Abstract
Cisplatin is a highly effective treatment for malignant cancers and has become a cornerstone in chemotherapeutic regimens. Unfortunately, its use in the clinic is often coupled with a high incidence of severe hearing loss. Over the past few decades, enormous effort has been put forth to find protective agents that selectively protect against the ototoxic side effects of cisplatin and do not interfere with its antitumoral activity. Many therapies have been successful in preclinical work, but only a few have shown any protection in the clinic, and none have been approved by the FDA. This review summarizes the clinical and preclinical studies of the most effective small-molecule candidates currently in clinical trials, while also detailing their molecular mechanisms of action, to gain insight for future drug development in the field.
Since its approval by the FDA in 1978, cisplatin has become a cornerstone in chemotherapeutic regimens and has been included on the World Health Organization’s list of essential medicines. However, its therapeutic benefits are often coupled with a potentially debilitating ototoxicity, from which some degree of hearing loss occurs in about 63% of patients.1 Despite the high incidence and enormous social and economic consequences of the ototoxic side effects, no FDA-approved therapies for the prevention of cisplatin-induced hearing loss have been developed. Still, numerous protective compounds have been identified in preclinical studies, and several are currently undergoing clinical trials (Table 1 and Figure 1).
Table 1. List of All Clinical Candidates for Protection Against Cisplatin-Induced Ototoxicity.
| name | mechanism | delivery | outcomes and considerations | completed | ref |
|---|---|---|---|---|---|
| sodium thiosulfate | inactivator of cisplatin, antioxidant, protector of antioxidant enzymes | IVa | lowered incidence of hearing loss, lowered survival for disseminated cancer | 2017 | (2) |
| IV | no results reported | ETCc 2018 | (3) | ||
| IAb | no impact on incidence of hearing loss | 2007 | (4) | ||
| acetylcysteine | inactivator of cisplatin, antioxidant, protector of antioxidant enzymes, promoter of glutathione synthesis | IV | dose finding study, no results reported | ETC 2019 | (5) |
| local | significant hearing protection only at 8 kHz | 2013 | (6) | ||
| local | no significant hearing protection | 2014 | (7) | ||
| d-methionine | inactivator of cisplatin, antioxidant, protector of antioxidant enzymes | oral | reported hearing protection at >10 kHz, no peer-reviewed data published | 2009 | (8) |
| amifostine | inactivator of cisplatin, antioxidant | IV | nonrandomized, hearing protection in average-risk but not high-risk medulloblastomas | 2014 | (9) |
| IV | 9% ototoxicity in amifostine group vs 16% in untreated group | 1996 | (10) | ||
| IV | (5 trials) no significant hearing protection | 1999–2009 | (11−15) | ||
| ebselen | antioxidant, glutathione peroxidase mimic, anti-inflammatory | oral | ongoing study, no results reported | ETC 2018 | (16) |
| dexamethasone | regulator of cytokines, protector of antioxidant enzymes | local | negative efficacy results in a related study | terminated | (17) |
| local | significant hearing protection only at 6 kHz | 2013 | (18) | ||
| flunarizine | inhibitor of MPTd and NF-κB activation | oral | significant hearing protection at <4 kHz | 2016 | (19) |
| lipoic acid | antioxidant, regulator of cytokines | N/A | no results reported | 2011 | (20) |
| aspirin | antioxidant, anti-inflammatory | oral | no significant hearing protection reported | 2016 | (21) |
| vitamin Ee | antioxidants | oral | significant hearing protection at 2 and 8 kHz | 2016 | (22) |
| statin drugse | N/A | N/A | ongoing study, no results reported | ETC 2022 | (23) |
| GBE 761e,f | antioxidants | oral | significant hearing protection only at 8 kHz | 2015 | (24) |
IV, intravenous.
IA, intra-arterial.
ETC, estimated time of completion.
MPT, mitochondrial permeability transition.
These compound mixtures will not be discussed in detail.
GBE, Ginkgo biloba extract.
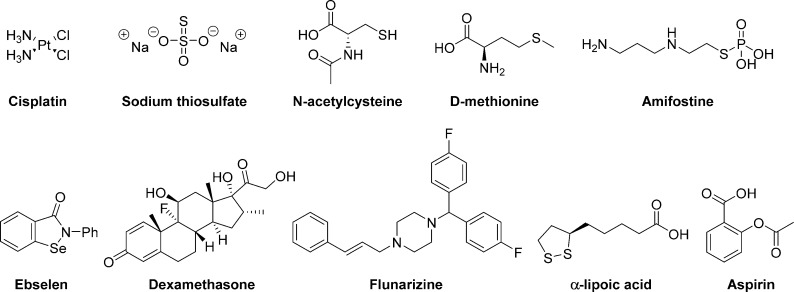
Figure 1.
Molecular structures of cisplatin and the clinical candidates discussed.
One of the most fundamental considerations in developing an otoprotective drug is the route of administration. Although systemic delivery is generally the most convenient method, the possibility of the otoprotectant interfering with the antineoplastic activity of cisplatin poses a significant challenge. In addition, the otoprotectant must be capable of crossing the blood–labyrinth barrier (BLB) that separates the inner ear from the rest of the body.25 Alternatively, the drug can be administered locally to the ear by using such drug-delivery techniques as transtympanic injection.26 In this case, the drug is administered by using a syringe with an ultrafine needle to penetrate the tympanic membrane and deliver the drug directly into the middle-ear cavity (Figure 2a). The drug can then permeate through the round-window membrane (RWM), located on the cochlea, and into the perilymph, the ionic cochlear fluid that runs throughout the cochlea, and reach the target auditory cells that reside within the cochlea (Figure 2b). This strategy reduces the potential for the compound to interfere with the antineoplastic activity of cisplatin in other parts of the body, avoids the need for the drug to cross the blood–brain barrier and BLB, and circumvents problems common to systemic delivery, such as those arising from drug metabolism and toxicity in other parts of the body. However, because cisplatin treatment is administered over a period of several weeks and can sometimes cause hearing loss even shortly after discontinuation, many transtympanic injections would be needed throughout the treatment period, and these would potentially be damaging and costly. One way to minimize the number of injections over the treatment course is to use drug-delivery systems with long residence times, such as hydrogels, which can adhere to the round window and allow the compound to slowly permeate into the perilymph over time.26 Many strategies for both systemic and local delivery have been used in clinical and preclinical studies of the compounds listed in Table 1. This review summarizes the progress of these compounds in both the clinical and preclinical settings and details the biomolecular mechanisms by which they exert their effects.
Figure 2.
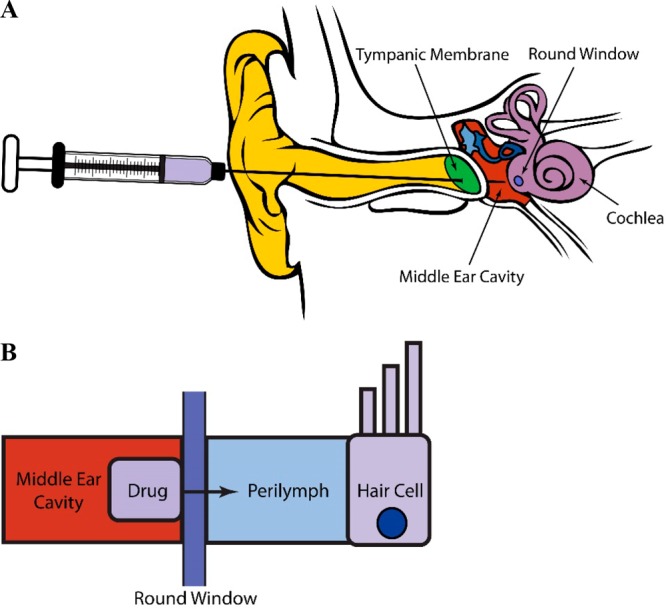
Examples of (a) transtympanic injection for the local administration of a drug to the inner ear and (b) permeation of a drug across the round-window membrane of the cochlea and into the perilymph to reach the targeted auditory cells.
General Mechanistic Pathways of Cisplatin-Induced Cytotoxicity in Auditory Cells
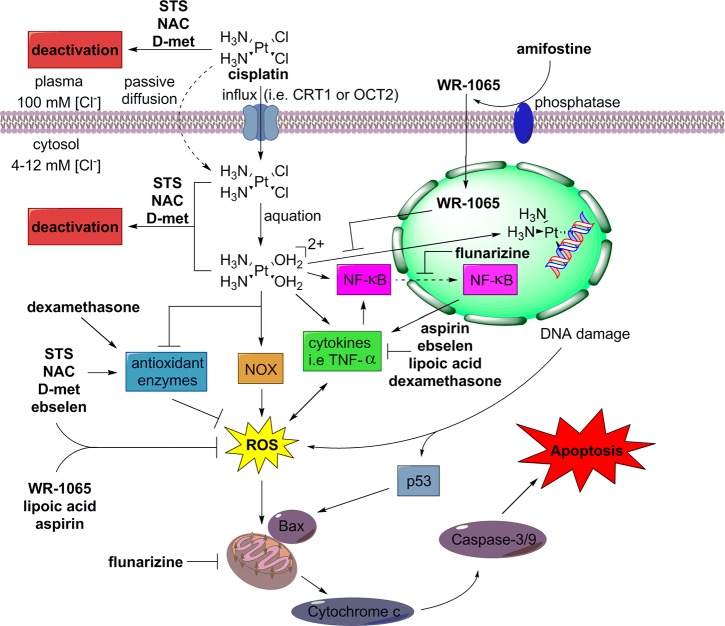
Figure 3 illustrates some of the general pathways by which cisplatin induces cytotoxicity that damages auditory cells, and it shows how the otoprotective clinical candidates combat this toxicity. The cellular and molecular mechanisms by which cisplatin causes ototoxicity are not fully understood. It is thought that cisplatin is first taken up into a cell by transporters such as copper transporter 1 (CTR1) or, in the case of inner-ear hair cells, organic cation transporters (OCT1–3).27 Once in the cytosol, where chloride concentrations are low, most of the cisplatin is hydrolyzed by the displacement of one or both of its chlorine atoms by water to form aqua–cisplatin complexes.28 The aqua complexes can react with N-donors in DNA bases to cause DNA damage and DNA cross-linking.28 DNA damage triggers the molecular sensor ataxia telangiectasia mutated (ATM), which initiates a downstream response that includes the activation of the major tumor suppressor p53.29 Activation of p53 increases the expression of Bcl-2-associated X protein (Bax), which triggers the release of cytochrome c from the mitochondria and causes apoptosis through caspase 3 activation.30
Figure 3.
General mechanistic pathways of cisplatin-induced cytotoxicity in auditory cells and the mechanistic pathways by which the otoprotective clinical candidates combat cisplatin toxicity.
Another major pathway by which cisplatin exerts its cytotoxicity is through the generation of reactive oxygen species (ROS). Cisplatin activates NADPH oxidases, such as NOX-3, an enzyme that is highly expressed in the cochlea, which causes an increase in lipid peroxidation and the accumulation of ROS.31 Cisplatin also reduces the levels of antioxidant enzymes, including superoxide dismutase (SOD), glutathione reductase (GR), glutathione S-transferase (GST), and glutathione peroxidase (GSH-Px).32 Among other effects, high levels of ROS can cause the release of cytochrome c from the mitochondria, leading to apoptosis, as previously described.30
Lastly, cisplatin increases the release of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, and nuclear factor NF-κB into the cytoplasm.33 Proinflammatory cytokines are linked to the generation of ROS and the degradation of IκB proteins, which are responsible for maintaining NF-κB in an inactive state within the cytoplasm.33 Once activated, the active heterodimer of NF-κB is translocated to the nucleus to precipitate a series of actions within the cell, including the induction of the de novo synthesis of proinflammatory cytokines, the activation of the proapoptotic caspases 3 and 9, and the increased expression of inducible nitric oxide synthase (iNOS), a producer of the free radical nitric oxide (NO).34
Sodium Thiosulfate
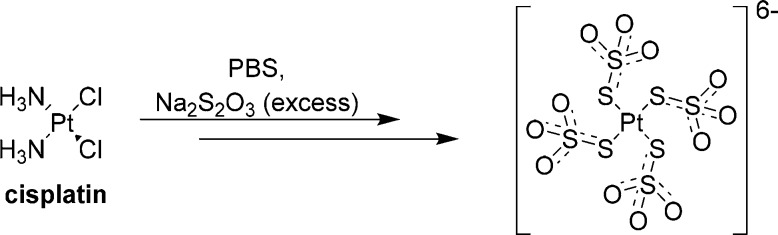
Sodium thiosulfate (STS) can reduce cisplatin-induced toxicity by quenching ROS (e.g., H2O2), preserving the activity of antioxidant enzymes (e.g., SOD), and forming biologically inactive complexes with cisplatin to effectively reduce the systemic exposure to cisplatin.35,36 Of the thiol-containing compounds that have been shown to protect against cisplatin-induced ototoxicity, STS is by far the most nucleophilic and forms complexes with cisplatin faster than any other sulfur-containing otoprotectant.37 The structural basis for the formation of the Pt–STS complexes has been recently described by Sooriyaarachchi et al., and a Pt–STS complex has been characterized as a four-coordinate Pt(II) species, [Pt(S2O3)4]6–, by the use of X-ray absorption spectroscopy (Scheme 1).36 The results of these studies indicate that the mode of binding to Pt occurs through the external sulfur of STS and that complexes with fewer bound thiosulfate groups probably exhibit this same type of binding. In addition, these studies have also demonstrated in vitro that STS decreases the amount of free cisplatin in human plasma, with 31% of free cisplatin remaining in plasma within 10 min of STS incubation, as compared with 87% with no STS. Furthermore, the free cisplatin remains in human plasma for less than 50 min in the presence of excess STS, as compared with more than 3 h in the absence of STS.36
Scheme 1. Depiction of a Cisplatin Complex with STS As Observed by X-ray Crystallography.
Given this mechanism, it is unsurprising that the concurrent systemic administration of STS and cisplatin results in the loss of a substantial portion of the antineoplastic activity of cisplatin.38 Interestingly, Dickey et al. demonstrated that if STS administration was delayed until 4 h after cisplatin treatment, there was only a modest loss of the anticancer activity of cisplatin, and the resulting protection against cisplatin-induced ototoxicity was significant.38 However, less protection against hearing loss was observed if STS administration was delayed until 8 h after cisplatin treatment, and no hearing protection was observed after a 12 h delay.38 Nevertheless, caution should be used when administering STS and cisplatin nonconcurrently in clinical studies, as such an approach could put patients with cancer at serious risk if the timing and dosing of the compounds are not carefully controlled.
Unfortunately, in a randomized phase 3 clinical trial (NCT00716976) that was completed in 2017, the 3 year survival rates for participants with disseminated cancers were substantially lower for those patients who received STS 6 h after cisplatin administration (45%) than they were for those who received cisplatin alone (84%).2 In contrast, for participants with localized diseases, there was no significant difference in overall survival between the two groups.2 Some protection against cisplatin-induced ototoxicity was observed: hearing loss was observed in 56.4% of the patients who received cisplatin alone but only in 28.6% of those who received STS 6 h after cisplatin administration.2 Preliminary results for the use of STS with cisplatin in patients with localized diseases have been reported for SIOPEL 6, a multicenter, randomized, open-label, phase 3 clinical trial (NCT00652132) that is expected to be completed by the end of 2017.3
Alternative approaches using local deliveries of STS to avoid the attenuation of the antineoplastic activity of cisplatin have been explored. Unfortunately, topical application of STS to the round window of guinea pigs failed to provide protection against cisplatin-induced ototoxicity.39 However, administration of STS by a highly intrusive intracochlear-perfusion technique or transtympanic injection of a thiosulfate-containing high-viscosity formulation of sodium hyaluronan (HYA gel) provided significant protection against cisplatin-induced ototoxicity in guinea pigs.40 In the latter case, the HYA gel adhered to the round-window membrane, and high concentrations of STS were achieved through the sustained release of STS.40 However, hearing function was not tested, and only a morphologic study of the survival of the outer hair cells (OHCs) in the cochlea was performed.40 In conclusion, caution should be used in future clinical studies involving the systemic administration of sodium sulfate, as this compound is not safe for systemic administration to patients with disseminated diseases. Accordingly, practical local delivery routes should be further investigated with a view to providing a safer alternative.
N-Acetylcysteine
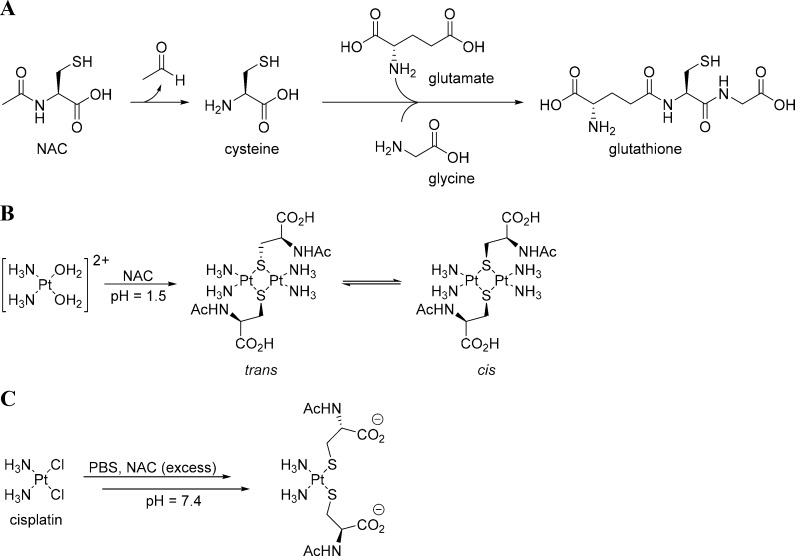
N-Acetylcysteine (NAC), an acetylated variant of l-cysteine, can act in a variety of ways to protect against cisplatin-induced ototoxicity. NAC is thought to have dual antioxidative potential, enabling it to combat ROS generated from cisplatin. As a thiol, NAC can serve directly as an antioxidant. Alternatively, as a precursor for l-cysteine, a building block in GSH synthesis, NAC can promote the endogenous antioxidant system (Scheme 2a).41 The latter scenario is the more likely mechanism, as NAC is a much weaker antioxidant than glutathione, and the concentrations of free NAC achievable in vivo are probably insufficient to make meaningful contributions to antioxidant defenses.41 NAC can also inhibit the activation of JNK, p38 MAPK, and NF-κB transcription-factor activities; promote cell survival; and prevent apoptosis by activating ERK pathways.42 Lastly, like other otoprotectants displaying a sulfur, NAC or its primary metabolite, cysteine, can form complexes with cisplatin to reduce cytotoxicity. Two molecular structures that explain how NAC and cisplatin can form complexes have been described in the literature. Appleton et al. observed the reaction of NAC and a labeled hydrolysis product of cisplatin, [Pt(15NH3)2(H2O)]2+, by using multinuclear NMR (15N, 195Pt, 13C, and 1H) and reported a sulfur-bridged dimer, [Pt(NH3)2(μ-NAC-S)2]2+, as the predominant product (Scheme 2b).43 However, when an excess of NAC was used at physiological pH, an [(NAC)2Pt(NH3)2]− complex was observed as the primary product by liquid-chromatography electrospray-ionization tandem mass spectrometry (LC-ESI-MS, Scheme 2c).36 Similar to the observations made in vitro with STS, Sooriyaarachchi et al. reported that NAC also decreases the amount of free cisplatin in human plasma, albeit to a lesser extent than does STS.36
Scheme 2. Molecular Mechanisms by Which NAC Combats Cisplatin Cytotoxicity.
(A) Deacetylation of NAC yielding cysteine, an important building block in glutathione synthesis. (B) Complex of NAC with cisplatin at a low pH as a mixture of sulfur-bridged dimers as observed by 15N, 195Pt, 13C, and 1H NMR. (C) Complex of NAC with cisplatin at physiological pH as observed by LC-ESI-MS.
Similar to STS, delayed administration of NAC is more clinically relevant, as its concurrent administration with cisplatin attenuates the antitumoral activity of the latter.38 Indeed, Muldoon et al. demonstrated that pretreatment with NAC ameliorates the antitumoral activity of cisplatin in rat models of neuroblastoma and medulloblastoma and that the antitumoral activity is uninhibited when NAC is administered 4 h after cisplatin.44 A phase 1 clinical trial, NCT02094625, is underway to determine the dose of NAC needed for otoprotection as well as how well it is tolerated in combination with chemotherapy.5 According to the study design, 30 patients with cancer aged 1 to 21 years will receive NAC intravenously over a period of 30 min, commencing 4 h after cisplatin administration, for their first three cycles of chemotherapy.5 These clinical trials are expected to conclude in February 2018.5
The use of local delivery for the administration of NAC to circumvent its interference with the antitumoral activity of cisplatin has yielded mixed results in preclinical and clinical studies. For instance, although a transtympanic injection of a 2% solution of NAC has been successful in animal models, a 2014 clinical study of patients with head and neck cancer found no statistically significant difference between the NAC-treated group and the untreated group with regard to protection against cisplatin-induced ototoxicity.7 In contrast, a 2013 clinical study determined that a transtympanic injection of a 10% solution of NAC protected hearing in patients receiving cisplatin, although only the protection of hearing at a frequency of 8 kHz reached statistical significance.6 Overall, ototoxicity was observed in two of the NAC-treated ears, as compared to seven of the untreated ears.6 Increasing the concentration of the NAC solution to 20% resulted in inflammation of the inner ear and intolerable pain in all patients.6 Indeed, the major limitation of this strategy is that it is not possible to obtain sufficient concentrations of NAC in the inner-ear to provide otoprotection, as the solution is rapidly cleared from the middle ear cavity through the Eustachian tube. Patient compliance could also be an issue, as multiple transtympanic injections of NAC, up to 8 injections over a period of 2 weeks, would be required to provide protection over the course of a cisplatin treatment.6 Recently, Ciftci et al. have developed a novel hydrogel system for NAC delivery.45 This system uses two solutions that form the gel upon coming into contact with one another.45 The preliminary results suggest that this controlled-delivery system will form the gel more quickly than traditional gels, which normally require a waiting period for the gel to solidify.45 The compound can be released from the gel over the desired amount of time, and the gel will degrade without the need for the excessive water that other gels may require.45 In conclusion, caution should be exercised when using systemic deliveries, especially in patients with disseminated diseases, because of the similarities between the mechanisms of action of NAC and STS. Local delivery is preferable, and efforts to improve local-delivery techniques might result in the provision of more complete protection against cisplatin-induced ototoxicity in the future.
d-Methionine
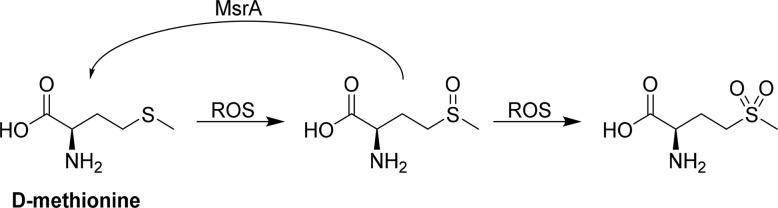
d-Methionine is the enantiomer of the amino acid l-methionine and has proved to be a potent protectant against cisplatin-induced ototoxicity in preclinical trials. Its mechanism of action can be conceptualized by its ability to serve as an antioxidant, protect the endogenous antioxidant system in the body, and form inactive complexes with cisplatin. As an antioxidant, methionine molecules are first oxidized by ROS to generate a mixture of R- and S-sulfoxides, which can be further oxidized to sulfones in the presence of a second equivalent of ROS.46 Alternatively, Moskovitz et al. reported that d-methionine sulfoxides, as well as other sulfoxide-containing xenobiotics, can be reduced by methionine sulfoxide reductase A, MsrA (Scheme 3).47
Scheme 3. Oxidation of d-Methionine and Reduction of the Sulfoxide by MsrA.
In addition to serving directly as an antioxidant, d-methionine also helps to protect the enzyme-activity levels of the endogenous antioxidant system of the body from cisplatin-induced decrements. Campbell et al. demonstrated that rats treated with cisplatin had increased levels of malondialdehyde (MDA), a lipid peroxidation marker, and significantly reduced activities of SOD, catalase (CAT), GR, and GSH-Px.32 Administering d-methionine 30 min before cisplatin treatment preserved the activity levels of GR, SOD, and CAT, while also causing a noticeable decrease in MDA levels. However, no protection of GSH-Px activity was observed.32 In addition, Cheng et al. reported that cisplatin significantly decreased Na+, K+-ATPase, and Ca2+-ATPase activities in the cochlear lateral wall and that d-methionine was able to reverse the reductions in these enzyme-activity levels.48
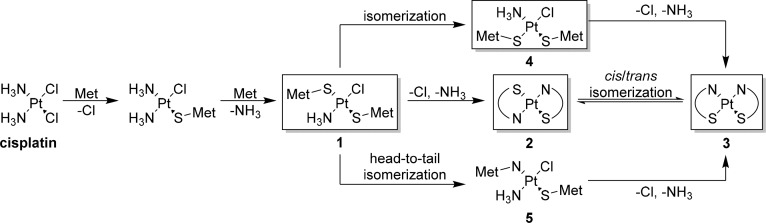
Although the mechanisms surrounding ROS mitigation explain how d-methionine protects against noise-induced and aminoglycoside-induced hearing loss, they do not fully explain how methionine protects against cisplatin-induced ototoxicity. In addition to promoting free-radical formation, cisplatin can also bind to proteins and DNA and cause damage to cells. To combat this action, methionine can reduce the concentration of free cisplatin by forming substantially less active methionine–cisplatin complexes. The cis-[Pt(Met-S,N)2] complex is considered to be the most stable and chemically inert complex of methionine and cisplatin, and it has been detected in the urine of patients receiving cisplatin and methionine.49 Sooriyaarachchi et al. detailed the formation of Pt–S(Met) complexes by LC-ESI-MS at various time points during reactions of l- or d-methionine with cisplatin.36 Between 1 and 4 h, the [Pt(NH3)Cl(S-Met)] complex was the dominant product, but after 21 h, the cis-[Pt(Met-S,N)2] complex was the sole product for both enantiomers.36 Similarly, Norman et al. monitored reactions involving a 2:1 ratio of l-methionine to cisplatin by using multinuclear NMR techniques and reported the formation of several cisplatin–methionine complexes that eventually converged to the cis-[Pt(Met-S,N)2] complex upon reaching equilibrium, with a 10:1 ratio of cis to trans isomers.50 With regard to the formation of the final complex, they reported that there were no major differences as to whether cisplatin had aqua or chloride ligands.50 The mechanism can be rationalized as shown in Scheme 4, in which the boxed intermediates were observed by NMR. After the coordination of the first methionine to platinum through its sulfur atom, a second methionine can coordinate through its sulfur atom and displace the amine trans to the first methionine to generate complex 1. The selectivity is driven by the trans effect, in which the sulfur of the first methionine most strongly destabilizes the bond of the amine trans to it.51 Chelation to platinum through the nitrogen of each methionine generates two stable six-member rings and provides the trans isomer, 2, which isomerizes to the preferred cis isomer, 3.50 However, the exact mechanism by which the cis isomer forms is unclear. El-Khateeb et al. observed both complex 1 and complex 4 by multinuclear NMR at a low pH, and they proposed that under their experimental conditions, isomerization from complex 1 to cis isomer 4 occurs before chelation.52 The chloride and amine of cis complex 4 are both trans to sulfur, and chelation would occur rapidly as a result of the trans effect to generate cis isomer 3. Alternatively, on the basis of observations of methionine complexes with transplatinum, Pinato et al. proposed that one of the S-bound methionines could undergo a head-to-tail isomerization to an N-bound methionine to generate complex 5 before the formation of cis isomer 3.53
Scheme 4. Proposed Mechanism for the Formation of cis-[Pt(Met-S,N)2], 3.
Met, methionine. Boxed intermediates have been observed by 15N, 195Pt, 13C, and 1H NMR.
When compared to other compounds, such as ebselen and GSH, in organotypic cultures of the organs of Corti from rats, d-methionine provided the highest preservation of hair cells, with nearly 100% of the mean hair-cell density being conserved in explants treated with cisplatin and d-methionine.54d-methionine also protected against cisplatin-induced toxicity in the stria vascularis, crista ampullaris, and otolith organs.48 Indeed, d-methionine has exhibited exceptional protective capabilities by preserving auditory-brainstem-response (ABR)-threshold shifts when administered systemically in multiple animal models.32 A phase 2 clinical study showed that the oral formulation of d-methionine (MRX-1024) was safe to use concurrently with combined radiation and chemotherapy and that it provided significant hearing-threshold protection at frequencies of 10 kHz and higher.8 However, no peer-reviewed results showing the extent of the hearing protection have been published, and it remains unclear how safe the oral formulation would be if administered in the absence of radiation therapy, as d-methionine could compromise the antitumoral activity of cisplatin.
As with other sulfur-containing otoprotectants, there is concern as to whether d-methionine interferes with the antineoplastic activity of cisplatin. Compared with compounds that display a primary thiol, such as cysteine or NAC, for which S–Pt coordination is considered to be irreversible, the S–Pt coordination of the thioether of methionine is weaker and considered to be reversible.55 Therefore, although the S,N-chelate is relatively inert, a monodentate S-methionine complex is susceptible to displacement by nucleophiles such as monomeric 5′-guanosine monophosphate (GMP).56 However, when short synthetic DNAs containing guanine residues were used instead of monomeric GMP, the relative speed of displacement slowed significantly, and only 25% of the synthetic DNA was platinated after 72 h in the presence of methionine and cisplatin.56 Ekborn et al. detailed the pharmacokinetics of d-methionine and cisplatin administered together and reported that d-methionine could cause a decrease of up to 30% in the area under the concentration–time curve for free cisplatin.57 These observations could explain why methionine inhibits the anticancer activity of cisplatin, albeit to a lesser extent than is seen with otoprotectants displaying a primary thiol, such as NAC. For example, two studies using rats implanted with MTLN-3 breast-cancer tumors found that the tumors in rats that received l- or d-methionine 30 min before cisplatin treatment showed minimal changes in size after 7 days, whereas the tumors in untreated rats underwent significant growth, and the tumors in rats treated only with cisplatin were completely reduced.58,59 Deegan et al. demonstrated that d-methionine inhibition of cisplatin activity was closely related to the dosing ratio of methionine to cisplatin, with any dose above a 1:1 ratio resulting in noticeable changes in cisplatin activity.60 Melvik et al. reported that a combination of cisplatin and methionine was less lethal to human NHIK 3025 cells in terms of their colony-forming abilities than was cisplatin alone.61 However, in an ovarian cancer model, Cloven et al. observed only a subjective (i.e., not statistically significant) decrease in the survival rates of mice that received cisplatin with d-methionine, as compared with those that received cisplatin alone.62 Jones et al. reported that rats bearing the Walker 256 carcinoma had their tumors completely reduced when various thiols and thioethers, including NAC and d-methionine, were administered before cisplatin treatment.63 However, the lack of attenuation of the antitumoral activity of cisplatin in the presence of primary thiols, such as NAC, is surprising, and the authors noted that it is probably due to the well-known sensitivity of the Walker 256 carcinoma to cisplatin as well as the rapid uptake of cisplatin by the tumor.63 The extent to which systemic d-methionine contributes to an attenuation of the antineoplastic activity of cisplatin varies between models, and more studies are needed to understand the interactions between d-methionine and cisplatin.
As a small molecule, d-methionine is an excellent candidate for local delivery. Indeed, Laurell et al. demonstrated in rats that locally administered [14C] d-methionine readily passes through the RWM and has a terminal half-life of 0.57 h by using a liquid scintillation counter and autoradiography to analyze the distribution of the tracer in the cochlea.64 Administration onto the round window would avoid the possible attenuation of the antitumoral activity of cisplatin by d-methionine, while retaining the otoprotective effects of methionine in the cochlea. Indeed, l- or d-methionine delivered onto the round window provides significant protection against cisplatin-induced ototoxicity in multiple animal models.39,59 In conclusion, d-methionine is protective when administered systemically or locally in animal models, although the local delivery method is to be preferred for clinical studies until there is a clearer understanding of how d-methionine influences the antineoplastic activity of cisplatin in patients with different cancers.
Amifostine
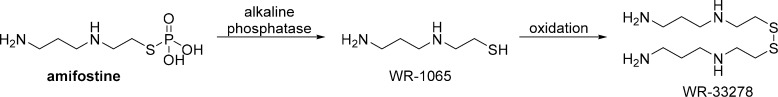
Amifostine is a thiophosphate prodrug that provides selective cytoprotection for normal tissues without attenuating the antitumoral effect of cisplatin. The active metabolite, WR-1065, is generated by the dephosphorylation of amifostine by a membrane-bound alkaline phosphatase at a neutral pH and is rapidly taken up by a carrier-mediated, facilitated-diffusion process in normal tissues (Scheme 5).65 In contrast, the formation and uptake of WR-1065 in tumors is slow to negligible, as poor vascularization in the tumor results in decreased alkaline phosphatase activity, hypoxia, and an acidic pH.65 WR-1065 has displayed a notable ability to protect against cisplatin-induced ototoxicity by scavenging ROS. Specifically, WR-1065 exerts strong, preferential antioxidant activity against highly reactive hydroxyl radicals, but exhibits only limited activity against spontaneous lipoperoxidation and no activity against superoxides.66 As a thiol, WR-1065 can form inactive complexes with cisplatin; however, significant inactivation of cisplatin in circulation is not expected, because of the significantly lower reactivity of WR-1065 as compared with that of STS and the pharmacokinetic behavior of amifostine.65 Instead, WR-1065 inactivates cisplatin in close proximity to DNA before damage can occur; WR-1065 has been shown to significantly decrease DNA platination by cisplatin and to accumulate in the vicinity of DNA through the counterion condensation of its protonated form and the negatively charged phosphate backbone of DNA at physiological pH.65 Given this mechanism, it is not surprising that numerous studies have demonstrated that amifostine does not interfere with the antitumoral activity of cisplatin.65
Scheme 5. Metabolism of Amifostine to WR-1065 and Oxidation of WR-1065 to the Disulfide, WR-33278.
Preclinical studies of the effectiveness of amifostine in preventing cisplatin-induced ototoxicity have yielded variable results. In a guinea pig model, Hyppolito et al. demonstrated that systemically delivered amifostine protects against cisplatin-induced ototoxicity, insofar as it helps to preserve the number and architecture of outer hair cells in the cochlea as well as cochlear function, as measured by distortion-product otoacoustic emissions (DPOAEs).67 In contrast, two separate studies found that systemically administered amifostine provided no protection against cisplatin-induced ototoxicity and that it provided only minimal protection to outer hair cells in the cochlea of hamsters.68
Similar to the preclinical investigations, clinical studies have led to divergent conclusions as to whether amifostine provides any protection against cisplatin-induced ototoxicity. A randomized study by Kemp et al., completed in 1996, demonstrated that IV administration of amifostine 35 min before cisplatin treatment resulted in a 43% reduction in the incidence of the protocol-specified ototoxicity.10 In another case, two nonrandomized, multi-institutional clinical studies, SJMB96 and SJMB03, were combined to evaluate amifostine as an otoprotectant against cisplatin-induced ototoxicity.9 Amifostine was administered intravenously before cisplatin treatment and again 3 h afterward. Amifostine provided significant hearing protection in patients with average-risk medulloblastomas but not in patients with high-risk medulloblastomas.9 The investigators suggested that the lack of protection in the high-risk medulloblastoma group might be due to the study having insufficient statistical power to detect a significant difference between the groups of amifostine-treated (n = 99) and untreated (n = 17) patients with high-risk medulloblastomas.9 In contrast, after a smaller clinical study, Fisher et al. reported that amifostine failed to provide significant protection against cisplatin-induced ototoxicity in patients with average-risk or high-risk medulloblastomas.13 Three separate small studies have, likewise, shown no significant hearing protection in patients that received amifostine in combination with cisplatin treatments.11,12,14 Lastly, a larger randomized study by Katzenstein et al. found that amifostine provided no protection in children with hepatoblastomas and increased the incidence of hypocalcemia.15 In conclusion, the results from the clinical studies are highly variable with regard to the extent to which amifostine protects against cisplatin-induced hearing loss. It is likely that this variability is the result of differences between the studies in terms of administration protocols, treatment cocktails, hearing evaluations, ototoxicity grading methods, cohort sizes, randomization, and disease types. Therefore, more uniform study designs are needed to evaluate amifostine as a potential protective agent against cisplatin-induced ototoxicity.
Ebselen
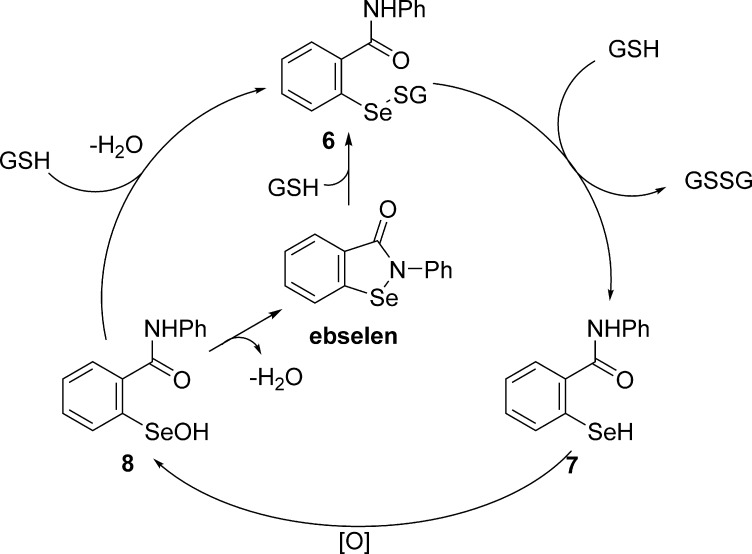
Ebselen, a seleno-organic compound, has been shown to provide significant protection against cisplatin-induced ototoxicity by serving as an antioxidant, a glutathione peroxidase mimic by scavenging ROS, an anti-inflammatory agent, and a nuclear-factor-(erythroid-derived 2)-like-2 (Nrf2) activator by promoting endogenous antioxidant responses and glutathione synthesis.69 As a glutathione peroxidase mimic, ebselen acts as a procatalyst, in which it is initially reduced by GSH to a selenyl sulfide, 6, which initiates a catalytic, three-step cycle (Scheme 6). A second equivalent of GSH reacts with the selenyl sulfide, 6, to generate a selenol, 7, which is subsequently converted to a selenenic acid, 8, by oxidation with ROS. The selenic acid, 8, can then react with available GSH to continue the cycle or, in the absence of available thiols, cyclize through the elimination of water to regenerate ebselen.70
Scheme 6. Proposed Catalytic Cycle of Ebselen as a Glutathione Mimic.
The first studies performed using organotypic cultures of the organs of Corti of rats revealed that treatment with ebselen resulted in nearly complete protection of auditory hair cells from cisplatin ototoxicity.54 Early in vivo experiments with rats demonstrated that ebselen treatment resulted in substantially lower ABR-threshold shifts than were seen in the cisplatin-treated controls.71 However, more recent work with ebselen has shown that it provides only modest protection in terms of ABR-threshold shifts, by comparison with that described in previous reports, with one study even finding no significant difference between the ABR shifts of rats receiving ebselen with cisplatin and those of rats receiving cisplatin alone.69,72 In another study, Lynch et al. demonstrated that ebselen combined with allopurinol exhibited some synergistic effects with regard to protection against cisplatin-induced ototoxicity in rats, although differences in the ABR shifts remained modest.73 Follow-up studies in models of breast and ovarian cancers revealed that the combination of ebselen and allopurinol not only did not interfere with the antineoplastic properties of cisplatin but actually improved the antitumoral activity in the ovarian model.73 A phase 2 clinical trial, NCT01451853, will investigate the prevention and treatment of chemotherapy-induced hearing loss with ebselen, but this trial is still in the recruitment phase.16 In conclusion, ebselen does not interfere with the antitumoral activity of cisplatin and provides variable protection against cisplatin-induced ototoxicity in animal models, but it has not yet been evaluated in clinical studies.
Dexamethasone
Dexamethasone is a glucocorticosteroid that has been reported to protect against cisplatin-induced ototoxicity through a variety of mechanisms, including the downregulation of proinflammatory cytokines, the inhibition of apoptosis, and the upregulation of antioxidant enzymes. Dexamethasone binds to glucocorticoid receptors to form activated GR–glucocorticoid complexes that translocate to the nucleus, where they bind to glucocorticoid-response elements of target genes.74 This binding regulates the transcription of anti- and proinflammatory genes.74 The proinflammatory genes downregulated by glucocorticosteroids are responsible for the expression of cytokines, such as IL-1β, IL-2, IL-3, IL-6, IL-11, and TNF-α, and chemokines, such as IL-8, RANTES, and MCP-1(CCL2).74 Dexamethasone also activates antiapoptotic pathways, which results in protection against TNF-α cytotoxicity.74 Lastly, dexamethasone decreases cell death caused by ROS by upregulating antioxidant-enzyme activities.75
Systemic delivery of dexamethasone has yielded conflicting results with regard to protection against cisplatin-induced ototoxicity, probably because the poor solubility, poor BLB penetration, and rapid clearance of dexamethasone make it difficult to achieve high concentrations of the drug in the inner ear.76 In addition, because glucocorticoids cause the downregulation of apoptosis genes, concerns have been raised about the consequences of systemically coadministering these compounds with cisplatin.77 To avoid the need for the frequent administration of high doses of dexamethasone, Sun et al. used dexamethasone-loaded nanoparticles to provide sustained systemic deliveries of dexamethasone in guinea pigs.76 The dexamethasone-loaded nanoparticles were administered intraperitoneally 1 h before cisplatin treatment and proved to be as effective at attenuating cisplatin-induced hearing loss as a multidose administration of dexamethasone over 3 days.76 Similarly, Salehi et al. systemically administered dexamethasone- and curcumin-loaded nanoparticles to guinea pigs and reported that dexamethasone and curcumin provided partial protection against cisplatin-induced hearing loss when administered together but not when administered separately.78
The dexamethasone-mediated resistance of cancer cells toward the apoptotic effects of cisplatin has prompted the use of local delivery techniques.77 Both single-dose and multidose strategies have been employed. For instance, the administration of a single dose of dexamethasone by intratympanic injection 30 min before cisplatin treatments resulted in the complete preservation of DPOAEs in guinea pigs, although similar protection was not achieved in rats treated with this regimen.79 Multidose approaches have yielded mixed results, with complete or partial protection being obtained when dexamethasone was administered daily for several days by intratympanic injection after a single dose of cisplatin on the first day but not when cisplatin was administered with dexamethasone each day.77 A phase 2 clinical trial to examine the efficacy of dexamethasone delivered by intratympanic injection against cisplatin ototoxicity, NCT01372904, found that the treatment provided only partial protection at pure tone thresholds, with protection being observed at frequencies of 6 and 8 kHz, but only the protection at 6 kHz reached statistical significance.18 Significant hearing loss was not observed at frequencies of 0.5, 1, 2, 3, or 4 kHz in both the control and treated groups.18
Recently, more advanced delivery systems have been evaluated for local dexamethasone administration. One such strategy employs self-assembled nanoparticles loaded with dexamethasone, with the goal of increasing the bioavailability of dexamethasone in the inner ear. This approach has been successfully demonstrated in rats, in which the dexamethasone-loaded nanoparticles, administered by bullostomy, enabled high concentrations of dexamethasone to be achieved in the inner ear and provided significant protection against cisplatin-induced ototoxicity.80 Alternatively, a sustained-release strategy using OTO-104, a poloxamer hydrogel containing dexamethasone, has been reported to dramatically increase the residence time of dexamethasone in the inner ear and, consequently, alleviate the need for multiple intratympanic injections. A single intratympanic injection of 6% OTO-104 a day before cisplatin treatment was effective in protecting against cisplatin ototoxicity, even when the cisplatin was administered at weekly intervals over a period of 3 weeks.81 Unfortunately, a phase 2 clinical study using OTO-104, NCT02997189, was terminated because of the negative efficacy of the compound in a recently completed phase 3 study, 104-201506.17 In conclusion, the local delivery of dexamethasone is preferred to minimize the dexamethasone-mediated resistance of cancer cells to cisplatin, although there is some variability in the extent to which locally delivered dexamethasone protects against cisplatin-induced ototoxicity.
Flunarizine
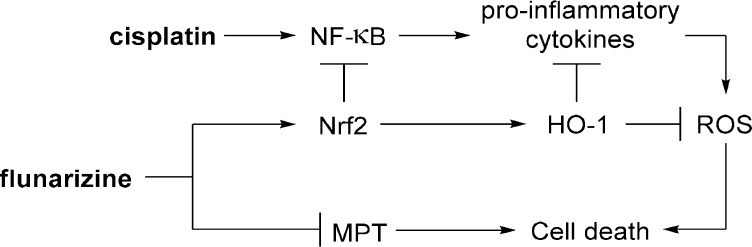
Flunarizine (Sibelium) is a T-type-calcium-channel antagonist that attenuates cisplatin-induced ototoxicity through the prevention of lipid peroxidation, inhibition of mitochondrial-permeability transition (MPT), and suppression of proinflammatory-cytokine secretion (Figure 4). Flunarizine downregulates NF-κB and MAPKs through the activation of Nrf2 and thereby reduces proinflammatory-cytokine secretion.33 Nrf2 drives the transcriptional activation of heme oxygenase 1 (HO-1), which among other effects, generates bilirubin and carbon monoxide (CO) through heme degradation.33 Bilirubin plays a role in lipid-peroxidation inhibition and, together with CO, suppresses proinflammatory-cytokine production due to cisplatin.33 Flunarizine also inhibits changes in MPT in the presence of cisplatin and thereby prevents the release of cytochrome c.33 The mechanism by which flunarizine inhibits changes in MPT is unclear and might be unrelated to its calcium-channel-blocking ability.82 Surprisingly, flunarizine has been reported to increase [Ca2+] levels, despite its function as a T-type-calcium-channel blocker.33 Furthermore, neither the calcium chelator BAPTA-AM nor the calcium ionophore A23187 affected cisplatin-induced cytotoxicity in the House-Ear-Institute organ-of-Corti 1 cell line (HEI-OC1).33 Similarly, Elimadiab et al. reported that high concentrations of flunarizine increased mitochondrial swelling induced by increased [Ca2+] levels, and they proposed that flunarizine could instead bind to mitochondrial hydrophobic sites to inhibit MPT.82
Figure 4.
Proposed mechanism by which flunarizine combats cisplatin-induced cytotoxicity.
Flunarizine ameliorated cisplatin-induced cytotoxicity in both HEI-OC1 cells and in cochlear explants from postnatal-day-2 rats.33 The administration of flunarizine significantly reduced proinflammatory-cytokine levels in the sera and cochleae of mice treated with cisplatin, as compared with those in mice treated with cisplatin alone.33 Unfortunately, ototoxicity and hearing were not evaluated in the mice, and it is unclear how effectively flunarizine protects against cisplatin-induced hearing loss in animal models. A clinical study of 40 patients with cancer demonstrated that orally administered flunarizine was effective at preventing cisplatin-induced ototoxicity at lower frequencies but not at frequencies above 4 kHz.19 In conclusion, flunarizine is an effective protectant against cisplatin-induced ototoxicity in vitro, but it has only limited efficacy in vivo. It is unclear how effectively flunarizine crosses the BLB to reach the inner ear, and local-delivery strategies may improve the treatment’s efficacy.
Other Compounds Studied in Clinical Trials
Lipoic acid and aspirin have been shown to protect against cisplatin-induced ototoxicity in animal models; however, no such protection has been observed in clinical studies.20,21 Although vitamin E, Ginkgo biloba extract (GBE 761), and statin drugs are each currently under investigation in clinical trials, they have not been covered in detail in this review, as they exist as compound mixtures, rather than pure individual compounds. Importantly, the preliminary results of the clinical studies showed that vitamin E provided significant protection against cisplatin-induced ototoxicity 1 month after treatment and that GBE 761 provided significant protection at a frequency of 8 kHz.22,24
Future Directions
It is difficult to prioritize a protective strategy moving forward, as the development and identification of new compounds for both new and established drug targets are being pursued in parallel. From a mechanistic point of view, it would seem intuitive to develop new compounds to target upstream pathways, such as approaches that focus on cisplatin inactivation. However, targeting this pathway has only led to partial otoprotection in the clinic, despite the extensive investigation of four structurally unique cisplatin inactivators, STS, NAC, d-methionine, and amifostine. Furthermore, the potential interference of the antitumoral activity of cisplatin by future compounds designed for this strategy introduces additional complications for drug development and poses significant risks for both investors and patients. To address these issues, future endeavors on the development of more advanced local-delivery strategies would be preferable to the development of new cisplatin inactivators, as recent innovations in local-delivery systems for STS, NAC, and dexamethasone have been shown to avoid the attenuation of the antitumoral activity of cisplatin, optimize drug distribution, and increase the residence time of the drug in animal models, all of which could ultimately lead to improved efficacy in the clinic.40,45,80,81
Currently all of the candidates in clinical trials share similar mechanisms of action, operating as cisplatin inactivators, antioxidants, cytokine regulators, or a combination of these modes of action, and none have provided complete protection against cisplatin-induced hearing loss. Another approach to developing a successful protective strategy could be through the identification and development of new compounds with novel mechanisms of action. For instance, Kaur et al. recently described that the adenosine-A1-receptor agonist, R-phenylisopropyladenosine, provides complete protection against cisplatin-induced hearing loss in rats by causing the suppression of NOX-3 and the attenuation of STAT1.83 In another example, Benkfadar et al. demonstrated that cisplatin activation of the ATM-Chk2-p53 pathway was a key contributor to ototoxicity, and the reversible inhibitor of p53-mediated apoptosis, pifithrin-α, provided significant hearing preservation in mice without interfering with the anticancer activity of cisplatin.29 Several other protective compounds and potential drug targets for combating cisplatin-induced ototoxicity have been discussed in a recent review by Sheth et al.84
Lastly, approaches utilizing combination therapies with the clinical candidates could be designed on the basis of reported mechanisms of action. For example, a combination of d-methionine and dexamethasone could provide increased efficacy, as the two compounds act on different mechanistic pathways of cisplatin-induced cytotoxicity in auditory cells. Furthermore, in clinical studies, dexamethasone provided significant hearing protection at lower frequencies, whereas d-methionine provided significant protection at higher frequencies, which suggests there could be a synergy between the compounds that could provide protection across a broader range of frequencies.
Conclusion
A total of nine compounds have been tested in clinical trials for their ability to prevent cisplatin-induced ototoxicity, and many more new compounds are in the preclinical phase of evaluation. All the compounds studied in clinical trials, except for aspirin and lipoic acid, have been proved to provide some protection against cisplatin-induced ototoxicity, but none have provided complete protection across all frequencies. Nearly half of the compounds studied in clinical trials have had conflicting results between studies in regard to their ability to protect against cisplatin-induced ototoxicity. Although some guidelines do exist for ototoxicity, none have been universally accepted, and the disagreement between studies is likely the result of many factors, such as administration protocols, treatment cocktails, hearing evaluations and ototoxicity grading methods, cohort sizes, patient ages, and cancer types.85 Therefore, the design of more uniform studies for future drug development would have a far-reaching impact upon the field. In conclusion, cisplatin-induced ototoxicity can have debilitating effects on a patient’s quality of life, and there is an urgent need to find safe and effective therapies via the development of more efficient drug-delivery methods, combinatorial drug cocktails, or compounds with novel mechanisms of action.
Acknowledgments
We thank Munia F. Sowaileh and Megan B. Wood for reviewing the manuscript and their suggestions, Tetsuji Yamashita for his help in creating the figures, Zoran Rankovic for advice, and Keith A. Laycock for scientific editing. We apologize for not including many relevant references because of space limitations. This work was supported by the National Institutes of Health (grant nos. 2R01DC006471, 1R01DC015010-01A1, 1R01DC015444-01, and 1R21DC013879-01 to J.Z.; and grant no. P30CA21765 to St. Jude), by ALSAC, and by the Office of Naval Research (grant nos. N000140911014, N000141210191, N000141210775, and N000141612315 to J.Z.).
Glossary
Abbreviations Used
- ABR
auditory brainstem response
- ATM
ataxia telangiectasia mutated
- Bax
Bcl-2-associated X protein
- BLB
blood–labyrinth barrier
- CAT
catalase
- CTR 1
copper transporter 1
- DPOAE
distortion-product otoacoustic emission
- ERK
extracellular signal-regulated kinases
- ETC
estimated time of completion
- GBE
Ginkgo biloba extract
- GMP
guanosine monophosphate
- GR
glutathione reductase
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- GST
glutathione S-transferase
- HEI-OC1
House-Ear-Institute organ-of-Corti 1 cell line
- HO-1
heme oxygenase 1
- HYA
sodium hyaluronan
- IA
intra-arterial
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IV
intravenous
- JNK
c-Jun N-terminal kinase
- LC-ESI-MS
liquid-chromatography electrospray-ionization tandem mass spectrometry
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MDA
malondialdehyde
- Met
methionine
- MPT
mitochondrial permeability transition
- MsrA
methionine sulfoxide reductase A
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear-factor κ-light-chain enhancer of activated B cells
- NO
nitric oxide
- NOX
NADPH oxidase
- NMR
nuclear magnetic resonance
- Nrf2
nuclear-factor (erythroid-derived 2)-like 2
- OCT
organic cation transporters
- OHC
outer hair cell
- RANTES
regulated on activation, normal-T-cell expressed, and secreted
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STS
sodium thiosulfate
- TNF-α
tumor necrosis factor α
Biographies
Robert A. Hazlitt graduated from Baylor University with a BS in chemistry in 2010 and went on to work as a chemical technologist at the DOW Chemical Company in Freeport, TX. In 2016, he received his PhD in organic chemistry from Purdue University, where he worked in the laboratory of David A. Colby on synthesis of complex fluorinated molecules for applications in the prevention of neurodegenerative diseases. In 2016, he joined St. Jude Children’s Research Hospital as a postdoctoral research associate in the laboratory of Jian Zuo, where his research project is focused on the development of small-molecule protective agents for the prevention of cisplatin-induced ototoxicity.
Jaeki Min obtained his BS (1990) and MS (1992) in chemistry from Hanyang University (South Korea), and then joined JW Pharmaceutical (South Korea) in 1992 as a medicinal chemist to work on small-molecule-drug discovery. In 2004, he received his PhD in organic chemistry from Seoul National University (South Korea) and had postdoctoral training in the field of chemical genetics at New York University. Then, he worked at Emory Chemical Biology Discovery Center. He joined St. Jude Children’s Research Hospital in 2009, and he is currently leading the Chemical Biology Center (CBC) in the Department of Chemical Biology and Therapeutics as a principal medicinal chemist and has been focused on the HTS, medicinal chemistry, and chemical-biology technology to develop novel in vitro and in vivo probes.
Jian Zuo obtained his PhD in Physiology in 1993 from the University of California San Francisco, studying the molecular genetics of Huntington’s Disease. He was then trained at Rockefeller University from 1993 to 1997 as a postdoctoral associate studying mouse cerebellar mutants. Since 1998, he has been a faculty member at St. Jude Children’s Research Hospital working on hearing. Recently, he has pursued studies on drug development for hearing loss.
Author Contributions
The manuscript was written by R. H. and revised by J. M. and J. Z.
The authors declare no competing financial interest.
References
- Greene J. B.; Standring R.; Siddiqui F.; Ahsan S. F. Incidence of cisplatin induced ototoxicity in adults with head and neck cancer. Adv. Otolaryngol. 2015, 56, 1–4. 10.1155/2015/245613. [DOI] [Google Scholar]
- Freyer D. R.; Chen L.; Krailo M. D.; Knight K.; Villaluna D.; Bliss B.; Pollock B. H.; Ramdas J.; Lange B.; Van Hoff D.; VanSoelen M. L.; Wiernikowski J.; Neuwelt E. A.; Sung L. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. 10.1016/S1470-2045(16)30625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock P. R.; Childs M.; Rajput K.; Maibach R.; Roebuck D.; Sullivan M. J.; Laithier V.; Ronghe M.; dall’Igna P.; Hiyama E.; Brichard B.; Skeen J.; Mateos M. E.; Fabre M.; Perilongo G.; Czauderna P.; Morland B.; Neuwelt E. A. Two-year results of clinical efficacy of cisplatin in combination with sodium thiosulfate (STS) vs cisplatin alone in a randomized phase III trial for standard risk hepatoblastoma (SR-HB): SIOPEL 6. J. Clin. Oncol. 2016, 34, 10514–10514. 10.1200/JCO.2016.34.15_suppl.10514. [DOI] [Google Scholar]
- Zuur C. L.; Simis Y. J.; Lansdaal P. E.; Hart A. A.; Schornagel J. H.; Dreschler W. A.; Rasch C. R.; Balm A. J. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J. Clin. Oncol. 2007, 25, 3759–3765. 10.1200/JCO.2006.08.9540. [DOI] [PubMed] [Google Scholar]
- Orgel E.NAC to Prevent Cisplatin-induced Hearing Loss, 2014. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02094625. (accessed Nov 6, 2017).
- Riga M. G.; Chelis L.; Kakolyris S.; Papadopoulos S.; Stathakidou S.; Chamalidou E.; Xenidis N.; Amarantidis K.; Dimopoulos P.; Danielides V. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity a feasible method with promising efficacy. Am. J. Clin. Oncol. 2013, 36, 1–6. 10.1097/COC.0b013e31822e006d. [DOI] [PubMed] [Google Scholar]
- Yoo J.; Hamilton S. J.; Angel D.; Fung K.; Franklin J.; Parnes L. S.; Lewis D.; Venkatesan V.; Winquist E. Cisplatin otoprotection using transtympanic L-N-Acetylcysteine: a pilot randomized study in head and neck cancer patients. Laryngoscope 2014, 124, E87–E94. 10.1002/lary.24360. [DOI] [PubMed] [Google Scholar]
- Campbell K. C. M.; Nayar R.; Borgonha S.; Hughes L.; Rehemtulla A.; Ross B.; Sunkara P.. Oral D-methionine (MRX-1024) Significantly Protects Against Cisplatin-Induced Hearing Loss: A Phase II Study in Humans. Proceedings of the 32nd Annual Midwinter Research Meeting Association for Research in Otolaryngology, Baltimore, MD, Feb 14–19, 2009; Santi P. A., Ed.; Association for Research in Otolaryngology, Mt. Royal, NJ, 2009; Abstract 22, p. 7. [Google Scholar]
- Gurney J. G.; Bass J. K.; Onar-Thomas A.; Huang J.; Chintagumpala M.; Bouffet E.; Hassall T.; Gururangan S.; Heath J. A.; Kellie S.; Cohn R.; Fisher M. J.; Panandiker A. P.; Merchant T. E.; Srinivasan A.; Wetmore C.; Qaddoumi I.; Stewart C. F.; Armstrong G. T.; Broniscer A.; Gajjar A. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro. Oncol. 2014, 16, 848–855. 10.1093/neuonc/not241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G.; Rose P.; Lurain J.; Berman M.; Manetta A.; Roullet B.; Homesley H.; Belpomme D.; Glick J. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J. Clin. Oncol. 1996, 14, 2101–2112. 10.1200/JCO.1996.14.7.2101. [DOI] [PubMed] [Google Scholar]
- Planting A. S. T.; Catimel G.; de Mulder P. H. M.; de Graeff A.; Höppener F.; Verweij J.; Oster W.; Vermorken J. B. Randomized study of a short course of weekly cisplatin with or without amifostine in advanced head and neck cancer. Ann. Oncol. 1999, 10, 693–700. 10.1023/A:1008353505916. [DOI] [PubMed] [Google Scholar]
- Ekborn A.; Hansson J.; Ehrsson H.; Eksborg S.; Wallin I.; Wagenius G.; Laurell G. High-dose cisplatin with amifostine: ototoxicity and pharmacokinetics. Laryngoscope 2004, 114, 1660–1667. 10.1097/00005537-200409000-00030. [DOI] [PubMed] [Google Scholar]
- Fisher M. J.; Lange B. J.; Needle M. N.; Janss A. J.; Shu H.-K. G.; Adamson P. C.; Phillips P. C. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr. Blood Cancer 2004, 43, 780–784. 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- Marina N.; Chang K. W.; Malogolowkin M.; London W. B.; Frazier A. L.; Womer R. B.; Rescorla F.; Billmire D. F.; Davis M. M.; Perlman E. J.; Giller R.; Lauer S. J.; Olson T. A. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors. Cancer 2005, 104, 841–847. 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- Katzenstein H. M.; Chang K. W.; Krailo M. D.; Chen Z.; Finegold M. J.; Rowland J.; Reynolds M.; Pappo A.; London W. B.; Malogolowkin M. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma. Cancer 2009, 115, 5828–5835. 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E.SPI-1005 for prevention and treatment of chemotherapy induced hearing loss, 2011. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01451853. (accessed Nov 6, 2017).
- Bishop K.Study of OTO-104 in subjects at risk from cisplatin-induced hearing loss, 2016. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02997189. (accessed Nov 6, 2017).
- Marshak T.; Steiner M.; Kaminer M.; Levy L.; Shupak A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: a randomized controlled study. Otolaryngol.--Head Neck Surg. 2014, 150, 983–990. 10.1177/0194599814524894. [DOI] [PubMed] [Google Scholar]
- Prinja S.; Singh G.; Vashisth M.; Arora T. Protective role of calcium channel blocker flunarizine on cisplatin induced ototoxicity: a clinical study. Int. J. of Contemp. Med. Res. 2016, 3, 1290–1292. [Google Scholar]
- Martin D. L.Alpha-lipoic acid in preventing hearing loss in cancer patients undergoing treatment with cisplatin, 2007. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00477607. (accessed Nov 6, 2017).
- King E.; Crabb S.; Martin K.; Abab J.; Ratcliffe I.; Thornton R.; Lineton B.; Ellis M.; Moody R.; Stanton L.; Geldart T.; Bayne M.; Davies J.; Lamb C.; Popat S.; Nutting C.; Chester J.; Hartley J.; Thomas G.; Ottensmeier C.; Huddart R.; Pugh K.. COAST (Cisplatin Ototoxicity Attenuated by Aspirin Trial): A Phase II Double Blind, Randomised Controlled Trial to Establish if Aspirin Reduces Cisplatin Induced Hearing Loss. Proceedings of the NCRI Cancer Conference, Liverpool, United Kingdom, Nov 6–9, 2016. [DOI] [PMC free article] [PubMed]
- Villani V.; Zucchella C.; Cristalli G.; Galiè E.; Bianco F.; Giannarelli D.; Carpano S.; Spriano G.; Pace A. Vitamin E neuroprotection against cisplatin ototoxicity: Preliminary results from a randomized, placebo-controlled trial. Head Neck 2016, 38, E2118–E2121. 10.1002/hed.24396. [DOI] [PubMed] [Google Scholar]
- Cunningham L. L.Hearing loss and the effects of statin drugs in people with head and neck squamous cell carcinoma treated with cisplatin chemoradiation, 2017. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03225157. (accessed Nov 6, 2017).
- Dias M. A.The protective effect of ginkgo biloba extract on cisplatin-induced ototoxicity in humans, 2010. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01139281. (accessed Nov 6, 2017).
- Sun W.; Wang W. Advances in research on labyrinth membranous barriers. J. Otol. 2015, 10, 99–104. 10.1016/j.joto.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kechai K.; Agnely F.; Mamelle E.; Nguyen Y.; Ferrary E.; Bochot A. Recent advances in local drug delivery to the inner ear. Int. J. Pharm. 2015, 494, 83–101. 10.1016/j.ijpharm.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G.; Deuster D.; Knief A.; Sperling M.; Holtkamp M.; Edemir B.; Pavenstädt H.; Lanvers-Kaminsky C.; am Zehnhoff-Dinnesen A.; Schinkel A. H.; Koepsell H.; Jürgens H.; Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T. C.; Suntharalingam K.; Lippard S. J. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkafadar N.; Menardo J.; Bourien J.; Nouvian R.; François F.; Decaudin D.; Maiorano D.; Puel J.-L.; Wang J. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med. 2017, 9, 7–26. 10.15252/emmm.201606230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Ladrech S.; Pujol R.; Brabet P.; Van De Water T. R.; Puel J.-L. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004, 64, 9217–9224. 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Bánfi B.; Malgrange B.; Knisz J.; Steger K.; Dubois-Dauphin M.; Krause K. H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Campbell K. C. M.; Meech R. P.; Rybak L. P.; Hughes L. F. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J. Am. Acad. Audiol. 2003, 14, 144–156. [PubMed] [Google Scholar]
- So H.-S.; Kim H.-J.; Kim Y.; Kim E.; Pae H.-O.; Chung H.-T.; Kim H.-J.; Kwon K.-B.; Lee K.-M.; Lee H.-Y.; Moon S.-K.; Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008, 9, 290–306. 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-H.; Boo S. H.; Chung M. K.; Lee H.-S.; Cho Y.-S.; Hong S. H. Proapoptotic effects of NF-kB on cisplatin-induced cell death in auditory cell line. Acta Oto-Laryngol. 2008, 128, 1063–1070. 10.1080/00016480701881811. [DOI] [PubMed] [Google Scholar]
- Bijarnia R. K.; Bachtler M.; Chandak P. G.; van Goor H.; Pasch A. Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats. PLoS One 2015, 10, e0124881. 10.1371/journal.pone.0124881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooriyaarachchi M.; George G. N.; Pickering I. J.; Narendran A.; Gailer J. Tuning the metabolism of the anticancer drug cisplatin with chemoprotective agents to improve its safety and efficacy. Metallomics 2016, 8, 1170–1176. 10.1039/C6MT00183A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videhult P.; Laurell G.; Wallin I.; Ehrsson H. Kinetics of cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp. Biol. Med. 2006, 231, 1638–1645. 10.1177/153537020623101009. [DOI] [PubMed] [Google Scholar]
- Dickey D. T.; Wu W. J.; Muldoon L. L.; Neuwelt E. A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005, 314, 1052–1058. 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- Wimmer C.; Mees K.; Stumpf P.; Welsch U.; Reichel O.; Suckfüll M. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol. Neurotol. 2004, 25, 33–40. 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Berglin C. E.; Pierre P. V.; Bramer T.; Edsman K.; Ehrsson H.; Eksborg S.; Laurell G. Prevention of cisplatin-induced hearing loss by administration of a thiosulfate-containing gel to the middle ear in a guinea pig model. Cancer Chemother. Pharmacol. 2011, 68, 1547–1556. 10.1007/s00280-011-1656-2. [DOI] [PubMed] [Google Scholar]
- Rushworth G. F.; Megson I. L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Wu Y. J.; Muldoon L. L.; Neuwelt E. A. The hemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J. Pharmacol. Exp. Ther. 2005, 312, 424–431. 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- Appleton T. G.; Connor J. W.; Hall J. R.; Prenzler P. D. NMR study of the reactions of the cis -diamminediaquaplatinum(II) cation with glutathione and amino acids containing a thiol group. Inorg. Chem. 1989, 28, 2030–2037. 10.1021/ic00310a007. [DOI] [Google Scholar]
- Muldoon L. L.; Wu Y. J.; Pagel M. A.; Neuwelt E. A. N-acetylcysteine chemoprotection without decreased cisplatin antitumor efficacy in pediatric tumor models. J. Neuro-Oncol. 2015, 121, 433–440. 10.1007/s11060-014-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci Z.; Deniz M.; Yilmaz I.; Ciftci H. G.; Sirin D. Y.; Gultekin E. In vitro analysis of a novel controlled release system designed for intratympanic administration of N-acetylcysteine: a preliminary report. Am. J. Otolaryngol. 2015, 36, 786–793. 10.1016/j.amjoto.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Nakao L. S.; Iwai L. K.; Kalil J.; Augusto O. Radical production from free and peptide-bound methionine sulfoxide oxidation by peroxynitrite and hydrogen peroxide/iron(II). FEBS Lett. 2003, 547, 87–91. 10.1016/S0014-5793(03)00674-4. [DOI] [PubMed] [Google Scholar]
- Moskovitz J.; Weissbach H.; Brot N. Cloning and expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 2095–2099. 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W.-C.; Chang C.-M.; Liao L.-J.; Wang C.-T.; Young Y.-H.; Chang Y.-L.; Cheng P.-W. Assessment of D-methionine protecting cisplatin-induced otolith toxicity by vestibular-evoked myogenic potential tests, ATPase activities and oxidative state in guinea pigs. Neurotoxicol. Teratol. 2015, 51, 12–20. 10.1016/j.ntt.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Riley C. M.; Sternson L. A.; Repta A. J.; Slyter S. A. Monitoring the reactions of cisplatin with nucleotides and methionine by reversed-phase high-performance liquid chromatography using cationic and anionic pairing ions. Anal. Biochem. 1983, 130, 203–214. 10.1016/0003-2697(83)90671-1. [DOI] [PubMed] [Google Scholar]
- Norman R. E.; Ranford J. D.; Sadler P. J. Studies of platinum(II) methionine complexes: metabolites of cisplatin. Inorg. Chem. 1992, 31, 877–888. 10.1021/ic00031a033. [DOI] [Google Scholar]
- Lau J. K.-C.; Deubel D. V. Loss of ammine from platinum(II) complexes: implications for cisplatin inactivation, storage, and resistance. Chem. - Eur. J. 2005, 11, 2849–2855. 10.1002/chem.200401053. [DOI] [PubMed] [Google Scholar]
- El-Khateeb M.; Appleton T. G.; Gahan L. R.; Charles B. G.; Berners-Price S. J.; Bolton A. M. Reactions of cisplatin hydrolytes with methionine, cysteine, and plasma ultrafiltrate studied by a combination of HPLC and NMR techniques. J. Inorg. Biochem. 1999, 77, 13–21. 10.1016/S0162-0134(99)00146-4. [DOI] [PubMed] [Google Scholar]
- Pinato O.; Musetti C.; Sissi C. Pt-based drugs: the spotlight will be on proteins. Metallomics 2014, 6, 380–395. 10.1039/C3MT00357D. [DOI] [PubMed] [Google Scholar]
- Kopke R. D.; Liu W.; Gabaizadeh R.; Jacono A.; Feghali J.; Spray D.; Garcia P.; Steinman H.; Malgrange B.; Ruben R. J.; Rybak L. P.; Van De Water T. R. Use of organotypic cultures of corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [PubMed] [Google Scholar]
- Zimmermann T.; Zeizinger M.; Burda J. V. Cisplatin interaction with cysteine and methionine, a theoretical DFT study. J. Inorg. Biochem. 2005, 99, 2184–2196. 10.1016/j.jinorgbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Vrana O.; Brabec V. L-methionine inhibits reaction of DNA with anticancer cis-diamminedichloroplatinum(II). Biochemistry 2002, 41, 10994–10999. 10.1021/bi0257134. [DOI] [PubMed] [Google Scholar]
- Ekborn A.; Laurell G.; Johnström P.; Wallin I.; Eksborg S.; Ehrsson H. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine infuences cisplatin pharmacokinetics. Hear. Res. 2002, 165, 53–61. 10.1016/S0378-5955(02)00277-0. [DOI] [PubMed] [Google Scholar]
- Reser D.; Rho M.; Dewan D.; Herbst L.; Li G.; Stupak H.; Zur K.; Romaine J.; Frenz D.; Goldbloom L.; Kopke R.; Arezzo J.; Van De Water T. L- and D-methionine provide equivalent long term protection against CDDP-induced ototoxicity in vivo, with partial in vitro and in vivo retention of antineoplastic activity. NeuroToxicology 1999, 20, 731–748. [PubMed] [Google Scholar]
- Li G.; Frenz D.; Brahmblatt S.; Feghali J.; Ruben R. J.; Berggren D.; Arezzo J.; Van De Water T. R. Round window membrane delivery of L-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. NeuroToxicology 2001, 22, 163–176. 10.1016/S0161-813X(00)00010-3. [DOI] [PubMed] [Google Scholar]
- Deegan P. M.; Pratt I. S.; Ryan M. P. The nephrotoxicity, cytotoxicity and renal handling of a cisplatin-methionine complex in male Wistar rats. Toxicology 1994, 89, 1–14. 10.1016/0300-483X(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Melvik J. E.; Pettersen E. O. Reduction of cis-dichlorodiammineplatinum-induced cell inactivation by methionine. Inorg. Chim. Acta 1987, 137, 115–118. 10.1016/S0020-1693(00)87128-5. [DOI] [Google Scholar]
- Cloven N. G.; Re A.; McHale M. T.; Burger R. A.; DiSaia P. J.; Rose G. S.; Campbell K. C. M.; Fan H. Evaluation of D-methionine as a cytoprotectant in cisplatin treatment of an animal model for ovarian cancer. Anticancer Res. 2000, 20, 4205–4210. [PubMed] [Google Scholar]
- Jones M. M.; Basinger M. A. Thiol and thioether suppression of cis-platin-induced nephrotoxicity in rats bearing the Walker 256 carcinoma. Anticancer Res. 1989, 9, 1937–1942. [PubMed] [Google Scholar]
- Laurell G.; Teixeira M.; Sterkers O.; Bagger-Sjöbäck D.; Eksborg S.; Lidman O.; Ferrary E. Local administration of antioxidants to the inner ear kinetics and distribution. Hear. Res. 2002, 173, 198–209. 10.1016/S0378-5955(02)00613-5. [DOI] [PubMed] [Google Scholar]
- Treskes M.; Nijtmans L. G. J.; Fichtinger-Schepman A. M. J.; van der Vijgh W. J. F. Effects of the modulating agent WR2721 and its main metabolites on the formation and stability of cisplatin-DNA adducts in vitro in comparison to the effects of thiosulphate and diethyldithiocarbamate. Biochem. Pharmacol. 1992, 43, 1013–1019. 10.1016/0006-2952(92)90607-K. [DOI] [PubMed] [Google Scholar]
- Marzatico F.; Porta C.; Moroni M.; Bertorelli L.; Borasio E.; Finotti N.; Pansarasa O.; Castagna L. In vitro antioxidant properties of amifostine (WR-2721, Ethyol). Cancer Chemother. Pharmacol. 2000, 45, 172–176. 10.1007/s002800050026. [DOI] [PubMed] [Google Scholar]
- Hyppolito M. A.; Oliveira A. A. d.; Lessa R. M.; Rossato M. Amifostine otoprotection to cisplatin ototoxicity: a guinea pig study using otoacoustic emission distortion products (DPOEA) and scanning electron microscopy. Rev. Bras. Otorrinolaringol. 2005, 71, 268–273. 10.1590/S0034-72992005000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach J. A.; Church M. W.; Blakley B. W.; McCaslin D. L.; Burgio D. L. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol.--Head Neck Surg. 1997, 117, 493–500. 10.1016/S0194-5998(97)70020-2. [DOI] [PubMed] [Google Scholar]
- Kim S.-J.; Park C.; Han A. L.; Youn M.-J.; Lee J.-H.; Kim Y.; Kim E.-S.; Kim H.-J.; Kim J.-K.; Lee H.-K.; Chung S.-Y.; So H.; Park R. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear. Res. 2009, 251, 70–82. 10.1016/j.heares.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Antony S.; Bayse C. A. Modeling the mechanism of the glutathione peroxidase mimic ebselen. Inorg. Chem. 2011, 50, 12075–12084. 10.1021/ic201603v. [DOI] [PubMed] [Google Scholar]
- Rybak L. P.; Whitworth C.; Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 1999, 109, 1740–1744. 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Lorito G.; Hatzopoulos S.; Laurell G.; Campbell K. C. M.; Petruccelli J.; Giordano P.; Kochanek K.; Sliwa L.; Martini A.; Skarzynski H. Dose-dependent protection on cisplatin-induced ototoxicity – an electrophysiological study on the effect of three antioxidants in the Sprague-Dawley rat animal model. Med. Sci. Monit. 2011, 17, BR179–186. 10.12659/MSM.881894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E. D.; Gu R.; Pierce C.; Kil J. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anti-Cancer Drugs 2005, 16, 569–579. 10.1097/00001813-200506000-00013. [DOI] [PubMed] [Google Scholar]
- Haake S. M.; Dinh C. T.; Chen S.; Eshraghi A. A.; Van De Water T. R. Dexamethasone protects auditory hair cells against TNFa-initiated apoptosis via activation of PI3K/Akt and NFjB signaling. Hear. Res. 2009, 255, 22–32. 10.1016/j.heares.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Palmer R. M.; Bridge L.; Foxwell N. A.; Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br. J. Pharmacol. 1992, 105, 11–12. 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.; Wang X.; Chen D.; Lin X.; Yu D.; Wu H. Dexamethasone loaded nanoparticles exert protective effects against cisplatin-induced hearing loss by systemic administration. Neurosci. Lett. 2016, 619, 142–148. 10.1016/j.neulet.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Özel H. E.; Özdoğan F.; Gürgen S. G.; Esen E.; Genç S.; Selçuk A. Comparison of the protective effects of intratympanic dexamethasone and methylprednisolone against cisplatin-induced ototoxicity. J. Laryngol. Otol. 2016, 130, 225–234. 10.1017/S0022215115003473. [DOI] [PubMed] [Google Scholar]
- Salehi P.; Akinpelu O. V.; Waissbluth S.; Peleva E.; Meehan B.; Rak J.; Daniel S. J. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol. Neurotol. 2014, 35, 1131–1139. 10.1097/MAO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- Paksoy M.; Ayduran E.; Sanli A.; Eken M.; Aydin S.; Oktay A. Z. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med. Oncol. 2011, 28, 615–621. 10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- Martín-Saldaña S.; Palao-Suay R.; Aguilar M. R.; Ramírez-Camacho R.; San Román J. Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017, 53, 199–210. 10.1016/j.actbio.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Fernandez R.; Harrop-Jones A.; Wang X.; Dellamary L.; LeBel C.; Piu F. The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against cisplatin-induced hearing loss. Audiol. Neuro-Otol. 2016, 21, 22–29. 10.1159/000441833. [DOI] [PubMed] [Google Scholar]
- Elimadi A.; Bouillot L.; Sapena R.; Tillement J.-P.; Morin D. Dose-related inversion of cinnarizine and flunarizine effects on mitochondrial permeability transition. Eur. J. Pharmacol. 1998, 348, 115–121. 10.1016/S0014-2999(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Kaur T.; Borse V.; Sheth S.; Sheehan K.; Ghosh S.; Tupal S.; Jajoo S.; Mukherjea D.; Rybak L. P.; Ramkumar V. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J. Neurosci. 2016, 36, 3962–3977. 10.1523/JNEUROSCI.3111-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S.; Mukherjea D.; Rybak L. P.; Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 2017, 11, 338. 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. C. M.; Fox D. J.. Cisplatin-Induced Hearing Loss. In Translational Research in Audiology, Neurotology, and the Hearing Sciences; Le Prell C., Lobarinas E., Popper A., Fay R., Eds.; Springer Handbook of Auditory Research; Springer: Cham, Switzerland, 2016; Vol. 58, pp 141–164. [Google Scholar]