Abstract
Background
Chronic opioid misuse is associated with reduced sensitivity to natural rewards and social motivation deficits that include impaired caregiving. The neurobiological mechanisms underlying these deficits and their response to treatment are not well understood. Baby schema (Kindchenschema) is a set of juvenile physical features, which is perceived as “cute” and triggers motivation for caregiving. Recent studies suggest that the “baby schema effect” is mediated by the brain “reward” network. We studied the impact of opioid antagonist treatment on the baby schema response in patients with opioid use disorder.
Methods
Forty-seven (24 F) recently detoxified patients with opioid use disorder underwent functional magnetic resonance imaging (fMRI) while viewing infant portraits that were parametrically manipulated for baby schema content and rating them for cuteness, at baseline and during treatment with the injectable extended release opioid antagonist naltrexone (XRNTX). The study was not placebo-controlled.
Results
The behavioral effect of baby schema, indexed by “cuteness” ratings, was present and unaffected by XRNTX. The brain response to baby schema was absent at baseline, but present in the bilateral ventral striatum after two weeks of XRNTX treatment. The decline in self-reported craving for opioids was positively correlated with the brain fMRI response to baby schema in the bilateral ventral striatum.
Conclusions
Opioid antagonist treatment modulated the brain reward system response to a marker of caregiving motivation in patients with opioid use disorder. Neural response to baby schema may offer a novel probe of social motivation and affiliative behaviors in this population.
Keywords: naltrexone, opioid, baby schema, functional magnetic resonance imaging, social motivation, caregiving, cuteness
1. Introduction
Opioid misuse is a growing global problem affecting millions in the US alone (Han et al., 2017). In addition to direct health risks, opioid addiction is associated with social dysfunction. Observational studies in humans and non-human primates suggest that opioid addiction contributes to deficits in pro-social behaviors culminating in dysfunctional parenting (Bridges & Grimm, 1982; Conroy, Degenhardt, Mattick, & Nelson, 2009; Lee, Wang, Tang, Liu, & Bell, 2015; Ragen, Maninger, Mendoza, & Bales, 2015; Wells, 2009), as well as reduced responses to natural rewards (Lubman et al., 2009). The relevance of neurobiology of attachment to opioid dependence is supported by the notion that opioids and social attachment share neural mechanisms (Insel, 2003) that converge on the mesocorticolimbic pathways implicated in both approach behavior and reward (Curtis, Liu, Aragona, & Wang, 2006; Hansen, Bergvall, & Nyiredi, 1993; Nelson & Panksepp, 1998; Saltzman & Maestripieri, 2011). Particularly conspicuous is the impairment of the maternal instinct by exogenous opioids in laboratory animals (Barr et al., 2008; Bridges & Grimm, 1982) (Mann, Pasternak, & Bridges, 1990; Rubin & Bridges, 1984; Sukikara, Platero, Canteras, & Felicio, 2007) and humans (Lawson & Wilson, 1980). Experimental studies in humans have not yet addressed the neurobiology of the observed deficits and their response to therapeutic interventions, perhaps due to paucity of laboratory paradigms that consistently engage social cognition systems and reliably elicit the caregiving instinct.
Caring for the young is a fundamental social phenomenon that may be triggered by typical external appearance characteristics of juvenile animals and humans that are perceived as “cute”. Konrad Lorenz defined “Baby schema” (Kindchenschema), as a set of physical features characteristic of juvenile animals and humans and postulated that it is a “key stimulus” that “releases” a caregiving instinct (Lorenz, 1971). The baby schema response (Lehmann, Huis in’t Veld, & Vingerhoets, 2013) precedes mother-infant bonding and is hypothesized to have evolved as an adaptation to cooperative rearing of offspring (M.L. Glocker et al., 2009; Hrdy, 2005; Preston, 2013). Baby schema features in portraits can be quantified and manipulated using computerized graphics. Recent studies reported that baby schema content in infant portraits enhances motivation for caretaking, cuteness appraisal and striatal fMRI activation (M. L. Glocker et al., 2009b; Parsons, Young, Kumari, Stein, & Kringelbach, 2011). For example, in healthy nulliparous women viewing a set of portraits of unrelated infants, “cuteness” appraisals and fMRI signal in the ventral striatum increased proportionately to the baby schema content of the portraits (M. L. Glocker et al., 2009b). In another group of healthy non-parents of both sexes, cuteness appraisals were highly correlated with motivation for caretaking (M.L. Glocker et al., 2009). These findings support the ethological concept of baby schema as an unconditional “releaser” (Lorenz, 1971; Schleidt, Schiefenhovel, Stanjek, & Krell, 1980) of behaviors fundamental to parenting, and suggest that this process is mediated by the mesocorticolimbic approach and reward system (Ikemoto & Panksepp, 1999). Given the importance of opioids to social cognition, the baby schema response could be sensitive to the impact of opioid use disorder and its pharmacotherapy on social cognition (Machin & Dunbar, 2011). Basic research points to specific effects of exogenous opioid modulators (agonists or antagonists) on social motivation and cognition that vary with species, dose, prior socialization and opioid exposure (Bridges & Grimm, 1982; Cinque et al., 2012; Loseth, Ellingsen, & Leknes, 2014; Nocjar & Panksepp, 2007; Ragen et al., 2015; Rubin & Bridges, 1984; Zaaijer et al., 2015). Naltrexone is a competitive opioid receptor antagonist that is used to prevent relapse in detoxified patients with opioid use disorder (Kleber, 2007). An injectable extended-release preparation (XRNTX, Vivitrol ®; Alkermes plc, Dublin, Ireland) gradually releases naltrexone from polymer microspheres and produces stable therapeutic levels that are pharmacologically effective for approximately one month (Krupitsky & Blokhina, 2010). Therapeutic advantages of the monthly injectable XRNTX over the daily oral preparation are stable plasma levels, greatly improved compliance profile (Hulse, Morris, Arnold-Reed, & Tait, 2009) (P. Lobmaier, Kornor, Kunoe, & Bjorndal, 2008; P. P. Lobmaier, Kunoe, Gossop, Katevoll, & Waal, 2010), and reduction in expectation of opioid effects leading to reduced conditioned withdrawal and craving (Krupitsky & Blokhina, 2010; O’Brien, Testa, O’Brien, Brady, & Wells, 1977; Wilson, Sayette, & Fiez, 2004). There have been no reports of experimental studies of the sustained effects of opioid antagonists on social cognition in patients with opioid use disorder. In the present study we tested the effects of XRNTX on the brain and behavioral response to baby schema in patients with opioid use disorder. We hypothesized that XRNTX would modulate the mesocorticolimbic brain fMRI and behavioral effects of baby schema.
2. Materials and methods
2.1. Participants
Forty-seven opioid-dependent individuals (24 females, 41 Caucasian, 3 African American, 3 Asian, age 28.9 ±7.5, mean ±SD) were recruited through local advertising and gave written informed consent to participate in the University of Pennsylvania Institutional Review Board-approved study. The study was registered as a clinical trial NCT02324725.
The DSM-IV-TR diagnosis of opioid dependence was established using the best estimate format, based on all available sources of information, including history, structured clinical interview for DSM-IV-TR (First, 2002) and the Addiction Severity Index (ASI) 5th Edition (McLellan et al., 1992). Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). The average ASI Drug Composite Score was 0.29 ± 0.10 (range 0.09–0.47). Participants were abstinent (by self-report) for 20.73 ± 17.99 days (range 5–69) before the 1st XRNTX injection. Duration of opioid dependence was 6.09 ± 6.90 years (range 1–34). Thirteen participants had children, 29 had no children, 5 declined to answer.
Inclusion criteria were: 1) 18–55 years of age; 2) DSM-IV-TR diagnosis of opioid dependence with documented daily opioid use for at least 2 out of the last 12 weeks; 3) Complete detoxification from opioids demonstrated by a negative naloxone challenge test, i.e. absence of withdrawal response within 30 min after the administration of 0.6 mg of naloxone hydrochloride intravenously (IV) and last documented use of opioids more than 5 days ago; 4) good physical health ascertained by history and physical examination, blood chemistry and urinalysis.
Exclusion criteria were: 1) chronic medical illness; 2) current medications potentially confounding vascular or electrophysiologic response; 3) current DSM-IV-TR Axis I psychiatric disorders other than opioid and nicotine dependence; 4) lifetime history of concurrent IV cocaine and heroin (speedball) administration; 5) pregnancy or breastfeeding; 6) history of clinically significant (i.e. followed by loss of consciousness or facial or skull fracture) head trauma; 7) contraindications for XRNTX treatment such as medical conditions requiring opioid analgesics, e.g. chronic pain or planned surgery, obesity, hepatic enzymes > 3 times upper limit of normal, failure to complete opioid detoxification and 8) contraindications for MRI, e.g. indwelling magnetically active foreign bodies or fear of enclosed spaces.
2.2. Study medication
To ensure completeness of opioid detoxification, XRNTX was preceded by a naloxone challenge. Participants then received an injection of XRNTX (380 mg of naltrexone HCL gradually released from dissolvable polymer microspheres over a period of one month, manufactured by Alkermes plc, Dublin, Ireland, under the brand name Vivitrol®). As part of the consent procedure, participants were briefed about the loss of pharmacological effects of opioids resulting from the XRNTX treatment, and the dangers of attempting to overcome the opiate receptor blockade with higher than usual opioid doses (Paronis & Bergman, 2011; Ruan, Chen, Gudin, Couch, & Chiravuri, 2010).
2.3. Procedures
MRI sessions were conducted immediately prior to (Pre-XRNTX), as well as approximately 2 weeks (12 ± 7 days) after the first XRNTX injection (On-XRNTX). Subjective craving for and withdrawal from opioids was recorded prior to each fMRI session using self-report on a 0–9 scale. Plasma concentrations of naltrexone and 6-beta-naltrexol (an active metabolite) were ascertained at the second MRI session 12 ± 7 days after injection, using established liquid chromatography and tandem mass spectrometry techniques (Langleben et al., 2014; Slawson et al., 2007). During the study, continuation of care was discussed with the participants and they were given referrals to treatment providers in the community. A urine drug screen (UDS, Redwood Toxicology Laboratory, Santa Rosa, CA) for opiates (e.g. heroin and codeine metabolites), oxycodone, methadone, buprenorphine, cocaine, amphetamine, methamphetamine, benzodiazepines and phencyclidine was conducted prior to each MRI scan and each XRNTX injection.
2.4. Baby schema task
The baby schema task was previously reported and validated in healthy young adults of both sexes using a behavioral paradigm (Glocker, 2009) and in young nulliparous women using a behavioral paradigm in the fMRI scanner (M. L. Glocker et al., 2009a). Briefly, baby schema was operationalized in infant faces using facial features that comprise the baby schema, i.e. face width, forehead height, and eye, nose and mouth size and shape (Fullard & Reiling, 1976; Sternglanz, Gray, & Murakami, 1977). Using Photoshop (Adobe Systems, San Jose, CA), these facial parameters were established in a sample of 40 original infant photographs courtesy of Dr. Katherine Karraker (20 boys and 20 girls aged 7–13 months with a neutral facial expression and on a black background (Karraker & Stern, 1990) and the mean values of each facial baby schema parameter in this sample were calculated. Anthropometric and morphing techniques and software (Morph Age, eX-cinder, www.creaceed.com; Face Filter Studio, Reallusion Inc, www.reallusion.com) were used to manipulate these facial parameters in 17 portraits, randomly selected from the sample of 40 (Farkas, 1994; Steyvers, 1999). The manipulation produced 17 “Low” (narrow face, low forehead, small eyes, big nose and mouth) and 17 “High” (round face, high forehead, big eyes, small nose and mouth) versions of the original “Unmanipulated” baby schema portraits of each infant (M. L. Glocker et al., 2009b).
Prior to the session, participants were familiarized with the baby schema task using unmanipulated images of juvenile animals and the use of a response device. During fMRI scan acquisition, participants were presented with a pseudo-random, event-related sequence of the 17 Low, 17 Unmanipulated and 17 High baby schema faces (optseq2, http://surfer.nmr.mgh.harvard.edu/optseq/) (Figure 1). Each image was presented once for 3 s, followed by a variable inter-stimulus interval (6–18 s) during which a crosshair fixation point was displayed on a black background (total 543 s). Participants rated each face for cuteness (1=“not very cute”, 2=“cute” and 3=“very cute”) with a button-press response device using a fiber-optic response pad (FORP™ Current Design, Inc., Philadelphia, PA). Total task duration was 11 min and 36 s. The baby schema task was always the first task in the imaging sessions that included additional tasks that varied across participants.
Figure 1.
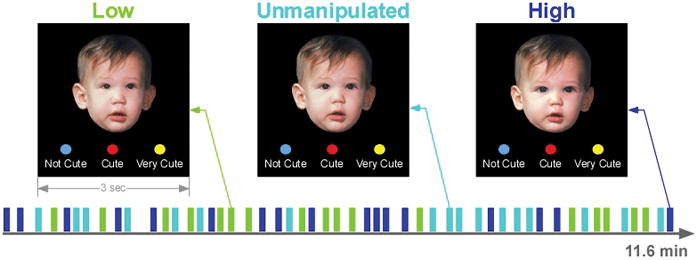
Paradigm of the Baby Schema Task.
2.5. Imaging data acquisition
Blood oxygenation level-dependent (BOLD) fMRI was acquired with a Siemens Trio 3 Tesla system (Erlangen, Germany) using a whole-brain, single-shot gradient-echo (GE) echoplanar (EPI) sequence with the following parameters: TR/TE = 3000/30 ms, FOV = 220 mm, matrix = 64 x 64, slice thickness/gap = 3/0mm, 40 slices, effective voxel resolution of 3.4 x 3.4 x 3 mm. To reduce susceptibility artifacts in orbital frontal regions, EPI was acquired obliquely (axial/coronal). Prior to time-series acquisition, a 5-minute magnetization-prepared, rapid acquisition gradient echo T1-weighted image (MPRAGE, TR 1620ms, TE 3.87 ms, FOV 250 mm, Matrix 192 x 256, effective voxel resolution of 1 x 1 x 1mm) was collected for anatomic overlays of functional data and to aid spatial normalization to a standard atlas space (Talairach & Tournoux, 1988).
2.6. Data analysis
Statistical analysis was conducted in SPSS (IBM SPSS version 19, Armonk, NY, USA). The effect of XRNTX treatment (Pre vs On) on baby schema perception (Low, Unmanipulated, High) was examined with 2 x 3 repeated-measures ANOVA on the subjective cuteness ratings, using Baby Schema (Low, Unmanipulated, High) and Treatment (Pre vs On) as within-subject factors.
The fMRI data were subjected to quality control, preprocessing and statistical analysis using FEAT (fMRI Expert Analysis Tool) version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Subject-level preprocessing included slice-time correction, motion correction to the median image using MCFLIRT, high-pass temporal filtering (100 s), spatial smoothing (6 mm FWHM, isotropic) and scaling using mean-based intensity normalization. The median functional image was co-registered to the corresponding high-resolution T1-weighted structural image and transformed into standard anatomical space (T1 Montreal Neurological Institute template) using FLIRT. Transformation parameters were applied to statistical maps before group analyses. The brain extraction tool (BET) was used to remove non-brain areas.
Subject-level time-series analyses were carried out using FILM (FMRIB’s Improved Linear Model) with local auto-correlation (Woolrich, Ripley, Brady, & Smith, 2001). Brain responses to three events: Low, Unmanipulated and High baby schema (versus crosshair), were modeled using a double-gamma haemodynamic response function. At the group-level analysis, subject-level contrast maps were entered into paired group test to identify brain activation in response to baby faces across baby schema levels as a function of XRNTX treatment (i.e. Pre-XRNTX > On-XRNTX, Pre-XRNTX < On-XRNTX). Group z (Gaussianized T) statistic images were generated for Pre- vs. On-XRNTX. Group maps were thresholded at the voxel level of z = 3.1 and cluster corrected at p < 0.005 using family-wise error correction based on Gaussian Random Field theory. Anatomic assignment of clusters was based on the peak z-score within the cluster using the Talairach Daemon Database confirmed by visual inspection. Then we extracted the mean BOLD fMRI percent signal change from the activated cluster for off-line analysis (Buchel, Holmes, Rees, & Friston, 1998) and graphic presentation. The repeated measures ANOVA on BOLD percent signals change and subjective ratings was performed using the Low, High, and Unmanipulated baby schema as within-subject factors for the Pre- and On-XRNTX sessions respectively. The relationships between percent BOLD signal change in response to Low, Unmanipulated, and High baby schema portraits after the XRNTX injection, and the change in craving score (i.e. Pre-XRNTX craving minus On-XRNTX craving) were explored using Pearson correlation tests. Finally, we explored whether gender or opioid abstinence period prior to the XRNTX treatment affected brain response to baby schema, by including them as covariates in the analysis.
3. Results
Demographic and clinical characteristics of participants were summarized in Table 1 below.
Table 1.
Demographic and clinical characteristics of participants.
| Group | Female | Male | |
|---|---|---|---|
| N | 47 (100%) | 24 (51%) | 23 (49%) |
| Age (years) | 28.94 ± 7.47 | 28.25 ± 8.54 | 29.65 ± 6.26 |
| Race | |||
| Caucasian | 41 (87%) | 22 (92%) | 19 (83%) |
| African American | 3 (6%) | 1 (4%) | 2 (9%) |
| Asian | 3 (6%) | 1 (4%) | 2 (9%) |
| Duration of heroin use (years) | 5.22 ± 6.53 | 6.30 ± 8.57 | 4.07 ± 3.21 |
| Duration of prescription opioid use (years) | 4.27 ± 3.27 | 3.41 ±3.18 | 5.00 ±3.24 |
| Route of opioid use | |||
| Heroin (IV) | 35 (74%) | 20 (83%) | 15 (65%) |
| Heroin (intranasal, IN) | 2 (4%) | 1 (4%) | 1 (4%) |
| Pill (oral, PO) | 5 (11%) | 1 (4%) | 4 (17%) |
| Pill (IN) | 6 (13%) | 2 ( 8%) | 4 (17%) |
| Average daily use (bags heroin) | 6.51 ± 8.07 | 6.24 ± 7.84 | 6.82 ±8.51 |
| Average daily use (mg oxycodone equivalents) | 10.47 ± 25.29 | 14.43 ± 29.35 | 6.12 ± 19.75 |
| Abstinence before XRNTX injection (days) | 15.08 ± 17.07 | 11.19 ± 16.47 | 17.78 ±17.32 |
| Other drug use in the last 30 days | |||
| Cocaine | 5.67 ± 11.82 | 6.54 ± 13.97 | 4.68 ± 8.98 |
| Amphetamines | 0 | 0 | 0 |
| Benzodiazepines | 1.81 ± 4.11 | 2.51 ± 5.27 | 1.01 ± 1.97 |
| Marijuana | 25.62 ± 74.82 | 34.46 ± 91.30 | 15.45 ± 50.18 |
| Alcohol | 1.63 ± 3.27 | 1.49 ± 3.34 | 1.80 ± 3.27 |
| Tobacco | 14.47 ± 9.08 | 16.45 ± 10.62 | 12.28 ± 6.61 |
Repeated-measures ANOVA revealed a significant main effect of baby schema on subjective cuteness ratings in the Pre-XRNTX session (F(2, 74) = 151.32 p < 0.0001), as well as in the On-XRNTX session (F(2, 74) = 97.84 p < 0.0001). Post-hoc pairwise comparisons with Bonferroni correction showed that the High baby schema portraits were rated as cuter than the Unmanipulated and the Low baby schema portraits (High vs. Unmanipulated p < 0.0001, High vs. Low p < 0.0001). Unmanipulated baby schema portraits were rated as cuter than Low baby schema ones (Unmanipulated vs. Low p < 0.0001) in both sessions. At the Pre-XRNTX MRI session, two participants were positive for opiates or buprenorphine, four for cocaine and seven for tetrahydrocannabinol (THC). At the On-XRNTX session, two participants were positive for opiates, eight for cocaine and thirteen for THC. Subjective withdrawal was significantly lower in the On-XRNTX session (paired t-test, t = 5.014, df = 36, p < 0.0001). When subjects were on XRNTX treatment, naltrexone plasma levels were 2.86 ± 1.41 ng/ml and 6-beta-naltrexol levels were 7.31 ± 4.40 ng/ml.
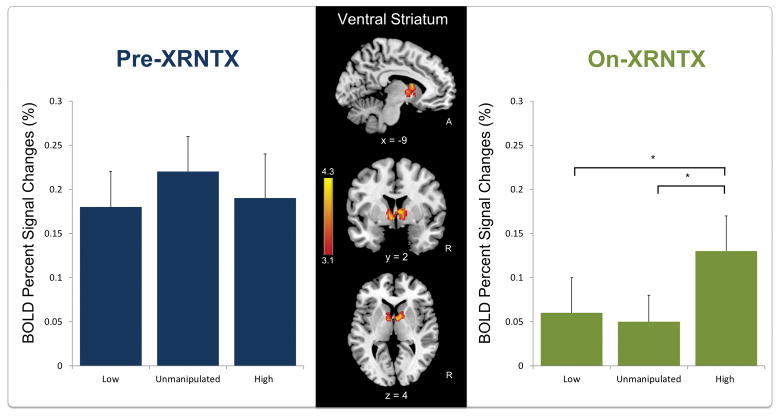
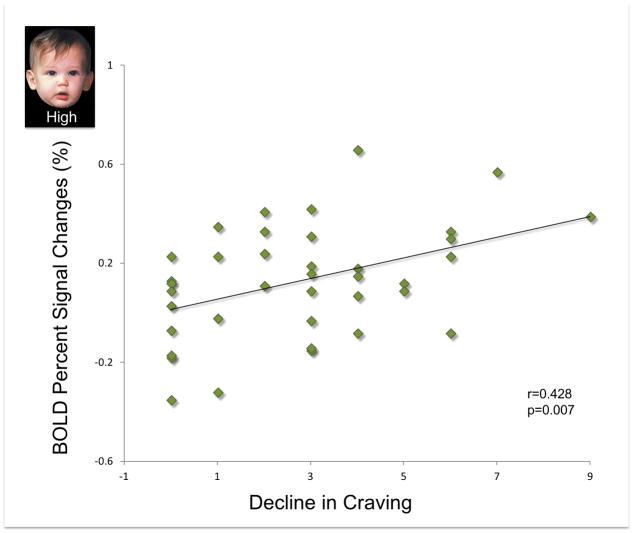
During XRNTX treatment, brain response to infant faces across all baby schema levels was reduced in the cluster with peaks in the bilateral caudate head, accumbens and anterior thalamic nuclei, (i.e. ventral striatum) (Table 2). Before XRNTX treatment (Figure 2, left panel), there was no baby schema effect (F(2, 74) = 0.688, p = 0.509). During the treatment, the baby schema effect was present (F(2, 74 )= 4.137, p = 0.020). Also, the decline in craving (i.e. Pre-XRNTX craving minus On-XRNTX craving) was positively correlated with the brain response to High baby schema (On-XRNTX) in the ventral striatum (r = 0.428, p =0.007) (Figure 3), while it did not affect the brain response to Low (r = 0.288 p = 0.079) or Unmanipulated baby schema (r = 0.051 p = 0.759) stimuli. Gender had no effect on the brain response to baby schema in either the Pre-XRNTX session (F(1, 74) = 0.36 p=0.55) or the On-XRNTX session (F(1, 74) = 0.90 p = 0.35). Duration of abstinence from opioids prior to the XRNTX injection also had no effect on brain responses either in the Pre-XRNTX session (F(1, 74) = 1.04 p = 0.31) or in the On-XRNTX session (F(1, 74) = 1.86 p = 0.18).
Table 2.
Greater brain response to infant faces across all baby schema levels before Pre-XRNTX than On-XRNTX (Figure 2 middle panel).
| Regiona | Hem | Size (vox) | Z-MAXb | Xc | Yc | Zc |
|---|---|---|---|---|---|---|
| Thalamus | Right | 403 | 4.33 | 3 | −1 | 9 |
| Caudate (head) | Left | 3.97 | −7 | 5 | 2 | |
| Caudate (body) | Right | 3.88 | 8 | 2 | 7 | |
| Caudate (body) | Left | 3.87 | −11 | 6 | 13 | |
| Caudate (body) | Left | 3.85 | −11 | 4 | 16 |
Location of the clusters and the global and local maxima of BOLD fMRI signal change.
Please refer to Figure 2 to visualize the full extent of each cluster.
z≥ 3.1 and (corrected) cluster significance p< 0.005.
Z-MAX values represent peak activation for the cluster.
Talairach (1988) coordinates.
Figure 2.
Images: Brain response to baby faces across all baby schema levels (High, Unmanipulated and Low) before naltrexone treatment (Pre-XRNTX) shows greater activation in the bilateral ventral striatum compared to brain response during treatment (On-XRNTX). Bar charts: Percent BOLD fMRI signal change (s) in the ventral striatum as function of baby schema in the Pre- and On-XRNTX sessions respectively. Error bars presented standard error of the mean (SEM).
Figure 3.
Positive correlations between decline in craving (i.e. Pre-XRNTX craving – On-XRNTX craving) and the brain response to High baby schema faces in bilateral ventral striatum during XRNTX treatment.
4. Discussion
We found that in the recently detoxified individuals addicted to opioids, treatment with an extended release opioid antagonist was associated with reduced brain fMRI response to infant portraits in the ventral striatum and thalamus. The behavioral baby schema effect, indexed by “cuteness” ratings was present and unchanged by XRNTX. The brain baby schema effect, i.e. differentiation between High and Low baby schema portraits in the ventral striatum, was absent before treatment and appeared after two weeks of XRNTX. Finally, change in self-reported craving was positively correlated with the brain response to High baby schema in the bilateral ventral striatum.
The frontal brain regions that mediate reward and motivation and regulate affect, project primarily to the rostral striatum that includes nucleus accumbens, medial caudate nucleus (i.e. caudate head), and medial and ventral rostral putamen, collectively referred to as the ventral striatum (Haber, 2011). Ventral striatum contains neuron bodies rich in opioid receptors that send dopaminergic projections to other parts of the mesocorticolimbic system. Ventral striatum is also part of the distributed system mediating social cognition that includes hippocampus, medial orbitofrontal cortex, periaqueductal gray and the insulae (Depue & Morrone-Strupinsky, 2005; M. L. Glocker et al., 2009b; Kringelbach, 2005; Kringelbach et al., 2008; Nocjar & Panksepp, 2007; Parsons et al., 2011). Infant faces are postulated to be instinctively hedonic stimuli that activate the ventral striatum and other components of the extended limbic system in healthy individuals (M. L. Glocker et al., 2009b; Kringelbach, 2005; Kringelbach et al., 2008; Nocjar & Panksepp, 2007; Parsons et al., 2011). The absent brain fMRI response to baby schema in the ventral striatum in our subjects at baseline is consistent with complex changes in reward sensitivity of the mesocorticolimbic system in opioid dependence (Kalivas & Volkow, 2005; Koob, 2015; Volkow & Fowler, 2000). By the third week of XRNTX, the nonspecific brain response to infant faces regardless of their cuteness had declined while the striatal sensitivity to baby schema levels had returned. These findings extend the limited evidence that opioid antagonism reduces hedonic responses and enhances prosocial activities in animals (Alcaro & Panksepp, 2011; Martel, Nevison, Rayment, Simpson, & Keverne, 1993) and modulates these domains in humans (Inagaki, Ray, Irwin, Way, & Eisenberger, 2016; Johnson et al., 2014; Shi et al., 2016; Wardle, Bershad, & de Wit, 2016). Prior studies that used sweet taste perception as a model of hedonic processing in detoxified heroin addicts (Kampov-Polevoy, Garbutt, & Janowsky, 1997) found that XRNTX treatment reduced self-reported liking of the sweet solutions and lowered the threshold of sweet taste perception in detoxified opioid addicts, making them similar to healthy controls and suggesting that naltrexone “reset” the hedonic processing to normal levels (Green et al., 2013; Langleben, Busch, O’Brien, & Elman, 2012). The contribution of XRNTX to the restoration of the baby schema response is also supported by the positive relationship between the reduction in self-reported craving and the brain response to the High baby schema in the ventral striatum (Figure 3).
At the molecular level, interpretation of the changes in sensitivity to baby schema during XRNTX treatment must take into account both the acute effects of naltrexone and the long-term adaptations to chronic opioid exposure in our subjects, which are certain to persist beyond detoxification (Koob & Volkow, 2010) (Shaham & Hope, 2005). As mentioned earlier, the ventral striatum contains opioid-sensitive dopaminergic neurons that project to the rest of the mesocorticolimbic system and engage in processing natural rewards (Tempel, Gardner, & Zukin, 1985). The affinity of naltrexone to the opioid receptors and its long-term effects vary with receptor subtypes, and the quantity and duration of exposure (Bilkei-Gorzo, Mauer, Michel, & Zimmer, 2014). In addition, data from non-human primates show that pre-treatment with opioids changes naltrexone receptor function from neutral antagonism to inverse agonism (Li, McMahon, & France, 2008), a situation that would apply to our opioid-addicted participants. Limited data suggest that the mu-opioid receptor (MOR) endorphin system, and the kappa opioid receptor (KOR) dynorphin system are involved in social cognition (Bilkei-Gorzo et al., 2014; Wardle et al., 2016). In rodents, naltrexone is associated with a two-fold upregulation of the MORs that reaches a plateau after approximately eight days, while there is little change in the KORs density or sensitivity to opioids (Tempel et al., 1985). Thus, sustained naltrexone administration may increase the ventral striatum sensitivity to baby schema by changing the balance between the MOR and KOR systems’ modulation of the dopaminergic activity in the mesocorticolimbic system (Ragen et al., 2015). Alternatively, opioid receptor blockade could enable greater contribution to the baby schema effect by the oxytocin/vasopressin system, which has also been strongly implicated in social cognition (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007; Heinrichs, von Dawans, & Domes, 2009; Higham et al., 2011; Kelley, 2004).
Unlike the brain response, the subjective hedonic appraisal of the baby schema was present and unchanged by the treatment. The dissociation between the baseline brain and behavioral response could be explained by the latter being sensitive to cognitive bias (Orne, 1962) or participants’ ability to appraise cuteness without emotionally relating to it through the baby schema effect. Our within-subject design was unable to fully dissociate the pharmacological effects of naltrexone per se, from the effect of continued abstinence. However, previous studies reported that social cognition deficits were independent of the duration of opioid abstinence (Kornreich et al., 2003). Additionally, since participants had been abstinent from opioids for an average of three weeks at the time of baseline fMRI session, pharmacodynamic effects are likely to have contributed to the overall findings. The study was uncontrolled, limiting our interpretation of the effects of naltrexone. However, placebo-controlled studies of naltrexone are difficult to conduct because most patients “test the blockade” by trying opioids in the beginning of treatment, and thus know what group have they been randomized to (Sullivan et al., 2013; Wang et al., 2015). Therefore, in an outpatient setting, a placebo XRNTX arm would have limited validity and increase the risk of opioid overdose. All study participants were polysubstance users. Excluding polysubstance use could have yielded a more homogeneous sample but would not be representative of the clinical OUD population (Wang et al., 2015). Finally, the between-subject variability in the menstrual cycle phase across scan sessions in this outpatient study could obscure the possible reproductive hormone-related effects (Carroll, Lynch, Roth, Morgan, & Cosgrove, 2004; Roche & King, 2015; Sprengelmeyer et al., 2009). Controlling for the menstrual cycle phase in a patient population may require a more structured environment than an outpatient setting could offer.
5. Conclusions
In conclusion, we found that extended release naltrexone treatment affects the neural system that supports social cognition in detoxified opioid dependent individuals. These preliminary findings set the stage for the future controlled studies of the effects of opioid modulators on the brain and behavioral correlates of social cognition, and for greater attention to social cognition variables in clinical research on opioids. The importance of such research is magnified by the very high lifetime rates of exposure to therapeutic and illicit opioids worldwide.
Acknowledgments
Funding
The study was supported by the National Institutes on Drug Abuse grants (DA-024553, PI: Dr. C. P. O’Brien; N01DA-14-7788, PI: Dr. D. Moody; R21 DA043983, PI: Dr. D. D. Langleben), the Commonwealth of Pennsylvania grant (SAP#4100055577, PI: Dr. A. R. Childress), National Institute of Child Health and Human Development grant (5K99HD084746, PI: A.L. Wang). Study medicine was donated by the manufacturer (Alkermes plc).
Melanie L. Glocker, Dr. rer. nat (1977–2010) created the baby schema task and performed the fundamental work that made the present study possible. Charles P. O’Brien, MD, PhD, James W. Loughead, PhD and Wen Cao, MS provided valuable comments on the study or the manuscript. Shira J. Blady, BS and Emily Dowd, BA coordinated the project.
References
- Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neuroscience and Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2011.03.002. S0149-7634(11)00045-5 [pii] [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, … Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Mauer D, Michel K, Zimmer A. Dynorphins regulate the strength of social memory. Neuropharmacology. 2014;77:406–413. doi: 10.1016/j.neuropharm.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Grimm CT. Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science. 1982;218(4568):166–168. doi: 10.1126/science.7123227. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8(2):140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cinque C, Pondiki S, Oddi D, Di Certo MG, Marinelli S, Troisi A, … D’Amato FR. Modeling socially anhedonic syndromes: genetic and pharmacological manipulation of opioid neurotransmission in mice. Transl Psychiatry. 2012;2:e155. doi: 10.1038/tp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy E, Degenhardt L, Mattick RP, Nelson EC. Child maltreatment as a risk factor for opioid dependence: Comparison of family characteristics and type and severity of child maltreatment with a matched control group. Child Abuse and Neglect. 2009;33(6):343–352. doi: 10.1016/j.chiabu.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Research. 2006;1126(1):76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav Brain Sci. 2005;28(3):313–350. doi: 10.1017/S0140525X05000063. discussion 350–395. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Farkas LG. Anthropometry of the Head and Face. 2. NY: Raven Press; 1994. [Google Scholar]
- First M, editor. Diagnostic and statistical manual of mental disorders, 4th Edition, Text Revision. Washington, DC: American Psychiatric Association; 2002. [Google Scholar]
- Fullard W, Reiling AM. An investigation of Lorenz’s “babyness”. Child Dev. 1976;47(4):1191–1193. [Google Scholar]
- Glocker M, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sachser N. Baby Schema in Infant Faces Induces Cuteness Perception and Motivation for Caretaking in Adults. Ethology. 2009;115(3):257–263. doi: 10.1111/j.1439-0310.2008.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sachser N. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology. 2009;115:257–263. doi: 10.1111/j.1439-0310.2008.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, … Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106(22):9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, … Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci U S A. 2009b;106(22):9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Kaul A, O’Shea J, Sharma E, Bennett L, Mullings EL, … Donaldson LF. Opiate agonists and antagonists modulate taste perception in opiate-maintained and recently detoxified subjects. J Psychopharmacol. 2013;27(3):265–275. doi: 10.1177/0269881112472567. [DOI] [PubMed] [Google Scholar]
- Haber S. Neuroanatomy of Reward: A View from the Ventral Striatum. In: Gottfried J, editor. Neuroanatomy of Reward: A View from the Ventral Striatum. Boca Raton (FL): CRC Press; 2011. [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017 doi: 10.7326/M17-0865. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacology, Biochemistry and Behavior. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behavioral Neuroscience. 2011;125(2):131–136. doi: 10.1037/a0022695. 2011-06370-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. Evolutionary context of human development: the cooperative breeding model. In: Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, Sachser N, editors. Attachment and Bonding: A New Synthesis. Cambridge, Massachusestts: The MIT Press; 2005. [Google Scholar]
- Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Archives of General Psychiatry. 2009;66(10):1108–1115. doi: 10.1001/archgenpsychiatry.2009.130. 66/10/1108 [pii] [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI. Opioids and social bonding: naltrexone reduces feelings of social connection. Soc Cogn Affect Neurosci. 2016;11(5):728–735. doi: 10.1093/scan/nsw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology and Behavior. 2003;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ulberg S, Shivale S, Donaldson J, Milczarski B, Faraone SV. Fibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Discov Med. 2014;18(99):209–220. [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. American Journal of Psychiatry. 1997;154(2):269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Karraker KH, Stern M. Infant physical attractiveness and facial expression: Effects on adult perceptions. Basic and Applied Social Psychology. 1990;11(4):371–385. [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The dark side of emotion: the addiction perspective. European Journal of Pharmacology. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Foisy ML, Philippot P, Dan B, Tecco J, Noel X, … Verbanck P. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Research. 2003;119(3):251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, … Stein A. A specific and rapid neural signature for parental instinct. PLoS ONE. 2008;3(2):e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Blokhina EA. Long-acting depot formulations of naltrexone for heroin dependence: a review. Curr Opin Psychiatry. 2010 doi: 10.1097/YCO.0b013e3283386578. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Busch EL, O’Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology. 2012;220(3):559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Loughead JW, Busch EL, Cornish J, … O’Brien CP. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addiction Biology. 2014;19(2):262–271. doi: 10.1111/j.1369-1600.2012.00462.x. [DOI] [PubMed] [Google Scholar]
- Lawson MS, Wilson GS. Parenting among women addicted to narcotics. Child Welfare. 1980;59(2):67–79. [PubMed] [Google Scholar]
- Lee CH, Wang TJ, Tang HP, Liu YH, Bell J. Familial expressed emotion among heroin addicts in methadone maintenance treatment: does it matter? Addictive Behaviors. 2015;45:39–44. doi: 10.1016/j.addbeh.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Lehmann V, Huis in’t Veld EM, Vingerhoets AJ. The human and animal baby schema effect: correlates of individual differences. Behav Processes. 2013;94:99–108. doi: 10.1016/j.beproc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, France CP. Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology. 2008;195(4):479–486. doi: 10.1007/s00213-007-0914-9. [DOI] [PubMed] [Google Scholar]
- Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008;(2):CD006140. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier PP, Kunoe N, Gossop M, Katevoll T, Waal H. Naltrexone implants compared to methadone: outcomes six months after prison release. European Addiction Research. 2010;16(3):139–145. doi: 10.1159/000313336. 000313336 [pii] [DOI] [PubMed] [Google Scholar]
- Lorenz K. Studies in Animal and Human Behavior. Cambridge, Massachusetts: Harvard University Press; 1971. [Google Scholar]
- Loseth GE, Ellingsen DM, Leknes S. State-dependent mu-opioid modulation of social motivation. Front Behav Neurosci. 2014;8:430. doi: 10.3389/fnbeh.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66(2):205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148:40. [Google Scholar]
- Mann PE, Pasternak GW, Bridges RS. Mu 1 opioid receptor involvement in maternal behavior. Physiology and Behavior. 1990;47(1):133–138. doi: 10.1016/0031-9384(90)90051-5. [DOI] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Rayment FD, Simpson MJ, Keverne EB. Opioid receptor blockade reduces maternal affect and social grooming in rhesus monkeys. Psychoneuroendocrinology. 1993;18(4):307–321. doi: 10.1016/0306-4530(93)90027-i. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Prior morphine experience induces long-term increases in social interest and in appetitive behavior for natural reward. Behav Brain Res. 2007;181(2):191–199. doi: 10.1016/j.bbr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195(4282):1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orne MT. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. American Psychologist. 1962;17(11):776–783. doi: 10.1037/h0043424. [DOI] [Google Scholar]
- Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2011;336(2):488–495. doi: 10.1124/jpet.110.173823. jpet.110.173823 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Kumari N, Stein A, Kringelbach ML. The motivational salience of infant faces is similar for men and women. PLoS One. 2011;6(5):e20632. doi: 10.1371/journal.pone.0020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD. The origins of altruism in offspring care. Psychol Bull. 2013;139(6):1305–1341. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and kappa opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus) Neuroscience. 2015;287:32–42. doi: 10.1016/j.neuroscience.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, King AC. Sex differences in acute hormonal and subjective response to naltrexone: The impact of menstrual cycle phase. Psychoneuroendocrinology. 2015;52:59–71. doi: 10.1016/j.psyneuen.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Chen T, Gudin J, Couch JP, Chiravuri S. Acute opioid withdrawal precipitated by ingestion of crushed embeda (morphine extended release with sequestered naltrexone): case report and the focused review of the literature. J Opioid Manag. 2010;6(4):300–303. doi: 10.5055/jom.2010.0028. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS. Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Research. 1984;307(1–2):91–97. doi: 10.1016/0006-8993(84)90464-5. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(5):1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleidt M, Schiefenhovel W, Stanjek K, Krell R. “Caring for a baby” behavior: Reactions of passersby to a mother and baby. Man-Environment Systems. 1980;10(2):73–82. [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nature Neuroscience. 2005;8(11):1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Shi Z, Fairchild V, Wang A, Aronowitz C, Jagannathan K, Childress A, Langleben D. Extended-Release Naltrexone Specifically Reduces the Brain Response to Drug-Related Visual Stimuli in Opioid-Dependent Patients. College on Problems of Drug Dependence annual meeting 2016 [Google Scholar]
- Slawson MH, Chen M, Moody D, Comer SD, Nuwayser ES, Fang WB, Foltz RL. Quantitative analysis of naltrexone and 6beta-naltrexol in human, rat, and rabbit plasma by liquid chromatography-electrospray ionization tandem mass spectrometry with application to the pharmacokinetics of Depotrex in rabbits. Journal of Analytical Toxicology. 2007;31(8):453–461. doi: 10.1093/jat/31.8.453. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Perrett DI, Fagan EC, Cornwell RE, Lobmaier JS, Sprengelmeyer A, … Young AW. The cutest little baby face: a hormonal link to sensitivity to cuteness in infant faces. Psychol Sci. 2009;20(2):149–154. doi: 10.1111/j.1467-9280.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- Sternglanz SH, Gray JL, Murakami M. Adult preferences for infantile facial features: An ethological approach. Anim Behav. 1977;25(1):108–115. doi: 10.1016/0003-3472(77)90072-0. [DOI] [PubMed] [Google Scholar]
- Steyvers M. Morphing techniques for manipulating face images. Behavior Research Methods, Instruments & Computers. 1999;31(2):359–369. doi: 10.3758/bf03207733. [DOI] [PubMed] [Google Scholar]
- Sukikara MH, Platero MD, Canteras NS, Felicio LF. Opiate regulation of behavioral selection during lactation. Pharmacology, Biochemistry and Behavior. 2007;87(3):315–320. doi: 10.1016/j.pbb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD, Nunes EV. Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade? Drug and Alcohol Dependence. 2013;133(1):80–85. doi: 10.1016/j.drugalcdep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3 Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tempel A, Gardner EL, Zukin RS. Neurochemical and functional correlates of naltrexone-induced opiate receptor up-regulation. Journal of Pharmacology and Experimental Therapeutics. 1985;232(2):439–444. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wang AL, Elman I, Lowen SB, Blady SJ, Lynch KG, Hyatt JM, … Langleben DD. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl Psychiatry. 2015;5:e531. doi: 10.1038/tp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Bershad AK, de Wit H. Naltrexone alters the processing of social and emotional stimuli in healthy adults. Soc Neurosci. 2016;11(6):579–591. doi: 10.1080/17470919.2015.1136355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K. Substance abuse and child maltreatment. Pediatric Clinics of North America. 2009;56(2):345–362. doi: 10.1016/j.pcl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neuroscience. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zaaijer ER, de Bruin K, la Fleur SE, Goudriaan AE, van den Brink W, Booij J. Subchronic administration of short-acting naltrexone has no effect on striatal dopamine transporter availability, food intake or body weight gain in rats. J Psychopharmacol. 2015;29(3):344–348. doi: 10.1177/0269881114565380. [DOI] [PubMed] [Google Scholar]