV1.
Fig. 2.
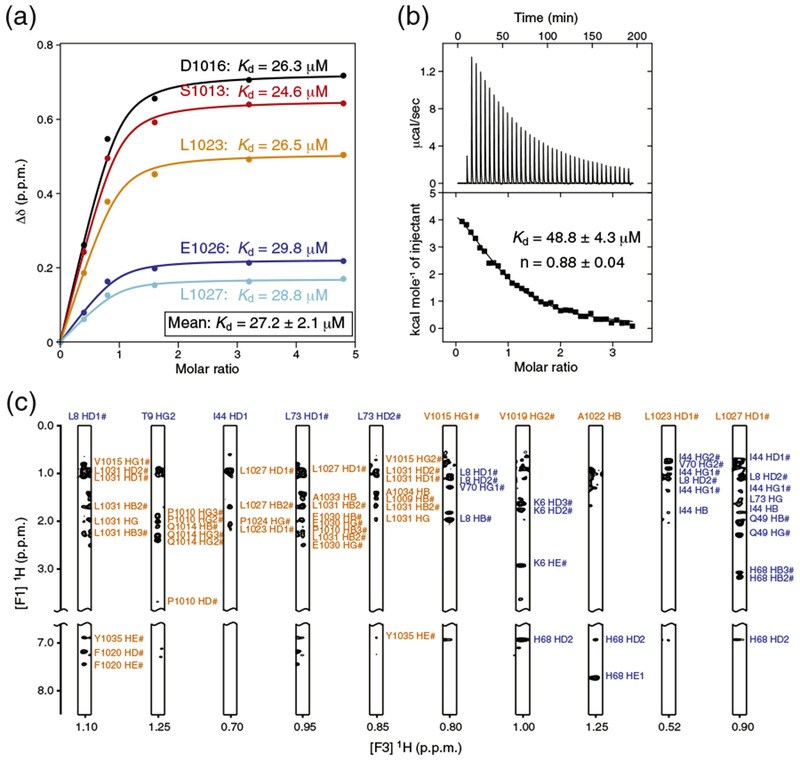
Estimation of the binding affinity of human REV1 (932–1039) for ubiquitin. (a) NMR spectroscopy-based titration of 15N-labeled REV1 with non-labeled ubiquitin. REV1 was at initial concentration of 0.5 mM. Multiple apparent Kd values were determined from the changes in chemical shifts for five REV1 residues by nonlinear least-squares analysis using equation: where [L] is the concentration of ubiquitin, [P] is the concentration of REV1, Δδ is the observed normalized chemical shift change as defined in Fig. 1b, and Δδmax is the normalized chemical shift change at saturation. (b) ITC of REV1 with ubiquitin. The integrated heat measurements from raw titration data and curve fitting using a one-site binding model are shown. The Kd and stoichiometry (n) values are indicated with the standard deviations determined by nonlinear least-squares fitting. (c) Representative intermolecular NOE signals identified in F1 13C-filtered, F2 13C-edited NOESY-HSQC spectra. The resonance labels are colored orange for REV1 and blue for ubiquitin.
Using NMR spectroscopy-derived interatomic distances and dihedral angles, we determined the 3D structure of REV1 bound to ubiquitin (Figs. 2c and 3a). The structure calculation statistics are presented in Table 1. In the structure, the most ordered region of REV1 spans residues Ile1006 to Arg1038, corresponding to the UBM2 domain (Fig. 3a). On the basis of TALOS+ angle predictions [43], in the calculated structure, there are also short helical motifs in the region corresponding to REV1 UBM1 (data not shown). REV1 UBM2 is composed of an N-terminal loop region (Ile1007–Val1015) followed by two α-helices separated by a leucine and trans-proline residues (Leu1023–Pro1024) (Fig. 3b). The trans conformation is supported by predictions done on the basis of backbone chemical shift values and amino acid sequence using the program Promega [44]. The first α-helix (α1, Asp1016-Ala1022) consists of hydrophobic residues with the exception of Asp1016 and Glu1018. The helix is stabilized by a canonical N-cap motif (Asp1016–Pro1017) [45] with the carboxyl group of Asp1016 forming hydrogen bonds with the amide groups of Glu1018 and Val1019. The second α-helix (α2, Ala1025–Arg1038) is amphipathic. The two α-helices are at an angle of 43° ± 4° relative to one another. The overall UBM2 fold resembles a triangle with one site formed by the long-range interaction of Leu1009 with Ala1034. UBM2 contacts the surface of ubiquitin through hydrophobic residues from the N-terminal loop, the two α-helices, and the Leu1023–Pro1024 intervening motif (Fig. 3c). The UBM2 triangle is centered on ubiquitin Leu8, which has intermolecular contacts with Val1015, Val1019, Phe1020, Leu1023, and Leu1031. Other interfacial residues are ubiquitin Ile44, which contacts Pro1024 and Leu1027; ubiquitin His68, which contacts Val1019 and Leu1023; ubiquitin Val70, which contacts Leu1027; and ubiquitin Leu73, which contacts Leu1031, Glu1030, and Leu1009. In addition, ubiquitin Arg42 is favorably positioned to form a salt bridge with Glu1030.
Fig. 3.
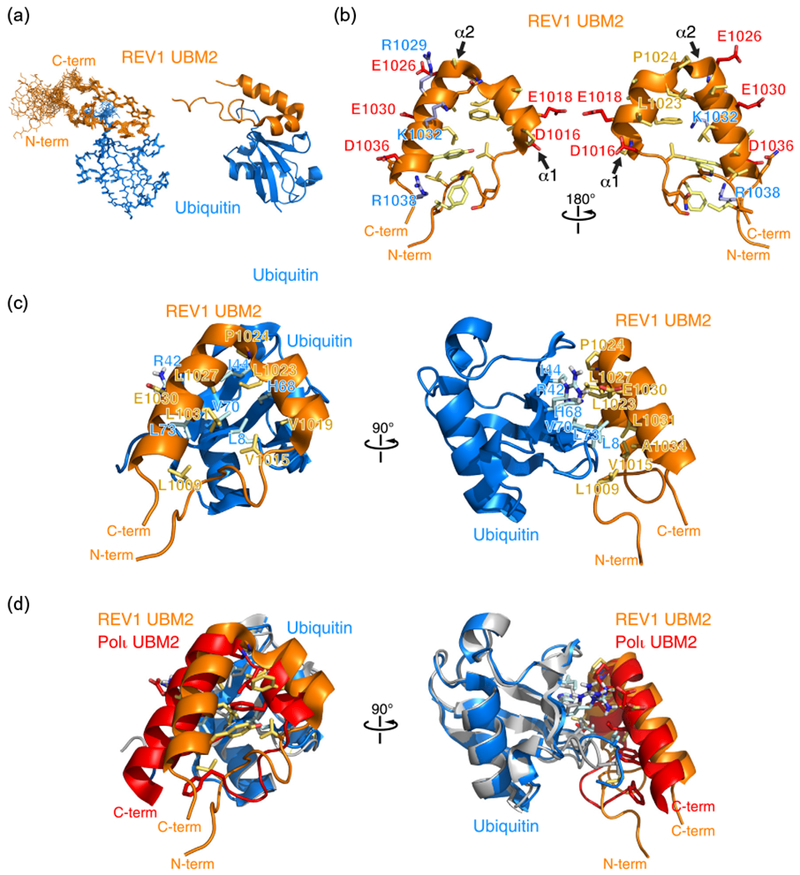
Three-dimensional structure of human REV1 (932–1039) in complex with ubiquitin. (a) Cartoon representation of the overlay of 20 REV1–ubiquitin complex structures representing the NMR ensemble (left) and a cartoon representation (right) of REV1 UBM2 in complex with ubiquitin. While structure calculations were done with REV1 (932–1039), the N-terminal disordered region is not shown. Only REV1 (1000–1039) is shown. (b) Cartoon and stick representation of the UBM2 structure highlighting negatively charged (blue), positively charged (red), neutral (orange), and hydrophobic (yellow) amino acid side chains. (c) Cartoon and stick representation of the REV1 UBM2–ubiquitin complex. Selected amino acid side chains at the binding interface are shown and labeled. (d) Overlay of the ubiquitin structures in the REV1 UBM2–ubiquitin and Polι UBM2–ubiquitin (PDB accession number 2KTF) complexes. Ubiquitin bound to REV1 is shown in blue, while ubiquitin bound to Polι is shown in gray.
Table 1.
NMR and refinement statistics for human REV1 (932–1039)–ubiquitin
| NMR distance and dihedral angle restraintsa | |
|---|---|
| Distance restraints | |
| Total NOE | 5903 |
| Intra-residues | 902 |
| Inter-residues | |
| Sequential (|i − j| = 1) | 851 |
| Medium (|i − j| < 5) | 606 |
| Long-range (|i − j| > 4) | 995 |
| Ambiguous | 2379 |
| Inter-molecular | 170 |
| Hydrogen bond restraints | 57 |
| Total dihedral angle restraints | 183 |
| φ | 90 |
| ψ | 93 |
| Structure statistics | |
| Violations (mean ± SD) | |
| Distance restraints (Å) | 0.11 ± 0.03 |
| Dihedral angle restraints (°) | 2.51 ± 0.75 |
| Max. dihedral angle violation (°) | 3.40 ± 0.46 |
| Max. distance violation (Å) | 0.22 ± 0.01 |
| Deviations from idealized geometry | |
| Bond lengths (Å) | 0.0086 ± 0.0001 |
| Bond angles (°) | 2.09 ± 0.02 |
| Impropers (°) | 0.22 ± 0.01 |
| Average pairwise r.m.s. deviation (Å)b | |
| Heavy | 1.00 ± 0.11 |
| Backbone | 0.35 ± 0.08 |
| Ramachandran plot (%)b | |
| Most favored | 91.1 |
| Additionally allowed | 8.6 |
| Generously allowed | 0.3 |
| Disallowed | 0 |
The number of NOEs corresponds to the non-redundant distance restraints generated from all NOESY spectra.
The r.m.s. deviations and Ramachandran plot parameters were calculated for residues REV1 (1009–1039) and ubiquitin (1–73) using an ensemble of 20 structures.
There is no interaction between REV1 UBM1 and ubiquitin (Fig. 1c), which can be tentatively explained by a number of structural differences between UBM1 and UBM2 in human and S. cerevisiae REV1 (Fig. 4a). The putative helix α1 may be less stable in REV1 UBM1 than in UBM2 as it lacks the N-terminal Asp-Pro capping motif. Yeast UBM2 also lacks this motif (Fig. 4a), but the negatively charged Glu-Glu sequence replacing Asp-Pro is expected to stabilize the helix dipole. In human UBM1, a proline residue follows the inter-helical Leu-Pro linker motif and may change the orientation of helix α2 in comparison to UBM2. Human UBM1 also lacks the glutamate residue (Glu1030 in UBM2) involved in a salt bridge with ubiquitin Arg42 (Fig. 4a).
Fig. 4.
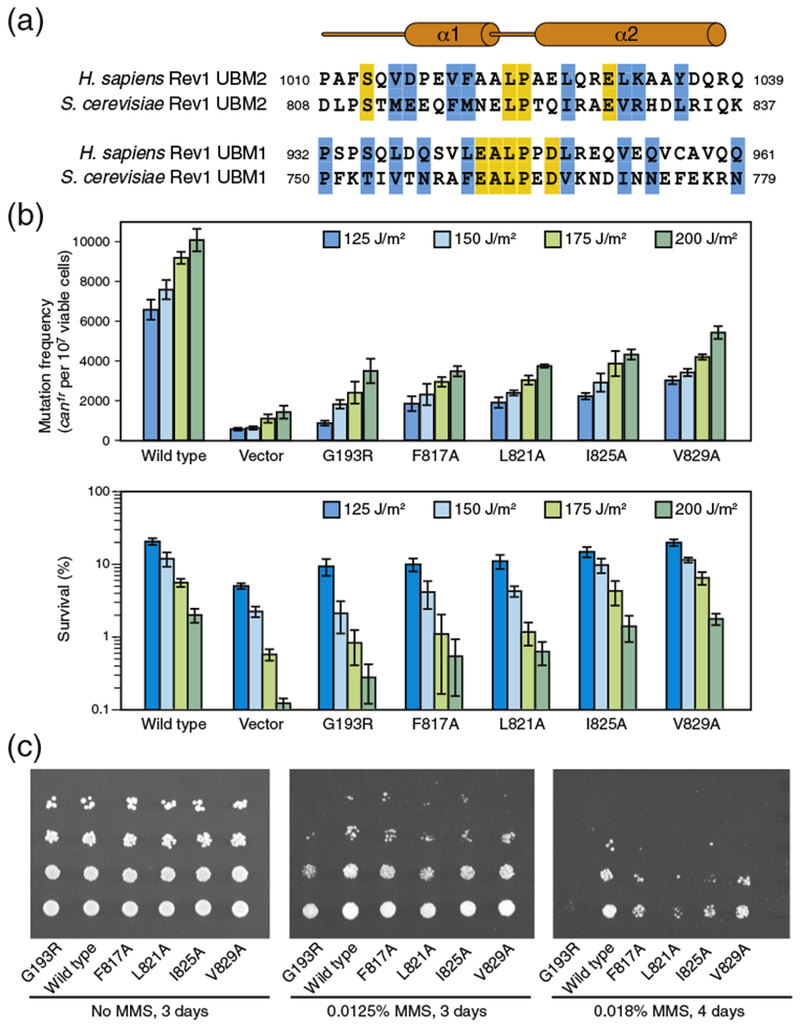
Effects of single-point mutations in REV1 UBM2 using S. cerevisiae as a model system. (a) Amino acid sequences of human and S. cerevisiae REV1 UBM2 and UBM1 regions. Similar and identical residues are highlighted in blue and yellow, respectively. (b) Frequencies of mutagenesis (top) and cell viability (bottom) following UV irradiation of yeast cells expressing wild-type or mutated REV1. (c) Viability of yeast cells expressing wild-type or mutated REV1 in presence of different concentrations of MMS.
The key interacting residues in the structure of REV1 UBM2 bound to ubiquitin are conserved in the Polι UBM2–ubiquitin structure [39,41,42] and are involved in the same intermolecular contacts in the two complexes. Conserved residues are as follows for REV1/Polι: Val1019/Val687, Phe1020/Phe688, Leu1023/Leu691, Pro1024/Pro692, Leu1027/Val695, and Glu1030/Glu698. The helix-stabilizing N-cap motif is also conserved in Polι (Asp1016–Pro1017/Asp684–Pro685). As expected, REV1 UBM2 and Polι UBM2 have similar ubiquitin binding modes where ubiquitin Leu8 is at the center of the binding interface. The angle between the two UBM2 α-helices is a little larger in Polι (52° ± 2°) and the domain is slightly shifted relative to ubiquitin compared to REV1 (Fig. 3d). The difference between the two complexes is most pronounced toward the C-terminal end of the second α-helix of UBM2 including residues Leu699 and Trp703 in Polι, corresponding to Leu1031 and Tyr1035 in REV1, and contact residue Leu73 at the C-terminus of ubiquitin (Fig. 3d). It is, however, not possible to conclude that these differences are significant owing to the experimental uncertainties inherent to NMR protein structure determination.
Our in vitro studies identifying UBM2, and not UBM1, as a ubiquitin binding domain in human REV1 parallels previous work with S. cerevisiae where only REV1 UBM2 was found to bind ubiquitin and to be necessary for REV1-mediated mutagenesis [18,38-40]. In mammals, however, UBM1 and UBM2 are both required for optimal association of REV1 with replication factories in UV-irradiated cells [40]. The function of UBM1 remains unknown. Because the amino acid sequences of REV1 UBM2 are well conserved in S. cerevisiae and human (Fig. 4a), guided by the structure of human REV1 UBM2, we introduced mutations in yeast REV1 at the UBM2–ubiquitin interface to further probe the importance of UBM2 ubiquitin-binding function on UV-induced mutagenesis. We mutated to alanine Phe817 (Val1019 in human), Leu821 (Leu1023 in human), Ile825 (Leu1027 in human), and Val829 (Leu1031 in human). Using an established assay, we examined the frequency of UV-induced CAN1S to can1r forward mutations in an S. cerevisiae rev1Δ strain carrying the wild-type or mutated REV1 gene in a plasmid [46]. The rate of UV-induced mutagenesis was markedly reduced for cells expressing any of the four REV1 mutants, the most defective being F817A and L821A (Fig. 4b). The defects were statistically significant for all mutants (with P values less than 0.05). The corresponding amino acids in human REV1 (Val1019 and Leu1023) are both in the vicinity of Ile8 and His68, two residues at the center of the binding interface in ubiquitin. As a control, we verified that the G193R mutation, which unfolds the BRCA1 C-terminus (BRCT) domain in S. cerevisiae REV1, dramatically reduced UV-induced mutagenesis [10,38,47]. In parallel to investigating the mutagenesis frequency, we analyzed the survival of UV-induced cells. In comparison to UV-irradiated yeast cells expressing wild-type REV1, cell survival was decreased when REV1 was mutated in the UBM2 domain, with the strongest effect observed for the F817A and L821A mutants (Fig. 4b). Decrease in cell survival was statistically significant for G193R, F817A, L821A, and F825A (P values less than 0.05), but not for the V829A mutant. A similar trend in cell survival was obtained after exposing yeast cells expressing wild-type or UBM2-mutated REV1 to DNA damaging agent methyl methanesulfonate (MMS) (Fig. 4c).
Taken together, our structural and functional studies further highlight the key role of REV1 UBM2 in TLS. Nonetheless, the mechanism by which REV1 activates TLS remains unclear. For example, REV1 is thought to constitutively associate with PCNA independently of PCNA ubiquitylation at Lys164, but what drives this interaction is unknown and what role PCNA ubiquitylation would play in an already pre-formed REV1-PCNA complex is puzzling. REV1 does not harbor any PCNA-interacting peptide box, a motif found in virtually all other PCNA-binding proteins. It was suggested that the N-terminal BRCT domain of REV1 could mediate the interaction with PCNA [48,49], while other studies hinted that the N-terminal region of REV1, which includes the BRCT domain, bound DNA and not PCNA [50]. It was also reported that PCNA interactions require the REV1 C-terminal region [51] or catalytic core [52]. Future structural studies in the context of full-length REV1 will be required to understand how PCNA ubiquitylation and UBM2-mediated recognition of PCNAub triggers the activation of REV1. Much more work remains to be done to understand the mode of action of this fascinating protein.
Materials and Methods
Protein preparation
The REV1 (932–1039) construct included an N-terminal His6 tag cleavable by TEV protease. Two ubiquitin constructs were used, one without a tag and another with an N-terminal non-cleavable His6 tag. The proteins were purified using previously reported protocols [41,53].
NMR spectroscopy and structure determination
The NMR spectra were recorded using a Bruker Avance III 700 MHz NMR spectrometer equipped with a cryoprobe. The proteins were in 20 mM sodium phosphate buffer (pH 7.0), 30 mM NaCl, and 90% H2O/10% D2O. The NMR measurements were done at 30 °C using samples of the REV1–ubiquitin complex composed of 15N/13C-labeled REV1 at 1 mM and unlabeled ubiquitin at 3 mM, and 15N/13C-labeled ubiquitin at 1 mM and unlabeled REV1 at 3 mM. A series of standard 2D and 3D spectra were recorded to generate the data required for structure determination [41,54,55]. For structure determination, nuclear Overhauser enhancement (NOE)-based restraints were categorized into distance ranges with upper limits of 2.8, 3.5, 4.0, 4.5, 5.0, and 5.5 Å. For very weak signals, an upper limit of 7.5 Å was used to take into account possible spin diffusion. Backbone hydrogen bond distance restraints were included in the calculations on the basis of amide 1H/2H exchange measurements. The upper limit was set to 3.2 Å for the distance between N and O, and 2.2 Å for the distance between HN and O. Dihedral angle restraints φ and ψ were used in the calculations with a 30° tolerance based on TALOS+ predictions [43]. The NMR spectra were processed using NMRPipe [56] and analyzed using NMRView [57]. The structures were calculated with CYANA 2.1 [58] and refined with AMBER 8 [59] using previously reported protocols [41,60].
ITC
The ITC measurements were done at 10 °C in 50 mM Tris–HCl (pH 7.0) and 30 mM NaCl using a VP-ITC instrument (Malvern MicroCal). Ubiquitin was in the injection syringe at a concentration of 1.5 mM. REV1 was in the calorimeter cell at an initial concentration of 100 μM.
UV irradiation-induced mutagenesis and cell survival in S. cerevisiae
To study the effect of single-point mutations in S. cerevisiae REV1 on cell survival and mutagenesis after UV irradiation, we used a rev1Δ strain derived from the EMY74.7 (MATa his3–1Δ leu2–3 leu2–112 ura3–52) to generate YREV1.14 in which deletion of the REV1 gene is marked by the URA3+ gene [46]. Wild-type and mutant REV1 proteins were expressed from plasmid pVP21, which carries the LEU2 gene [46]. Yeast cells were cultured as previously reported [46]. To probe cell viability following UV irradiation, serial dilutions of yeast cultures were spread on plates prepared with synthetic defined (SD) media lacking leucine (SD − leu) for plasmid selection. The frequencies of CAN1S to can1r mutations caused by UV exposure were determined by plating the cells on SD media lacking leucine and arginine, but containing canavanine (SD − leu − arg + can). The plates were UV-irradiated at 125, 150, 175, and 200 J/m2, using a Stratalinker 2400 irradiator (Stratagene) operated in energy mode. Plates were incubated in the dark at 30 °C for 3 to 5 days. Three independent experiments were conducted with the assays done in triplicate. The cell survival and CAN1S to can1r mutation assays were done in parallel. The P values were calculated using the multiple-comparisons Dunnett’s test implemented in GraphPad Prism 6.
To test the viability of yeast cells expressing wild-type or mutated REV1 in the presence of MMS, the cells were spotted onto SD − leu media prepared without and with MMS. Plates were incubated in the dark at 30 °C for 3 to 5 days.
Acknowledgments
We are extremely grateful to Drs. Louise Prakash and Peter M. Burgers for providing reagents, and to Dr. Zhenkun Lou for access to a UV irradiator. We thank Dr. Chao Xu for many helpful discussions and Drs. Prasanna Mishra and Slobodan Macura for assistance with NMR spectroscopy. This work was supported by a National Institutes of Health grant (CA132878) to G.M. and by a Fellowship Award from the Mayo Clinic Cancer Center Fraternal Order of Eagles Funds to G.C.
Abbreviations used:
- BRCT
BRCA1 C-terminus
- HSQC
heteronuclear single quantum coherence
- ITC
isothermal titration calorimetry
- PCNA
proliferating cell nuclear antigen
- TLS
translesion synthesis
- UBM
ubiquitin-binding motif
Footnotes
Accession Numbers
Coordinates have been deposited in the Protein Data Bank with accession number 5VZM. NMR chemical shift assignments have been deposited in the Biological Magnetic Resonance Data Bank with accession number 30300.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1]. Muller HJ Artificial transmutation of the gene Science 1927. 66 84–87 [DOI] [PubMed] [Google Scholar]
- [2]. Friedberg EC Suffering in silence: the tolerance of DNA damage Nat. Rev. Mol. Cell Biol 2005. 6 943–953 [DOI] [PubMed] [Google Scholar]
- [3]. Dumstorf CA Mukhopadhyay S Krishnan E Haribabu B Mcgregor WG REV1 is implicated in the development of carcinogen-induced lung cancer Mol. Cancer Res 2009. 7 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Friedberg EC Wagner R Radman M Specialized DNA polymerases, cellular survival, and the genesis of mutations Science 2002. 296 1627–1630 [DOI] [PubMed] [Google Scholar]
- [5]. Ling H Boudsocq F Woodgate R Yang W Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication Cell 2001. 107 91–102 [DOI] [PubMed] [Google Scholar]
- [6]. Vaisman A Woodgate R Translesion DNA polymerases in eukaryotes: what makes them tick? Crit. Rev. Biochem. Mol. Biol 2017. 52 274–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Hoege C Pfander B Moldovan GL Pyrowolakis G Jentsch S RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO Nature 2002. 419 135–141 [DOI] [PubMed] [Google Scholar]
- [8]. Bergink S Jentsch S Principles of ubiquitin and SUMO modifications in DNA repair Nature 2009. 458 461–467 [DOI] [PubMed] [Google Scholar]
- [9]. Wang Z Huang M Ma X Li H Tang T Guo C REV1 promotes PCNA monoubiquitylation through interacting with ubiquitylated RAD18 J. Cell Sci 2016. 129 1223–1233 [DOI] [PubMed] [Google Scholar]
- [10]. Lemontt JF Mutants of yeast defective in mutation induced by ultraviolet light Genetics 1971. 68 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Haracska L Johnson RE Unk I Phillips B Hurwitz J Prakash L et al. Physical and functional interactions of human DNA polymerase eta with PCNA Mol. Cell. Biol 2001. 21 7199–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Guo C Fischhaber PL Luk-Paszyc MJ Masuda Y Zhou J Kamiya K et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis EMBO J 2003. 22 6621–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Garg P Burgers PM Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1 Proc. Natl. Acad. Sci. U. S. A 2005. 102 18361–18366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Friedberg EC Lehmann AR Fuchs RP Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 2005. 18 499–505 [DOI] [PubMed] [Google Scholar]
- [15]. Haracska L Unk I Prakash L Prakash S Ubiquitylation of yeast proliferating cell nuclear antigen and its implications for translesion DNA synthesis Proc. Natl. Acad. Sci. U. S. A 2006. 103 6477–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. D’Souza S Walker GC Novel role for the C terminus of Saccharomyces cerevisiae Rev1 in mediating protein-protein interactions Mol. Cell. Biol 2006. 26 8173–8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Friedberg EC Reversible monoubiquitination of PCNA: a novel slant on regulating translesion DNA synthesis Mol. Cell 2006. 22 150–152 [DOI] [PubMed] [Google Scholar]
- [18]. D’Souza S Waters LS Walker GC Novel conserved motifs in Rev1 C-terminus are required for mutagenic DNA damage tolerance DNA Repair (Amst) 2008. 7 1455–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Kosarek JN Woodruff RV Rivera-Begeman A Guo C D’Souza S Koonin EV et al. Comparative analysis of in vivo interactions between Rev1 protein and other Y-family DNA polymerases in animals and yeasts DNA Repair (Amst) 2008. 7 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Washington MT Carlson KD Freudenthal BD Pryor JM Variations on a theme: eukaryotic Y-family DNA polymerases Biochim. Biophys. Acta 2010. 1804 1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Boehm EM Powers KT Kondratick CM Spies M Houtman JC Washington MT The proliferating cell nuclear antigen (PCNA)-interacting protein (PIP) motif of DNA polymerase eta mediates its interaction with the C-terminal domain of Rev1 J. Biol. Chem 2016. 291 8735–8744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Boehm EM Spies M Washington MT PCNA tool belts and polymerase bridges form during translesion synthesis Nucleic Acids Res 2016. 44 8250–8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Pozhidaeva A Pustovalova Y D’Souza S Bezsonova I Walker GC Korzhnev DM NMR structure and dynamics of the C-terminal domain from human Rev1 and its complex with Rev1 interacting region of DNA polymerase eta Biochemistry 2012. 51 5506–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Wojtaszek J Liu J D’Souza S Wang S Xue Y Walker GC et al. Multifaceted recognition of vertebrate Rev1 by translesion polymerases zeta and kappa J. Biol. Chem 2012. 287 26400–26408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Wojtaszek J Lee CJ D’Souza S Minesinger B Kim H D’Andrea AD et al. Structural basis of Rev1-mediated assembly of a quaternary vertebrate translesion polymerase complex consisting of Rev1, heterodimeric polymerase (Pol) zeta, and Pol kappa J. Biol. Chem 2012. 287 33836–33846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Northam MR Moore EA Mertz TM Binz SK Stith CM Stepchenkova EI et al. DNA polymerases zeta and Rev1 mediate error-prone bypass of non-B DNA structures Nucleic Acids Res 2014. 42 290–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Makarova AV Nick McElhinny SA Watts BE Kunkel TA Burgers PM Ribonucleotide incorporation by yeast DNA polymerase zeta DNA Repair (Amst) 2014. 18 63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Nelson JR Lawrence CW Hinkle DC Deoxycytidyl transferase activity of yeast REV1 protein Nature 1996. 382 729–731 [DOI] [PubMed] [Google Scholar]
- [29]. Wiltrout ME Walker GC The DNA polymerase activity of Saccharomyces cerevisiae Rev1 is biologically significant Genetics 2011. 187 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Choi JY Guengerich FP Kinetic analysis of translesion synthesis opposite bulky N2- and O6-alkylguanine DNA adducts by human DNA polymerase REV1 J. Biol. Chem 2008. 283 23645–23655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Choi JY Lim S Kim EJ Jo A Guengerich FP Translesion synthesis across abasic lesions by human B-family and Y-family DNA polymerases alpha, delta, eta, iota, kappa, and REV1 J. Mol. Biol 2010. 404 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Pryor JM Washington MT Pre-steady state kinetic studies show that an abasic site is a cognate lesion for the yeast Rev1 protein DNA Repair (Amst) 2011. 10 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Nair DT Johnson RE Prakash L Prakash S Aggarwal AK Rev1 employs a novel mechanism of DNA synthesis using a protein template Science 2005. 309 2219–2222 [DOI] [PubMed] [Google Scholar]
- [34]. Nair DT Johnson RE Prakash L Prakash S Aggarwal AK Protein-template-directed synthesis across an acrolein-derived DNA adduct by yeast Rev1 DNA polymerase Structure 2008. 16 239–245 [DOI] [PubMed] [Google Scholar]
- [35]. Nair DT Johnson RE Prakash L Prakash S Aggarwal AK DNA synthesis across an abasic lesion by yeast REV1 DNA polymerase J. Mol. Biol 2011. 406 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Gabel SA Derose EF London RE XRCC1 interaction with the REV1 C-terminal domain suggests a role in post replication repair DNA Repair (Amst) 2013. 12 1105–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Bienko M Green CM Crosetto N Rudolf F Zapart G Coull B et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis Science 2005. 310 1821–1824 [DOI] [PubMed] [Google Scholar]
- [38]. Wood A Garg P Burgers PM A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage J. Biol. Chem 2007. 282 20256–20263 [DOI] [PubMed] [Google Scholar]
- [39]. Bomar MG D’Souza S Bienko M Dikic I Walker GC Zhou P Unconventional ubiquitin recognition by the ubiquitin-binding motif within the Y family DNA polymerases iota and Rev1 Mol. Cell 2010. 37 408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Guo C Tang TS Bienko M Parker JL Bielen AB Sonoda E et al. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage Mol. Cell. Biol 2006. 26 8892–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Cui G Benirschke RC Tuan HF Juranic N Macura S Botuyan MV et al. Structural basis of ubiquitin recognition by translesion synthesis DNA polymerase iota Biochemistry 2010. 49 10198–10207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Burschowsky D Rudolf F Rabut G Herrmann T Peter M Wider G Structural analysis of the conserved ubiquitin-binding motifs (UBMs) of the translesion polymerase iota in complex with ubiquitin J. Biol. Chem 2011. 286 1364–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Shen Y Delaglio F Cornilescu G Bax A TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts J. Biomol. NMR 2009. 44 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Shen Y Bax A Prediction of Xaa–Pro peptide bond conformation from sequence and chemical shifts J. Biomol. NMR 2010. 46 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Richardson JS Richardson DC Amino acid preferences for specific locations at the ends of alpha helices Science 1988. 240 1648–1652 [DOI] [PubMed] [Google Scholar]
- [46]. Pages V Santa Maria SR Prakash L Prakash S Role of DNA damage-induced replication checkpoint in promoting lesion bypass by translesion synthesis in yeast Genes Dev 2009. 23 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Pryor JM Gakhar L Washington MT Structure and functional analysis of the BRCT domain of translesion synthesis DNA polymerase Rev1 Biochemistry 2013. 52 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Guo C Sonoda E Tang TS Parker JL Bielen AB Takeda S et al. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo Mol. Cell 2006. 23 265–271 [DOI] [PubMed] [Google Scholar]
- [49]. Pustovalova Y Maciejewski MW Korzhnev DM NMR mapping of PCNA interaction with translesion synthesis DNA polymerase Rev1 mediated by Rev1-BRCT domain J. Mol. Biol 2013. 425 3091–3105 [DOI] [PubMed] [Google Scholar]
- [50]. de Groote FH Jansen JG Masuda Y Shah DM Kamiya K de Wind N et al. The Rev1 translesion synthesis polymerase has multiple distinct DNA binding modes DNA Repair (Amst) 2011. 10 915–925 [DOI] [PubMed] [Google Scholar]
- [51]. Ross AL Simpson LJ Sale JE Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1 Nucleic Acids Res 2005. 33 1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Sharma NM Kochenova OV Shcherbakova PV The non-canonical protein binding site at the monomer-monomer interface of yeast proliferating cell nuclear antigen (PCNA) regulates the Rev1-PCNA interaction and Polzeta/Rev1-dependent translesion DNA synthesis J. Biol. Chem 2011. 286 33557–33566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Hu Q Botuyan MV Cui G Zhao D Mer G Mechanisms of ubiquitin-nucleosome recognition and regulation of 53BP1 chromatin recruitment by RNF168/169 and RAD18 Mol. Cell 2017. 66 473–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Ferentz AE Wagner G NMR spectroscopy: a multifaceted approach to macromolecular structure Q. Rev. Biophys 2000. 33 29–65 [DOI] [PubMed] [Google Scholar]
- [55]. Zwahlen C Legault P Vincent SJF Greenblat J Konrat R Kay LE Methods of measurements of intermolecular NOEs by multinuclear NMR spectroscopy: application to a bacteriophage N-peptide/box B RNA complex J. Am. Chem. Soc 1997. 119 6711–6721 [Google Scholar]
- [56]. Delaglio F Grzesiek S Vuister GW Zhu G Pfeifer J Bax A NMRPipe: a multidimensional spectral processing system based on UNIX pipes J. Biomol. NMR 1995. 6 277–293 [DOI] [PubMed] [Google Scholar]
- [57]. Johnson BA Blevins RA NMRView: a computer program for visualization and analysis of NMR data J. Biomol. NMR 1994. 4 603–614 [DOI] [PubMed] [Google Scholar]
- [58]. Güntert P Automated NMR structure calculation with CYANA Methods Mol. Biol 2004. 278 353–378 [DOI] [PubMed] [Google Scholar]
- [59]. Case DA Cheatham TE III Darden T Gohlke H Luo R Merz KM Jr. et al. The Amber biomolecular simulation programs J. Comput. Chem 2005. 26 1668–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Botuyan MV Mer G Yi GS Koth CM Case DA Edwards AM et al. Solution structure and dynamics of yeast elongin C in complex with a von Hippel–Lindau peptide J. Mol. Biol 2001. 312 177–186 [DOI] [PubMed] [Google Scholar]


