Abstract
Weight gain in early pregnancy may influence a woman’s risk of developing pre-eclampsia. However, the consequences of weight gain throughout pregnancy up to the diagnosis of pre-eclampsia are unknown. The aim of this study was to determine if pregnancy weight gain before the diagnosis of pre-eclampsia is associated with increased risks of pre-eclampsia (overall and by pre-eclampsia subtype).
The study population included nulliparous pregnant women in the Swedish counties of Gotland and Stockholm, 2008-2013, stratified by early-pregnancy body mass index category. Electronic medical records were linked with population inpatient and outpatient records to establish date of pre-eclampsia diagnosis (classified as: any, early preterm <34 weeks, late preterm 34-36 weeks, term ≥37 weeks). Antenatal weight gain measurements were standardised into gestational age-specific z-scores.
Among 62,705 nulliparous women, 2,770 (4.4%) developed pre-eclampsia. Odds of pre-eclampsia increased by approximately 60% with every 1 z-score increase in pregnancy weight gain among normal weight and overweight women, and by 20% among obese women. High pregnancy weight gain was more strongly associated with term pre-eclampsia than early preterm pre-eclampsia (e.g., 64% vs. 43% increased odds per 1 z-score difference in weight gain in normal-weight women, and 30% vs. 0% in obese women, respectively). By 25 weeks, the weight gain of women who subsequently developed pre-eclampsia was significantly higher than women who did not [e.g. 0.43kg in normal-weight women].
In conclusion, high pregnancy weight gain before diagnosis increases the risk of pre-eclampsia in nulliparous women, and is more strongly associated with later-onset pre-eclampsia than early onset pre-eclampsia.
Keywords: Gestational weight gain, pre-eclampsia, z-scores, BMI, normal weight, overweight, obesity
Introduction
Pre-eclampsia is a predictor of cardiovascular disease and other metabolic disorders in later life1. Identifying modifiable risk factors for pre-eclampsia is an important priority for preventing disease onset during pregnancy and potentially reducing longer-term health risks. While pre-pregnancy obesity is a well-established modifiable risk factor for pre-eclampsia2-4, the role of high weight gain during pregnancy is less clear.
A number of studies have found that women with high pregnancy weight gain are more likely to develop pregnancy hypertensive disorders, including pre-eclampsia5-14. However, many of these studies only measured total pregnancy weight gain (i.e., weight gain up to the time of delivery). This introduces a serious methodological flaw15, since one of the symptoms of pre-eclampsia is oedema, leading to higher weight gain due to water retention16. Past findings therefore likely suffer from reverse causality (i.e., higher weight gain is the result of, rather than a cause of, pre-eclampsia).
A major challenge in overcoming bias due to reverse causation is that information on timing of pre-eclampsia onset is rarely available in population perinatal databases. Typically, only the woman’s final pre-eclampsia status at delivery is available. As a result, identifying weight gain patterns before disease diagnosis has been difficult. Several studies have examined weight gain in early or mid pregnancy11-14, as by definition, pre-eclampsia cannot be diagnosed until after 20 weeks. However, this analytic approach does not consider the role of weight gain during the second half of pregnancy, when the majority of weight gain occurs17. Further, these studies did not investigate the role of weight gain according to pre-eclampsia subtypes, which may be etiologically distinct18.
The aims of this study were to:
Determine if pregnancy weight gain before diagnosis is associated with increased risks of pre-eclampsia, overall and according to disease subtype (early preterm diagnosis <34 weeks, late preterm diagnosis 34-36 weeks, term diagnosis ≥37 weeks)
Establish the gestational age at which the pregnancy weight gain trajectories of women who go on to develop pre-eclampsia diverge from those of women without pre-eclampsia
Methods
Population
Our study population was drawn from singleton deliveries in the Stockholm-Gotland Obstetrical database between 2008-201317. The database contains electronic medical records from all antenatal and delivery visits for births in the counties of Stockholm and Gotland, Sweden, and has been linked with the national Prescribed Drug Register and the National Patient Register, which contains summaries of all diagnoses and procedures during inpatient and outpatient visits. We excluded women with pre-pregnancy hypertension, and restricted our cohort to nulliparous women, as pre-eclampsia status in a woman’s first pregnancy strongly predicts her risks of pre-eclampsia in subsequent pregnancies19. We excluded pregnancies of women without early pregnancy body mass index (BMI; kg/m2) or no gestational weight gain measurement and cases of pre-eclampsia where the date of diagnosis was unknown. Our study was approved by The Regional Ethics Committee in Stockholm and all clinics in the database consented to medical record access. Sharing of these data is not permissible as according to Swedish law and The Stockholm Regional Ethical Review Board, it is prohibited to publicly share data with personal information. Statistical code is available on request from the corresponding author.
Weight and weight gain
Early pregnancy BMI was calculated as the first measured weight in kilograms <14 completed gestational weeks divided by height in metres squared [median age at first measurement at 9 weeks´ gestation, interquartile range (IQR): 8-10]. We defined normal weight as an early pregnancy BMI of 18.5-24.9 kg/m2, overweight as 25-29.9 kg/m2, and obese as ≥30 kg/m2 20. Gestational weight gain was calculated as the measured weight at the time of antenatal care or delivery minus early pregnancy weight. In Sweden, the typical schedule of routine antenatal visits are at weeks 8–12, week 24, week 28, and weeks 31–32 and, thereafter, every second week until birth. Women are weighed at most, but not all, visits as part of routine antenatal care. For women with pre-eclampsia, we calculated weight gain using only measurements prior to the date of diagnosis. Gestational weight gain measurements were standardised for gestational duration into z-scores using previously-published, BMI-specific weight-gain-for-gestational-age charts derived for our population17. The use of z-scores ensures that the weight gain before pre-eclampsia diagnosis is compared to the weight gain of women without pre-eclampsia at the same point in pregnancy (instead of to the weight gain at delivery, which is usually at a later gestational age). That is, they account for the fact that women who remain pregnant longer have a greater opportunity to gain weight than women who deliver earlier due to pre-eclampsia. Z-scores are the WHO-recommended approach to assess change in anthropometry (e.g., weight gain)21. They have conventionally been used to assess pediatric growth, but charts have recently been produced for pregnancy weight gain17.
Outcome
Pre-eclampsia was defined using International Classification of Diseases, tenth revision (ICD-10). Preeclampsia was defined using ICD-10 codes O14 or O15 in the National Patient Register at either 1) an inpatient admission, or 2) an outpatient visit followed by either a) one or more outpatient visits or b) one or more inpatient admissions with a pre-eclampsia code or c) a pre-eclampsia diagnosis at delivery. That is, diagnosis could not be based only on a single outpatient visit, reflecting that in clinical practice patients with pre-eclampsia would be followed-up either at delivery or in clinic. The clinical definition of pre-eclampsia during the study period was a new onset of hypertension (blood pressure of ≥140/90) combined with proteinuria (≥0.3 grams per 24 h or ≥1 on a urine dipstick on at least two subsequent occasions) at 20 weeks gestation or later.
The date of pre-eclampsia diagnosis was defined as the first outpatient visit or inpatient admission with a diagnosis of O14 or O15. In accordance with International Society for the Study of Hypertension in Pregnancy guidelines, we defined early preterm onset pre-eclampsia as cases diagnosed (although not necessarily delivered) before 34 weeks gestation, late preterm onset pre-eclampsia as cases diagnosed between 34 and 36 weeks gestation, and term onset pre-eclampsia as cases diagnosed at or beyond 37 weeks22.
Statistical analysis
We described the characteristics of women with vs. without pre-eclampsia, overall and according to pre-eclampsia subtype, using means with standard deviations or counts with percentages. We examined the association between the last measured weight prior to delivery (or diagnosis, for pre-eclampsia cases), expressed as a weight gain z-score, and pre-eclampsia using multivariable logistic regression. Results were estimated for each pre-pregnancy BMI category. We did not build models for underweight women due to the small number of cases (n=56). We assessed linearity by regressing pre-eclampsia against quintiles of weight gain and visually examining the pattern of the resulting coefficients with 95% confidence intervals.
We adjusted for maternal age at delivery (years), maternal height (cm), smoking status (non-smoker, 1-9 cigarettes per day, ≥10 cigarettes per day), cohabitation status (living with partner, not living with partner), and pre-pregnancy diabetes mellitus. We further adjusted for early pregnancy BMI to account for potential residual confounding within each BMI category. We did not control for gestational diabetes as we hypothesized that is was a downstream consequence of pregnancy weight gain.
The analyses were repeated using the outcomes of early preterm, late preterm, and term onset pre-eclampsia. For early preterm (<34 weeks) models, the entire cohort was included in the denominator, while for late preterm and term onset pre-eclampsia, only women remaining pregnant at or beyond 34 and 37 weeks were retained in the denominator, respectively.
We used a multi-level random effects model with a random intercept and random slope to express the serial pregnancy weight gain measurements as a function of gestational age. The random intercept allowed each woman’s weight gain measurements to vary above or below the population average weight gain, while the random slope allowed each woman’s rate of weight gain to vary above or below the population average. We used all available weight measurements for women without pre-eclampsia, and weight gain measurements before diagnosis for women with pre-eclampsia. Gestational age was modelled as a restricted cubic spline with 5 knots to allow a smoothed, curvilinear weight gain curve. The same confounders were adjusted for as in the logistic regression models.
We first examined weight gain patterns for women with vs. without pre-eclampsia separately and visually compared the estimated curves in each group. We then built a combined model that included an interaction term between pre-eclampsia status and gestational age to allow the shapes of the weight gain curves to vary according to pre-eclampsia status. We used this model to estimate the adjusted difference in pregnancy weight gain between women with and without pre-eclampsia at select periods in gestation with 95% confidence intervals using the ‘margins’ command in Stata23.
As a sensitivity analysis, we repeated our primary analyses replacing the last measured weight prior to diagnosis with the weight gain at the time of the last normal blood pressure documented in the antenatal and/or delivery admission records. This excluded weight gain measurements in which the woman was being prescribed antihypertensive medication (defined as ATC-codes: C02, C03, C07, C08 and C09). This conservative approach ensured that weight gain measurements taken shortly before diagnosis (e.g., when a woman had a high blood pressure but did not meet the diagnostic criteria for pre-eclampsia due to lack of proteinuria) were not included.
Results
Study Characteristics
There were 151,710 singleton pregnancies in Stockholm-Gotland from 2008 to 2013. Excluding 15,916 pregnancies due to missing early pregnancy BMI, missing weight gain, pre-pregnancy hypertension and without a known date of pre-eclampsia data left 135,794 pregnancies. Further restricting to nulliparous women left 62,705 women with 2,770 cases of pre-eclampsia (4.4%) for analysis. Exclusions are detailed in Figure 1. Among women with pre-eclampsia, 1920 (69%) had term-onset pre-eclampsia (≥37 weeks).
Figure 1.
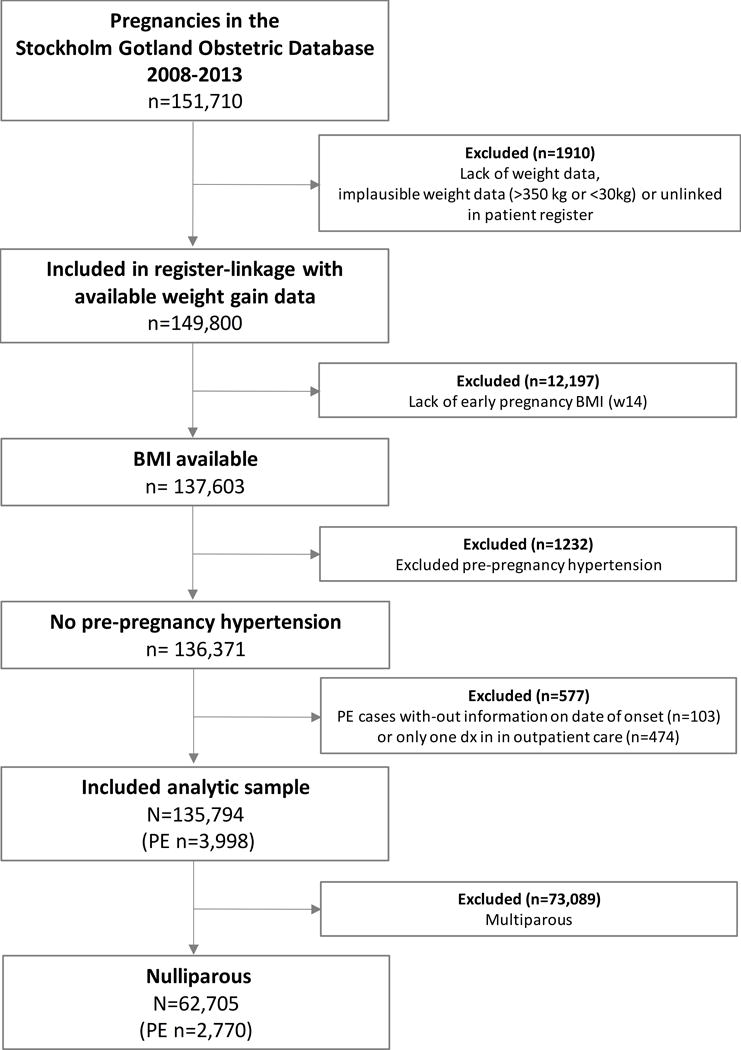
Flow chart of women with and without pre-eclampsia (PE) by early pregnancy BMI status in 62,705 nulliparous women in Stockholm-Gotland, 2008-2013
Table 1 compares the characteristics of women with vs. without pre-eclampisa. Women who developed pre-eclampsia had systematically higher BMI in early pregnancy than women who did not develop pre-eclampsia (15.0% vs. 6.3% obese, respectively), and were more likely to have pre-pregnancy diabetes (2.0% vs. 0.4%). Women with preeclampsia delivered a median of six days earlier than women without pre-eclampsia (275 and 281 days, respectively). The median gestational age at diagnosis was 269 days, and the median diagnosis-to-delivery interval was 3 days [interquartile range (IQR): 1-10].
Table 1.
Descriptive characteristics in women with vs. without pre-eclampsia among 62,705 nulliparous women in Stockholm-Gotland, 2008-2013.
| Characteristic | No pre-eclampsia N=59,935 |
Any pre-eclampsia N=2,770 |
Early preterm onset pre-eclampsia (<34 weeks) N=330 |
Late preterm onset pre-eclampsia (34-36 weeks) N=520 |
Term onset pre-eclampsia (≥37 weeks) N=1,920 |
|---|---|---|---|---|---|
| Maternal Age, yrs | 29.9 (5.0) | 30.4 (5.3) | 31.1 (5.9) | 30.4 (5.5) | 30.2 (5.1) |
| Maternal Height, cm | 167 (6) | 166 (6.0) | 165 (6.0) | 166 (6.0) | 166 (7) |
| BMI in early pregnancy, kg/m2 | |||||
| Underweight (<18.5) | 2,131 (3.6%) | 56 (2.0%) | 6 (1.1%) | 15 (2.9%) | 35 (1.8%) |
| Normal weight (18.5-24.9) | 42,897 (71.6%) | 1,617 (58.4%) | 196 (59.4%) | 310 (59.6%) | 1,111 (57.9%) |
| Overweight (25.0-29.9) | 11,105 (18.5%) | 683 (24.7%) | 71 (21.5%) | 125 (24.0%) | 487 (25.4%) |
| Obese (≥30.0) | 3,802 (6.3%) | 414 (15.0%) | 57 (17.3%) | 70 (13.5%) | 287 (14.9%) |
| Living with Partner | 55,127 (92.0%) | 2,532 (91.4%) | 302 (91.5%) | 467 (89.8%) | 1,763 (91.8%) |
| Pre-pregnancy Diabetes | 235 (0.4%) | 54 (2.0%) | 7 (2.1%) | 21 (4.0%) | 26 (1.4%) |
| Early Smoking Status | |||||
| Nonsmoker | 57,156 (95.4%) | 2,661 (96.1%) | 319 (96.7%) | 492 (94.6%) | 1850 (96.4%) |
| 1-9cig/d | 2,086 (3.5%) | 76 (2.7%) | 8 (2.4%) | 21 (4.0%) | 47 (2.4%) |
| ≥10cig/d | 374 (0.6%) | 20 (0.7%) | 3 (0.9%) | 4 (0.8%) | 13 (0.7%) |
| Missing | 319 (0.5%) | 13 (0.5%) | - | 3 (0.6%) | 10 (0.5%) |
| Gestational age at delivery (days) Median [IQR] | 281 [274-288] | 275 [264-284] | 231 [213-249] | 262 [252-270] | 280 (8.8) |
| Gestational age at diagnosis (days) Median [IQR] | - | 269 [253-280] | 221 [204-231] | 250 [245-255] | 276 (9.0) |
| Gestational weight gain, kg | |||||
| Total | 13.0 (5.5) | 14.5 (6.8) | 10.3 (6.4) | 13.8 (6.8) | 15.4 (6.6) |
| Prior to diagnosis of pre-eclampsia | - | 13.4 (6.7) | 8.4 (5.4) | 12.1 (6.3) | 14.6 (6.5) |
| Gestational weight gain, z-scores | |||||
| Total | 0.14 (0.96) | 0.60 (1.03) | 0.51 (1.12) | 0.62 (1.08) | 0.61 (1.00) |
| Prior to diagnosis of pre-eclampsia | - | 0.54 (1.02) | 0.36 (1.06) | 0.54 (1.06) | 0.57 (0.99) |
| No. of weight measurements Median [IQR] | 5 [3-7] | 5 [3-7] | 4 [3-5] | 5 [3-7] | 5 [3-8] |
Values are mean (SD) or N (%) unless otherwise stated.
Total pregnancy weight gain was 1.5 kg higher in women with vs. without pre-eclampsia, but given that women with pre-eclampsia delivered nearly a week earlier, this corresponded to a meaningful difference in gestational-age standardised weight gain z-scores: 0.60 vs. 0.14, respectively. When weights measured after a woman’s pre-eclampsia diagnosis were excluded, the z-score for the last measured weight gain in women with pre-eclampsia decreased to 0.54, suggesting that the rate of weight gain in women with pre-eclampsia increased following diagnosis (potentially due to oedema).
As expected, the interval between diagnosis and delivery was longer among women diagnosed with pre-eclampsia at preterm ages compared with term diagnoses (median interval between diagnosis and delivery of 10 days [IQR: 3-23] in early preterm, 11 days [IQR: 5-20] in late preterm, and 2 days [IQR: 1-5] at term) (Table 1). After restricting to weight gain before diagnosis, women with late preterm and term pre-eclampsia gained relatively more weight than women with early term pre-eclampsia (z-scores of 0.54 and 0.57 vs. 0.36, respectively). Supplementary Table S1 details the differences in weight gain in women with vs. without pre-eclampsia by early-pregnancy BMI status.
Association between gestational weight gain z-scores and pre-eclampsia
The associations between pregnancy weight gain z-score before diagnosis and risk of pre-eclampsia by early-pregnancy BMI status is shown in Table 2. After adjusting for confounders, higher pregnancy weight gain was associated with increased odds of pre-eclampsia in normal weight, overweight, and obese women, although the magnitude of risk was higher in normal weight and overweight women (both approximately 60% for every 1 z-score increase in weight gain than in obese women (19% for every 1 z-score increase in weight gain). These z-score differences correspond to 4.6 kg for normal weight, 5.9 kg for overweight, and 6.9 kg for obese at 37 weeks’ gestation. A spreadsheet calculator for conversion of z-scores into kilograms is provided in the Supplemental Material.
Table 2.
Association between pregnancy weight gain z-score before pre-eclampsia diagnosis in 62,705 nulliparous women in Stockholm-Gotland, 2008-2013.
| Pre-eclampsia status | BMI-category | N case/N total | Odd Ratios (95 % CI) of a z-score increase in gestational weight gain | |
|---|---|---|---|---|
| Crude | Adjusted* | |||
| Pre-eclampsia, any | Normal | 1,617/44,514 | 1.61 (1.53-1.70) | 1.64 (1.55-1.73) |
| Overweight | 683/11,788 | 1.52 (1.39-1.66) | 1.59 (1.45-1.74) | |
| Obese | 414/4,216 | 1.13 (1.01-1.26) | 1.19 (1.06-1.33) | |
| Early onset pre-eclampsia, <34 weeks | Normal | 196/44,514 | 1.35 (1.16-1.56) | 1.43 (1.23-1.67) |
| Overweight | 71/11,788 | 1.11 (0.86-1.44) | 1.21 (0.92-1.58) | |
| Obese | 57/4,216 | 0.87 (0.67-1.14) | 0.97 (0.74-1.27) | |
| Late preterm onset pre-eclampsia, 34-36 weeks | Normal | 310/43,934 | 1.67 (1.49-1.88) | 1.70 (1.51-1.92) |
| Overweight | 125/11,592 | 1.50 (1.22-1.83) | 1.58 (1.28-1.94) | |
| Obese | 70/4,112 | 0.88 (0.69-1.12) | 0.90 (0.71-1.16) | |
| Term onset pre-eclampsia, ≥37 weeks | Normal | 1,111/42,321 | 1.62 (1.52-1.73) | 1.64 (1.53-1.75) |
| Overweight | 487/11,122 | 1.59 (1.43-1.77) | 1.65 (1.48-1.84) | |
| Obese | 287/3,917 | 1.24 (1.09-1.42) | 1.30 (1.14-1.50) | |
Adjusted for maternal age, height, smoking, cohabiting with partner, pre-pregnancy diabetes and earl-pregnancy BMI.
The odds ratios for the association between weight gain and pre-eclampsia were generally stronger for pre-eclampsia cases with a later pregnancy onset than for early onset preeclampsia (Table 2). In normal weight women, every 1 z-score increase in weight gain was associated with 64-70% increased odds for late preterm and term pre-eclampsia, but only 43% increased odds for early preterm pre-eclampsia. Similar patterns were observed in overweight women. In obese women, associations were close to the null for early preterm and late preterm pre-eclampsia, but 30% higher odds for term pre-eclampsia.
Gestational weight gain trajectories and pre-eclampsia risk
The estimated weight gain trajectories of women with vs. without pre-eclampsia are shown in Figure 2, and the adjusted differences in weight gain between these two groups of women at select gestational ages are shown in Table 3. Weight gain patterns were similar in the first half of pregnancy for women with vs. without pre-eclampsia across all early-pregnancy BMI groups. For normal weight and overweight women, significant differences in weight gain were apparent by 25 weeks’ gestation, with differences of 0.43 and 0.52 kg, respectively. By 40 weeks, these differences had increased to 2.62 kg and 2.54 kg, respectively. In obese women, the weight gain trajectories of women with and without pre-eclampsia diverged at a later gestational age, with no significant differences at 25 weeks’ gestation (0.20 kg [−0.18, 0.59]), but a difference of 0.81 kg emerging by 30 weeks, and a difference of 2.02 kg at 40 weeks.
Figure 2.
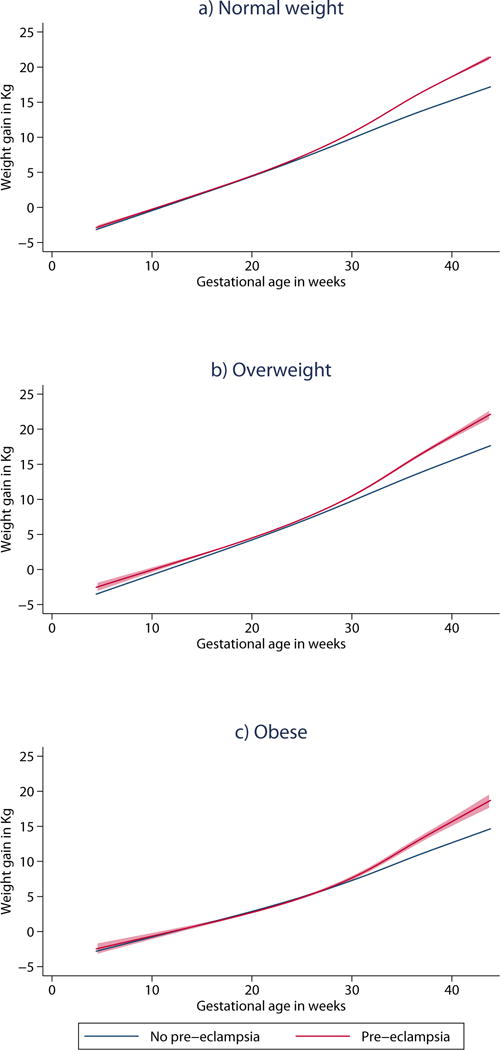
Estimated weight gain (Kg) trajectories with 95% confidence intervals of women with and without pre-eclampsia by early pregnancy BMI status in 62,705 nulliparous women in Stockholm-Gotland, 2008-2013. Trajectories are adjusted for maternal age, height, smoking, cohabiting with partner, pre-pregnancy diabetes and early-pregnancy BMI.
Table 3.
Estimated differences in pregnancy weight gain between women with and without pre-eclampsia at select gestational ages in 62,705 nulliparous women in Stockholm-Gotland, 2008-2013.
| Gestational age | Adjusted difference* (kg) [95% CI] between women with and without pre-eclampsia | ||
|---|---|---|---|
| Normal weight | Overweight | Obese | |
| 20 weeks | −0.30 (−0.43 to −0.17) | −0.16 (−0.40 to 0.08) | −0.40 (−0.74 to −0.06) |
| 25 weeks | 0.43 (0.29 to 0.57) | 0.52 (0.26 to 0.78) | 0.20 (−0.18 to 0.59) |
| 30 weeks | 1.17 (1.00 to 1.33) | 1.19 (0.88 to 1.50) | 0.81 (0.34 to 1.28) |
| 35 weeks | 1.90 (1.70 to 2.11) | 1.87 (1.49 to 2.24) | 1.42 (0.85 to 1.98) |
| 40 weeks | 2.62 (2.39 to 2.88) | 2.54 (2.09 to 3.00) | 2.02 (1.34 to 2.70) |
Adjusted for for maternal age, height, smoking, cohabiting with partner, pre-pregnancy diabetes and early-pregnancy BMI.
Results were essentially unchanged in our sensitivity analysis replacing the last measured weight prior to diagnosis with the weight gain at the time of the last normal blood pressure documented in the antenatal and/or delivery admission records (Supplementary Table S2).
Discussion
Main Findings
This is the first study to examine how pregnancy weight gain before diagnosis is linked with risk of pre- eclampsia subtypes. In this large population-based cohort of women with serial weight gain measurements and known date of pre-eclampsia diagnosis, we found that high weight gain was more strongly associated with later-onset pre-eclampsia. Further, risks associated with high weight gain were more pronounced in leaner women. The weight gain trajectories of women with pre-eclampsia began to diverge at approximately 25 weeks and continued to diverge until delivery, highlighting the importance of weight gain in mid and late pregnancy in disease etiology and in antenatal care.
Previous literature
The 2009 Committee to Reexamine IOM Pregnancy Weight Gain Guidelines reviewed the literature on the consequences of pregnancy weight gain for maternal and child health15. Six of 10 studies linking total pregnancy weight gain with pre-eclampsia found increased risks among women with higher weight gain, but the studies were deemed “inconclusive and problematic due to methodological flaws” (page 187) and excluded from consideration in the guidelines15. Our finding that weight gain z-scores at delivery for women with pre-eclampsia were systematically higher than their z-scores for weight gain before diagnosis confirms that use of total pregnancy weight gain likely introduces some degree of bias due to reverse causation. More recent studies based on total pregnancy weight gain are therefore likely equally inconclusive8-10.
Four studies have examined the association between weight gain in the first or second trimester and risk of pre-eclampsia11-14. A large population-based study from China (n=84,656) found that weight gain ≥600 g/week between 8 and 18 weeks was associated with a 69% higher risk of pre-eclampsia compared with women gaining <200 grams week in a cohort of predominantly normal-weight women11. No increased risks were observed among women gaining 200-399 or 400-599 grams per week. In the ALSPAC cohort from the United Kingdom, there was a 31% increase in risk for every 200 grams per week increase in weight gained prior to 18 weeks, with no differences according to pre-pregnancy BMI13.
In contrast, the Generation R cohort from the Netherlands (n=6956) found no significant association between first trimester or second trimester pregnancy weight gain and pre-eclampsia12. A smaller cohort study of 1441 women from the United States examined rate of pregnancy weight gain up to 28 weeks in relation to mild and severe pre-eclampsia14. The study found no association between a rate of weight gain above the IOM recommendations (vs. within recommendations) and mild or severe pre-eclampsia. However, with only 17 cases of mild pre-eclampsia and 58 cases of severe pre-eclampsia, the study was likely underpowered to detect small-moderate effect sizes. The study did find a 70% increased risk for any hypertensive disorder of pregnancy with weight gain above recommendations.
Our study agrees with the studies from China and the United Kingdom demonstrating that higher pregnancy weight gain increases risk of pre-eclampsia. However, one major difference between these studies and ours is that we did not find a difference in weight gain between those with vs. without pre-eclampsia until week 25. Hence our results suggest that later pregnancy weight gain is more important, potentially explaining the null findings of the Dutch12 and US studies14.
Mechanisms
Our finding of a stronger association between excessive pregnancy weight gain and late pre-eclampsia may be explained by differences in the pathophysiology of the disease phenotypes. Early pre-eclampsia has a strong association with poor placentation24, 25, which occurs early in pregnancy and is regulated in part by immunological factors26. Late pre-eclampsia is usually accompanied by normal placental function24, 25, but is linked to maternal factors. One important predisposing factor for late preeclampsia is high BMI4. Pre-eclampsia is characterised by a generalised maternal inflammatory systemic response incorporating a substantive component of endothelial cell dysfunction26. Adipose tissue is a hormonally active tissue and produces e.g. several inflammatory mediators that can act to alter endothelial function27, making the mother more vulnerable to develop pre-eclampsia. Consequently, not only obesity, but also excessive weight gain during pregnancy has been associated with increased concentrations of inflammatory factors28, which might predispose the woman to pre-eclampsia.
Excessive pregnancy weight gain seemed to be a stronger risk factor of pre-eclampsia in lean than in obese women in our study. The mechanism underlying this finding is unknown, but the relative increase in levels of inflammatory factors might act differently depending on state of inflammation in early pregnancy. Further, it is unknown if distribution of fat mass accrual differs in lean and obese women. Since fat distribution is important concerning cardiovascular risk, we could expect that fat distribution may also important for pre-eclampsia27.
Strengths and limitations
Strengths of this study included our linkage of multiple population registries, including electronic medical records and inpatient/outpatient visit records, to produce a novel population-based cohort with information on date of pre-eclampsia diagnosis. Our large sample size allowed us to examine pre-eclampsia subtypes while retaining good statistical precision.
Several limitations should be noted. First, the date of pre-eclampsia diagnosis is not the same as the date of disease onset. As a result, use of the last measured weight before diagnosis could have included weight measurements influenced by early stages of disease. Nevertheless, our sensitivity analysis restricting weight measurements to the time of last normal blood pressure measurement reduces the likelihood that this bias influenced our findings. Second, early pregnancy BMI is an insufficient proxy for pre-pregnancy BMI. However, it reduces the measurement error associated with self-reported pre-pregnancy BMI29, 30 and is a pragmatic measure because it reflects the information available to the clinician at the time of the first antenatal visit. Third, our diagnosis of pre-eclampsia was based on ICD coding in hospital and outpatient administrative databases. Previous validation studies from Swedish and other Scandinavian data registries have found that pre-eclampsia is coded with reasonable accuracy in population databases.31-33 Our outcome definition required confirmation of an initial outpatient diagnosis with subsequent visit or diagnosis at the delivery admission. Nevertheless, we cannot rule out outcome misclassification due to other factors such as variability in physician diagnostic practices or error in blood pressure measurement34. Finally, our population consisted of predominantly white women attending publicly-funded antenatal care with an uptake of nearly 100%. For this reason, the generalizability to other races or medical insurance systems is uncertain. This relative homogeneity is also strength, however, as it reduces the likelihood of confounding by differences in patient characteristics and access to care.
Perspectives
High pregnancy weight gain before diagnosis is an important risk factor for pre-eclampsia in nulliparous women, particularly in leaner women. Our finding that the association between pregnancy weight gain and pre-eclampsia was more pronounced in pre-eclampsia developing later in gestation supports the hypothesis that early preterm and term onset pre-eclampsia may have different etiologic pathways. Randomised trials assessing if interventions to reduce pregnancy weight gain lower the risk of pre-eclampsia would be an appropriate next step in our efforts to reduce the burden of this disease.
Supplementary Material
Novelty and Significance.
What Is New?
High weight gain during pregnancy may increase a woman’s risk of developing pre-eclampsia. However, previous studies have only investigated the effect of total pregnancy weight gain or early pregnancy weight gain and risk of pre-eclampsia. Further, studies have not had information on the timing of diagnosis in order to examine if the risks associated with high weight gain differ according to pre-eclampsia subtypes (early preterm, late preterm, or term diagnosis).
What Is Relevant?
This is the first study to examine how pregnancy weight gain before diagnosis is linked with risk of pre- eclampsia and its subtypes.
Summary
We found that high pregnancy weight gain increases the risk of pre-eclampsia, and that risks were more strongly associated with later-onset pre-eclampsia and among leaner women.
Acknowledgments
Sources of Funding: The study was supported by grants from Swedish Research Council for Health, Working Life and Welfare (grant No. 2014-0073 to SC, and 2015-00251 to OS and KJ), Stockholm County Council (ALF project No.20140105 to SC and 20150179 to OS). JAH is the recipient of New Investigator awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. OS was supported by the Swedish Research Council (grant No 2013-2429) and the Strategic Research Program in Epidemiology at Karolinska Institutet. KJ was the recipient of a grant from Karolinska Institutet Research Foundations. AKW was supported by the Swedish Research Council (grant No 2014-3561). SC was supported from an unrestricted grant from Karolinska Institutet (Distinguished Professor Award). LMB is supported by the National Institute of Child Health & Human Development (R01 HD072008).
Footnotes
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work
Author Contribution to Authorship (Alphabetical Order)
Designed research; Hutcheon, Johansson, Stephansson, Wikström
Conducted research; Bodnar, Cnattingius, Hutcheon, Johansson, Stephansson, Wikström
Analysed data or performed statistical analysis; Hutcheon, Johansson
Drafting of the manuscript; Hutcheon, Johansson
Critical revision of the manuscript for important intellectual content; Bodnar, Cnattingius, Hutcheon, Johansson, Stephansson, Wikström
Had primary responsibility for final content; Hutcheon, Johansson
All authors read and approved the final manuscript
Details of Ethics Approval: The was approved by the regional ethics committee at Karolinska Institutet Stockholm, Sweden (DNR: 2009/275-31 with amendment 2013/792-32).
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, Gillman MW. Preconceptional and maternal obesity: Epidemiology and health consequences. The lancet Diabetes & endocrinology. 2016;4:1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 3.Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: Weighing in on the mechanisms. American journal of physiology Regulatory, integrative and comparative physiology. 2015;309:R1326–1343. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohlberg S, Stephansson O, Cnattingius S, Wikstrom AK. Maternal body mass index, height, and risks of preeclampsia. American journal of hypertension. 2012;25:120–125. doi: 10.1038/ajh.2011.175. [DOI] [PubMed] [Google Scholar]
- 5.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in sweden. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006;93:269–274. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstetrics and gynecology. 2007;110:745–751. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- 7.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstetrics and gynecology. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 8.Barton JR, Joy SD, Rhea DJ, Sibai AJ, Sibai BM. The influence of gestational weight gain on the development of gestational hypertension in obese women. American journal of perinatology. 2015;32:615–620. doi: 10.1055/s-0034-1386634. [DOI] [PubMed] [Google Scholar]
- 9.Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2009;31:28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- 10.Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among latina women. American journal of obstetrics and gynecology. 2009;200:167 e161–167. doi: 10.1016/j.ajog.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhou A, Xiong C, Hu R, Zhang Y, Bassig BA, Triche E, Yang S, Qiu L, Zhang Y, Yao C, Xu S, Wang Y, Xia W, Qian Z, Zheng T, Zhang B. Pre-pregnancy bmi, gestational weight gain, and the risk of hypertensive disorders of pregnancy: A cohort study in wuhan, china. PloS one. 2015;10:e0136291. doi: 10.1371/journal.pone.0136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity. 2013;21:1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. American journal of obstetrics and gynecology. 2013;209:327 e321–317. doi: 10.1016/j.ajog.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhstaller KE, Bastek JA, Thomas A, McElrath TF, Parry SI, Durnwald CP. The effect of early excessive weight gain on the development of hypertension in pregnancy. American journal of perinatology. 2016;33:1205–1210. doi: 10.1055/s-0036-1585581. [DOI] [PubMed] [Google Scholar]
- 15.Institute of medicine (us) national research council (us) committee to reexamine iom pregnancy weight guidelines. Weight gain during pregnancy; Washington (DC): 2009. [Google Scholar]
- 16.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vascular health and risk management. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson K, Hutcheon JA, Stephansson O, Cnattingius S. Pregnancy weight gain by gestational age and bmi in sweden: A population-based cohort study. The American journal of clinical nutrition. 2016;103:1278–1284. doi: 10.3945/ajcn.115.110197. [DOI] [PubMed] [Google Scholar]
- 18.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertension in pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: Prospective cohort study. Bmj. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity : Preventing and managing the global epidemic : Report of a who consultation. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 21.World Health Organization. The z-score or standard deviation classification system. Retrieved 2018-04-17, from http://www.who.int/nutgrowthdb/about/introduction/en/index4.html.
- 22.Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (isshp) Pregnancy hypertension. 2013;3:44–47. doi: 10.1016/j.preghy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. The Stata Journal. 2012;12:308–331. [Google Scholar]
- 24.Sohlberg S, Mulic-Lutvica A, Lindgren P, Ortiz-Nieto F, Wikstrom AK, Wikstrom J. Placental perfusion in normal pregnancy and early and late preeclampsia: A magnetic resonance imaging study. Placenta. 2014;35:202–206. doi: 10.1016/j.placenta.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG : an international journal of obstetrics and gynaecology. 2006;113:580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 26.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JM, Bodnar LM, Patrick TE, Powers RW. The role of obesity in preeclampsia. Pregnancy hypertension. 2011;1:6–16. doi: 10.1016/j.preghy.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hrolfsdottir L, Schalkwijk CG, Birgisdottir BE, Gunnarsdottir I, Maslova E, Granstrom C, Strom M, Olsen SF, Halldorsson TI. Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity. 2016;24:2133–2139. doi: 10.1002/oby.21617. [DOI] [PubMed] [Google Scholar]
- 29.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: Findings from the third national health and nutrition examination survey, 1988-1994. Journal of the American Dietetic Association. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. quiz 35-26. [DOI] [PubMed] [Google Scholar]
- 30.Craig BM, Adams AK. Accuracy of body mass index categories based on self-reported height and weight among women in the united states. Maternal and child health journal. 2009;13:489–496. doi: 10.1007/s10995-008-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. American journal of epidemiology. 2007;166:117–124. doi: 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
- 32.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scandinavian journal of social medicine. 1990;18:143–148. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 33.Klungsoyr K, Harmon QE, Skard LB, Simonsen I, Austvoll ET, Alsaker ER, Starling A, Trogstad L, Magnus P, Engel SM. Validity of pre-eclampsia registration in the medical birth registry of norway for women participating in the norwegian mother and child cohort study, 1999-2010. Paediatric and perinatal epidemiology. 2014;28:362–371. doi: 10.1111/ppe.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. The New England journal of medicine. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


