Abstract
Importance
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by loss of upper and lower motor neurons. Although novel ALS genetic variants have been identified, the shared genetic risk between ALS and other neurodegenerative disorders remains poorly understood.
Objectives
To examine whether there are common genetic variants that determine the risk for ALS and other neurodegenerative diseases and to identify their functional pathways.
Design, Setting, and Participants
In this study conducted from December 1, 2016, to August 1, 2017, the genetic overlap between ALS, sporadic frontotemporal dementia (FTD), FTD with TDP-43 inclusions, Parkinson disease (PD), Alzheimer disease (AD), corticobasal degeneration (CBD), and progressive supranuclear palsy (PSP) were systematically investigated in 124 876 cases and controls. No participants were excluded from this study. Diagnoses were established using consensus criteria.
Main Outcomes and Measures
The primary outcomes were a list of novel loci and their functional pathways in ALS, FTD, PSP, and ALS mouse models.
Results
Among 124 876 cases and controls, genome-wide conjunction analyses of ALS, FTD, PD, AD, CBD, and PSP revealed significant genetic overlap between ALS and FTD at known ALS loci: rs13302855 and rs3849942 (nearest gene, C9orf72; P = .03 for rs13302855 and P = .005 for rs3849942) and rs4239633 (nearest gene, UNC13A; P = .03). Significant genetic overlap was also found between ALS and PSP at rs7224296, which tags the MAPT H1 haplotype (nearest gene, NSF; P = .045). Shared risk genes were enriched for pathways involving neuronal function and development. At a conditional FDR P < .05, 22 novel ALS polymorphisms were found, including rs538622 (nearest gene, ERGIC1; P = .03 for ALS and FTD), which modifies BNIP1 expression in human brains (35 of 137 females; mean age, 59 years; P = .001). BNIP1 expression was significantly reduced in spinal cord motor neurons from patients with ALS (4 controls: mean age, 60.5 years, mean [SE] value, 3984 [760.8] arbitrary units [AU]; 7 patients with ALS: mean age, 56 years, mean [SE] value, 1999 [274.1] AU; P = .02), in an ALS mouse model (mean [SE] value, 13.75 [0.09] AU for 2 SOD1 WT mice and 11.45 [0.03] AU for 2 SOD1 G93A mice; P = .002) and in brains of patients with PSP (80 controls: 39 females; mean age, 82 years, mean [SE] value, 6.8 [0.2] AU; 84 patients with PSP: 33 females, mean age 74 years, mean [SE] value, 6.8 [0.1] AU; β = –0.19; P = .009) or FTD (11 controls: 4 females; mean age, 67 years; mean [SE] value, 6.74 [0.05] AU; 17 patients with FTD: 10 females; mean age, 69 years; mean [SE] value, 6.53 [0.04] AU; P = .005).
Conclusions and Relevance
This study found novel genetic overlap between ALS and diseases of the FTD spectrum, that the MAPT H1 haplotype confers risk for ALS, and identified the mitophagy-associated, proapoptotic protein BNIP1 as an ALS risk gene. Together, these findings suggest that sporadic ALS may represent a selectively pleiotropic, polygenic disorder.
This genome-wide association study examines whether there are common genetic variants that determine risk for amyotrophic lateral sclerosis and other neurodegenerative diseases and identifies novel shared genetic variants and their functional pathways.
Key Points
Question
Are there genome-wide genetic risk factors for amyotrophic lateral sclerosis that are shared with other neurodegenerative diseases?
Findings
This study of combined genome-wide association data identified selective genetic overlap between amyotrophic lateral sclerosis and neurodegenerative diseases within the frontotemporal dementia spectrum.
Meaning
These findings identify common genetic pathways between amyotrophic lateral sclerosis and frontotemporal dementia and suggest that MAPT and BNIP1 influence the pathogenesis of amyotrophic lateral sclerosis.
Introduction
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are thought to represent a continuous disease spectrum.1 Clinically, ALS presents as progressive muscle wasting, hyperreflexia, and spasticity, whereas FTD is defined by cognitive and behavioral dysfunction. Between 40% and 50% of patients with ALS present with FTD-associated clinical phenotypes, including progressive aphasia, language impairment, and executive dysfunction.1 Neuropathologically, ALS is defined by the loss of upper and lower motor neurons and the formation of TDP-43, SOD1, and ubiquitin-positive inclusions within motor neurons. Frontotemporal dementia is defined by atrophy of the frontal and temporal lobes, and subtypes of FTD are distinguished by the types of inclusions in these regions (tau, FUS, TDP-43, and ubiquitin).2 Comparatively less is known about the shared pathobiology between ALS and other neurodegenerative diseases, such as Alzheimer disease (AD), corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), and Parkinson disease (PD).
Genetic factors offer insights into the molecular mechanisms underlying disease. Rare mutations in TDP43 (GenBank 3435) are associated with ALS and FTD.3,4,5 Genetic studies have revealed expansions of the hexanucleotide repeat within the noncoding promoter region of C9orf72 (GenBank 203228) as the cause of ALS and FTD.6,7 However, beyond C9orf72 and TDP43, the genetic overlap across sporadic forms of ALS, FTD, and other neurodegenerative diseases remains poorly understood.
One approach to assessing additional genetic risk among these diseases is to identify single-nucleotide polymorphisms (SNPs) that are jointly associated with multiple traits.8,9,10,11 Using previously validated methods, we investigated the genetic overlap between ALS, FTD, PSP, CBD, AD, and PD. We then used molecular and bioinformatics approaches to begin to define the role that these shared risk genes play in neurodegeneration.
Methods
Participant Samples
We evaluated summary statistics (P values and odds ratios) from genome-wide association studies (GWASs) for ALS, PD, AD, CBD, PSP, sporadic FTD, and autosomal dominant FTD with TDP-43 inclusions (eTable 1 in the Supplement). The GWASs were performed for individuals of European descent. Samples have been previously described in detail.12,13,14,15,16,17,18 The data set on ALS represents 31 independent cohorts of participants with ALS and control participants.14 Amyotrophic lateral sclerosis was diagnosed as probable or definite according to the 1994 El Escorial Criteria by neurologists specializing in motor neuron diseases.19 The data set on sporadic FTD included multiple subtypes within the FTD spectrum: behavioral variant FTD, semantic dementia, progressive nonfluent aphasia, and FTD overlapping with motor neuron disease. The relevant institutional review boards or ethics committees approved the research protocol of the individual GWASs used in the present analysis, and all human participants gave written informed consent. The Human Research Protection Program Institutional Review Board at University of California San Francisco waived consent for all participants. The Institutional Review Board determined that the use or disclosure of the information does not adversely affect the rights and welfare of the individuals and involves no more than a minimal risk to their privacy.
Statistical Analysis
Genetic Enrichment
We applied previously validated statistical methods to assess shared genetic risk and identify ALS susceptibility loci.9,10,20,21,22 We evaluated SNPs associated with increased risk for ALS and FTD, ALS and PD, ALS and AD, ALS and CBD, ALS and PSP, and ALS and FTD with TDP-43 inclusions. Using this approach, the genetic enrichment of phenotype A with phenotype B exists if the proportion of SNPs or genes associated with phenotype A increases as a function of the increased association with phenotype B. To evaluate enrichment, we constructed fold-enrichment and quantile-quantile plots of nominal –log10 P values for all ALS SNPs and for subsets of SNPs determined by the significance of their association with PD, AD, CBD, PSP, and FTD (sporadic FTD and FTD with TDP-43 inclusions) (eFigure 1 in the Supplement). Enrichment can be directly interpreted in terms of the true discovery rate, which is equal to 1 minus the false discovery rate (FDR) (eAppendix 1 in the Supplement).9,10,20,21,22 To minimize false positives, we used a 100-iteration random pruning with a linkage disequilibrium (LD) r2 < 0.2.21
Identification of Shared Risk Loci-Conjunction FDR
To identify specific loci jointly shared between ALS and PD, AD, CBD, PSP, or FTD (sporadic FTD and FTD with TDP-43 inclusions), we computed the conjunction FDR.8,9,20,21 The conjunction FDR is defined as the posterior probability that an SNP is null for either phenotype or for both simultaneously, given that the P values for both traits are as small, or smaller, than the P values for each trait individually (eAppendix 1 in the Supplement).8,9 We used an overall FDR threshold of P < .05 to indicate statistical significance. Manhattan plots were constructed based on the ranking of the conjunction FDR to illustrate the genomic location of the shared genetic risk loci.
Identification of Novel Risk Loci-Conditional FDR
To identify specific ALS susceptibility loci as a function of genetic variants associated with the 6 neurodegenerative disorders, we computed conditional FDRs.20,21 The conditional FDR is an extension of the standard FDR, which incorporates information from GWAS summary statistics of a second phenotype to adjust its significance level. The conditional FDR is defined as the probability that an SNP is null in the first phenotype given that the P values in the first and second phenotypes are as small as or smaller than the observed ones. Ranking SNPs by the standard FDR or by P values gives the same ordering of SNPs. In contrast, if the primary and secondary phenotypes are related genetically, the conditional FDR reorders SNPs and results in a different ranking than that based on P values alone. We used an overall FDR threshold of P < .05 to indicate statistical significance, which means 5 expected false discoveries per 100 reported. In addition, we constructed Manhattan plots based on the ranking of the conditional FDR to illustrate the genomic location. In all analyses, we controlled for the effects of genomic inflation by using intergenic SNPs (eAppendix 1 in the Supplement). Detailed information on the conditional FDR can be found in prior reports.20,21
Functional Evaluation of Shared Risk Loci
To determine whether the conjunction and conditional SNPs shared across ALS, PD, AD, CBD, PSP, and FTD (sporadic FTD and FTD with TDP-43 inclusions) modify gene expression, we evaluated cis–expression quantitative trait loci (eQTL) in a publicly available data set from neuropathologically confirmed control brains (UK Brain Expression Consortium, http://braineac.org/).23 To minimize multiple comparisons, we analyzed eQTL for the mean P value derived across the following brain regions: the cerebellum, frontal cortex, hippocampus, medulla, occipital cortex, putamen, substantia nigra, temporal cortex, thalamus, and white matter. To minimize false positives, we applied a Bonferroni-corrected P value of 1.5 × 10−3. To test for association between genotypes and gene expression, we used an analysis of covariance. We tested SNPs using an additive model in SAS (SAS Institute Inc). To evaluate cis-acting splicing quantitative trait loci (sQTL), we examined the associations of our shared risk SNPs with alternative splicing in control human brains.24 Each study reported genetic and expression data on brains from individuals of European descent.
Differential Expression of Shared Genetic Risk Variants in Tissues of Patients With ALS, PSP, FTD, AD, or PD
To determine whether shared risk genes identified by the conjunction FDR, the conditional FDR, and genes in cis-eQTL were differentially expressed in tissue from patients with ALS compared with controls, we analyzed the gene expression of the target genes in motor neurons isolated from 11 patients with ALS and controls (Gene Expression Omnibus [GEO] accession number GSE833).25 To validate the genes identified in GSE833, we examined expression data from a well-characterized mouse model.26 The RNA expression data were analyzed in nontransgenic SOD1 WT and SOD1 G93A mice at 75 and 110 days (GEO accession number GSE4390).27 SOD1 G93A mice are presymptomatic at 75 days and exhibit hindlimb paralysis at 110 days.26,27
To determine whether differentially expressed genes in ALS were altered across brains with neurodegenerative disease, we analyzed the gene expression of the target genes using publicly available data sets. Differential gene expression was analyzed from the following data sets: the temporal cortices from patients with PSP and control brains (Synapse ID No. syn6090802)28 and the frontal, hippocampus, and cerebellum from patients with FTD and controls (GEO accession number E13162).29 Each data set of control and disease tissue was obtained from individuals of European descent. All analyses were performed using analysis of covariance in SAS.
Gene Ontologic Features and Network-Based Functional Association Analyses
To identify enrichments in gene ontologic features associated with the ALS, PD, AD, CBD, PSP, and FTD (sporadic FTD and FTD with TDP-43 inclusions) shared risk genes identified by the conjunction FDR, the conditional FDR, and genes in cis-eQTL, we used the Consensus Path Database, which compares gene ontologic terms between background and candidate gene sets using the hypergeometric test and generates P values that are corrected for multiple testing using the FDR. Gene ontologic analyses were performed using the Consensus Path Database (Release 31; http://cpdb.molgen.mpg.de/).30,31 We used the default background gene set, which includes 18 043 genes. Biological, cellular, and molecular gene ontologic terms were included in a single analysis.
Results
Selective Shared Genetic Risk Between ALS, PD, AD, CBD, PSP, and FTD
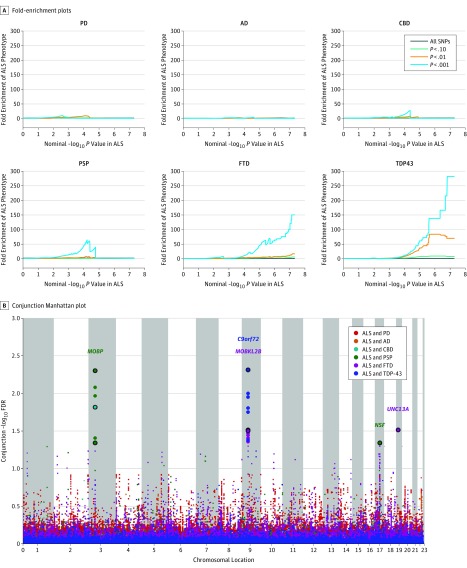
We identified enrichment in ALS SNPs across different levels of significance with FTD, PSP, and CBD (Figure 1). Applying progressively stringent P value thresholds for ALS SNPs (ie, increasing values of nominal –log10 P value), we found up to 300-fold enrichment using FTD with TDP-43 inclusions, 150-fold enrichment using FTD, 75-fold enrichment using PSP, and 25-fold enrichment using CBD (Figure 1). In contrast, we found minimal or no enrichment in ALS SNPs as a function of AD or PD (Figure 1).
Figure 1. Genetic Enrichment Across the Amyotrophic Lateral Sclerosis (ALS)–Frontotemporal Dementia (FTD) Spectrum.
A, Fold-enrichment plots. Graphs depict enrichment vs nominal −log10 P values (corrected for inflation) in amyotrophic lateral sclerosis (ALS) below the standard genome-wide association study threshold of P < 5 × 10−8 as a function of significance of association with Parkinson disease (PD), Alzheimer disease (AD), corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), frontotemporal dementia (FTD) (sporadic and FTD with TDP-43 inclusions [TDP43]) and at the level of −log10 P ≥ 0 corresponding to −log10 P ≤ 1, −log10 P ≥ 1 corresponding to P ≤ .10, and –log10 P ≥ 2 corresponding to P ≤ .01. B, Conjunction Manhattan plot showing shared risk loci. A plot of conjunction −log10 (false discovery rate [FDR]) values for ALS given PSP, CBD, TDP-43, and FTD. Single-nucleotide polymorphisms (SNPs) with conjunction −log10 FDR > 1.3 (ie, FDR P < .05) are shown as large points. A black line around the large points indicates the most significant SNP in each linkage disequilibrium block. This SNP was annotated with the nearest gene, which is listed above the symbols in each locus.
At a conjunction FDR P < .05, we identified 5 SNPs that were jointly associated with increased risk for ALS and FTD with TDP-43 inclusions, ALS and FTD, or ALS and PSP (Figure 1 and Table 1). These SNPs included the following: (1) rs9820623 (nearest gene, MOBP [GenBank 17433]; FDR ALS and PSP, P = 4.99 × 10−3); (2) rs13302855 (nearest gene, C9orf72; FDR ALS and FTD, P = 3.13 × 10−2); (3) rs3849942 (nearest gene, C9orf72; FDR ALS and FTD with TDP-43 inclusions, P = 4.88 × 10−3); (4) rs7224296 (nearest gene, NSF [GenBank 4905]; FDR ALS and PSP, P = 4.52 × 10−2); (5) rs4239633 (nearest gene, UNC13A [GenBank 23025]; FDR ALS and FTD, P = 3.05 × 10−2).
Table 1. Shared Risk SNPs Between ALS and FTD, PSP, CBD, TDP43, AD, and PD at a Conjunction FDR < 0.05.
| SNP | Chr | Nearest Gene | Associated Phenotype | Minimum Conjunction FDR | ALS P Value |
|---|---|---|---|---|---|
| rs9820623 | 3 | MOBP | PSP | 4.99 × 10−3 | 1.69 × 10−5 |
| rs13302855 | 9 | C9orf72 | FTD | 3.13 × 10−2 | 4.04 × 10−6 |
| rs3849942 | 9 | C9orf72 | TDP43 | 4.88 × 10−3 | 6.29 × 10−19 |
| rs7224296 | 17 | NSF | PSP | 4.54 × 10−2 | 5.90 × 10−4 |
| rs4239633 | 19 | UNC13A | FTD | 3.05 × 10−2 | 1.98 × 10−6 |
Abbreviations: AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; CBD, corticobasal degeneration; Chr, chromosome; FDR, false discovery rate; FTD, frontotemporal dementia; PD, Parkinson disease; PSP, progressive supranuclear palsy; SNP, single-nucleotide polymorphism; TDP43, FTD with TDP-43 inclusions.
Conditional FDR Analysis and Novel Risk Loci
Conditional FDR analysis revealed 29 additional risk loci at an FDR P < .05 (eFigure 1 and eTable 2 in the Supplement). Signals at rs3849943, rs10511816, and rs13302855 (nearest gene, C9orf72; rs3849943: FDR for ALS and FTD, P = 5.30 × 10−9, ALS P = 4.56 × 10−19; rs10511816: FDR for ALS and FTD, P = 4.97 × 10−9, ALS P = 6.08 × 10−11; rs13302855: FDR for ALS and FTD, P = 2.03 × 10−4; ALS P = 4.04 × 10−6); rs12608932 (nearest gene, UNC13A; FDR for ALS and FTD, P = 1.04 × 10−6; ALS P = 1.83 × 10−8); rs1768208 and rs13079368 (nearest gene, MOBP; rs1768208: FDR for ALS, FTD, and PSP, P = 6.89 × 10−3, ALS P = 4.04 × 10−5; rs13079368: FDR for ALS, FTD, and PSP, P = 1.99 × 10−3; ALS P = 4.11 × 10−5); and rs7813314 (nearest gene, BC045738 [GenBank 101927815]; FDR for ALS and FTD, P = 4.86 × 10−3; ALS P = 7.78 × 10−7) have been described previously to be associated with ALS.14 In addition, we identified 22 additional novel risk SNPs, including rs538622 (nearest gene, ERGIC1 [GenBank 57222]; FDR for ALS and FTD, P = 3.07 × 10−2; ALS P = 1.37 × 10−5; eTable 2 in the Supplement). Among these novel risk SNPs, we identified rs7224296 (nearest gene, NSF), which is located on chromosome 17 and occurs within the 1-megabase (Mb) inversion of the MAPT (GenBank 4137) haplotype.
eQTL and sQTL
To begin to define the functional effects of these shared risk SNPs, we evaluated cis-eQTL in human brains free of neuropathologic characteristics (Table 2). The SNP rs7224296 near NSF has been previously reported to tag the MAPT H1 haplotype.9 The MAPT H1 haplotype is associated with increased risk for FTD, PSP, CBD, AD, and PD.10,11,13,16,17,18 However, the most significant cis-eQTL with 24296 occurred with KIAA1267 (GenBank 284058) (also known as KANSL1) (Table 2). rs7224296 is in high LD with rs199533 (D′ = –0.97), which was previously reported to be associated with shared risk for PSP, CBD, and FTD.9 Consistent with previous findings for rs199533,9 rs7224296 is significantly associated with the altered expression of exon 3 within the MAPT gene (Table 2). MAPT H1 is associated with decreased expression of messenger RNA transcripts containing exons 2 and 3, which results in the 2N tau protein.32 Together, these findings point to an association between the MAPT H1 haplotype and the risk for ALS.
Table 2. eQTL That Reveal Functional Effects of Shared Risk SNPs in a Human Brain Tissue (UK Brain Expression Consortium).
| SNP | Chr | Nearest Gene | eQTL | |
|---|---|---|---|---|
| P Value | Gene | |||
| rs9820623 | 3 | MOBP | 4.40 × 10−3 | SCN11A |
| rs13302855 | 9 | C9orf72 | 1.30 × 10−2 | LRRC19 |
| rs3849942 | 9 | C9orf72 | 4.80 × 10−3 | MOBKL2B |
| rs7224296 | 17 | NSF | 3.30 × 10−18 | KIAA1267 |
| 6.5 × 10−5 | MAPT | |||
| 6.9 × 10−11 | MAPT exon 3 | |||
| rs4239633 | 19 | UNC13A | 1.00 × 10−3 | ELL |
Abbreviations: Chr, chromosome; eQTL, cis-expression quantitative trait loci; SNP, single-nucleotide polymorphism.
We also identified 2 SNPs sharing genetic overlap between ALS and FTD or ALS and FTD with TDP-43 inclusions near C9orf72: rs13302855 and rs3849942 (Table 1). These SNPs are not in LD (r2 < 0.02). The SNP rs13302855 produced distinct eQTL with LRRC19 (GenBank 64922), and rs3849942 produced distinct eQTL with MOBKL2B (GenBank 79817) (Table 2). Thus, our findings suggest that there are 2 independent signals within the C9orf72 locus that confer risk. In addition to cis-eQTL, we examined the association of shared risk SNPs with sQTL. We found that rs2282241 (nearest gene, C9orf72; conditional FDR ALS and FTD with TDP-43 inclusions, P = 3.68 × 10−5; ALS P = 1.55 × 10−7) was significantly associated with alternative splicing of the C9orf72 gene (alternative splicing ID, HsaINT0025532; FDR P = 1.08 × 10−3), specifically intron retention. The sQTL SNP rs2282241 is in high LD with rs3849942 (D′ = 0.99; Table 1).
Among the novel ALS risk SNPs identified by conditional FDR analysis, we identified cis-eQTL in human brains (eTable 3 in the Supplement). Most ALS risk SNPs produced cis-eQTL with genes within the associated locus but not with the nearest named gene. We found that rs538622, which is associated with ALS and FTD and falls near the ERGIC1 gene, is significantly associated with BNIP1 (GenBank 662) such that the minor allele (G) is associated with the lower expression of BNIP1 in human brains (P = 1.1 × 10−3).
Attenuation of Genetic Enrichment After Removing C9orf72 and MAPT
We identified several SNPs in C9orf72 (on chromosome 9) and in LD with MAPT (on chromosome 17), suggesting that variants associated with C9orf72 and MAPT were critical in driving our enrichment results. To test this hypothesis, we repeated our enrichment analysis after removing all SNPs in LD with r2 > 0.2 within 1 Mb of C9orf72 and MAPT variants (based on 1000 Genomes Project33 LD structure). After removing C9orf72 and MAPT SNPs, we observed considerable attenuation of genetic enrichment in ALS as a function of FTD with TDP-43 inclusions (eFigure 2 in the Supplement). However, we still found robust enrichment between ALS and PSP (100-fold enrichment) and sporadic FTD (800-fold enrichment; eFigure 2 in the Supplement), suggesting that the observed overlap between ALS and FTD was not driven by the C9orf72 and MAPT regions.
Shared Genetic Risk Genes Reveal Dysregulation of Neuronal Networks
To determine whether the shared risk genes fall within common biological pathways, we used bioinformatics approaches to identify common pathways. Because most risk SNPs occur within intergenic regions, we used 2 approaches to associate a risk SNP with a gene: genes nearest the SNPs and genes producing eQTL with the SNPs. Pathway analysis reveals that shared risk genes, from conjunction and conditional analyses, fall within pathways directly involved in neuronal function: axon guidance, myelin sheath, synaptic vesicle pathways, neuronal action potential, and regulation of postsynaptic membrane potential, among others (Table 3; eTables 4 and 5 in the Supplement).
Table 3. Gene-Based Analysis of Shared Risk Genesa.
| GOID | GO Term | FDR |
|---|---|---|
| GO:0017075 | Syntaxin-1 binding | 2.59 × 10−6 |
| GO:0000149 | SNARE binding | 2.78 × 10−4 |
| GO:0048278 | Vesicle docking | 3.35 × 10−4 |
| GO:0034706 | Sodium channel complex | 1.46 × 10−3 |
| GO:0051648 | Vesicle localization | 2.17 × 10−3 |
| GO:0043209 | Myelin sheath | 2.28 × 10−3 |
| GO:0006887 | Exocytosis | 3.22 × 10−3 |
| GO:0016050 | Vesicle organization | 3.22 × 10−3 |
| GO:0051046 | Regulation of secretion | 3.90 × 10−3 |
| GO:0001518 | Voltage-gated sodium channel complex | 4.07 × 10−3 |
| GO:0015629 | Actin cytoskeleton | 5.45 × 10−3 |
| GO:0014854 | Response to inactivity | 5.65 × 10−3 |
| GO:0016684 | Oxidoreductase activity | 6.76 × 10−3 |
| GO:0019226 | Transmission of nerve impulse | 9.05 × 10−3 |
| GO:0051656 | Establishment of organelle localization | 0.01 |
| GO:0051640 | Organelle localization | 0.01 |
| GO:0004601 | Peroxidase activity | 0.01 |
| GO:0043169 | Cation binding | 0.01 |
| GO:0043198 | Dendritic shaft | 0.01 |
| GO:1903561 | Extracellular vesicle | 0.02 |
| GO:0005911 | Cell-cell junction | 0.02 |
| GO:0030055 | Cell-substrate junction | 0.02 |
| GO:0042744 | Hydrogen peroxide catabolic process | 0.02 |
| GO:0043005 | Neuron projection | 0.02 |
| GO:0046872 | Metal ion binding | 0.02 |
| GO:0050678 | Regulation of epithelial cell proliferation | 0.03 |
| GO:0048705 | Skeletal system morphogenesis | 0.03 |
| GO:0070161 | Anchoring junction | 0.03 |
| GO:0005925 | Focal adhesion | 0.03 |
| GO:0051174 | Regulation of phosphorus metabolic process | 0.03 |
| GO:0042743 | Hydrogen peroxide metabolic process | 0.04 |
| GO:0086010 | Membrane depolarization during action potential | 0.04 |
| GO:0019228 | Neuronal action potential | 0.04 |
| GO:0030424 | Axon | 0.05 |
Abbreviations: FDR, false discovery rate; GO, Gene Ontology; GOID, Gene Ontology Identifier; SNARE, soluble N-ethylmaleimide sensitive fusion attachment protein receptor.
Shared risk genes include genes nearest the single-nucleotide polymorphism and genes producing a cis-expression quantitative trait loci with the single-nucleotide polymorphism in conjunction and conditional FDR analyses.
Differential Expression of Shared Risk Genes in Tissues of Patients With ALS, FTD, PSP, AD, or PD
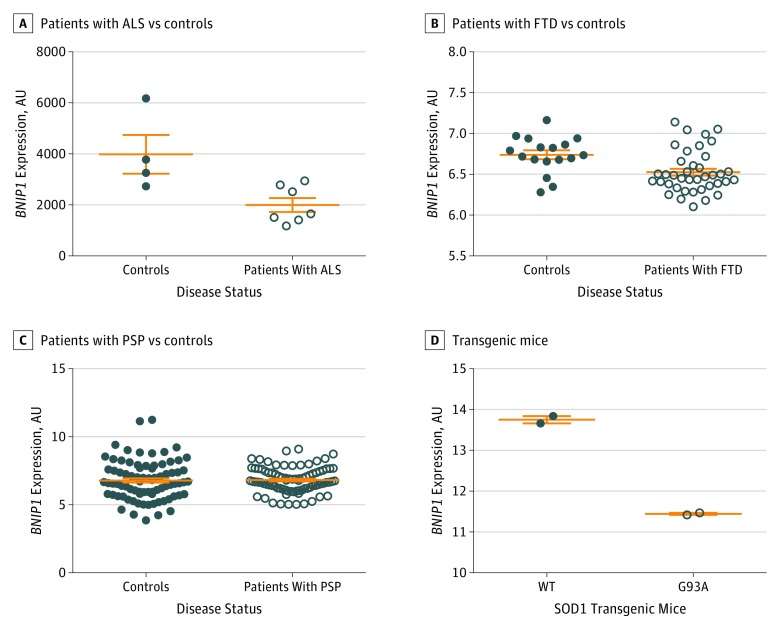
We next sought to determine whether the risk genes shared across ALS, FTD, PSP, and CBD were differentially expressed in disease tissues. To make this determination, we assessed the differential expression of the genes nearest the top SNP from conditional and conjunction FDR analyses and of the genes that produced the strongest eQTL in our functional analyses in motor neurons isolated from spinal cords of patients with ALS and controls (genes included in the analysis were taken from Tables 1 and 2 and from eTables 2 and 3 in the Supplement).25 Only 15 genes fitting these criteria were present in the ALS data set: BNIP1, C20orf24 (GenBank 55969), CAT (GenBank 847), CD59 (GenBank 966), ELL (GenBank 8178), GPX3 (GenBank 2878), HTRA2 (GenBank 27429), MOBP, MAPT, NFASC (GenBank 114), NSF, SCN5A (GenBank 6331), TEK (GenBank 7010), TNFAIP1 (GenBank 7126), and TNIP1 (GenBank 10318). We found that BNIP1 was significantly lower in motor neurons isolated from patients with ALS compared with controls (Figure 2A; eTable 6 in the Supplement). MAPT and MOBP were not differentially expressed in the motor neurons in patients with ALS and controls (eTable 6 in the Supplement).
Figure 2. Reduced BNIP1 Expression in Neurodegenerative Tissue.
A, Differential expression in motor neurons isolated from patients with amyotrophic lateral sclerosis (ALS) (GSE833 [Gene Expression Omnibus accession number]; mean [SEM] value, 3984 [760.8] arbitrary units [AU] for 4 controls and 1999 [274.1] AU for 7 patients with ALS; P = .02). B, Differential expression in homogenates from brains of patients with frontotemporal dementia (FTD) (GSE13162; mean [SEM] value, 6.7 [0.05] AU for 11 controls and 6.5 [0.04] AU for 17 patients with FTD; P = .005). C, Differential expression in homogenates from brains of patients with progressive supranuclear palsy (PSP) (syn6090802; mean [SEM] value, 6.8 [0.2] AU for 80 controls and 6.8 [0.1] AU for 84 patients with PSP; P = .009). D, Differential expression in homogenates from spinal cords of SOD1 WT and SOD1 G93A transgenic mice (GSE4390; mean [SEM] value, 13.8 [0.09] AU for 2 SOD1 WT mice and 11.5 [0.025] AU for 2 SOD1 G93A mice; P = .002).
Given the genetic overlap, we next examined BNIP1 expression in the brains of patients with FTD and PSP. Compared with controls, the BNIP1 expression was significantly reduced in the brains of patients with a neuropathologic diagnosis of FTD and PSP (Figure 2B and C). MAPT expression was not significantly altered in the brains of patients with FTD or PSP relative to controls (eTable 6 in the Supplement).
To further assess whether BNIP1 expression is associated with ALS pathologic characteristics, we examined BNIP1 expression in the spinal cords from a transgenic mouse model of ALS.26,27 BNIP1 expression was significantly reduced in the spinal cord of SOD1 G93A mice compared with SOD1 WT mice (Figure 2G). Thus, BNIP1 expression is associated with ALS pathologic characteristics.
Discussion
Using summary statistics from large GWASs (124 876 individuals) and established genetic methods, we investigated the genetic overlap between ALS, FTD (sporadic FTD and FTD with TDP-43 inclusions), PD, AD, CBD, and PSP. At a conjunction FDR of P < .05, we identified up to 300-fold enrichment in genetic risk for ALS across different levels of significance for FTD and PSP. Conjunction FDR analyses revealed shared loci previously associated with ALS risk as well as several loci not previously implicated in disease risk but that point to genetic drivers of neuronal function and mitophagy. Using this approach, we report novel genetic overlap between ALS and diseases of the FTD spectrum within the MAPT H1 haplotype.
We observed multiple signals within chromosome 9 that were associated with risk between ALS and FTD (the cohort defined by TDP-43 pathologic characteristics) and ALS and PSP. Among these, rs3849942 is associated with C9orf72 repeat expansions that cause ALS and is used as a surrogate marker for the C9orf72 expansion haplotype.34,35,36 Consistent with these reports, our sQTL findings suggest that SNPs in LD with rs3849942 modify C9orf72 splicing. Thus, it is likely that C9orf72 expansion carriers are present in multiple data sets and are driving some of the association. However, given that rs3849942 is not in LD with a second SNP near C9orf72, 302855, we may be detecting an independent signal on chromosome 9 that is associated with the risk for ALS, FTD, and PSP.
Mutations in MAPT cause autosomal dominant forms of FTD.37 Among FTD, PSP, and CBD, common variants in MAPT that tag the H1 haplotype represent the strongest genetic predictor of disease.10,11,13,16,17,18 In addition, the MAPT H1 haplotype has been associated with PD and AD.10,11,13,16,17,18 The MAPT H1 haplotype has recently been implicated in ALS risk in a meta-analysis of publications on neurodegenerative disease.38 The risk SNP tagging the MAPT H1 haplotype, rs7224296, is associated with altered splicing of MAPT of exon 3, which, together with exon 2, encodes 2N-containing transcripts. Although the role that individual MAPT transcripts play in normal physiology and disease remains poorly understood, a recent study of human-induced pluripotent stem cell–derived neurons from MAPT haplotype carriers suggests that the H1 haplotype influences axonal transport velocities.39 In ALS, these potential gene-induced deficits in axonal transport could alter disease onset and/or progression.
Conditional FDR analyses offer the opportunity to begin to reveal novel ALS risk loci. Using this approach, we identified 29 SNPs at a conditional FDR of P < .05. Within chromosome 5, we identified a risk locus at rs538622 (nearest gene, ERGIC1) that produced a significant eQTL in human brains with BNIP1. BNIP1 is a proapoptotic protein (Bcl-2 family member) involved in the regulation of endoplasmic reticulum structure and mitophagy.40,41 BNIP1 is highly expressed in neurons (eFigure 3 in the Supplement). More important, we demonstrate that BNIP1 is significantly lower in central nervous system tissues from patients with ALS, FTD, and PSP. Because neuronal cell loss is a hallmark feature of neurodegenerative disease and because BNIP1 is a neuronally expressed gene, we find that BNIP1 is specifically reduced in both motor neurons isolated from the spinal cords of patients with ALS compared with the motor neurons from matched controls. BNIP1 plays a critical role in mitophagy, a homeostatic mechanism for the selective degradation of damaged mitochondria.42 Depletion of BNIP1 in a cell model results in the disintegration of the endoplasmic reticulum network.41 Given that endoplasmic reticulum stress and mitochondrial dysfunction have been implicated in ALS at the genetic, molecular, and cellular levels,43 BNIP1 may represent an important driver of pathologic characteristics.44,45,46 Altered endoplasmic reticulum stress and mitochondrial dysfunction have been implicated in PSP and FTD.47,48,49,50,51,52 The MAPT H1 haplotype has been shown to alter the axonal transport velocities of mitochondria, providing a biological connection to 2 of our most interesting genetic signals.39
Functionally, beyond mitophagy, we observed enrichment in shared risk genes occurring in pathways involved in neuronal health and maintenance. This finding, taken together with the relative lack of enrichment of genes in ALS, AD, and PD, points to the important role that genes involved in neuronal heath and function play in driving ALS, FTD, and PSP. Together, this study provides genetic, molecular, and functional insights into the effects of risk variants shared across the ALS-FTD spectrum.
Limitations
Beyond BNIP1, by leveraging statistical power from large neurodegenerative GWASs, we identified numerous novel ALS genetic variants. Although these SNPs warrant replication in an independent cohort, our findings suggest that sporadic ALS may represent a polygenic disorder characterized by numerous genetic variants, each of which has a small association with disease risk. Although no single common variant may be informative clinically, the additive combination of risk variants may help identify individuals who are at greatest genetic risk for ALS. The GWASs used in these analyses were performed for participants of European decent; thus, our findings of the genetic architecture of ALS and FTD spectrum disorders may be biased for individuals of European decent. Future studies conducted in large non-European populations will be critical for gaining a more complete understanding of the genetic architecture underlying ALS and FTD spectrum disorders.
Conclusions
By integrating GWAS data with gene expression data from neurodegenerative disease and transgenic mouse models, our multimodal findings implicate the MAPT H1 haplotype in ALS and BNIP1 in the ALS-FTD spectrum. Additional work will be required to understand the role that tau plays in ALS and the relationship between BNIP1, mitophagy, and neurodegenerative diseases.
eTable 1. Summary Data From GWAS Used in the Current Study
eTable 2. ALS Risk SNPs Conditional on FTD, PSP, CBD, TDP43 at a Conditional FDR <0.05
eTable 3. eQTLs Reveal Functional Effects of Novel ALS Risk SNPs (From Conditional FDR Analysis) in a Human Brain Tissue (UKBEC)
eTable 4. Gene-Based Analysis of Genes From Conjunction and Conditional FDR Analysis
eTable 5. Pathway Analysis of Shared Risk Genes From Conjunction and Conditional FDR Analyses
eTable 6. Differential Expression of Shared ALS-FTD Risk Genes in Diseased Central Nervous System Tissues
eFigure 1. Conditional Manhattan Plots Reveal Novel Risk Loci
eFigure 2. Genetic Enrichment Between ALS, Sporadic FTD, and FTD-TDP43 After Controlling for LD in chr 9 and chr 17
eFigure 3. Cell-Type Specific Expression of BNIP1
eAppendix 1. Methods
eAppendix 2. Acknowledgments
References
- 1.Ferrari R, Kapogiannis D, Huey ED, Momeni P. FTD and ALS: a tale of two diseases. Curr Alzheimer Res. 2011;8(3):273-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cairns NJ, Bigio EH, Mackenzie IRA, et al. ; Consortium for Frontotemporal Lobar Degeneration . Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gitcho MA, Bigio EH, Mishra M, et al. TARDBP 3′-UTR variant in autopsy-confirmed frontotemporal lobar degeneration with TDP-43 proteinopathy. Acta Neuropathol. 2009;118(5):633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. [DOI] [PubMed] [Google Scholar]
- 5.Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17(23):3631-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renton AE, Majounie E, Waite A, et al. ; ITALSGEN Consortium . A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21–linked ALS-FTD. Neuron. 2011;72(2):257-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p–linked FTD and ALS. Neuron. 2011;72(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama JS, Wang Y, Schork AJ, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Association between genetic traits for immune-mediated diseases and Alzheimer disease. JAMA Neurol. 2016;73(6):691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama JS, Karch CM, Fan CC, et al. ; International FTD-Genomics Consortium (IFGC) . Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol. 2017;133(5):825-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desikan RS, Schork AJ, Wang Y, et al. ; ADNI, ADGC, GERAD, CHARGE and IPDGC Investigators . Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry. 2015;20(12):1588-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari R, Wang Y, Vandrovcova J, et al. ; International FTD-Genomics Consortium (IFGC); International Parkinson’s Disease Genomics Consortium (IPDGC); International Genomics of Alzheimer’s Project (IGAP) . Genetic architecture of sporadic frontotemporal dementia and overlap with Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurg Psychiatry. 2017;88(2):152-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalls MA, Plagnol V, Hernandez DG, et al. ; International Parkinson Disease Genomics Consortium . Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rheenen W, Shatunov A, Dekker AM, et al. ; PARALS Registry; SLALOM Group; SLAP Registry; FALS Sequencing Consortium; SLAGEN Consortium; NNIPPS Study Group . Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat Genet. 2016;48(9):1043-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Deerlin VM, Sleiman PM, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42(3):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouri N, Ross OA, Dombroski B, et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015;6:7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höglinger GU, Melhem NM, Dickson DW, et al. ; PSP Genetics Study Group . Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43(7):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari R, Hernandez DG, Nalls MA, et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13(7):686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(suppl):96-107. [DOI] [PubMed] [Google Scholar]
- 20.Andreassen OA, Djurovic S, Thompson WK, et al. ; International Consortium for Blood Pressure GWAS; Diabetes Genetics Replication and Meta-analysis Consortium; Psychiatric Genomics Consortium Schizophrenia Working Group . Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92(2):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreassen OA, Thompson WK, Schork AJ, et al. ; Psychiatric Genomics Consortium (PGC); Bipolar Disorder and Schizophrenia Working Groups . Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desikan RS, Schork AJ, Wang Y, et al. ; Inflammation working group, IGAP and DemGene Investigators . Polygenic overlap between C-reactive protein, plasma lipids, and Alzheimer disease. Circulation. 2015;131(23):2061-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramasamy A, Trabzuni D, Guelfi S, et al. ; UK Brain Expression Consortium; North American Brain Expression Consortium . Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17(10):1418-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takata A, Matsumoto N, Kato T. Genome-wide identification of splicing QTLs in the human brain and their enrichment among schizophrenia-associated loci. Nat Commun. 2017;8:14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dangond F, Hwang D, Camelo S, et al. Molecular signature of late-stage human ALS revealed by expression profiling of postmortem spinal cord gray matter. Physiol Genomics. 2004;16(2):229-239. [DOI] [PubMed] [Google Scholar]
- 26.Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR. Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci U S A. 2009;106(19):7774-7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukas TJ, Luo WW, Mao H, Cole N, Siddique T. Informatics-assisted protein profiling in a transgenic mouse model of amyotrophic lateral sclerosis. Mol Cell Proteomics. 2006;5(7):1233-1244. [DOI] [PubMed] [Google Scholar]
- 28.Allen M, Carrasquillo MM, Funk C, et al. Human whole genome genotype and transcriptome data for Alzheimer’s and other neurodegenerative diseases. Sci Data. 2016;3:160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen-Plotkin AS, Geser F, Plotkin JB, et al. Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet. 2008;17(10):1349-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamburov A, Pentchev K, Galicka H, Wierling C, Lehrach H, Herwig R. ConsensusPathDB: toward a more complete picture of cell biology. Nucleic Acids Res. 2011;39(Database issue):D712-D717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamburov A, Stelzl U, Lehrach H, Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013;41(Database issue):D793-D800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trabzuni D, Wray S, Vandrovcova J, et al. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012;21(18):4094-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones AR, Woollacott I, Shatunov A, et al. Residual association at C9orf72 suggests an alternative amyotrophic lateral sclerosis-causing hexanucleotide repeat. Neurobiol Aging. 2013;34(9):2234.e1-2234.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobson-Stone C, Hallupp M, Bartley L, et al. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology. 2012;79(10):995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng ASL, Tan EK. Intermediate C9orf72 alleles in neurological disorders: does size really matter? J Med Genet. 2017;54(9):591-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702-705. [DOI] [PubMed] [Google Scholar]
- 38.Zhang CC, Zhu JX, Wan Y, et al. Meta-analysis of the association between variants in MAPT and neurodegenerative diseases. Oncotarget. 2017;8(27):44994-45007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beevers JE, Lai MC, Collins E, et al. MAPT genetic variation and neuronal maturity alter isoform expression affecting axonal transport in iPSC-derived dopamine neurons. Stem Cell Reports. 2017;9(2):587-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang F, Wang B, Li N, et al. RNF185, a novel mitochondrial ubiquitin E3 ligase, regulates autophagy through interaction with BNIP1. PLoS One. 2011;6(9):e24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima K, Hirose H, Taniguchi M, et al. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23(16):3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci U S A. 2014;111(42):E4439-E4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirulli ET, Lasseigne BN, Petrovski S, et al. ; FALS Sequencing Consortium . Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freischmidt A, Wieland T, Richter B, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18(5):631-636. [DOI] [PubMed] [Google Scholar]
- 47.Ehrlich M, Hallmann AL, Reinhardt P, et al. Distinct neurodegenerative changes in an induced pluripotent stem cell model of frontotemporal dementia linked to mutant tau protein. Stem Cell Reports. 2015;5(1):83-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallmann AL, Araúzo-Bravo MJ, Mavrommatis L, et al. Astrocyte pathology in a human neural stem cell model of frontotemporal dementia caused by mutant tau protein. Sci Rep. 2017;7:42991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoica R, Paillusson S, Gomez-Suaga P, et al. ALS/FTD-associated FUS activates GSK-3β to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO Rep. 2016;17(9):1326-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrari R, Forabosco P, Vandrovcova J, et al. ; UK Brain Expression Consortium (UKBEC) . Frontotemporal dementia: insights into the biological underpinnings of disease through gene co-expression network analysis. Mol Neurodegener. 2016;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79(3):416-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stutzbach LD, Xie SX, Naj AC, et al. ; PSP Genetics Study Group . The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary Data From GWAS Used in the Current Study
eTable 2. ALS Risk SNPs Conditional on FTD, PSP, CBD, TDP43 at a Conditional FDR <0.05
eTable 3. eQTLs Reveal Functional Effects of Novel ALS Risk SNPs (From Conditional FDR Analysis) in a Human Brain Tissue (UKBEC)
eTable 4. Gene-Based Analysis of Genes From Conjunction and Conditional FDR Analysis
eTable 5. Pathway Analysis of Shared Risk Genes From Conjunction and Conditional FDR Analyses
eTable 6. Differential Expression of Shared ALS-FTD Risk Genes in Diseased Central Nervous System Tissues
eFigure 1. Conditional Manhattan Plots Reveal Novel Risk Loci
eFigure 2. Genetic Enrichment Between ALS, Sporadic FTD, and FTD-TDP43 After Controlling for LD in chr 9 and chr 17
eFigure 3. Cell-Type Specific Expression of BNIP1
eAppendix 1. Methods
eAppendix 2. Acknowledgments