SUMMARY
Background
Fibrotic stricture is a common complication of Crohn’s disease (CD) affecting approximately half of all patients. No specific anti-fibrotic therapies are available; however, several therapies are currently under evaluation. Drug development for the indication of stricturing CD is hampered by a lack of standardized definitions, diagnostic modalities, clinical trial eligibility criteria, endpoints and treatment targets in stricturing CD.
Methods
An interdisciplinary expert panel consisting of 15 gastroenterologists and radiologists was assembled. Using modified RAND/University of California Los Angeles appropriateness methodology, 109 candidate items derived from systematic review and expert opinion focusing on small intestinal strictures were anonymously rated as inappropriate, uncertain or appropriate). Survey results were discussed as a group before a second and third round of voting.
Results
Fibrotic strictures are defined by the combination of luminal narrowing, wall thickening and pre-stenotic dilation. Definitions of anastomotic (at site of prior intestinal resection with anastomosis) and naïve small bowel strictures were similar, however there was uncertainty regarding wall thickness in anastomotic strictures. Magnetic resonance imaging is considered the optimal technique to define fibrotic strictures and assess response to therapy. Symptomatic strictures are defined by abdominal distension, cramping (i.e. colicky abdominal pain), dietary restrictions, nausea, vomiting, abdominal pain (duration and intensity) and postprandial abdominal pain (duration and intensity). Need for intervention (endoscopic balloon dilation or surgery) within 24 to 48 weeks is considered the appropriate endpoint in pharmacological trials.
Conclusions
Consensus criteria for diagnosis and response to therapy in stricturing Crohn’s disease should inform both clinical practice and trial design.
Keywords: Fibrosis, Inflammatory Bowel Disease, Complications
INTRODUCTION
The lifetime risk of stricture is approximately 50% among patients with Crohn’s disease (CD).1 In addition to causing abdominal pain, distension, bloating and vomiting, evidence suggests that stricturing CD may precede the development of internal penetrating disease with fistula formation.2, 3
Whilst there has been an unprecedented expansion in CD drug development over the last decade, novel and established treatments are primarily directed toward reducing inflammation.4, 5 Anti-inflammatories may be effective in patients with small bowel strictures,6 however, they do not specifically target or reverse fibrosis. Most often, stricturing CD is treated with surgical resection.7 Unfortunately, post-operative disease recurrence and re-stricturing are common.8 Effective drug therapy to prevent and treat CD-associated strictures is therefore a substantial unmet medical need.
Multiple anti-fibrotic compounds are currently under evaluation for the treatment of liver, skin, kidney, heart and lung disease,9 with two agents approved for use in patients with lung fibrosis (pirfenidone and nintedanib).10, 11 In contrast, there have been no trials of anti-fibrotics in inflammatory bowel diseases (IBD). This lack of progress is potentially attributable to several factors, including heterogeneous disease definitions, diagnostic methods, clinical trial eligibility criteria and endpoints, and treatment targets.12
We assembled a global, multidisciplinary panel of experts (the CrOhN’S disease anti-fibrotic STRICTure Therapies [CONSTRICT] group) and conducted a three-round consensus process using modified RAND/University of California Los Angeles (UCLA) appropriateness methodology13, 14 with the aim of standardizing assessment of CD strictures and treatment targets. Additionally, we developed a conceptual framework for the conduct of early phase clinical trials of anti-fibrotic agents.
MATERIALS AND METHODS
Systematic Review of Literature
The systematic review and consensus process focused solely on small bowel strictures, since these are most common.7 Furthermore, colonic strictures harbor the risk for malignancy15 and accordingly, may not be a primary initial target for anti-fibrotic therapies. PubMed, EMBASE and the Cochrane Library (CENTRAL) were searched from inception to July 31, 2017 to identify definitions, instruments and trial design features used for assessment of CD-associated strictures. Keywords included (‘Crohn’s disease’ OR ‘small bowel’) AND (‘stricture’ OR ‘fibrosis’ OR ‘stenosis’ OR ‘dilation’). A recursive search of bibliographies of relevant articles was also performed. Eligible studies enrolled adult patients (>18 years) and provided information on how stricture was defined, the modality of diagnosis, and treatment target(s). Controlled trials, cohort studies, case-control studies and cross-sectional studies were included. Non-English language publications, case series and case reports were excluded.
Four reviewers (FR, DB, CM and CP) independently screened citations and abstracts. The full-text publications of potentially eligible studies were reviewed in duplicate by two pairs of researchers (FR and DB, CM and CP). Variables pertaining to clinical, endoscopic and radiologic definitions of strictures, diagnostic modality, and clinical trial design were extracted independently and in duplicate by the same two pairs. Disagreements regarding inclusion or extraction were resolved through discussion, or arbitration was performed by VJ.
Expert Consensus Process
Recruitment of Experts
Ten experienced gastroenterologists and five experienced radiologists from the United States, Canada, the Netherlands, Belgium, Spain, Italy, France, Switzerland, UK and Germany were chosen to participate. Panelists were selected based on publication record, international reputation in stricturing CD, and experience in trial design, drug development and clinical epidemiology; these criteria took precedence over global representation. After reviewing a list of experts in the above areas the final selection of participants was performed by FR, BF and VJ. Given that this project had the purpose of providing a framework for the development of medical therapy for CD-associated strictures, surgeons and pathologists were not included.
Modified RAND/UCLA appropriateness methodology was used to assess the face validity (the extent to which an item appears to address the concept it purports to measure) and feasibility of items identified in the systematic review. Additional items were included based upon the opinion of the panelists after distribution of the initial item list. RAND/UCLA appropriateness methodology employs a modified Delphi panel approach to combine the best available evidence with the clinical experience of relevant experts.16 This process is widely accepted, iterative and evidence-based.
First Panel Meeting and Initial Survey
Items identified by systematic review and an introductory panel meeting were circulated via an online survey. Panelists anonymously rated the appropriateness of each item on a scale from 1 to 9 (1 = inappropriate, 9 = highly appropriate).
Second and Third Panel Meeting and Final Survey
Results of the initial survey were distributed to panelists and discussed in a moderated teleconference. Areas of disagreement regarding item appropriateness were identified and panelists were asked to explain the rationale behind their responses. In accordance with RAND/UCLA appropriateness methodology, no attempt was made to force the panel to consensus. The survey was revised based on the second panel meeting to improve clarity and a second survey was circulated. One key item (#30) was chosen by the panel for re-discussion based on an unexpected disagreement in survey round two. The item was discussed via e-mail, and a third survey consisting only of this item was circulated.
Analysis of Panel Results
Each survey item was classified as inappropriate, uncertain or appropriate based on the median panel rating and degree of panel disagreement (median 1 to 3 without disagreement = inappropriate; median 4 to 6 or any median with disagreement = uncertain; median 7 to 9 without disagreement = appropriate).14 Disagreement was considered present when two or more panelists rated appropriateness in each extreme 3-point region (1 to 3 and 7 to 9).
RESULTS
Systematic Review
The literature search retrieved a total of 2238 citations. After removing duplicates, 1518 citations were screened using predefined eligibility criteria. Of these, 1270 citations were deemed not applicable based on title and abstract review. Ninety studies were excluded during full-text review, leaving a total of 158 included studies (Supplementary Figure 1).
Data obtained from the systematic review were arranged into four tables: 1) radiologic definitions and diagnosis; 2) clinical definitions and diagnosis; 3) endoscopic definitions and diagnosis; and 4) endpoint assessment in pharmacological studies (Supplementary Tables 1–4). These data, in addition to other items of potential importance, were subsequently incorporated into a survey and sent to panelists for appropriateness rating (Supplementary Table 5).
Consensus Process
Panel discussion resulted in minimal edits to the proposed items and the addition of two new statements. Item #30 revealed an unexpected disagreement in round two and was re-discussed in a third round.
In the literature, the terms ‘stenosis’ and ‘stricture’ are used interchangeably. In this article, we defined ‘stricture’ based upon the recommendation of the Consensus of the American Gastroenterology Association (AGA).17 The term stricture encompasses the possibility of the coexistence of inflammatory and fibrotic components.
Appropriateness of Items
Definition of naïve small bowel stricture
The panelists felt that a naïve small bowel stricture (strictures arising in parts of the intestine that do not contain a bowel anastomosis) on cross sectional imaging is optimally defined by the combination of three features: 1) localized luminal narrowing; 2) bowel wall thickening; and 3) pre-stricture dilation. Panelists were uncertain about other combinations of radiologic features for stricture definition.
Specific criteria were generated for each of the radiologic features. With respect to bowel wall thickening, panelists felt that a 25% increase in wall thickness relative to the adjacent non-affected bowel was an appropriate definition. Relating to the definition of pre-stricture dilation in cross sectional imaging, a luminal diameter greater than 3 cm was regarded as appropriate. The definition of luminal narrowing as a luminal diameter reduction of at least 50%, measured relative to the normal adjacent bowel loop was considered appropriate. The inability to pass an adult colonoscope through the narrowed area without prior endoscopic dilation with a reasonable amount of pressure applied was felt to be an appropriate definition of stricture on endoscopy.
Obstructive symptoms alone were determined to be insufficient to define a stricture (Supplementary Table 5; Table 1).
Table 1.
Selectconsensus definitions for diagnosis and improvement of Crohn’s disease-associated small bowel strictures. Detailed definitions for key features on radiology and endoscopy are provided.
| Definition of naïve small bowel strictures | ||
|---|---|---|
| Item | Median, panel score interquartile range | Appropriateness |
| A naïve small bowel stricture on cross sectional imaging (CTE, MRE or ultrasound) is optimally defined as: | ||
| Localized luminal narrowing and bowel wall thickening with pre-stricture dilation. | 8.0, 4.0 | Appropriate |
| Definitions for luminal diameter in a naïve stricture: | ||
| Luminal diameter reduction by at least 50%, measured relative to a normal adjacent appropriately distended bowel loop. | 7.0, 2.0 | Appropriate |
| Luminal diameter of < 1 cm in an appropriately distended lumen. | 5.0, 4.0 | Uncertain |
| Definitions for bowel wall thickening in a naïve stricture: | ||
| Increase in wall thickness of 25% in the maximally thickened area, in an appropriately distended lumen, measured relative to a normal, adjacent, appropriately distended bowel loop. | 7.0, 3.0 | Appropriate |
| > 3mm with luminal distension in the maximally thickened area, in an appropriately distended lumen. | 8.0, 2.0 | Uncertain |
| Definitions for pre-stricture dilation in a naïve stricture: | ||
| Bowel diameter that is 20% greater than the normal diameter in an appropriately distended lumen. | 7.0, 2.0 | Uncertain |
| Bowel diameter of greater than 3 cm | 8.0, 2.0 | Appropriate |
| Definition for naïve stricture on endoscopy | ||
| Inability to pass an adult colonoscope through the narrowed area without prior endoscopic dilation and with a reasonable amount of pressure applied. | 8.0, 2.0 | Appropriate |
| Definition of anastomotic small bowel strictures | ||
|---|---|---|
| Item | Median panel score, interquartile range | Appropriateness |
| An anastomotic small bowel stricture on cross sectional imaging (CTE, MRE or ultrasound) is optimally defined as: | ||
| Localized luminal narrowing and bowel wall thickening with pre-stricture dilation. | 8.0, 2.0 | Appropriate |
| Definitions for luminal diameter in an anastomotic stricture: | ||
| Luminal diameter reduction by at least 50%, measured relative to a normal adjacent appropriately distended bowel loop. | 7.0, 1.0 | Appropriate |
| Luminal diameter of < 1 cm in an appropriately distended lumen. | 5.0, 3.0 | Uncertain |
| Definitions for bowel wall thickening in an anastomotic stricture: | ||
| Increase in wall thickness of 25% in the maximally thickened area, in an appropriately distended lumen, measured relative to a normal, adjacent, appropriately distended bowel loop. | 7.0, 4.0 | Uncertain |
| > 3mm with luminal distension in the maximally thickened area, in an appropriately distended lumen. | 7.0, 3.0 | Appropriate |
| Definitions for pre-stricture dilation: | ||
| Bowel diameter that is 20% greater than the normal diameter in an appropriately distended lumen. | 6.0, 1.0 | Uncertain |
| Bowel diameter of greater than 3 cm. | 8.0, 2.0 | Appropriate |
| Definition for anastomotic stricture on endoscopy: | ||
| Inability to pass an adult colonoscope through the narrowed area without prior endoscopic dilation and with a reasonable amount of pressure applied. | 8.0, 2.0 | Appropriate |
| Definitions for successful treatment of a small bowel stricture | ||
|---|---|---|
| Item | Median panel score, interquartile range | Appropriateness |
| Successful treatment of a small bowel stricture requires improvement in: | ||
| Clinical symptoms and endoscopic features | 8.0, 2.0 | Appropriate |
| Clinical symptoms and radiologic features | 8.0, 2.0 | Appropriate |
| Radiologic and endoscopic features | 7.0, 3.0 | Appropriate |
| Radiologic features alone | 7.0, 3.0 | Uncertain |
| Clinical symptoms alone | 3.0, 2.0 | Inappropriate |
| The radiologic features that improve upon successful anti-fibrotic treatment of a small bowel stricture are: | ||
| Localized luminal narrowing | 8.0, 2.0 | Appropriate |
| Wall thickening | 8.0, 3.0 | Appropriate |
| Pre-stricture dilation | 8.0, 1.0 | Appropriate |
| Stricture length | 7.0, 3.0 | Appropriate |
| An improvement in localized luminal narrowing of the small bowel on cross sectional imaging is defined as: | ||
| Luminal diameter > 1cm in an appropriately distended small bowel | 7.0, 2.0 | Uncertain |
| Luminal diameter reduction of less than 50% in an appropriately distended small bowel. | 7.0, 2.0 | Appropriate |
| Improvement of the luminal narrowing by 50%. | 8.0, 2.0 | Appropriate |
| An improvement in wall thickness of the small bowel on cross sectional imaging is defined as: | ||
| < 3mm with luminal distension in the maximally thickened area in an appropriately distended small bowel. | 7.0, 3.0 | Uncertain |
| Reduction of the bowel wall thickening by 50%. | 8.0, 1.0 | Appropriate |
| An improvement in pre-stricture dilation of the small bowel on cross sectional imaging is defined as: | ||
| Bowel diameter less than 2.5 cm | 7.0, 1.0 | Appropriate |
| Reduction of the pre-stricture dilation by 50% with measurements performed compared to a non-affected adjacent well distended bowel loop | 8.0, 3.0 | Appropriate |
| Bowel diameter equal to normal bowel | 8.0, 0.0 | Appropriate |
| An improvement in stricture length of the small bowel on cross sectional imaging is defined as: | ||
| Reduction in length by 50% | 7.0, 2.0 | Appropriate |
| The endoscopic features that improve upon successful anti-fibrotic treatment are: | ||
| Increase in luminal diameter | 7.0, 1.0 | Uncertain |
| Ability to pass an adult endoscope | 8.0, 0.5 | Appropriate |
 Green: Median 7–9
Green: Median 7–9
 Blue: Median 4–6
Blue: Median 4–6
 Yellow: Median 1–3
Yellow: Median 1–3
 Orange: Appropriate
Orange: Appropriate
 Purple: Uncertain
Purple: Uncertain
 Pink: Inappropriate
Pink: Inappropriate
The depicted interquartile range is a measure of statistical dispersion, being equal to the difference between 75th and 25th percentiles.
Definition of anastomotic small bowel stricture
Appropriateness ratings were similar for definitions of anastomotic (at site of prior intestinal resection with anastomosis) and naïve small bowel strictures, however there was uncertainty regarding the definitions of wall thickness (Supplementary Table 5; Table 1). The authors were cautious about evaluation of small bowel anastomosis, as these definitions only apply to proximal small bowel unaltered by surgical intervention, not enteroenterostomy associated with side-to-side small bowel anastomosis.
Diagnosis of small bowel stricturing CD
Cross sectional imaging or ileocolonoscopy alone were considered appropriate to diagnose a small bowel stricture. Symptoms alone were considered inappropriate to diagnose a stricture. Moreover, most panelists felt that symptoms are not required to diagnose a stricture. Panelists felt that MR enterography (MRE) is the preferred diagnostic modality (sensitivity 55–100%; specificity 91–100%).18 There was uncertainty about whether CT enterography (CTE) and ultrasound with or without oral contrast are the preferred diagnostic modalities. The high accuracy of both MRE and CTE was considered appropriate for detection of a single or multiple small bowel stricture(s), with CTE and MRE felt to have comparable accuracy. Ultrasound with or without oral contrast was deemed uncertain by the panel for detection of single or multiple small bowel stricture(s). MRE was preferred over CTE due to lack of radiation exposure in non-acutely ill, clinically stable patients (Supplementary Table 5).
Clinical symptoms of stricturing CD
Clinical symptoms are not highly correlated with the presence of small bowel strictures on cross sectional imaging or endoscopy and there is a disconnect between clinical symptoms and the severity of small bowel strictures on cross sectional imaging or endoscopy. Symptoms considered appropriate for collection were acute abdominal distension, cramping, dietary restrictions, nausea, vomiting, abdominal pain (duration and intensity) and postprandial abdominal pain (duration and intensity) (Supplementary Table 5).
Detection of inflammation and fibrosis
In advanced small bowel strictures, extensive overlap between fibrotic and inflammatory components can be found on histopathology.8 To detect the inflammatory component of a small bowel stricture, MRE and CTE were deemed to be highly accurate and clinical symptoms were felt to be highly inaccurate. There was uncertainty about ultrasound, colonoscopy, C-reactive protein (CRP) and fecal calprotectin for detection of the inflammatory component of a small bowel stricture. It was uncertain whether the degree of inflammation should optimally be determined using validated endoscopic scores. Panelists rated the following imaging features, reflecting the inflammatory component of a small bowel stricture on cross sectional imaging, as appropriate: mural hyperenhancement, presence of ulcers, co-existence with penetrating disease, perienteric fat stranding, comb sign, and intramural T2 hyperintensity (for MRE only).18–23
It was uncertain whether delayed enhancement MRI, magnetization transfer MRI, ultrasound elastography, contrast enhanced ultrasound, bowel ultrasound, MRE and CTE are most accurate for confidently quantifying the fibrotic component of a small bowel stricture.18, 21, 24–26 Colonoscopy with endoscopic mucosal biopsies was considered inappropriate. Currently, no technique can accurately distinguish the inflammatory from the fibrotic component of a small bowel stricture (Supplementary Table 5).
Treatment targets for anti-fibrotic treatment of a small bowel stricture
No precedent for a trial of an anti-fibrotic in CD exists. Panelists considered it appropriate that successful treatment of a small bowel stricture requires improvement in clinical symptoms combined with radiologic features, clinical symptoms combined with endoscopic features or radiologic features combined with endoscopic features. Improvement in clinical symptoms alone was considered inappropriate as a clinical trial endpoint. When symptoms are used in combination with radiologic or endoscopic features to indicate successful treatment, then absence of acute abdominal distention, cramping, dietary restrictions, vomiting, abdominal pain and post-prandial abdominal pain, were considered reflective of successful anti-fibrotic treatment of small bowel strictures.
Radiologic features considered to indicate improvement were localized luminal narrowing, wall thickening, pre-stricture dilation and stricture length. Panelists also felt that the following individual radiologic features should improve with successful anti-fibrotic treatment: 1) a greater than 50% improvement in luminal narrowing or luminal diameter reduction of less than 50%; 2) reduction in bowel wall thickening by 50%; 3) reduction in pre-stricture dilation by 50%, a pre-stricture bowel diameter equal to normal bowel or a bowel diameter less than 2.5 cm; and 4) improvement in stricture length by 50%. The ability to pass an adult endoscope through the stricture was felt to indicate successful anti-fibrotic treatment of a small bowel obstruction (Table 1).
In terms of time points to evaluate the efficacy of medical therapies for CD stricture on cross sectional imaging, 24 and 48 weeks were considered appropriate, with 24 weeks chosen as the optimal primary efficacy endpoint for a clinical trial. Twenty-four weeks was also considered the only acceptable time point to evaluate endoscopic treatment success. Twelve weeks was considered the optimal time point to evaluate treatment success based on clinical symptom improvement (Supplementary Table 5).
Endoscopic treatment of a stricture as a starting point for a clinical trial in Crohn’s disease
Endoscopic balloon dilation may be useful for treatment of symptomatic patients with obstruction and may be used to temporize symptoms in an anti-fibrotic trial. The following items were judged to be appropriate: 18 mm as the maximal luminal diameter after dilation in one or several sessions; a balloon inflation time of at least 1 minute; and 5 cm as the maximum stricture length that should be dilated. Technical success after dilation is defined as the ability to pass an adult ileocolonoscope through a previously non-traversable stricture with reasonable amount of pressure applied, clinical efficacy for dilation is defined as the relief of clinical symptoms of bowel obstruction after dilation. Comparable items were considered appropriate for anastomotic strictures. Graded-through-the-scope balloons should be the preferred tool for endoscopic dilation (Supplementary Table 5).
Endpoints for failure of stricture therapy after initial response
There optimal clinical symptoms indicative of treatment failure or re-obstruction of a small bowel stricture are abdominal distention, cramping, vomiting, dietary restrictions, abdominal pain and post-prandial abdominal pain. A combination of pre-stricture dilation, wall thickening and luminal narrowing on radiology and the inability to pass an adult endoscope were felt to be signs of treatment failure or re-obstruction.
Time to re-stricturing on imaging, endoscopic re-dilation or surgery were considered acceptable and optimal long-term endpoints in a clinical trial of an anti-fibrotic drug. There was uncertainty about time to symptom recurrence as an optimal endpoint for failure of stricture therapy after initial response. A trial endpoint for an anti-fibrotic in stricturing Crohn’s disease was recommended to include cross sectional imaging, endoscopy and clinical symptoms (Supplementary Table 5).
Procedure preparation and reporting of cross sectional imaging
To standardize procedure preparation and reporting, panelists assessed the appropriateness of cross sectional imaging procedures. These results can be found in Supplementary Table 5 and Supplementary Appendix 1.
Expert Consensus-Based Development of Clinical Trial Prototype
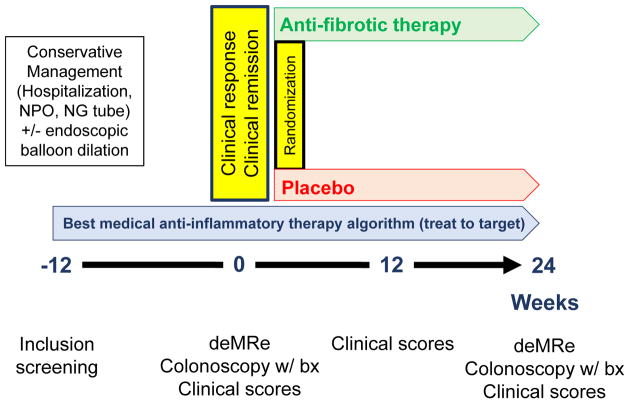
Based on items considered appropriate by the CONSTRICT group, we propose a clinical trial outline to be used in the first anti-fibrotic trial in CD (Figure 1). Primary prevention of a stricture is a large unmet clinical need that novel anti-fibrotic therapies may address. However, the duration from CD diagnosis to stricture formation can be several years27, 28 and there is a lack of validated biomarkers to risk stratify patients29, 30. Hence, pharmaceutical companies are unlikely to embark on primary prevention trials during the first wave of anti-fibrotic drug development. The panelists therefore felt that eligible patients should be clinically symptomatic, with single, naïve or anastomotic ileal strictures that are in reach of endoscopy and confirmed on cross sectional imaging (CT or MR enterography). This approach was chosen since the current ‘gold standard’ (surgical specimen analysis) is not feasible in this situation and mucosal biopsy specimen are superficial and will not detect transmural disease. The panel recommended the inclusion of only symptomatic strictures given that new anti-fibrotic agents are likely to have limited safety data. Therefore, patients and investigators would be reluctant to participate in a trial of therapy that did not offer the possibility of improving symptoms. Furthermore, it is highly unlikely any regulatory agency would agree to a trial evaluating asymptomatic patients at this juncture. Stricture presence on cross-sectional imaging should require all three identified features: localized luminal narrowing (luminal diameter reduction of at least 50%), bowel wall thickening (increase in wall thickness of 25%), and pre-stenotic dilation (luminal diameter less than 3 cm). Patients with internal penetrating disease should be excluded, as internal penetrating disease associated with stricturing disease is an indication for surgery.29 On the basis of these three criteria, all eligible patients should initially receive optimal anti-inflammatory therapy to control symptoms and treat mucosal healing31 with or without endoscopic balloon dilation (using graded-through-the-scope balloons). The maximal diameter of balloon should be 18 mm with a minimal inflation time of 1 minute. Strictures longer than 5 cm should not be dilated. Anti-inflammatory therapy optimization should be performed based on a pre-specified algorithm that reflects optimal standard of care. If patient symptoms improve or subside within 12 weeks, the patients should undergo MRE with inclusion of experimental sequences, such as delayed enhancement or magnetization transfer. The technical details about preparation for MR can be found in Supplementary Table 5 and Supplementary Appendix 1. If symptoms do not improve within the 12 weeks lead-in phase, the patient should be excluded. The 12 weeks mark does not reflect an endpoint, but rather allows selection of patients with symptomatic improvement for inclusion into the trial. The minimum number of symptoms that should be recorded are acute abdominal distension, cramping, dietary restrictions, nausea, vomiting, abdominal pain (duration and intensity) and postprandial abdominal pain (duration and intensity). Given the current lack of validated tools the authors recommend using a Likert scale or 100 mm visual analogue scale to quantitate these items. Imaging features representing the inflammatory component of a stricture on cross sectional imaging are mural hyperenhancement, presence of ulcers, perienteric fat stranding, comb sign, and intramural T2 hyperintensity. In addition, an ileocolonoscopy (adult ileocolonoscope only, to standardize the approach in the setting of a clinical trial) should be performed to assess passability of the stricture. This approach allows for direct visualization of mucosal disease activity and sampling in biomarker studies, while also restricting clinical trial inclusion to patients with the most distal ileal strictures. This should be followed by randomization to placebo or anti-fibrotic drug, given in combination with optimal anti-inflammatory therapy.
Figure 1.
Proposed approach to early development of anti-fibrotics in stricturing Crohn’s disease. It is presumed that strictures in the patient population consist of a mix of inflammation and fibrosis. The optimal primary endpoint is 24 weeks, however a later timepoint (52 weeks) may also be advantageous. At each endpoint data relevant to objective assessment of disease activity, such as C-reactive protein and fecal calprotectin, should be collected. No patient reported outcome (PRO) tool for stricturing Crohn’s disease exists and we recommend inclusion of clinical symptoms found appropriate in this consensus statement into the clinical trial until PROs are available.
Abbrevitions: NPO: Nothing per mouth; NG: Nasogastric; expMRe: experimental magnetic resonance enterography (including delayed enhancement and magnetization transfer sequences)
While there is high accuracy for the detection of inflammation on cross sectional imaging18, currently no imaging technique is able to accurately measure the amount of fibrosis in a stricture.29 Given that anti-fibrotic therapy approaches may modulate the inflammatory component of a stricture1, serial objective parameters of inflammatory activity (i.e. serum and fecal biomarkers) throughout the observation should also be collected. This process will facilitate the greatest possible distinction between the anti-fibrotic versus anti-inflammatory effects of an anti-fibrotic drug. This distinction is important as inflammation may be necessary for the development of fibrosis, however the progression of fibrosis may become independent of inflammation as the disease progresses.32
Co-primary endpoints should be recurrence or worsening of clinical symptoms (following randomization) and documented intestinal obstruction on MRE, with inclusion of experimental sequences signaling the need for endoscopic intervention or surgery. At the end of follow-up, asymptomatic patients should undergo MRE with experimental sequences and ileocolonoscopy. Success of anti-fibrotic treatment should be defined as an asymptomatic patient with reduction in luminal narrowing (greater than 50% improvement and/or luminal diameter reduction of less than 50%), pre-stenotic dilation (reduction in pre-stricture dilation by 50%, bowel diameter equal to normal bowel and/or improvement in pre-stricture dilation to less than 2.5cm), wall thickening (improvement in bowel wall thickening by 50%) and stricture length (improvement of 50%). On ileocolonoscopy, successful anti-fibrotic treatment should be defined as an increase in luminal diameter or ability to pass an adult endoscope. Twenty-four weeks is considered the optimal time point to evaluate treatment success on cross sectional imaging and ileocolonoscopy.
DISCUSSION
Management of small bowel strictures associated with CD is a challenging clinical problem.1 Accordingly, there is an urgent need to develop targeted anti-fibrotic therapies. Drug development has been hampered by the lack of well-defined diagnostic modalities, eligibility criteria and endpoints.12 Furthermore, disagreement surrounding stricture definition, clinical symptoms and what constitutes improvement has led to heterogeneous studies and clinical practices.1, 18, 33–35
Despite the availability of several indices to measure intestinal stenosis and corresponding symptoms6, 18, 36, 37, descriptors are not consistently applied (particularly within the context of treatment response), and none of these instruments are fully validated. There is also lack of clarity on preparation and recording of cross sectional imaging procedures.
The CONSTRICT consensus is an initial step towards establishing valid stricture definitions, diagnostic modalities, eligibility criteria and endpoints for use in CD trials and clinical care. We compiled a comprehensive list of items based on a systematic literature review and expert opinion to assess appropriateness using modified RAND/UCLA methodology. This approach combines the best available evidence with personal clinical experience of international experts and is widely accepted.
Based on the appropriateness rating results, The CONSTRICT group devised detailed recommendations for defining small bowel strictures in CD, including specific radiologic and endoscopic features indicative of stricture, and what constitutes therapeutic improvement. Key definitions are summarized in Table 1. Naïve and anastomotic strictures were discussed separately, which revealed differences in the appropriateness criteria for bowel wall thickness. This is possibly explained by post-surgical changes and the potential for chronically dilated bowel that may fail to normalize following resection. Although clinically-relevant strictures without prestenotic dilation exist, the panelists found the inclusion of prestenotic dilation as a definition criterion for a stricture important due to its high specificity. This would provide a homogenous patient population, increasing the chances to see a meaningful difference with anti-fibrotic treatment. The panelists considered none of the existing cross-sectional imagining techniques appropriate to confidently quantify the fibrotic component of a small bowel stricture, which reflected the opinion of the group that existing technologies for quantification of fibrosis have not been validated.
In addition to having clinical applicability, the current initiative addresses the heterogeneity that prevents direct comparisons across stricturing CD trials. 1, 18, 33–35 Fully validated scoring instruments are particularly needed. While there is no validated patient-reported outcome (PRO) available, the successful launch of a novel anti-fibrotic drug will likely require PRO development. Patients with symptomatic strictures might be included in proof of concept studies with evaluation by imaging, endoscopy or biomarkers whereas registration studies will likely require studies conducted in symptomatic patients with co-primary endpoints of improvement in a PRO and reduction in imaging based outcomes of fibrosis.
To develop fully validated clinical trial endpoints, novel scoring indices must undergo responsiveness testing. However, this presents a challenge since there are no approved anti-fibrotic therapies for stricturing CD. In Figure 1, we propose a framework for proof of concept clinical trials in an ideal scenario where the study population exclusively consists of patients with terminal ileal CD. In this population, strictures consist of a mix of inflammation and fibrosis.19 While recruitment of such a homogenic population may be a challenge, this design would enhance trial rigor and allow for inclusion of endoscopy as an endpoint. Moreover, by targeting mucosal healing and using stratified randomization to ensure groups receiving placebo and anti-fibrotic therapy are balanced with respect to receipt of balloon dilation, it may be possible to measure reduction in fibrosis despite the lack of non-invasive techniques to separate inflammation from fibrosis. While the panel chose 24 weeks as the optimal desired primary endpoint in clinical trials, incorporation of an additional later timepoint (52 weeks) may help to understand the kinetics of imaging features in early phase trials.
Our study has limitations. Given that research in stricturing CD is limited, and no randomized controlled trials are available, most of our recommendations are based on observational data that are vulnerable to bias. For example, assessment of endpoint appropriateness was entirely subjective, given that no validated PROs or clinical instruments currently exist. A specific limitation of this work is the lack of patient representation on the panel. The initial process of evaluating the validity of the symptom items was initiated to identify items that might be considered in future PRO development. The list is not meant to be used in totality for a clinical trial endpoint. Ultimately, any PRO item must be patient-derived, however, the procedure we completed is a recommended exercise as a prelude to PRO development, an extensive and iterative process that may require several years.
The strength of our study lies in the inclusion of internationally recognized IBD radiologists and clinical experts and adoption of rigorous methodology to minimize bias. The individual items are not meant to be read in isolation (e.g. individual diagnostic modalities to detect a stricture), and while some items were rated highly (e.g. MRE), they may not perform with perfect accuracy. Additionally, it may be advantageous to combine items, for instance cross sectional imaging and symptoms, given the relevance of the latter in clinical practice.
In conclusion, we performed an international consensus process using modified RAND/UCLA appropriateness methodology to standardize CD stricture definitions, inclusion criteria and endpoints for use in routine clinical practice. Based on the items considered appropriate, we constructed a prototypic clinical trial design to be shared with the scientific community as a starting point for future investigations. Initiatives are underway to determine reliability of radiologic items identified in the current study and to create a PRO tool specifically for ileal CD-associated strictures. The ultimate goal is the development of a fully validated set of criteria for use in clinical practice and in drug development.
Supplementary Material
Acknowledgments
All authors approved the final version of the manuscript, including the authorship list.
We would like to thank Xin Sun and Leonardo Guizzetti (Robarts Clinical Trials, Inc.) for helping prepare the survey results.
This work was supported by grants from the National Institutes of Health [T32DK083251, P30DK097948 Pilot, K08DK110415] (held by FR).
STATEMENT OF INTEREST
FR is on the advisory board for AbbVie, Celgene, Receptos, Thetis and UCB, consultant to Samsung, UCB, Celgene, Pliant, Boehringer-Ingelheim, Helmsley, RedX, Thetis and Roche and on the speakers’ bureau of AbbVie.
DB is consultant to AbbVie, Takeda, Janssen-Cilag and Tillotts and received lecture fees from AbbVie, MSD, Takeda, Vifor Pharma and Falk Foundation.
CM has no known conflicts of interest.
CEP is an employee of Robarts Clinical Trials, Inc.
LAW is an employee of Robarts Clinical Trials, Inc.
SAN is an employee of Robarts Clinical Trials, Inc.
GVA has received financial support for research from Abbvie, MSD; lecture fees from Abbvie, Ferring, MSD, Janssen, and Takeda; and consultancy fees from Abbvie, MSD, and Takeda.
AD is consultant to MSD, AbbVie, Takeda, Ferring, Dr. Falk Pharma, Pharmacosmos, Vifor, Hospira, Mundipharma, Pfizer, Hexal, Janssen, Otsuka, Boehringer-Ingelheim, Celgene, Roche and received lecture fees from MSD, AbbVie, Takeda, Ferring, Falk Foundation, Pharmacosmos, Vifor, Otsuka, Roche, Janssen, Pfizer, Tillots, Immundiagnostik and Med Update GmbH.
YB has received consulting fees from Abbvie, Biogaran, Boehringer Ingelheim, Ferring, Hospira, Janssen, MSD, Norgine, Pfizer, Roche, Sanofi, Shire, Takeda, UCB, lecture fees from Abbvie, Ferring, Janssen, Mayoli Spindler, MSD, Norgine and Takeda and received research grant support from Pfizer and Takeda.
RWS has served as consultant for Abbvie, Merck and Janssen and has received grant support from Abbvie, UCB and Pfizer.
ADS has no known conflicts of interest.
GR is a consultant for Abbott, AbbVie, Augurix, Boehringer, Calypso, FALK, Ferring, Fisher, Genentech, Essex/MSD, Novartis, Pfizer, Phadia, Roche, Takeda, Tillots, UCB, Vifor, Vital Solutions and Zeller; received speaker honoraria from AstraZeneca, Abbott, AbbVie, FALK, MSD, Phadia, Tillots, UCB and Vifor; and received educational grants and research grants from Abbot, AbbVie, Ardeypharm, Augurix, Calypso, Essex/MSD, FALK, Flamentera, Novartis, Roche, Takeda, Tillots, UCB and Zeller.
ST is a consultant for Robarts Clinical Trials Inc. (central reading for research studies).
JS has no known conflicts of interest.
JR is a consultant for TiGenix and Robarts; received research grants from AbbVie and Genentech; and received speaker honoraria from Takeda, MSD and AbbVie.
MEB receives support from Siemens Healthineers in the form of salary, hardware and software for CT exposure reduction research.
JGF receives grants to his institution from Siemens Healthineers and Medtronic.
JP has received consultancy fees from Abbvie, Arena, Boehringer Ingelheim, Galapagos, Genentech, Janssen, MSD, Novartis, Pfizer, Robarts, Second Genome, Takeda, Theravance, TiGenix, and Topivert.
WJS has received grant support from Exact Sciences, the American College of Gastroenterology, and the Broad Foundation; grant support and personal fees from Receptos, Amgen, Prometheus Laboratories, AbbVie, Boehringer Ingelheim, Takeda, Atlantic Pharmaceuticals, Janssen, Bristol-Myers Squibb, Genentech, Pfizer, and Nutrition Science Partners; and personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Gilead Sciences, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Amgen, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune (AstraZeneca), Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, and the University of Western Ontario (owner of Robarts Clinical Trials Inc).
BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speakers bureaux fees from UCB, AbbVie, and J&J/Janssen.
VJ has received scientific advisory board fees from Abbvie, Takeda, Sandoz, Ferring and Janssen; speaker’s fees from Takeda, Abbvie, Shire, Ferring and Ferring.
Footnotes
AUTHORSHIP
Development of the study concept and design: FR, MEB, WJS, BGF and VJ; Study supervision: FR, WJS, BGF, VJ; Systematic review of the literature: FR, DB, CM, CEP; Participation in the panel: FR, GVA, ADS, YB, RS, AD, GR, ST, JS, JR, MEB, JGF, JP, WJS, BGF and VJ; Data collection and analysis: FR, LAW and SAN; Drafting of the manuscript: FR, CEP, BGF, VJ. All authors performed critical revision of the manuscript for intellectual content and approved the final draft.
References
- 1.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn’s disease. Virchows Arch. 2000;437:293–297. doi: 10.1007/s004280000226. [DOI] [PubMed] [Google Scholar]
- 3.Orscheln ES, Dillman JR, Towbin AJ, et al. Penetrating Crohn disease: does it occur in the absence of stricturing disease? Abdom Radiol (NY) 2017 doi: 10.1007/s00261-017-1398-7. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Jairath V, Levesque BG, Vande Casteele N, et al. Evolving concepts in phases I and II drug development for Crohn’s disease. J Crohns Colitis. 2017;11:246–255. doi: 10.1093/ecco-jcc/jjw137. [DOI] [PubMed] [Google Scholar]
- 5.Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 6.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53–60. doi: 10.1136/gutjnl-2016-312581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer RG, Hawk WA, Turnbull RB., Jr Clinical patterns in Crohn’s disease: a statistical study of 615 cases. Gastroenterology. 1975;68:627–635. [PubMed] [Google Scholar]
- 8.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair. 2014;7:5. doi: 10.1186/1755-1536-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 11.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 12.Rieder F. Toward an antifibrotic therapy for inflammatory bowel disease. United European Gastroenterol J. 2016;4:493–495. doi: 10.1177/2050640616660000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook RH. The RAND/UCLA appropriateness method. In: McCormick KA, Moore SR, Siegel RA, editors. Clinical practice guideline development: methodology practices. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; 1994. [Google Scholar]
- 14.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 15.Fumery M, de Chambrun GP, Stefanescu C, et al. Detection of dysplasia or cancer in 3. 5% of patients with inflammatory bowel disease and colonic strictures. Clin Gastroenterol Hepatol. 2015;13:1770–1775. doi: 10.1016/j.cgh.2015.04.185. [DOI] [PubMed] [Google Scholar]
- 16.Brook RH. Appropriateness: the next frontier. BMJ. 1994;308:218–219. doi: 10.1136/bmj.308.6923.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruining D, Zimmerman EM, Loftus EV, Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286:776–799. doi: 10.1148/radiol.2018171737. [DOI] [PubMed] [Google Scholar]; Gastroenterology. 2018;4:1172–1194. doi: 10.1053/j.gastro.2017.11.274. [DOI] [PubMed] [Google Scholar]
- 18.Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–145. doi: 10.1111/j.1365-2036.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 19.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012;18:849–856. doi: 10.1002/ibd.21801. [DOI] [PubMed] [Google Scholar]
- 20.Patel NS, Pola S, Muralimohan R, et al. Outcomes of computed tomography and magnetic resonance enterography in clinical practice of inflammatory bowel disease. Dig Dis Sci. 2014;59:838–849. doi: 10.1007/s10620-013-2964-7. [DOI] [PubMed] [Google Scholar]
- 21.Sconfienza LM, Cavallaro F, Colombi V, et al. In-vivo axial-strain sonoelastography helps distinguish acutely-inflamed from fibrotic terminal ileum strictures in patients with Crohn’s disease: preliminary results. Ultrasound Med Biol. 2016;42:855–863. doi: 10.1016/j.ultrasmedbio.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014;147:334–342. doi: 10.1053/j.gastro.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Wagner M, Ko HM, Chatterji M, et al. Magnetic resonance imaging predicts histopathologic composition of ileal Crohn’s disease. J Crohns Colitis. 2018 doi: 10.1093/ecco-jcc/jjx186. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazahr S, Blume I, Frei P, et al. Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. MAGMA. 2013;26:291–301. doi: 10.1007/s10334-012-0355-2. [DOI] [PubMed] [Google Scholar]
- 25.Quaia E, De Paoli L, Stocca T, et al. The value of small bowel wall contrast enhancement after sulfur hexafluoride-filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med Biol. 2012;38:1324–1332. doi: 10.1016/j.ultrasmedbio.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110(3):432–40. doi: 10.1038/ajg.2014.424. [DOI] [PubMed] [Google Scholar]
- 27.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J Crohns Colitis. 2016;10:873–885. doi: 10.1093/ecco-jcc/jjw055. [DOI] [PubMed] [Google Scholar]
- 30.Rieder F, de Bruyn JR, Bao Tung P, et al. Results of the 4th Scientific Workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;8:1166–1178. doi: 10.1016/j.crohns.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 32.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol. 1983;5:211–215. doi: 10.1097/00004836-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Bettenworth D, Lopez R, Hindryckx P, et al. Heterogeneity in endoscopic treatment of Crohn’s disease-associated strictures: an international inflammatory bowel disease specialist survey. J Gastroenterol. 2016;51:939–948. doi: 10.1007/s00535-016-1172-6. [DOI] [PubMed] [Google Scholar]
- 35.Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis. 2017;23(1):133–142. doi: 10.1097/MIB.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 36.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 37.Sipponen T, Nuutinen H, Turunen U, et al. Endoscopic evaluation of Crohn’s disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis. 2010;16:2131–2136. doi: 10.1002/ibd.21300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.