Abstract
Background
Perioperative vedolizumab (VDZ) and anti-tumor necrosis factor (TNFi) therapies are implicated in causing postoperative complications in inflammatory bowel disease (IBD).
Aim
To compare the risk of surgical site infections (SSIs) between VDZ- and TNFi-treated IBD patients in propensity-matched cohorts.
Methods
The Optum® Research Database was used to identify IBD patients who received VDZ or TNFi within 30 days prior to abdominal surgery between January 2015 and December 2016. The date of IBD-related abdominal surgery was defined as the index date. SSIs were determined by ICD-9/10 and CPT codes related to superficial wound infections or deep organ space infections after surgery. Propensity score 1:1 matching established comparable cohorts based on VDZ or TNFi exposure before surgery based on evidence-based risk-modifiers.
Results
The propensity-matched sample included 186 patients who received preoperative biologic therapy (VDZ, n=94; TNFi, n=92). VDZ and TNFi cohorts were similar based on age, gender, IBD type, concomitant immunomodulator exposure, chronic opioid or corticosteroid therapy, Charlson Comorbidity Index, and malnutrition. VDZ patients were more likely to undergo an open bowel resection with ostomy. After propensity score matching, there was no significant difference in postoperative SSIs (TNFi 12.0% vs. VDZ 14.9%, P=0.56). Multivariable analysis indicated that malnutrition was the sole risk factor for developing SSI (OR 3.1, 95% CI 1.11, 8.71) regardless of the type of biologic exposure.
Conclusion
In the largest, risk-adjusted cohort analysis to date, perioperative exposure to VDZ therapy was not associated with a significantly higher risk of developing an SSI compared to TNFi therapy.
Keywords: Inflammatory bowel disease, postoperative complications, vedolizumab, biologic
INTRODUCTION
Anti-tumor necrosis factor-α (TNFi) agents have traditionally been the biologic therapies of choice for moderate-to-severe inflammatory bowel diseases (IBD), consisting of ulcerative colitis (UC) and Crohn’s disease (CD). While the use of TNFi has effectively improved health outcomes such as corticosteroid dependency in patients with IBD, the risk of IBD-related abdominal surgery remains high in patients with medically refractory disease and those who lose their response to TNFi agents.1 Since the natural history of IBD is that of relapse and remittance, a significant proportion of patients with IBD rely on complementary or curative surgery as a cornerstone treatment option for CD or UC.2,3,4,5
Considering the need to optimize surgical outcomes in select patients, decisions about preoperative pharmacotherapy require judicious medical care and evidence-based recommendations. Although there is no clear consensus6 regarding TNFi exposure prior to major abdominal surgery,7 research supports the relationship between preoperative TNFi use and increased risk for surgical complications.8,9,10
Similar to TNFi, preoperative use of vedolizumab (VDZ) has more recently been implicated as a significant predictor of worse postoperative outcomes, including surgical site infections (SSI).11,12,13,14,15,16,17 VDZ is a fully humanized monoclonal antagonist targeting α4-β7 integrin receptors of the gastrointestinal tract.18,19 It received FDA-approval in May 2014 for moderate-to-severe UC and CD.20,21,22 Considering VDZ’s gut selectivity, the safety profile has been shown to be favorable.23 One study using single-center data showed that VDZ exposure was not associated with postoperative complications,24 including a recent meta-analysis.25 However, since VDZ’s mechanism of action blocks leukocyte migration into the intestinal lining,26 wound and anastomotic healing after IBD-related surgery remains a potential concern.27 If differences in mechanism of action for various biologics affect postoperative outcomes in IBD, methods to optimize pharmacotherapy options and surgical strategies remain a major area for future study.
Patients with IBD are at higher risk for infectious postoperative complications because of their underlying disease, malnutrition, and immunosuppression. SSIs include superficial wound infections, deep wound infections affecting the fascia, and organ space infections including abdominal and pelvic abscesses and anastomotic leaks28. The sequelae of these infections can be quite severe. In the short term, patients may develop life-threatening sepsis, require reoperations29 or procedures for abscess drainage,30,31 suffer from prolonged ileus or prolonged hospital stay, and require hospital readmission.32 Long-term effects include the need for an ostomy, development of hernias,33 and poor quality of life due to poor bowel function.34,35
Considering the devastating consequences of SSIs in this population, judicious and evidence-based selection of pharmacotherapy for patients at high risk for surgical intervention is critical. Given the clinical equipoise from published studies and the mechanistic concerns for postoperative SSI after VDZ exposure, we aimed to compare the risk of postoperative SSI between VDZ- and TNFi-treated patients in propensity-matched cohorts using a population-based dataset.
METHODS
Data Source
We performed a longitudinal retrospective cohort analysis of IBD patients in the Optum Research Database from years 2015 through 2016. The Optum database contains de-identified inpatient, outpatient, and pharmaceutical claims data as well as laboratory data from approximately 12–14 million privately insured patients per year across 50 states. The database includes demographic and socioeconomic characteristics (e.g., age, sex, geographic region, race, household income), encounter data (i.e., hospital admissions, outpatient visits, and associated procedures), pharmaceutical data (i.e., filled pharmaceutical claims, days supply, dose dispensed, strength, administration method), financial data (i.e., total cost, copayment, deductibles), and lab results (e.g., test description, result number and unit). The Stanford University Institutional Review Board (IRB) determined this study does not meet the definition of human subject research as defined in federal regulations 45 CFR 46.102. The study was therefore exempt from IRB review.
Cohort Identification and Classification
We selected a cohort of patients with: (1) at least one IBD-related inpatient or outpatient visit between 2015 and 2016, (2) at least one claim for VDZ or TNFi in 2015 or 2016 within 30 days preceding an abdominal surgery, and (3) continuous enrollment in Optum for at least six months preceding surgery. To classify each patient as CD, UC, or indeterminate colitis (IC), the total number of distinct encounters including CD and UC International Classification of Disease (ICD-9 and ICD-10) codes were summed (555.xx and K50.xx for Crohn’s disease, 556.xx and K51.xx for ulcerative colitis). Patients were classified as CD or UC if at least 80% of the ICD codes were 555.xx/K50.xx or 556.xx/K51.xx, respectively. The patient was classified as having IC if neither of the CD nor UC conditions were fulfilled. Patients were classified as receiving either VDZ or TNFi based on outpatient pharmaceutical claims or Healthcare Common Procedure Coding System (HCPCS) J-codes. TNFi included infliximab, adalimumab, certolizumab, and golimumab. Patients in the VDZ group and TNFi group were excluded if they had additionally taken a TNFi or VDZ, respectively, in the thirty days preceding surgery (3.4% for VDZ group and none for TNFi group). Surgical procedures were characterized as either laparoscopic or open, and by whether a primary bowel anastomosis or end ostomy was performed. Charlson comorbidity score was calculated using inpatient and outpatient claims from six months preceding surgery.36 Use of corticosteroids was identified by at least three corticosteroid refills covering at least 100 days in 2016.
Outcomes
The primary outcome of interest was the occurrence of postoperative SSI, including superficial wound infections and deep organ space infections, within 30 days after abdominal surgery (CPT codes are included in Supplementary Table 1). The primary predictor of interest was VDZ infusion within 30 days before surgery compared to TNFi prescription within 30 days before surgery. This 30 day preoperative window defining “drug exposure” is consistent with the published pharmacokinetics of VDZ.37 Clinically, this time frame is consistent with patients’ real-world TNFi or VDZ maintenance dosing frequency (every four weeks) if they were not responding to biologic therapy prior to surgery.
Propensity Score Matching
As patients prescribed VDZ may have already failed first-line biologic therapies (suggestive of more severe disease than patients prescribed anti-TNF agents alone), we performed propensity score matching to establish comparable cohorts using risk-modifying clinical determinants. Propensity scoring helps adjust for confounders resulting from baseline characteristics of the patients. Patients were matched by a Mahalanobis matching algorithm,38 based on age, gender, IBD type (UC, CD, IC), Charlson comorbidity score, diagnosed malnutrition (as captured by ICD-9 and ICD-10), and use of immunomodulators (i.e., small molecules used in severe colitis including azathioprine, 6-mercaptopurine, methotrexate sodium, ciclosporin, or tacrolimus within 30 days of surgery), corticosteroids, and opioids within 30 days before surgery. We utilized 1:1 matching with no replacement, and a maximum caliper of 0.007. Propensity score matching was implemented using the psmatch2 module in Stata/SE 14.2 (College Station, TX). Comparability of baseline characteristics between matched groups was assessed using chi-square tests for categorical variables and student’s independent sample t-tests for continuous variables.
Mulitvariable Model
A multivariable logistic regression model was calculated to estimate the association between preoperative VDZ use and SSIs. Covariates included age, sex, type of surgery performed, immunomodulator use, corticosteroid dependency (≥100 days of corticosteroid use39 per year), and malnutrition. Statistical significance was assessed at the level of α=0.05. All analyses were performed using Stata/SE 14.2 (College Station, TX).
RESULTS
Patient Cohort Identification & Characteristics
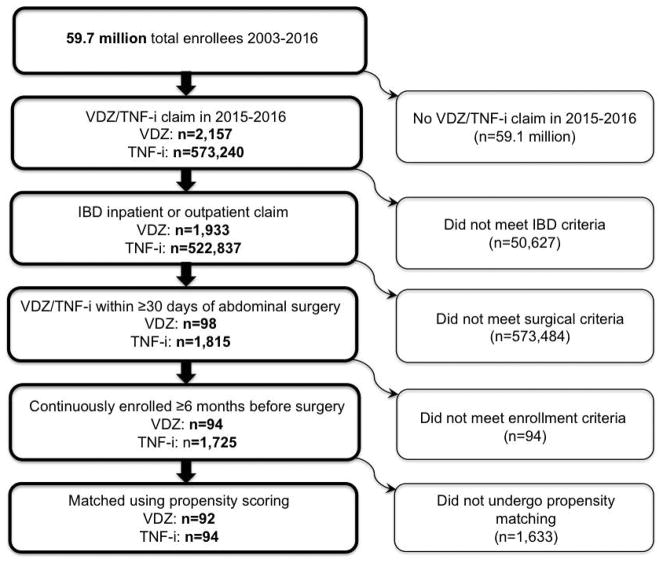
The data for 1,933 and 522,837 IBD patients receiving VDZ or TNFi, respectively, were retrieved from intpatient and outpatient claims dataset in Optum. Of these, 573,484 patients were excluded as they did not undergo surgery or had VDZ or TNFi exposure outside the 30-day abdominal surgery date. Ultimately, 94 VDZ and 1,725 TNFi IBD patients met criteria for study inclusion. After propensity score matching, we identified and compared 92 VDZ-exposed patients with 94 TNFi-exposed patients, creating a cohort of 186 patients. Figure 1 illustrates the patient cohort identification process.
Figure 1.
Patient Cohort Identification
Table 1 displays the patient characteristics of VDZ and TNFi patients. After propensity matching, there were no statistically significant differences in patient demographics, operative characteristics, and predictors of post-operative complications. The proportion of laparoscopic bowel resections with ostomies and the mean number of days of cumulative steroid use in 2016 were greater among patients using VDZ. The proportion of patients with open bowel resection with ostomy, laparoscopic bowel resection with anastomosis, open bowel resection with anastomosis, and the use of corticosteroids within 30 days of surgery were greater among patients using TNFi. These differences were not statistically significant.
Table 1.
Comparison of Anti-TNF vs VDZ Cohorts after Propensity Score Matching
| Variables | Anti-TNF Patients | Vedolizumab Patients | P-value |
|---|---|---|---|
| Age, years (mean) | 37.8 | 39.0 | 0.569 |
| Sex, female (%) | 41.3% | 43.6% | 0.751 |
| Disease type | |||
| Ulcerative Colitis (%) | 41.3% | 43.6% | 0.828 |
| Crohn’s Disease (%) | 55.4% | 48.9% | |
| Indeterminate Colitis (%) | 3.3% | 7.5% | |
| Operative characteristics | |||
| Laparoscopic bowel resection with ostomy | 21.7% | 30.9% | 0.160 |
| Open bowel resection with ostomy | 32.6% | 30.9% | 0.798 |
| Laparoscopic bowel resection with anastomosis | 10.9% | 7.5% | 0.421 |
| Open bowel resection with anastomosis | 34.8% | 30.9% | 0.570 |
| Systemic corticosteroids | |||
| Within 30 days surgery (%) | 52.1% | 45.8% | 0.383 |
| Cumulative steroid days (mean) | 158.7 | 167.5 | 0.743 |
| Immunomodulator (%) | 10.9% | 13.3% | 0.542 |
| Chronic Opioid Use (%)* | 63.0% | 61.6% | 0.737 |
| Charlson Comorbidity Index (mean, median) | 1.4, 1 | 1.7, 1 | 0.257 |
| Malnutrition (%) | 50.0% | 52.1% | 0.773 |
| Hemoglobin (g/dL) (mean) [median] | 12.7 [12.6] | 12.3 [12.4] | 0.454 |
| Albumin (g/dL) (mean) [median] | 3.8 [4.0] | 3.5 [3.9] | 0.503 |
Chronic opioid use was defined ≥3 separate opioid drug claims on distinct dates within a 2-year rolling window, as previously shown.49
Comparison of SSI Risk Based on TNFi vs VDZ Exposure
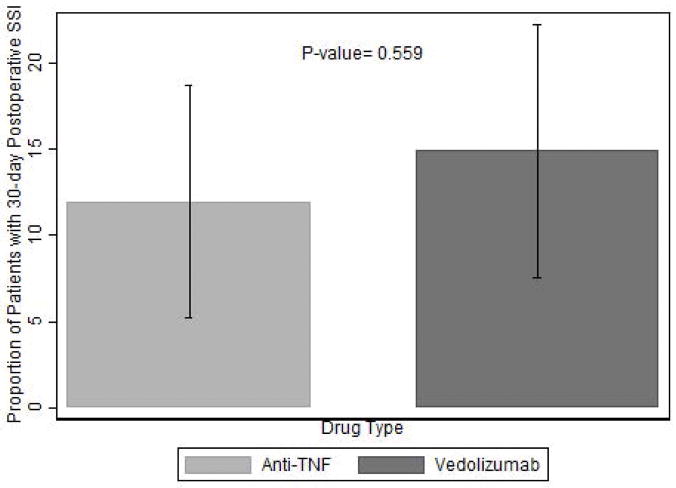
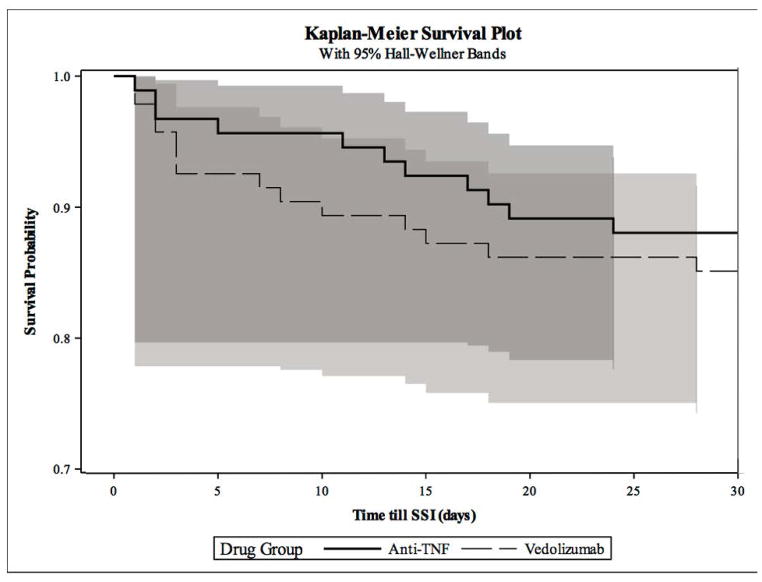
The probability of SSI within 30 days post-operatively was not statistically different between the VDZ versus the TNFi groups (VDZ 14.9% versus TNFi 12.0%, P=0.56) (Figure 2). The risk of postoperative SSI in the first 10 days was lower in the TNFi group, but the difference was not statistically significant. Of those who ultimately developed SSI within 30 days, 10 of the 14 VDZ patients (71.4%) compared to 5 of the 11 (45.5%) TNFi patients had SSIs in the first 10 days (Figure 3). There was no significant difference in the rates of deep organ space versus superficial SSIs between VDZ and TNFi groups (p=0.17).
Figure 2.
Comparison of SSI Risk Between TNFi vs VDZ
Figure 3.
KM Plot Comparing SSI Risk Over 30-days
Malnutrition is an Independent Risk Factor for SSI
Multivariable analysis showed malnutrition as the sole significant predictor for post-operative SSI (OR 3.1, 95% CI 1.1–8.7, P= 0.03) (Table 2). None of the other variables, including VDZ exposure (OR 1.8, 95% CI 0.7–3.7, P= 0.25), being female (OR 1.1, 95% CI 0.4–3.1, P= 0.8), or having open surgery (OR 1.4, 95% CI 0.5–4.2, P= 0.51), were associated with increased risk for SSI. Exposure to systemic corticosteroids prior to surgery trended towards increased risk but was not statistically significantly associated with SSI (OR 1.9, 95% CI 0.7–5.0, P= 0.2). In a post-hoc analysis, we modified the model to evaluate CD and UC separately. VDZ exposure did not increase SSI in either UC or CD patients, while malnutrition remained a significant predictor of SSI in UC and corticosteroids became a significant predictor of SSI in CD.
Table 2.
Assessment of Individual Risk Factors for SSI
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Vedolizumab | 1.785 | [0.671, 4.747] | 0.246 |
| Age | 0.995 | [0.965, 1.027] | 0.776 |
| Sex, female | 1.141 | [0.425, 3.058] | 0.794 |
| Operative characteristics | |||
| Open (vs. laparoscopic) | 1.441 | [0.493, 4.216] | 0.505 |
| Ostomy (vs. anastomosis) | 0.881 | [0.321, 2.415] | 0.805 |
| Concomitant Immunomodulators | 0.765 | [0.184, 3.185] | 0.713 |
| Corticosteroid dependency* | 1.914 | [0.728, 5.029] | 0.188 |
| Malnutrition | 3.110 | [1.110, 8.713] | 0.031* |
≥100 days of corticosteroid use in 1 year
DISCUSSION
Postoperative infectious complications in patients undergoing surgery for IBD-related indications are a major cause of morbidity and increased healthcare resource utilization.31–33 The effects of TNFi or VDZ exposure perioperatively on the risk of infectious complications remains unclear.6–14 Understanding the differences in operative risk posed by different biologic agents has become increasingly important, given the numerous competing therapy options after first-line treatment failure. In the largest risk-adjusted cohort analysis to date, we sought to answer this clinically relevant question.
Through propensity-score matching using potential predictors for postoperative infectious complications, we found that SSI risk was not statistically different between TNFi and VDZ exposed patients. Furthermore, in multivariable analysis, malnutrition was the only significant predictor of SSI among multiple well-established risk factors including age, surgical approach, presence of an ostomy, concomitant immunomodulatory use, and corticosteroid exposure. Patients who were classified as having malnutrition had over a three-fold increased risk of SSI as a complication.
Our study findings have significant clinical implications, demonstrating the need to optimize nutrition in patients with IBD, who are often chronically malnourished during states of prolonged disease exacerbation. Although the prevalence of malnutrition in IBD by ICD coding from a representative inpatient cohort of IBD patients is 5–10%,40 the probability of malnutrition just prior to IBD-related surgery is likely much higher.41 Previous literature suggests that more than half of all patients may meet criteria for malnutriton at the time of IBD diagnosis.42 Furthermore, it is likely that malnutrition especially in acute care settings may be under-reported and not diagnosed in patients with IBD.43,44 This may be more apparent in patients who have been hospitalized for surgical management of a primary diagnosis.45,46 Therefore, we recommend preoperative measures to optimize nutrition including supportive enteral feeds or parenteral nutrition as indicated.
Exposure to corticosteroids in the perioperative period is a potential risk factor for surgical site infections, although there is some debate in the literature about its effect.47,48 In this study, we found that perioperative corticosteroid exposure was associated with a greater risk for surgical site infections in patients with Crohn’s disease. Patients exposed to corticosteroids in the perioperative period are more likely to have steroid-refractory disease, so this may also be an indicator of greater clinical disease severity.
The limitations of this study are associated with the inherent limitations of insurance claims data. Ascertaining clinical status such as disease severity requires the use of surrogate markers of illness such as presence of diagnosis codes for malnutrition, lab values for hemoglobin and albumin, and the need for surgical intervention. Underreporting of comorbid conditions such as malnutrition could lead to falsely increase the effect on SSI risk. A potential weakness of our retrospective claims analysis is the reliance on data which may not adequately capture variables like malnutrition. Regardless, propensity matching served as a method of likely balancing any over- or under-estimation of malnutrition in the two groups. Another limitation of the dataset is the ability to determine cause of deep organ space infections (intra-abdominal abscesses). These infections may be due to anastomotic leaks, leaks from a Hartmann’s pouch (rectal stump), inadvertent injury to bowel, or contamination from the original operation or underlying condition, but these details are not available in insurance claims data. Finally, although we report on the largest risk-adjusted cohort to date comparing VDZ to TNFi exposure on post-operative complications, our final cohort size remains relatively small. As vedolizumab use becomes increasingly common in clinical practice, smaller differences undetected in this study may become apparent.
In conclusion, preoperative vedolizumab use does not increase postoperative SSI risk as compared to preoperative TNFi treatment. However, a chart diagnosis of malnutrition is a significant predictor of SSI. While a diagnosis of malnutrition certainly indicates severe overall disease, further prospective research is needed to determine whether this risk can be modified with perioperative interventions to address nutritional status.
Supplementary Material
Supplementary Table 1. Superficial Wound Infections and Deep Organ Space Infections
Acknowledgments
Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from Internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank Lesley Park, Isabella Chu, and Valerie Meausoone at the Population Health Sciences for their collaboration and technical support. We would like to thank Jason Bentley at Stanford University’s Quantitiative Statistical Unit for statistical expertise.
Footnotes
Conflict of Interest & Funding Source: KTP has received research support from Janssen, Takeda, AbbVie Inc. unrelated to this work, and is supported by NIDDK094868 and Bacher-English Family Grant for this research. The remaining authors have no conflicts of interests to disclose. No form of payment or honorarium was given to any contributor for manuscript preparation or production.
Authorship: KTP – obtaining funding surce, study concept and design, supporting the analysis, drafting/editing the manuscript. LS – study design, supporting and performing the analysis, drafting/editing the manuscript. MD – study design, supporting and performing the analysis, drafting/editing the manuscript. AWT – study design, supporting and performing the analysis, editing the manuscript. AW, JJW, RB, BNL, KK – study design, supporting the analysis, editing the manuscript. CK – obtaining funding source, study design, supporting the analysis, drafting/editing the manuscript.
References
- 1.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2018 Jan 23; doi: 10.1136/gutjnl-2017-315568. pii: gutjnl-2017–315568. [DOI] [PubMed] [Google Scholar]
- 2.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Alimentary pharmacology & therapeutics. 2016 Oct 1;44(8):807–16. doi: 10.1111/apt.13763. [DOI] [PubMed] [Google Scholar]
- 4.Park KT, Tsai R, Perez F, et al. Cost-effectiveness of early colectomy with ileal pouch-anal anastamosis versus standard medical therapy in severe ulcerative colitis. Ann Surg. 2012 Jul;256(1):117–24. doi: 10.1097/SLA.0b013e3182445321. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Stocchi L, Ashburn J, Remzi FH. Total abdominal colectomy vs. restorative total proctocolectomy as the initial approach to medically refractory ulcerative colitis. International journal of colorectal disease. 2017 Aug 1;32(8):1215–22. doi: 10.1007/s00384-017-2836-2. [DOI] [PubMed] [Google Scholar]
- 6.Holubar SD, Holder-Murray J, Flasar M, Lazarev M. Anti–Tumor Necrosis Factor-α Antibody Therapy Management Before and After Intestinal Surgery for Inflammatory Bowel Disease: A CCFA Position Paper. Inflammatory bowel diseases. 2015 Sep 29;21(11):2658–72. doi: 10.1097/MIB.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang MI, Cohen BL, Greenstein AJ. A review of the impact of biologics on surgical complications in Crohn’s disease. Inflammatory bowel diseases. 2015 Mar 25;21(6):1472–7. doi: 10.1097/MIB.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn’s disease patients. The American journal of gastroenterology. 2013 Apr;108(4):583. doi: 10.1038/ajg.2012.464. [DOI] [PubMed] [Google Scholar]
- 9.Billioud V, Ford AC, Del Tedesco E, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. Journal of Crohn’s and Colitis. 2013 Dec 1;7(11):853–67. doi: 10.1016/j.crohns.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Kulaylat AS, Kulaylat AN, Schaefer EW, Tinsley A, Williams E, Koltun W, Hollenbeak CS, Messaris E. Association of Preoperative Anti–Tumor Necrosis Factor Therapy With Adverse Postoperative Outcomes in Patients Undergoing Abdominal Surgery for Ulcerative Colitis. JAMA surgery. 2017 Aug 1;152(8):e171538. doi: 10.1001/jamasurg.2017.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lightner AL, Raffals LE, Mathis KL, Cima RR, Tse CS, Pemberton JH, Dozois EJ, Loftus EV. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. Journal of Crohn’s and Colitis. 2017 Feb 1;11(2):185–90. doi: 10.1093/ecco-jcc/jjw147. [DOI] [PubMed] [Google Scholar]
- 12.Lightner AL, McKenna NP, Moncrief S, Pemberton JH, Raffals LE, Mathis KL. Surgical outcomes in vedolizumab-treated patients with ulcerative colitis. Inflammatory bowel diseases. 2017 Aug 29;23(12):2197–201. doi: 10.1097/MIB.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 13.Lightner AL, McKenna NP, Tse CS, Raffals LE, Loftus EV, Mathis KL. Postoperative outcomes in vedolizumab-treated Crohn’s disease patients undergoing major abdominal operations. Alimentary pharmacology & therapeutics. 2017 Dec 18; doi: 10.1111/apt.14459. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante M, de Buck van Overstraeten A, Schils N, Moens A, Van Assche G, Wolthuis A, Vermeire S, D’hoore A. Perioperative use of vedolizumab is not associated with postoperative infectious complications in patients with ulcerative colitis undergoing colectomy. Journal of Crohn’s and Colitis. 2017 Oct 27;11(11):1353–61. doi: 10.1093/ecco-jcc/jjx095. [DOI] [PubMed] [Google Scholar]
- 15.Lightner AL, McKenna NP, Moncrief S, Pemberton JH, Raffals LE, Mathis KL. Surgical outcomes in vedolizumab-treated patients with ulcerative colitis. Inflammatory bowel diseases. 2017 Aug 29;23(12):2197–201. doi: 10.1097/MIB.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 16.Lightner AL, McKenna NP, Tse CS, Raffals LE, Loftus EV, Mathis KL. Postoperative outcomes in vedolizumab-treated Crohn’s disease patients undergoing major abdominal operations. Alimentary pharmacology & therapeutics. 2017 Dec 18; doi: 10.1111/apt.14459. [DOI] [PubMed] [Google Scholar]
- 17.Ferrante M, de Buck van Overstraeten A, Schils N, Moens A, Van Assche G, Wolthuis A, Vermeire S, D’hoore A. Perioperative use of vedolizumab is not associated with postoperative infectious complications in patients with ulcerative colitis undergoing colectomy. Journal of Crohn’s and Colitis. 2017 Oct 27;11(11):1353–61. doi: 10.1093/ecco-jcc/jjx095. [DOI] [PubMed] [Google Scholar]
- 18.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 19.von Andrian UH, Engelhardt B. α4 Integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 20.Entyvio®, vedolizumab [package insert] Takeda Pharmaceuticals America, Inc; Deerfield, IL: 2014. [Google Scholar]
- 21.Feagan BG, Rutgeerts P, Sands BE, et al. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Feagan BG, Rutgeerts P, et al. GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 23.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2016 doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada A, Komaki Y, Patel N, Komaki F, Aelvoet AS, Tran AL, Pekow J, Dalal S, Cohen RD, Cannon L, Umanskiy K. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. The American journal of gastroenterology. 2017 Sep;112(9):1423. doi: 10.1038/ajg.2017.201. [DOI] [PubMed] [Google Scholar]
- 25.Law CCY, Narula A, Lightner AL, et al. Systematic Review and Meta-Analysis: Preoperative Vedolizumab Treatment and Postoperative Complications in Patients with Inflammatory Bowel Disease. J Crohns Colitis. 2018 Apr 27;12(5):538–545. doi: 10.1093/ecco-jcc/jjy022. [DOI] [PubMed] [Google Scholar]
- 26.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- 27.Schleier L, Wiendl M, Binder MT, et al. α4β7 Integrin-dependent gut homing of non-classical monocytes is essential for intestinal wound healing mediated by M2 macrophages. ECCO. 2018 (Oral Presentation OP007) [Google Scholar]
- 28.National Healthcare Safety Network, Centers for Disease Control and Prevention. [Accessed 8 May 2018];Surgical site infection (SSI) event. http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Published January 2017.
- 29.Gomila A, Badia JM, Carratalà J, et al. Current outcomes and predictors of treatment failure in patients with surgical site infection after elective colorectal surgery. A multicentre prospective cohort study. J Infect. 2017 Jun;74(6):555–563. doi: 10.1016/j.jinf.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Kirat HT, Kiran RP, Oncel M, et al. Management of leak from the tip of the “J” in ileal pouch-anal anastomosis. Dis Colon Rectum. 2011 Apr;54(4):454–9. doi: 10.1007/DCR.0b013e31820481be. [DOI] [PubMed] [Google Scholar]
- 31.Kirat HT, Remzi FH, Shen B, Kiran RP. Pelvic abscess associated with anastomotic leak in patients with ileal pouch-anal anastomosis (IPAA): transanastomotic or CT-guided drainage? Int J Colorectal Dis. 2011 Nov;26(11):1469–74. doi: 10.1007/s00384-011-1272-y. [DOI] [PubMed] [Google Scholar]
- 32.Bliss LA, Maguire LH, Chau Z, Yang CJ, Nagle DA, Chan AT, Tseng JF. Readmission After Resections of the Colon and Rectum: Predictors of a Costly and Common Outcome. Dis Colon Rectum. 2015 Dec;58(12):1164–73. doi: 10.1097/DCR.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimann TM, Swaminathan S, Greenstein AJ, et al. Incidence and Factors Correlating With Incisional Hernia Following Open Bowel Resection in Patients With Inflammatory Bowel Disease: A Review of 1000 Patients. Ann Surg. 2018 Mar;267(3):532–536. doi: 10.1097/SLA.0000000000002120. [DOI] [PubMed] [Google Scholar]
- 34.Remzi FH, Aytac E, Ashburn J, et al. Transabdominal Redo Ileal Pouch Surgery for Failed Restorative Proctocolectomy: Lessons Learned Over 500 Patients. Ann Surg. 2015 Oct;262(4):675–82. doi: 10.1097/SLA.0000000000001386. [DOI] [PubMed] [Google Scholar]
- 35.Kiely JM1, Fazio VW, Remzi FH, et al. Pelvic sepsis after IPAA adversely affects function of the pouch and quality of life. Dis Colon Rectum. 2012 Apr;55(4):387–92. doi: 10.1097/DCR.0b013e318246418e. [DOI] [PubMed] [Google Scholar]
- 36.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994 Nov;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 37.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015 Jul;42(2):188–202. doi: 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu XS, Rosenbaum PR. Comparison of Multivariate Matching Methods: Structures, Distances, and Algorithms. Journal of Computational and Graphical Statistics. 2012;2(4):405–420. [Google Scholar]
- 39.Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013 Sep;206(3):410–7. doi: 10.1016/j.amjsurg.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008 Aug;14(8):1105–11. doi: 10.1002/ibd.20429. [DOI] [PubMed] [Google Scholar]
- 41.Bendersky V, Sun Z, Adam MA, et al. Determining the Optimal Quantitative Threshold for Preoperative Albumin Level Before Elective Colorectal Surgery. J Gastrointest Surg. 2017 Apr;21(4):692–699. doi: 10.1007/s11605-017-3370-9. Epub 2017 Jan 30. [DOI] [PubMed] [Google Scholar]
- 42.Goh J, O’Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther. 2003 Feb;17(3):307–20. doi: 10.1046/j.1365-2036.2003.01482.x. [DOI] [PubMed] [Google Scholar]
- 43.Bistrian BR, Blackburn GL, Vitale J, et al. Prevalence of malnutrition in general medical patients. JAMA. 1976;235:1567–1570. [PubMed] [Google Scholar]
- 44.Han PD, Burke A, Baldassano RN, et al. Nutrition and inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:423–43. ix. doi: 10.1016/s0889-8553(05)70063-7. [DOI] [PubMed] [Google Scholar]
- 45.Hill GL, Blackett RL, Pickford I, et al. Malnutrition in surgical patients. An unrecognised problem. Lancet. 1977;1:689–692. doi: 10.1016/s0140-6736(77)92127-4. [DOI] [PubMed] [Google Scholar]
- 46.Silk DB, Payne-James J. Inflammatory bowel disease: nutritional implications and treatment. Proc Nutr Soc. 1989;48:355–361. doi: 10.1079/pns19890051. [DOI] [PubMed] [Google Scholar]
- 47.Kohut AY, Liu JJ, Stein DE, et al. Patient-specific risk factors are predictive for postoperative adverse events in colorectal surgery: an American College of Surgeons National Surgical Quality Improvement Program-based analysis. Am J Surg. 2015 Feb;209(2):219–29. doi: 10.1016/j.amjsurg.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Aytac E, Londono JM, Erem HH, et al. Impact of stress dose steroids on the outcomes of restorative proctocolectomy in patients with ulcerative colitis. Dis Colon Rectum. 2013 Nov;56(11):1253–8. doi: 10.1097/DCR.0b013e3182a180b7. [DOI] [PubMed] [Google Scholar]
- 49.Buckley JP, Cook SF, Allen JK, Kappelman MD. Prevalence of Chronic Narcotic Use Among Children With Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology. 2015;13(2):310–U148. doi: 10.1016/j.cgh.2014.07.057. We now clarify this in the manuscript text. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Superficial Wound Infections and Deep Organ Space Infections