Abstract
Glucose excursion was assessed prior to and post hypoglycaemia to increase understanding of hypoglycaemia incidence and recovery during hybrid closed‐loop insulin delivery. We retrospectively analysed data from 60 adults with type 1 diabetes who received, in a crossover randomized design, day‐and‐night hybrid closed‐loop insulin delivery and insulin pump therapy, the latter with or without real‐time continuous glucose monitoring. Over 4‐week study periods, we identified hypoglycaemic episodes, defined as sensor glucose <3.0 mmol/L, and analysed sensor glucose relative to the onset of hypoglycaemia. We identified 377 hypoglycaemic episodes during hybrid closed‐loop intervention vs 662 during control intervention (P < .001), with a predominant reduction of nocturnal hypoglycaemia. The slope of sensor glucose prior to hypoglycaemia was steeper during closed‐loop intervention than during control intervention (P < .01), while insulin delivery was reduced (P < .01). During both day and night, participants recovered from hypoglycaemia faster when treated by closed‐loop intervention. At 120 minutes post hypoglycaemia, sensor glucose levels were higher during closed‐loop intervention compared to the control period (P < .05).
In conclusion, closed‐loop intervention reduces the risk of hypoglycaemia, particularly overnight, with swift recovery from hypoglycaemia leading to higher 2‐hour post‐hypoglycaemia glucose levels.
Keywords: continuous glucose monitoring (CGM), CSII, glycaemic control, hypoglycaemia, insulin delivery, type 1 diabetes
1. INTRODUCTION
In type 1 diabetes, hypoglycaemia is a major barrier to achieving euglycaemia using modern tight glycaemic control strategies.1 It may be accompanied by sweating, trembling and confusion, and may require assistance from another person.2
Insulin pump therapy has been shown to reduce glycated haemoglobin levels without increasing the risk of hypoglycaemia.3 Continuous glucose monitoring also leads to improved glucose control and reduces the risk of hypoglycaemia4 and severe hypoglycaemia in adults with type 1 diabetes.5 Closed‐loop glucose control, combining insulin delivery with real‐time glucose sensing to administer insulin in a glucose‐responsive fashion, further improves glucose control.6 However, hypoglycaemia continues to be of concern during closed‐loop insulin delivery. Detailed assessments of hypoglycaemia timing, incidence and other characteristics during home use of closed‐loop insulin delivery are undocumented.
In the present analysis, we retrospectively assessed hypoglycaemic episodes from a large dataset comprising sensor glucose and insulin delivery involving 60 adults with type 1 diabetes who participated in a randomized crossover study contrasting day‐and‐night hybrid closed‐loop insulin delivery and sensor‐augmented or conventional pump therapy. We report data over 4‐week intervention periods and describe diurnal distribution of hypoglycaemia events and describe glucose excursion and insulin delivery before, during and after hypoglycaemic episodes.
2. METHODS
2.1. Experimental data
We retrospectively analysed 4‐week‐long periods of sensor glucose and insulin delivery data collected in 60 adults (27 from Cambridge, UK; 22 from Graz, Austria; 11 from Profil, Germany) with type 1 diabetes (31 male; mean age, 40.0 [11.2] years; mean BMI, 25.2 [3.8] kg/cm2; baseline HbA1c, 7.7 [0.9]%; duration of diabetes, 22.1 [10.4] years; total daily insulin, 0.57 [0.14] U/kg).7, 8
Participants were randomly assigned to receive, in a crossover randomized fashion, hybrid day‐and‐night closed‐loop insulin delivery and sensor‐augmented (32 participants) or conventional (28 participants) pump therapy. The participants' pre‐study rapid‐acting insulin analogue (aspart or lispro) was used during the study. Real‐time (closed‐loop and sensor‐augmented pump therapy) or masked (conventional pump therapy) glucose levels were measured using a continuous glucose monitoring device (FreeStyle Navigator II, Abbott Diabetes Care, Alameda, California) calibrated according to the manufacturer's instructions. The built‐in bolus wizard of the study insulin pump (Dana Diabecare R, SOOIL, Seoul, Republic of Korea) was used by participants to calculate insulin boluses at mealtimes and when administering correction boluses. During the closed‐loop period, a model‐predictive control algorithm directed basal insulin delivery.7, 8
A hypoglycaemic episode was defined as sensor glucose <3 mmol/L for at least 10 minutes.9 Hypoglycaemic episodes that were at least 30 minutes apart were counted as separate events. We excluded episodes within 60 minutes of insulin bolus as these episodes may be predominantly attributable to bolus over‐delivery and unrelated to closed‐loop glucose control. The exclusion criterion was applied to both study periods.
2.2. Statistical analysis
We identified hypoglycaemic episodes for each participant separately. We evaluated for each participant the average sensor glucose and the average basal insulin infusion rates from −60 to 120 minutes in 10‐minute steps relative to the onset of hypoglycaemic episodes. We then calculated the mean sensor glucose excursions and mean basal insulin infusion across all participants. The minimum glucose levels during hypoglycaemia, the area‐under‐curve (AUC) hypoglycaemia and the duration of hypoglycaemia were also calculated. Hypoglycaemic episodes identified during the night (midnight to 6:00 am) and during the day (6:00 am to midnight) were analysed separately.
A Student's t‐test contrasted endpoints collected during closed‐loop and control periods. Statistical analyses were performed using SPSS, version 21 (IBM Software, Hampshire, UK). P values less than .05 were considered statistically significant. Data are presented as mean (SD) unless stated otherwise.
3. RESULTS
Data from 1680 days of closed‐loop insulin delivery and 1680 days of sensor‐augmented or conventional insulin pump therapy were analysed. We identified 377 hypoglycaemic episodes during the closed‐loop period, of which 87 were nocturnal (midnight to 6:00 am), as compared to 662 episodes during the control period, of which 205 were nocturnal (closed‐loop vs control arm, 1.27 [1.17] vs 2.48 [2.50] episodes per participant per week; P < .001).
Figure 1 shows the diurnal distribution of hypoglycaemia incidence during 2 treatment periods. A reduced incidence of hypoglycaemia was observed during the closed‐loop period as compared to the control period, with a predominant reduction between 10:00 pm and 8:00 am when the incidence of hypoglycaemia was halved. Figure S1 shows the risk of hypoglycaemia conditioned on ambient sensor glucose; with sensor glucose between 3 and 8 mmol/L, the risk of hypoglycaemia 60 minutes later is halved during closed‐loop intervention.
Figure 1.
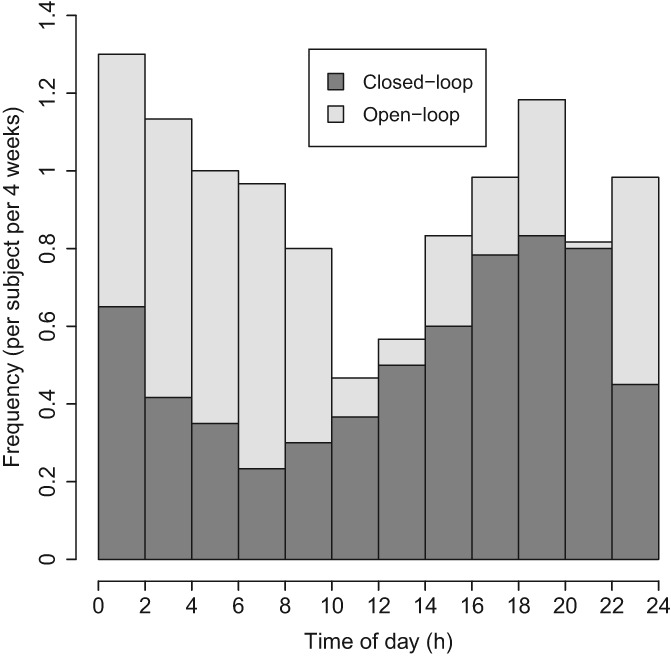
Incidence of hypoglycaemia events (sensor glucose <3.0 mmol/L for at least 10 minutes) during hybrid closed‐loop insulin delivery (dark grey bars) and control periods (light grey bars) (mean; N = 60)
Figure 2 summarizes sensor glucose levels before, during and after hypoglycaemic episodes during closed‐loop and control periods. Sensor glucose prior to hypoglycaemia had a steeper decline during closed‐loop intervention as compared to the control period (P = .002). During the day, participants recovered from hypoglycaemia more rapidly when treated by closed‐loop intervention (higher sensor glucose values from 20 to 120 minutes post hypoglycaemia; P < .05). A similar trend was observed during the night. Table S1 shows sensor glucose values from 30 to 120 minutes relative to the onset of hypoglycaemia.
Figure 2.
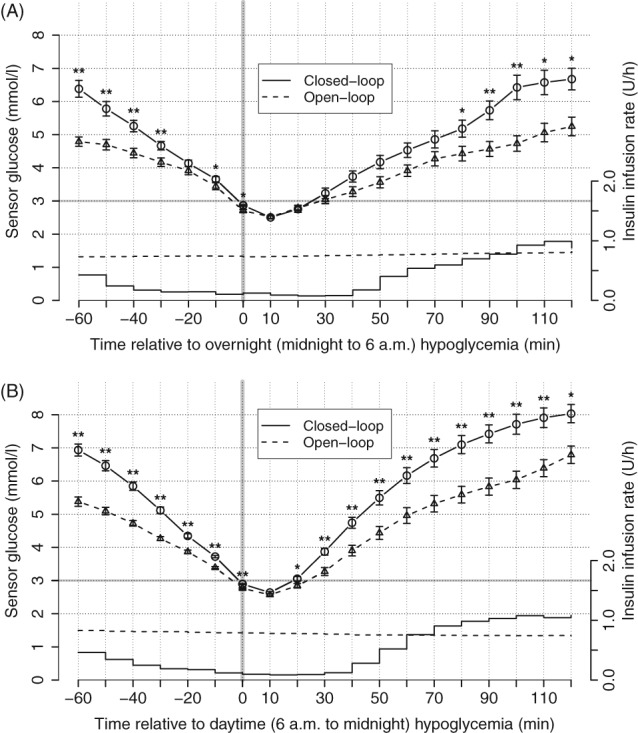
Sensor glucose values from −60 to 120 minutes relative to the onset of hypoglycaemia (sensor glucose <3.0 mmol/L; vertical bar) during the hybrid closed‐loop period (circles connected by solid line; mean ± SEM; N = 60; * P < .05, ** P < .01 compared to control therapy) and during the control period (triangles connected by dashed line). Piecewise‐constant lines without error bars represent mean insulin infusion rates during the closed‐loop period and dashed lines without error bars represent insulin infusions during the control period. Panel A, shows glycaemic and insulin infusion data during the night period (midnight to 6:00 am) and panel B, shows the day period (6:00 am to midnight)
Mean basal insulin infusion rates were lower during closed‐loop intervention as compared to control periods, from −60 to 80 minutes during the day (P = .001) and from −60 to 50 minutes during the day (P = .003), respectively.
Minimum glucose levels during hypoglycaemia did not differ between closed‐loop and open‐loop intervention (2.4 [0.4] vs 2.5 [0.4] mmol/L; P = .4 for the night period and 2.6 [0.2] vs 2.5 [0.4] mmol/L; P = .1 for the day period). AUC hypoglycaemia was reduced during closed‐loop compared to open‐loop intervention (40.3 [33.1] vs 52.8 [43.9] mmol/L min; P = .04 for the night period and 22.4 [8.8] vs 38.8 [52.9] mmol/L min; P = .02 for the day period). The duration of hypoglycaemia was reduced by 21 minutes during the closed‐loop night period (51.9 [30.3] vs 72.9 [37.8] minutes; P < .001), with no difference during the day (35.2 [11.9] vs 45.5 [24.4] minutes; P = .06].
4. DISCUSSION
The present analysis reports the incidence and diurnal distribution of hypoglycaemia in adults with type 1 diabetes during home use of hybrid closed‐loop insulin delivery and sensor‐augmented or conventional insulin pump therapy. We evaluated sensor glucose excursions and basal insulin infusion rates prior to and post hypoglycaemia. We found different patterns of hypoglycaemia incidence and hypoglycaemia recovery between the 2 interventions.
Many prospective and retrospective studies of hypoglycaemia incidence are based on self‐reported data, with considerable variation in reported outcomes (eg, 43 episodes per patient‐year,10 73 episodes per patient‐year11 and 94 episodes per patient‐year12). In the present analysis, we report the incidence of clinically significant hypoglycaemia at 144 episodes per patient‐year during insulin pump therapy using sensor glucose data. Continuous glucose monitoring provides comprehensive glucose levels over 24 hours and enables transparent definition and recording of hypoglycaemic episodes when device usage is high, as in the present analysis with median sensor wear time at 94% and 95% of the total time for closed‐loop and control periods, respectively.
Our analyses document the risk of hypoglycaemia being reduced during closed‐loop compared to control periods (377 vs 662 hypoglycaemic episodes) in adults with type 1 diabetes and with baseline HbA1c levels ranging from 5.8% to 9.7%. Figure 1 shows that the predominant reduction in hypoglycaemia was during the night. Figure 2 shows that, during closed‐loop periods, sensor glucose reduced more rapidly prior to hypoglycaemia as compared to control intervention. Our interpretation is that closed‐loop intervention was capable of preventing hypoglycaemia when sensor glucose was not decreasing rapidly. Thus, only a rapid decline in sensor glucose leads to hypoglycaemia during closed‐loop intervention. This is supported by reducing the risk of hypoglycaemia within 60 minutes, stratified according to ambient sensor glucose (Figure S1).
Two peaks of hypoglycaemia incidence were observed during 2 treatment periods, 1 at approximately 4:00–8:00 pm and the other at approximately 12:00–2:00 am (Figure 1). The former may be related to increased physical activity and the latter may result from post‐meal insulin corrections because of delayed effects following high‐fat evening meals.13
Previous studies have shown that closed‐loop intervention improves glycaemic control in type 1 diabetes through the system's ability to adjust insulin delivery in response to varying insulin requirements.14 Figure 2 demonstrates this paradigm; comparing the mean insulin delivery at approximately 0.8 U/h observed even during imminent onset of hypoglycaemia, closed‐loop intervention reduced insulin delivery from −60 to 80 minutes relative to onset of overnight hypoglycaemia and from −60 to 50 minutes relative to daytime hypoglycaemia. The reduced amount of insulin resulted in more rapid recovery and higher 2‐hour post‐hypoglycaemia glucose levels during closed‐loop intervention (Table S1 and Figure 2).
Glucose troughs during hypoglycaemia did not differ during closed‐loop and open‐loop interventions. However, during closed‐loop intervention, both AUC hypoglycaemia and the duration of hypoglycaemic events were reduced because of a more rapid recovery from hypoglycaemia.
An observational study reported that, in real‐life settings, a majority of patients over treated their hypoglycaemic episodes.15 Given that post‐hypoglycaemia glucose levels were higher during closed‐loop intervention as compared to control periods, a reasonable recommendation for clinical practitioners would be to reinforce, and possibly revise, patient education concerning hypoglycaemia correction, especially for those undergoing closed‐loop treatment. Further studies are warranted to explore optimal strategies for hypoglycaemia treatment during closed‐loop glucose control.
The strengths of our analysis are the multicentre, multinational, crossover, randomized study design, in which each subject serves as his/her own control, and the considerable volume of sensor glucose data used to identify the hypoglycaemic episodes. The data were collected during unsupervised home studies and, thus, glucose excursions reflect hypoglycaemia incidence and self‐treatment of hypoglycaemia under free‐living settings. The limitations include the lack of reliable data concerning the amount of rescue carbohydrates.
In conclusion, hybrid closed‐loop intervention reduces the risk of hypoglycaemia, particularly during the night, with a swift recovery from hypoglycaemia during the day, and leads to slightly elevated 2‐hour post‐hypoglycaemia glucose levels compared to those with insulin pump therapy.
Supporting information
Figure S1. Probability of observing a sensor glucose value <3 mmol/L (y‐axis) conditional on the sensor glucose value 60 minutes earlier (x‐axis).
Table S1. Sensor glucose values at 30, 60, 90 and 120 min following the onset of hypoglycaemia (sensor glucose <3.0 mmol/L for at least 10 min) during hybrid closed‐loop insulin delivery and control periods.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for their participation. We acknowledge the support of the staff at the Addenbrooke's Wellcome Trust Clinical Research Facility. Jasdip Mangat and John Lum (Jaeb Center) supported development and validation of the closed‐loop system. Josephine Hayes (University of Cambridge) provided administrative support. Karen Whitehead (University of Cambridge) provided laboratory support. We acknowledge the support of the staff at Profil Institut. Krisztina Schmitz‐Grozs provided support as a research physician; Martina Haase supported the study as an insulin pump expert; and Maren Luebkert, Kirstin Kuschma and Elke Przetak provided administrative, coordinating and documentation support.
Conflict of interest
R. H. reports having received speaker honoraria from Eli Lilly, Novo Nordisk and Astra Zeneca; serving on advisory panels for Eli Lilly and Novo Nordisk; receiving license fees from BBraun and Medtronic; and having served as a consultant to BBraun. M. E. W has received license fees from Becton Dickinson and has served as a consultant to Becton Dickinson. M. L. E. reports having received speaker honoraria from Abbott Diabetes Care, Novo Nordisk and Animas; serving on advisory panels for Novo Nordisk, Abbott Diabetes Care, Medtronic, Roche and Cellnovo; and holding stock options in Cellnovo. S. H. serves as a consultant for Novo‐Nordisk and for the ONSET group, and reports having received speaker/training honoraria from Medtronic. R. H. and M. E. W. report patents and patent applications. J. K. M. reports having received speaker honoraria from Abbott Diabetes Care, AstraZeneca, Eli Lilly & Co, NintaMed, NovoNordisk, Roche Diabetes Care, Sanofi, Servier, Takeda and serving on advisory panels for Becton Dickinson, MSD, Sanofi and Boehringer Ingelheim. T. R. P. is an advisory board member of Novo Nordisk A/S; a consultant for Roche, Novo Nordisk A/S, Eli Lilly & Co, Infineon, Carnegie Bank; and serves on speaker's bureaus of Novo Nordisk A/S and Astra Zeneca. L. L. reports having received speaker honoraria from Minimed Medtronic, Animas, Sanofi and Novo Nordisk; serving on advisory panels for Animas Minimed Medtronic and Novo Nordisk. L. B., H. T., S. D., C. B., M. H., H. K. and S. A. declare that no competing financial interests exist.
Author contributions
Y. R. and R. H. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R. H. coordinated the study. R. H., M. L. E., L. L., C. B., S. A., H. T., L. B., M. E. W., T. P. and J. K. M. co‐designed the study. H. T., L. B., S. H., S. D., J. K. M., M. H., H. K. and J. P. were responsible for screening and enrolment of participants, and arranged for informed consent from the participants. H. T., L. B., S. H., S. D., J. K. M., M. H. and H. K. provided patient care and/or took samples. Y. R. and R. H. carried out data analysis and wrote the manuscript. All authors critically reviewed the report.
Ruan Y, Bally L, Thabit H, et al. Hypoglycaemia incidence and recovery during home use of hybrid closed‐loop insulin delivery in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20:2004–2008. 10.1111/dom.13304
Funding information Funding was provided by the Seventh Framework Programme of the European Union (ICT FP7‐ 247138) and the Swiss National Science Foundation (P1BEP3_165297). Additional support for the Artificial Pancreas work by JDRF, the National Institute for Health Research Cambridge Biomedical Research Centre, Wellcome Strategic Award (100574/Z/12/Z), EC Horizon 2020 (H2020‐SC1‐731560), NIDDK (DP3DK112176 and 1UC4DK108520‐01), Efficacy and Mechanism Evaluation Programme of National Institute for Health Research (14/23/09).
REFERENCES
- 1. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937‐948. [DOI] [PubMed] [Google Scholar]
- 2. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bode B, Beck RW, Xing D, et al. G. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study: sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care. 2009;32:2047‐2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinemann L, Freckmann G, Ehrmann D, et al. Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018. 10.1016/S0140-6736(18)30297-6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta‐analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501‐512. [DOI] [PubMed] [Google Scholar]
- 7. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bally L, Thabit H, Kojzar H, et al. Day‐and‐night glycaemic control with closed‐loop insulin delivery versus conventional insulin pump therapy in free‐living adults with well controlled type 1 diabetes: an open‐label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5:261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. International Hypoglycaemia Study. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155‐157. [DOI] [PubMed] [Google Scholar]
- 10. Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in type 1 and insulin‐treated type 2 diabetes: a population‐based study. Diabet Med. 2005;22:749‐755. [DOI] [PubMed] [Google Scholar]
- 11. Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostenson CG, Geelhoed‐Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen‐Bjergaard U. Self‐reported non‐severe hypoglycaemic events in Europe. Diabet Med. 2014;31:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elleri D, Allen JM, Harris J, et al. Absorption patterns of meals containing complex carbohydrates in type 1 diabetes. Diabetologia. 2013;56:1108‐1117. [DOI] [PubMed] [Google Scholar]
- 14. Ruan Y, Thabit H, Leelarathna L, et al. Variability of insulin requirements over 12 weeks of closed‐loop insulin delivery in adults with type 1 diabetes. Diabetes Care. 2016;39:830‐832. [DOI] [PubMed] [Google Scholar]
- 15. Savard V, Gingras V, Leroux C, et al. Treatment of hypoglycemia in adult patients with type 1 diabetes: an observational study. Can J Diabetes. 2016;40:318‐323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Probability of observing a sensor glucose value <3 mmol/L (y‐axis) conditional on the sensor glucose value 60 minutes earlier (x‐axis).
Table S1. Sensor glucose values at 30, 60, 90 and 120 min following the onset of hypoglycaemia (sensor glucose <3.0 mmol/L for at least 10 min) during hybrid closed‐loop insulin delivery and control periods.


