Abstract
Previous studies have examined the concurrent relationship between posttraumatic stress disorder (PTSD) and a range of psychophysiological variables, including respiratory sinus arrhythmia (RSA). However, there is a lack of research examining the prospective development of trauma symptomatology, and the directionality of the association between RSA level and PTSD has yet to be determined. The current study is the first prospective study to examine whether RSA level and RSA reactivity are risk factors for PTSD symptoms in children. Assessments were conducted both prior to (Time 1) and following (Time 2) a natural disaster (i.e., Hurricane Katrina). Participants were 36 children who were 3-6 years-old during the Time 1 assessment. Structured diagnostic interviews were used to assess PTSD symptoms at both Time 1 and Time 2. RSA level during a neutral stimulus, RSA reactivity to emotional video stimuli (distress, joy, and trauma videos) and RSA reactivity to memory stimuli (remote happy memory, trauma memory, mother’s recall of the trauma memory) were also collected at both time points. Time 1 RSA level during a neutral stimulus was a significant predictor of Time 2 PTSD symptoms (controlling for age, Time 1 PTSD symptoms, Time 2 neutral RSA level), such that lower RSA during a neutral condition was related to higher PTSD symptoms. Also, Time 1 RSA reactivity in response to memory (but not video) stimuli, in the form of relatively less vagal withdrawal, was a significant predictor of more Time 2 PTSD symptoms (controlling for age, Time 1 PTSD symptoms, Time 2 RSA reactivity). This unique prospective study provides evidence for level of RSA and RSA reactivity as pre-existing clinical markers of stress sensitivity that predict psychopathology following a trauma.
Keywords: risk factor, PTSD, RSA, childhood
Posttraumatic stress disorder (PTSD) develops in a subset of individuals exposed to traumatic events. Symptoms include intrusions, avoidance, negative alterations in cognitions and mood, and increased arousal and reactivity (American Psychiatric Association; APA 2013). There has been a great deal of research examining the relationship between PTSD and an array of psychophysiological abnormalities (Zoladz and Diamond 2013); however, the directionality of these relationships is unknown. While some studies suggested altered physiology is a consequence of PTSD, others suggested it is a predisposing factor that increases the risk of developing PTSD. The current study is the first to examine the prospective relationship between level and reactivity of one psychophysiological marker, respiratory sinus arrhythmia (RSA), and later PTSD symptoms in children.
The autonomic nervous system (ANS), comprised of the parasympathetic (PNS) and sympathetic (SNS) branches, functions to coordinate physiological responses to the environment. At rest, or when the environment is perceived as safe, the PNS promotes growth and restoration by slowing the heart, inhibiting the SNS, and reducing inflammation (Porges 2007). The PNS is often referred to as a “brake” on sympathetic activity. During a stressor the PNS “brake” is deactivated, releasing the physiological effects controlled by the SNS. After a stressor, a primary function of the PNS is to counteract SNS response and again reduce physiological arousal. This role of the PNS in stress reaction and recovery appears to have straightforward relevance to PTSD (Sahar et al. 2001).
RSA is an index of PNS influences on the heart, or more specifically, vagal control of the heart. It has been conceptualized as a psychophysiological index representing an organism’s ability to maintain homeostasis (Graziano and Derefinko 2013), and a marker of emotion regulation (Beauchaine 2001; Porges 2007). Baseline RSA and RSA reactivity to a stressor are two different metrics of the same system, and both have been linked to a range of psychological disorders across development (Beauchaine 2001). Generally, high resting or baseline RSA is related to better adaptability and health (Thayer et al. 2012) and better social functioning (Porges 2007). An adaptive response in the face of stress is for PNS activity to decrease (vagal withdrawal) to allow the SNS to respond, resulting in a decrease in RSA.
Several studies in adults have found that individuals with PTSD had lower baseline RSA compared to healthy controls, suggesting decreased parasympathetic tone (Cohen et al. 2000; Blechert et al. 2007; Hauschildt et al. 2011). However, Sahar et al. (2001) found no differences between individuals with PTSD and trauma exposed controls. The results are more mixed when examining RSA reactivity in adults with PTSD. Some have found no significant change in RSA in response to a trauma recall or a stressful task, suggesting a maladaptive failure to respond (Cohen et al. 2000; Sahar et al. 2001). Others have found evidence of the adaptive response, a significant decrease in RSA in response to a trauma reminder (Sack et al. 2004). Still others have found consistently decreased RSA in individuals with PTSD across several different affective conditions, not specific to only trauma reminders (Hauschildt et al. 2011).
Only a few studies have examined the relationship between RSA and PTSD in childhood. Kirsch et al. (2015) compared baseline RSA and RSA reactivity during a trauma memory between children (ages 6-17) diagnosed with PTSD (n = 16) and trauma-exposed controls (n = 18). Results showed no differences between groups on either RSA measure. Scheeringa et al. (2004) examined RSA response to pleasant and traumatic memories in children (ages 20 months-6 years) with PTSD (n = 14), trauma-exposed controls (n = 43), and non-traumatized controls (n = 60). They also found no significant differences between groups on baseline RSA or RSA reactivity. However, when parenting was taken into account, a significant interaction was found such that children with high PTSD symptoms and low parental positive discipline showed the largest decrease in RSA during the trauma memory. Among 6-12 year-old children with mothers exposed to intimate partner violence, Katz and Gurtovenko (2015) found no association between baseline RSA and PTSD symptoms. However, they did find an interaction such that those children who had low baseline RSA and mothers experiencing PTSD symptoms showed the highest PTSD symptoms themselves. There appears to be a relationship between level of baseline RSA and RSA reactivity and PTSD in childhood, although contextual factors appeared influential in these cross-sectional studies.
Finally, although Scott and Weems (2014) did not examine PTSD symptoms, they examined the relationship between baseline RSA and RSA reactivity with anxiety in a sample of 80 children (age 11-17) who had been exposed to a traumatic event (including Hurricane Katrina). The results showed lower baseline RSA was predictive of higher child- and parent-reported anxiety. Further, children who reported high anxiety showed an increase in RSA in response to a stressful task, whereas children who reported low anxiety showed the adaptive RSA withdrawal.
The directionality of the association between RSA level and reactivity and posttraumatic stress has yet to be determined. Some researchers suggested that trauma causes physiological changes (Nemeroff and Seligman 2013; Teicher and Samson 2013; Vermetten and Bremner 2002), and others proposed models in which individual differences predispose children to be differentially sensitive to environmental stressors (Del Guidice et al. 2011; Ellis et al. 2011). In the latter models, rather than being an outcome of PTSD, low RSA level and maladaptive reactivity may be risk factors for the development of PTSD.
In line with this idea, McLaughlin et al. (2015) proposed that RSA level may be a moderator between environmental adversity and psychopathology. In a study of 157 children ages 13-17, they found that resting RSA was not predictive of internalizing or externalizing problems. However, when taking into account environmental adversity (i.e., exposure to abuse, community violence, peer victimization, and other traumatic events), they found an interaction such that individuals with high adversity and low resting RSA exhibited the most internalizing psychopathology. The authors concluded that low resting RSA may be a clinical marker of stress sensitivity that predicts psychopathology following adversity, whereas high resting RSA may buffer against the negative mental health outcomes following adversity.
The only way to determine if maladaptive physiological functioning is a risk factor or consequence of PTSD is to examine the phenomena longitudinally, beginning prior to trauma exposure. Researchers have circumvented this challenge by focusing on samples at high risk for experiencing trauma, or by following up with participants after the occurrence of a city-wide disaster. Researchers who examined physiological reactivity in police or firefighter recruits during training and again after having traumatic job experiences showed that pre-trauma physiological differences (i.e., cortisol reactivity, salivary MHPG, startle response) were predictive of variability in PTSD or distress following traumas (Apfel et al. 2011; Galatzer-Levy et al. 2014; Guthrie and Bryant 2005). Further, in a small sample of adolescents tested prior to and following the Boston Marathon terrorist attack, researchers showed that pre-trauma amygdala reactivity to negative emotional stimuli was predictive of later PTSD symptoms (McLaughlin et al. 2014).
The goal of the current study was to test for the first time whether RSA level and reactivity are risk factors for PTSD symptoms in children using prospective data. Similar to McLaughlin et al. (2014), we assessed participants prior to (Time 1) and following (Time 2) a regional disaster. Based on prior work suggesting that pre-trauma maladaptive physiological reactivity may be a risk factor for the development of psychopathology following a trauma (e.g., McLaughlin et al. 2014), we hypothesized that lower Time 1 RSA level during a neutral condition would be a risk factor, and predictive of greater PTSD symptoms at Time 2. Further, we expected that Time 1 RSA level would be a better predictor of Time 2 PTSD symptoms than Time 2 RSA level. In terms of RSA reactivity, we expected that a maladaptive response to a trauma stimulus at Time 1 (relatively less decrease or greater increase in RSA from neutral condition to trauma stimulus) would be predictive of more PTSD symptoms at Time 2. Also, we expected that Time 1 RSA reactivity would be a better predictor of Time 2 PTSD symptoms than Time 2 RSA reactivity.
Methods
Participants
Children were 36 to 83 months of age at time of enrollment. Children with a history of one or more traumas occurring after 36 months of age were recruited. Healthy controls with no history of trauma were also recruited. Children were excluded if they had experienced a head trauma, were intellectually disabled, had a pervasive developmental disorder, blindness, deafness, or were non-English speaking. Beginning in 2003, participants were recruited from a level 1 trauma center hospital, three local battered women’s programs, and a Head Start center in the greater New Orleans area. After 156 children participated in the study (Time 1), Hurricane Katrina devastated the area on August 29, 2005. The time between the Time 1 assessment and the hurricane ranged from 19-681 days (mean = 388). After the disaster, attempts were made to contact all of the 156 previous participants using the contact information they provided before the storm. However, there was a mandatory evacuation and many people were displaced after the storm making them difficult to contact. Between March 2006 and May 2008 a total of 36 participants were located and returned to the lab to repeat the assessment battery (Time 2). The time between the hurricane and Time 2 assessment ranged from 185 – 996 days (mean = 492). All of the returning children were displaced by the hurricane (range = 22-750 days, mean = 323 days, median = 290 days) and 36% had not moved back into their original home at the time of their participation. Almost two-thirds (61%) witnessed or directly experienced trauma, such as being trapped in a flooded house, sleeping on a street overnight, witnessing damage to their own home, or death of a loved one, 28% were separated from their primary caregiver at some point during evacuation or displacement, and 75% of children experienced personal property loss/damage. Those who returned at Time 2 did not differ from those who did not return on age, gender, ethnicity, race, whether the father lived with the child, father employment, mother’s age, father’s age, or mother’s highest level of schooling. Children who returned for Time 2 had fathers who had slightly higher education (Mann-Whitney U = 1485, z = −2.085, p = .037), and were marginally more likely to have a mother who was employed (Yate’s Continuity Corrected χ2 = 3.741, p = .053). Findings from the larger sample have been reported elsewhere (Scheeringa et al. 2012).
At Time 2, participants ranged in age from 4-9 years (M = 7.38, SD = 1.02). About two-thirds (n = 23) of participants were male, and a majority (91.7%) were non-Latino. Racial composition of the sample was 83.3% black, 5.6% white, 5.6% mixed, and 5.6% other. Half of the participants (n = 18) had experienced a trauma prior to Hurricane Katrina, and the other half had originally been recruited as healthy controls.
Procedures
This study was approved by the Tulane University Institutional Review Board (IRB). All procedures performed were in accordance with the ethical standards of the IRB and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Time 1 data were collected in two sessions. Caregivers attended the first 2.5 hour session alone and were compensated $50. Written informed consent was obtained from caregivers upon arrival to the lab. Caregivers completed a psychiatric interview about their children and completed several questionnaires. Both the caregivers and children attended the second session, which occurred approximately 1 week later and took 1.5 hours. Psychophysiological data were collected and child interviews were conducted. The caregiver was compensated $100. The Time 2 assessment followed the same two-session protocol.
Measures
Diagnostic Interview
The Preschool Age Psychiatric Assessment (PAPA; Egger and Angold 2004) is a structured diagnostic interview. Previous research showed good test-retest reliability for the PTSD diagnosis (kappa = .73) among a community sample of 2-5 year-old children (Egger et al. 2006). Caregivers were interviewed both Time 1 and Time 2 about their child’s symptoms across nine disorders, but only PTSD symptoms are of interest in the current study. Although based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA 2000), the PTSD module of the PAPA includes developmentally modified symptoms which were later incorporated into the age 6 years and younger PTSD criteria in DSM-5 (APA 2013). Interview questions assessing two symptoms (substantially increased frequency of negative emotional states and markedly diminished interest or participation in significant activities) were pulled from the major depression module of the PAPA. This allowed us to capture all DSM-5 symptoms of PTSD for children 6 years and younger. At the Time 2 assessment, children were also interviewed about their PTSD symptoms using a modified PAPA interview. Because the children were not asked about major depression, information regarding the two symptoms from that module (described above) were unavailable from child-report.
Symptom information gathered from the interviews were used to create PTSD sum scores. For the Time 1 assessment, only caregiver-report was available. For the Time 2 assessment, a joint-report PTSD sum score was created by considering a symptom present if either child or caregiver endorsed it, consistent with recommendations in the literature (Scheeringa et al. 2006). To be consistent, the DSM-5 PTSD symptoms for children 6 years and younger was used for all children at both Time 1 and Time 2, even though ages ranged from 4-9 years at the Time 2 assessment. Recent research has also supported the use of the 6 years and younger criteria for older children (Meiser-Stedman et al. 2017; Mikolajewski et al. 2017).
Psychophysiological Data
Electrocardiogram (ECG) data were recorded from children using software and equipment from the James Long Company. Three adhesive electrodes were placed on the children’s torsi (left and right rib cage, abdomen) to record upward-deflected R waves. Data were passed through a bioamplifier set for bandpass filtering at frequencies of .1 and 1000 Hz. R waves were identified automatically from digitized raw ECG using a multiple-pass, self-scaling algorithm. Identified R waves were visually inspected and corrected manually when necessary. Interbeat intervals (IBI) were calculated as distances between successive R waves. IBI analysis software was used to quantify respiratory sinus arrhythmia (RSA). Prorated and detrended IBIs were spectrally analyzed using discrete Fourier transformation and empirically established frequency bands (3-year-olds: 0.30-0.75 Hz; age 4 and older: 0.20-0.65 Hz; Bar-Haim et al. 2000).
Physiological data were collected while children were exposed to video and memory stimuli. The video stimuli were created from clips of theatrical movies. First, data were recorded while children watched an emotionally neutral video of old-fashioned biplanes flying, accompanied by soft music. (RSA obtained from this segment is considered neutral RSA level in the following analyses.) It should be noted that a traditional resting baseline involves the participant being asked to relax and sit calmly and quietly with their eyes closed. However, using this approach for a baseline condition has several drawbacks. First, there is no way to know the content or valence of the participants’ thoughts, making the baseline unstandardized across participants. Second, asking young children to sit calmly and quietly with eyes closed for several minutes is often too developmentally challenging and may not in fact result in a resting state. To remedy this and standardize the baseline, a neutral video was chosen. Next, children watched emotional clips depicting distressing (boy being bullied), joy (children opening presents), and trauma (woman running from attacker) scenes. Each video clip was 45 seconds long. The neutral biplanes video was shown between emotional clips. RSA reactivity for each video stimulus was calculated by subtracting the previous neutral RSA level from the RSA level during the stimulus. Thus, if RSA increased from the neutral condition to the stimulus, the reactivity score was positive; if RSA decreased from the neutral condition to the stimulus, the reactivity score was negative.
Directly after the trauma video, children watched the neutral airplane video again prior to presentation of the memory stimuli. Following this neutral condition, children were asked by interviewers to recall and discuss a remote happy memory identified by their caregivers which occurred within the past 2-6 months (e.g., trip to amusement park, zoo). Next, children were asked to recall a traumatic memory. Children with a history of trauma were asked to recall the event identified by their caregivers as their most significant trauma. Controls without a trauma history were asked to recall an unpleasant event from their past (e.g., trip to hospital, falling off bike). For the final memory stimulus, children listened while their caregivers recalled the children’s traumatic/unpleasant event. For each memory, the interviewers asked the participants to recall the location, children’s activity at the time, others present, method of injury (if applicable), how the children felt, and how the children reacted. The interviewers began with open-ended questions, progressed to more specific questions, and then asked yes-no questions, consistent with previous research (Merritt et al. 1994). RSA reactivity for each memory stimulus was calculated by subtracting the neutral condition RSA (during neutral video prior to memory interviews) from the RSA during each memory recall.
Psychophysiological data collection methods and stimuli were identical for Time 1 and Time 2 assessments with one exception. At the Time 2 assessment the interviewers read a script created from the caregiver’s description of recent pleasant and traumatic events, rather than prompting the children to discuss the memories.
Data Analysis
Preliminary analyses including descriptive statistics and correlations among study variables were conducted using IBM SPSS 22.0. Hypotheses were tested using a series of multiple linear regressions conducted in Mplus 7.0 (Muthen and Muthen, 1998-2015) using the robust maximum likelihood (MLR) estimator to handle non-Gaussian distributions. The outcome variable for all analyses was the number of Time 2 PTSD symptoms based on the joint-report of both caregivers and children, based on prior research that joint-report is more accurate because either respondent alone is an underestimate (Scheeringa et al., 2006). Because previous research shows a link between age and RSA (Agelink et al. 2001), age was controlled for in all analyses. Time 1 PTSD symptoms (parent-reported) were also controlled for to ensure the relationship between Time 1 RSA variables and Time 2 PTSD was not due to a relationship between Time 1 RSA variables and Time 1 PTSD. To test the first hypothesis that lower Time 1 RSA level would be a better predictor of Time 2 PTSD symptoms than Time 2 RSA level, we included Time 1 RSA level and Time 2 RSA level in the same regression model. To test the second hypothesis that maladaptive RSA reactivity to trauma stimuli at Time 1 would be a better predictor of Time 2 PTSD symptoms than Time 2 RSA reactivity, both Time 1 and Time 2 reactivity were included in the same model for each stimulus. We expected that only reactivity to trauma-related stimuli (trauma video, trauma memory, mother’s trauma memory) would be predictive of Time 2 PTSD. However, because previous research has found consistently decreased RSA in individuals with PTSD across several different affective conditions, not specific to only trauma reminders (Hauschildt et al. 2011), we included results from the other affective conditions as well.
Results
Preliminary analyses were conducted to check for violations of the assumptions of normality, linearity, multicollinearity, and homoscedasticity. Preliminary analyses revealed the existence of one outlier on Time 2 RSA reactivity during the mother’s trauma memory, which was brought in to 2 interquartile ranges from the median. Time 1 neutral RSA level was somewhat positively skewed (skew = 1.08, SE = 0.41). To address this, Time 1 and Time 2 neutral RSA level variables were transformed using a natural log transformation. Descriptive statistics following these corrections are presented in Table 1.
Table 1.
Descriptive Statistics
| Variable | N | Min | Max | Mean (SD) |
|---|---|---|---|---|
| Time 1 | ||||
| Age | 36 | 3.47 | 6.72 | 4.98 (0.93) |
| PTSD symptoms (parent report) | 36 | 0.00 | 13.00 | 3.64 (3.93) |
| Neutral RSA level (nlog) | 33 | 2.90 | 5.85 | 4.58 (0.67) |
| RSA reactivity (change score) | ||||
| Distress video | 33 | −118.85 | 110.35 | −2.79 (43.68) |
| Joy video | 32 | −116.63 | 71.80 | 4.85 (40.97) |
| Trauma video | 33 | −133.58 | 91.13 | −13.21 (43.99) |
| Remote happy memory | 32 | −111.11 | 41.05 | −23.76 (30.04) |
| Trauma memory | 32 | −106.10 | 47.07 | −26.66 (38.05) |
| Mother’s trauma memory | 32 | −81.30 | 81.65 | −14.19 (33.92) |
| Time 2 | ||||
| Age | 36 | 4.37 | 9.40 | 7.38 (1.02) |
| PTSD symptoms (joint report) | 24 | 0.00 | 12.00 | 6.00 (3.73) |
| Neutral RSA level (nlog) | 26 | 3.45 | 5.82 | 4.80 (0.66) |
| RSA reactivity (change score) | ||||
| Distress video | 26 | −124.45 | 72.62 | −2.82 (42.91) |
| Joy video | 26 | −72.11 | 73.80 | 0.07 (38.26) |
| Trauma video | 26 | −115.62 | 87.96 | −3.65 (38.94) |
| Remote happy memory | 26 | −122.85 | 144.47 | −0.64 (61.77) |
| Trauma memory | 26 | −98.79 | 79.86 | −13.38 (43.67) |
| Mother’s trauma memory | 26 | −119.39 | 118.22 | −8.20 (56.70) |
Note. PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia.
Our a priori decision to use joint-report from both caregivers and children for Time 2 PTSD symptoms was supported. Joint-reported PTSD (M = 6.00; Md = 6.00) was significantly (Wilcoxon Signed Rank Test z = −3.24, p = .001) higher than parent-reported PTSD symptoms (M = 3.91; Md = 3.00), and children reported fewer symptoms (M = 2.57; Md = 1.00) than parents. The amount of time between the hurricane and Time 2 assessment was not related to the child’s joint-reported PTSD symptoms (r = .08, p = .706).
Correlations among variables are presented in Table 2. There was no evidence for multicollinearity among the predictors for any of the multiple regression analyses. Of note, there was no significant association between Time 1 neutral RSA level and Time 1 PTSD symptoms (r = −0.08, p > .05) or Time 2 neutral RSA level and Time 2 PTSD symptoms (r = −0.33, p > .05). However, there was a significant negative relationship between Time 1 neutral RSA level and Time 2 PTSD symptoms (r = −0.49, p = .017).
Table 2.
Correlations among Variables
| Time 1
|
Time 2
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| Time 1 | |||||||||||||||||
| 1 Age | – | ||||||||||||||||
| 2 PTSD symptoms (parent) | −.06 | – | |||||||||||||||
| 3 Neutral RSA level (nlog) | −.17 | −.08 | – | ||||||||||||||
| 4 Distress video | −.11 | −.03 | −.43 | – | |||||||||||||
| 5 Joy video | −.20 | .18 | −.16 | −.08 | – | ||||||||||||
| 6 Trauma video | −.01 | .25 | −.05 | −.27 | .31 | – | |||||||||||
| 7 Remote happy memory | .10 | −.16 | −.18 | −.05 | −.13 | .11 | – | ||||||||||
| 8 Trauma memory | .09 | −.18 | −.23 | −.08 | −.11 | .22 | .90 | – | |||||||||
| 9 Mother’s trauma memory | −.04 | −.21 | .07 | −.07 | −.31 | −.02 | .83 | .79 | – | ||||||||
| Time 2 | |||||||||||||||||
| 10 Age | .68 | −.09 | −.25 | −.04 | .02 | .31 | .18 | .21 | −.04 | – | |||||||
| 11 PTSD symptoms (joint) | .23 | .64 | −.49 | .10 | .00 | .24 | .09 | .14 | .02 | .22 | – | ||||||
| 12 Neutral RSA level (nlog) | −.13 | −.31 | .67 | −.19 | −.36 | −.22 | −.03 | −.21 | .21 | −.08 | −.33 | – | |||||
| 13 Distress video | .10 | −.11 | −.16 | .09 | .04 | −.02 | .35 | .46 | .24 | −.05 | .13 | −.35 | – | ||||
| 14 Joy video | −.16 | −.28 | .02 | −.10 | .11 | −.07 | .44 | .34 | .43 | −.19 | −.44 | −.12 | .19 | – | |||
| 15 Trauma video | .15 | −.03 | −.02 | −.18 | .36 | .29 | .06 | −.07 | −.23 | .13 | .05 | .03 | .04 | −.17 | – | ||
| 16 Remote happy memory | −.02 | .18 | −.18 | .18 | .21 | .00 | .38 | .32 | .30 | .01 | .28 | −.12 | .26 | −.18 | .12 | – | |
| 17 Trauma memory | .12 | .27 | −.07 | .15 | .15 | −.11 | .05 | .22 | .09 | .00 | .19 | −.26 | .14 | −.19 | −.10 | .48 | – |
| 18 Mother’s trauma memory | .06 | −.01 | .02 | −.02 | .32 | −.10 | −.17 | −.09 | −.13 | .14 | .05 | −.13 | .14 | −.26 | −.12 | .37 | .47 |
Note. PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia.
In terms of RSA reactivity, there were no significant bivariate relationships between Time 1 reactivity variables and Time 1 or Time 2 PTSD symptoms. The only significant relationship between Time 2 reactivity and Time 2 PTSD symptoms was for reactivity during the joy video (r = −0.44, p = 0.03).
Time 1 neutral RSA level predicting Time 2 PTSD symptoms
To test the first hypothesis, a multiple linear regression was conducted with Time 1 neutral RSA level predicting Time 2 PTSD symptoms, controlling for Time 2 neutral RSA level, as well as Time 1 age and PTSD symptoms. The full model including all predictors explained 51% of variance in Time 2 PTSD symptoms (R squared = 0.51, SE = 0.15, z = 3.37, p = .001; Adjusted R squared = 0.45). Results presented in Table 3 show Time 1 neutral RSA level was a significant predictor (β = −0.38, z = −2.21, p = .027) of Time 2 PTSD symptoms, such that lower neutral RSA level was related to higher PTSD symptoms. The relationship between Time 1 neutral RSA level and Time 2 PTSD symptoms is depicted in Figure 1.
Table 3.
Results from a Multiple Linear Regression of Neutral RSA Level Predicting Time 2 PTSD Symptoms
| Variable | B | SE B | β | z | p |
|---|---|---|---|---|---|
| Time 1 age | 0.48 | 0.70 | .12 | 0.68 | .500 |
| Time 1 PTSD symptoms | 0.57 | 0.15 | .62 | 3.84 | <.001 |
| Time 1 neutral RSA level | −2.01 | 0.91 | −.38 | −2.21 | .027 |
| Time 2 neutral RSA level | 0.73 | 1.44 | .13 | 0.51 | .613 |
Note. PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia.
Figure 1.
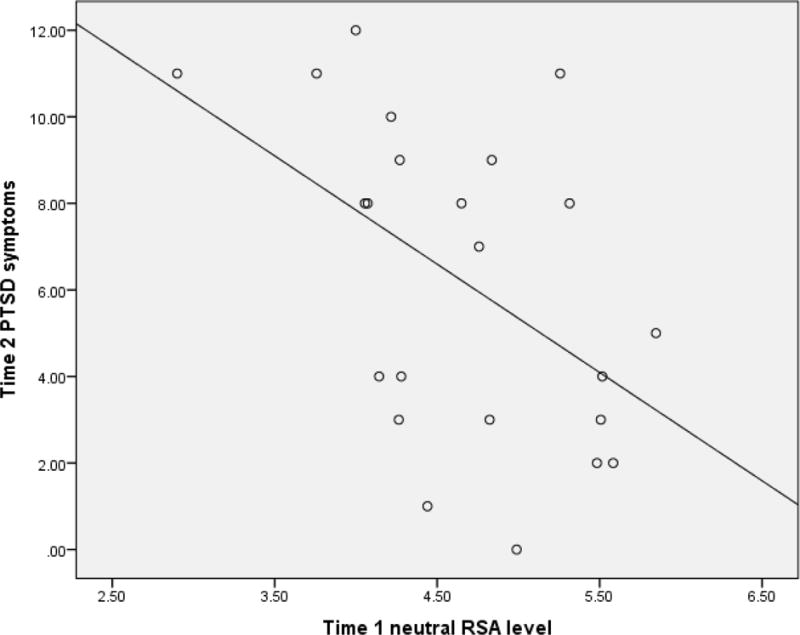
Graph depicting the relationship between Time 1 respiratory sinus arrhythmia (RSA) level during the neutral condition (natural log transformed) and Time 2 joint-reported posttraumatic stress disorder (PTSD) symptoms.
Time 1 RSA reactivity predicting Time 2 PTSD symptoms
To test the second hypothesis, six multiple linear regressions were conducted, one for each of the RSA reactivity stimuli. For each stimulus, the model included Time 1 RSA reactivity as a predictor of Time 2 PTSD symptoms, controlling for Time 2 RSA reactivity, as well as Time 1 age and PTSD symptoms. Table 4 shows the results from three models testing the video stimuli (distress, joy, and trauma videos). Time 1 RSA reactivity in response to video stimuli was not a significant predictor of Time 2 PTSD symptoms in any model. The only significant predictor of Time 2 PTSD symptoms was Time 1 PTSD symptoms. Table 5 shows the results from the three models testing the memory stimuli (remote happy memory, trauma memory, mother’s recall of the trauma memory). Time 1 RSA reactivity in response to memory stimuli and Time 1 PTSD symptoms were significant predictors of Time 2 PTSD symptoms in all three models. It is important to note that a negative reactivity (change score) value suggests a decrease in RSA from neutral condition to stimulus, whereas a positive value suggests an increase in RSA from neutral condition to stimulus. The pattern of results suggests that RSA increase from neutral condition to stimulus (or less vagal withdrawal) was related to higher PTSD symptoms. The relationship between Time 1 RSA reactivity during the trauma memory and Time 2 PTSD symptoms is depicted in Figure 2.
Table 4.
Results from Multiple Linear Regressions of RSA Reactivity to Video Stimuli Predicting Time 2 PTSD Symptoms
| Variable | B | SE B | β | z | p |
|---|---|---|---|---|---|
| Distress video | |||||
| Time 1 age | 0.66 | 0.70 | .17 | 0.94 | .347 |
| Time 1 PTSD symptoms | 0.62 | 0.13 | .66 | 4.65 | <.001 |
| Time 1 RSA reactivity | 0.01 | 0.01 | .06 | 0.46 | .648 |
| Time 2 RSA reactivity | 0.02 | 0.01 | .18 | 1.33 | .185 |
|
| |||||
| Joy video | |||||
| Time 1 age | 0.54 | 0.75 | .13 | 0.71 | .475 |
| Time 1 PTSD symptoms | 0.59 | 0.16 | .62 | 3.74 | <.001 |
| Time 1 RSA reactivity | −0.02 | 0.01 | −.17 | −1.08 | .281 |
| Time 2 RSA reactivity | −0.02 | 0.02 | −.21 | −1.40 | .161 |
|
| |||||
| Trauma video | |||||
| Time 1 age | 0.76 | 0.75 | .19 | 1.01 | .311 |
| Time 1 PTSD symptoms | 0.57 | 0.14 | .61 | 3.96 | <.001 |
| Time 1 RSA reactivity | 0.01 | 0.01 | .11 | 0.82 | .413 |
| Time 2 RSA reactivity | 0.00 | 0.01 | .02 | 0.14 | .892 |
Note. PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia.
Table 5.
Results from Multiple Linear Regressions of RSA Reactivity to Memory Stimuli Predicting Time 2 PTSD Symptoms
| Variable | B | SE B | β | z | p |
|---|---|---|---|---|---|
| Remote happy memory | |||||
| Time 1 age | 0.74 | 0.58 | .18 | 1.27 | .203 |
| Time 1 PTSD symptoms | 0.66 | 0.12 | .68 | 5.36 | <.001 |
| Time 1 RSA reactivity | 0.03 | 0.02 | .26 | 1.96 | .050 |
| Time 2 RSA reactivity | <0.01 | 0.01 | .06 | 0.44 | .659 |
|
| |||||
| Trauma memory | |||||
| Time 1 age | 0.80 | 0.60 | .20 | 1.34 | .180 |
| Time 1 PTSD symptoms | 0.69 | 0.13 | .73 | 5.31 | <.001 |
| Time 1 RSA reactivity | 0.03 | 0.01 | .30 | 2.37 | .018 |
| Time 2 RSA reactivity | −0.01 | 0.01 | −.09 | −0.68 | .497 |
|
| |||||
| Mother’s trauma memory | |||||
| Time 1 age | 0.85 | 0.59 | .21 | 1.43 | .152 |
| Time 1 PTSD symptoms | 0.67 | 0.12 | .70 | 5.50 | <.001 |
| Time 1 RSA reactivity | 0.03 | 0.01 | .27 | 2.35 | .019 |
| Time 2 RSA reactivity | 0.01 | 0.01 | .08 | 0.75 | .451 |
Note. PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia.
Figure 2.
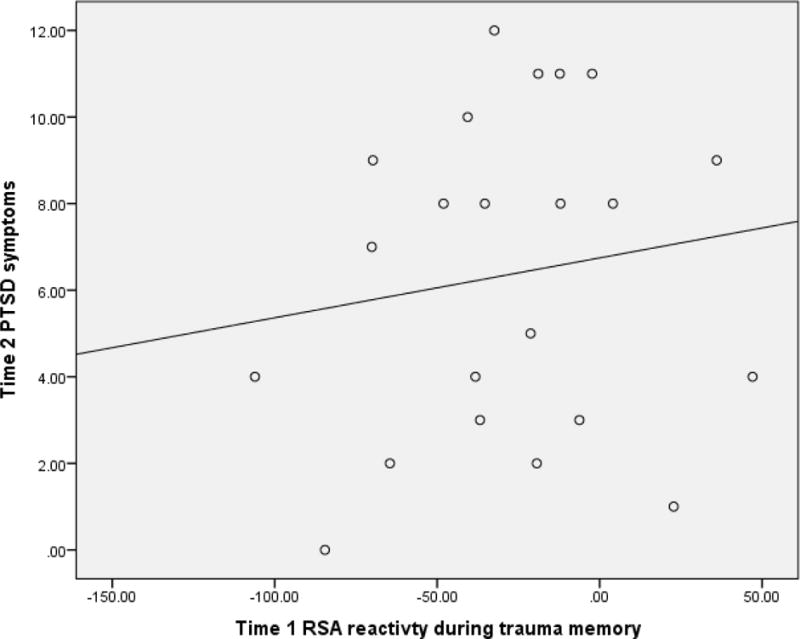
Graph depicting the relationship between Time 1 respiratory sinus arrhythmia (RSA) reactivity during the trauma memory condition and Time 2 joint-reported posttraumatic stress disorder (PTSD) symptoms. Negative values for RSA reactivity indicate a decrease from the neutral condition to the trauma memory condition.
Discussion
Despite interest in the relationship between psychophysiology and PTSD, there is a lack of studies examining the prospective relationships among pre-trauma functioning and post-trauma outcomes. Further, very few studies have examined the relationship between psychophysiological variables and PTSD in children. The goal of the current study was to begin to fill these gaps in the literature by examining the ability of RSA level and reactivity, potential clinical markers of stress sensitivity, to predict post-disaster psychopathology in a sample of children.
Previous cross-sectional studies in adults have generally shown lower baseline RSA among those with PTSD compared to healthy controls (Blechert et al. 2007; Cohen et al. 2000; Hauschlidt et al. 2011); however, the few previous studies of children have not shown significant differences without taking contextual factors into consideration (Scheeringa et al. 2004; Katz and Gurtovenko 2015). Similarly, the current study found no significant concurrent associations between neutral RSA level and PTSD symptoms. Rather, in line with predictions, lower Time 1 neutral RSA level was a significant prospective predictor of Time 2 PTSD symptoms when examining the simple bivariate relationship, and when controlling for other variables (i.e., Time 1 PTSD symptoms, age, and Time 2 neutral RSA level). These findings provide evidence to support what McLaughlin et al. (2015) concluded from their cross-sectional study, that low baseline RSA may be a clinical marker of stress sensitivity that predicts psychopathology following a stressor, whereas high baseline RSA may act as a buffer. This is consistent with other suggestions that higher baseline RSA is related to quicker physiological adaptation during stress (Lane et al. 1992).
No significant relationships were found between Time 1 RSA reactivity to video stimuli and Time 2 PTSD symptoms for any of the video stimuli (distress, joy, or trauma conditions). In contrast, significant relationships were found between Time 1 RSA reactivity to each of the memory stimuli and Time 2 PTSD symptoms when controlling for Time 2 RSA reactivity and other variables (i.e., Time 1 PTSD symptoms, age). The results showed that Time 1 RSA reactivity was a better predictor of Time 2 PTSD symptoms than Time 2 RSA reactivity. Further, individuals who showed the least decrease in RSA during the memory stimuli (least vagal withdrawal) had the highest PTSD symptoms. While there are no similar prospective studies of PTSD to compare these results to, these findings are consistent with the polyvagal theory, in that RSA suppression is conceptualized as a positive index of emotional regulation, and unreliable RSA modulation is a risk index for emotional dysregulation and psychiatric disorders (Porges 2007).
It is interesting that RSA reactivity to the memory stimuli (trauma memory, mother’s trauma memory) was prospectively related to PTSD symptoms (i.e., less vagal withdrawal was related to more PTSD symptoms) but RSA reactivity to the trauma video stimulus was not related to PTSD symptoms. It may be the case that the autobiographical nature of the memory stimulus is necessary for the relationship to trauma-related symptomatology. It was also somewhat surprising that RSA reactivity to all three memory stimuli was predictive of PTSD, regardless of the valence of the memory. That is, it was expected that RSA reactivity to the trauma-related memories would be predictive of PTSD symptoms, but it was unexpected that RSA reactivity to the happy memory would be predictive of PTSD symptoms and show the same pattern (i.e, less vagal withdrawal related to more PTSD symptoms). However, Hauschildt et al. (2011) also found similar RSA reactivity across several different affective conditions in adults with PTSD, regardless of valence.
The authors know of no previous studies that have examined baseline/neutral RSA level or RSA reactivity both prior to and following trauma in a sample of children. However, our findings are consistent with a growing body of evidence which suggest that some indicators of maladaptive physiological functioning may be premorbid risk factors, rather than consequences, of trauma and/or PTSD (Apfel et al. 2011; Galatzer-Levy et al. 2014; Guthrie and Bryant 2005; McLaughlin et al. 2014).
While the current study involved a rare opportunity for a natural experiment examining the prospective development of trauma symptomatology, there were limitations to note. First, in the assessment of PTSD symptoms, only the caregiver was interviewed at the Time 1 assessment due to the young age of the children. While caregivers are generally better reporters of symptomatology for very young children, they tend to underestimate symptoms (Scheeringa et al. 2006). At Time 2, both caregiver and child interviews were completed, however some symptoms were not assessed with child-report. Second, the PTSD criteria for children 6 years and younger were used to establish symptom counts for all children at both time points for the sake of consistency, but not all children were actually in this age range at Time 2. Although as previously noted, recent work has supported the use of these criteria in older children (Meiser-Stedman et al. 2017; Mikolajewski et al. 2017). Third, given the nature of the traumatic event which resulted in mandatory evacuation and displacement of the community, it was not possible to assess the effects of the traumatic event until several months after its occurrence. Additionally, contextual factors that might influence young children’s physiological states include parental factors (Skowron et al. 2013), and these may be important to investigate in future studies. Finally, another limitation is the small sample size. With limited power, it was not feasible to look at subgroups (e.g., initial status as control or trauma-exposed), to examine interaction effects, or to control for comorbid psychopathology. Sample size should be increased in future research for conducting more accurate explanatory analyses.
Designing the perfect prospective study of pre-trauma physiological variables predicting post-trauma symptoms is difficult, and maybe impossible. However, there are several important directions for future research. Whereas the current study examined PTSD as a sum of all symptoms present, given the heterogeneity of PTSD symptoms, it may be the case that there are more specific prospective relationships between baseline/neutral RSA level or RSA reactivity and certain clusters of PTSD symptoms (Michopoulos et al. 2015). Further, there is some evidence for an interaction between contextual factors (e.g., discipline style, parental PTSD symptoms) and baseline RSA level, and it may be important to elucidate what type of environmental factors lead to the greatest increase in risk. Finally, whereas the current study showed low neutral RSA level may be a risk factor for later psychopathology, no conclusions from these results can be made about the etiology of the low neutral RSA level. It may be that adverse environmental factors, genetic influences, or epigenetic effects initially lead to low baseline RSA, which then acts as a diathesis, putting those children exposed to a later trauma at higher risk for developing PTSD. Research specifically focused on the etiological factors affecting baseline RSA is needed to test these possibilities.
Conclusion
While a great deal of research has established a link between maladaptive psychophysiological functioning and PTSD, evidence of the directionality of these relationship remains inconclusive given the difficulty of conducting longitudinal studies involving trauma exposure. The current study was unique in that the researchers, by chance, began collecting data prior to a natural disaster that devastated the community, allowing for a prospective study of the ability of neutral RSA level and RSA reactivity to predict PTSD symptoms. The results showed that RSA level and reactivity measured prior to the disaster was a better predictor of later PTSD symptoms than RSA measured post-disaster, and provides evidence for RSA level and reactivity as pre-existing clinical markers of stress sensitivity that predict psychopathology following a trauma.
Acknowledgments
This research was supported by National Institute of Mental Health (R01 MH065884).
The authors thank the Medical Center of Louisiana Charity Hospital Trauma Center, Crescent House, Metropolitan Battered Women’s Program, St. Bernard Battered Women’s Program, and Children’s Bureau of Greater New Orleans for their cooperation.
Footnotes
ORCID: Amy J. Mikolajewski, 0000-0002-9085-1727
ORCID: Michael S. Scheeringa, 0000-0002-5775-313X
Contributor Information
Amy J. Mikolajewski, Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine
Michael S. Scheeringa, Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine
References
- Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clinical Autonomic Research. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, Virginia: American Psychiatric Publishing; 2013. [Google Scholar]
- Apfel BA, Otte C, Inslicht SS, McCaslin SE, Henn-Haase C, Metzler TJ, Makotkine I, Yehuda R, Neylan TC, Marmar CR. Pretraumatic prolonged elevation of salivary MHPG predicts preitraumatic distress and symptoms of post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45:735–741. doi: 10.1016/j.jpsychires.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A. The Preschool Age Psychiatric Assessment (PAPA): a structured parent interview for diagnosing psychiatric disorders in preschool children. In: DelCarmen-Wiggins R, Carter A, editors. Handbook of Infant, Toddler, and Preschool Mental Assessment. New York: Oxford University Press; 2004. pp. 223–243. [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psyciatric Assessment (PAPA) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Steenkamp MM, Brown AD, Qian M, Inslicht S, Henn-Haase C, Otte C, Yehuda R, Neylan TC, Marmar CR. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. Journal of Psychiatric Research. 2014;56:36–42. doi: 10.1016/j.jpsychires.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Auditory startle response in firefighters before and after trauma exposure. American Journal of Psychiatry. 2005;162:283–290. doi: 10.1176/appi.ajp.162.2.283. [DOI] [PubMed] [Google Scholar]
- Hauschildt M, Peters MJV, Moritz S, Jelinek L. Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biological Psychology. 2011;88:215–222. doi: 10.1016/j.biopsycho.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gurtovenko K. Posttraumatic stress and emotion regulation in survivors of intimate partner violence. Journal of Family Psychology. 2015;29:528–536. doi: 10.1037/fam0000128. [DOI] [PubMed] [Google Scholar]
- Kirsch V, Wilhelm FH, Goldbeck L. Psychophysiological characteristics of pediatric posttraumatic stress disorder during script-driven traumatic imagery. European Journal of Psychotraumatology. 2015;6 doi: 10.3402/ejpt.v6.25471. doi: 10.3402/ejpt.v6.25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Adcock A, Burnett RE. Respiratory sinus arrhythmia and cardiovascular responses to stress. Psychophysiology. 1992;29:461–470. doi: 10.1111/j.1469-8986.1992.tb01720.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green AG, Alves S, Way M, Sheridan MA. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Rith-Najarian L, Dirks MA, Sheridan MA. Low vagal tone magnifies the association between psychosocial stress exposure and internalizing psychopathology in adolescents. Journal of Clinical Child & Adolescent Psychology. 2015;44:314–328. doi: 10.1080/15374416.2013.843464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser-Stedman R, McKinnon A, Dixon C, Boyle A, Smith P, Dalgleish T. Acute stress disorder and the transition to posttraumatic stress disorder in children and adolescents: Prevalence, course, prognosis, diagnostic suitability, and risk markers. Depression and Anxiety. 2017 doi: 10.1002/da.22602. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt KA, Ornstein PA, Spicker B. Children’s memory for a salient medical procedure: Implications for testimony. Pediatrics. 1994;94:17–23. [PubMed] [Google Scholar]
- Michopoulos V, Norrhom SD, Jovanovic T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biological Psychiatry. 2015;78:344–353. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajewski AJ, Scheeringa MS, Weems CF. Evaluating Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) posttraumatic stress disorder diagnostic criteria in older children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2017 doi: 10.1089/cap.2016.0134. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide 7th edn. Los Angeles (CA): Muthen & Muthen; 1998–2015. [Google Scholar]
- Nemeroff CB, Seligman F. The pervasive and persistent neurobiological and clinical aftermath of child abuse and neglect. Journal of Clinical Psychiatry. 2013;74:999–1001. doi: 10.4088/JCP.13com08633. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biological Psychiatry. 2004;55:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biological Psychiatry. 2001;49:637–643. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Wright MJ, Hunt JP, Zeanah CH. Factors affecting the diagnosis and prediction of PTSD symptomatology in children and adolescents. The American Journal of Psychiatry. 2006;163:644–651. doi: 10.1176/ajp.2006.163.4.644. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Zeanah CH, Myers L, Putnum F. Heart period and variability findings in preschool children with posttraumatic stress symptoms. Biological Psychiatry. 2004;55:685–691. doi: 10.1016/j.biopsych.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Myers L, Putnam FW, Zeanah CH. Diagnosing PTSD in early childhood: An empirical assessment of four approaches. Journal of Traumatic Stress. 2012;25:359–367. doi: 10.1002/jts.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BG, Weems CF. Resting vagal tone and vagal response to stress: Associations with anxiety, aggression, and perceived anxiety control among youths. Psychophysiology. 2014;51:718–727. doi: 10.1111/psyp.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Benjamin LS, Pincus AL, Van Ryzin MJ. Cardiac vagal tone and quality of parenting show concurrent and time-ordered associations that diverge in abusive, neglectful and non-maltreating mothers. Couple and Family Psychology: Research and Practice. 2013;22:95–115. doi: 10.1037/cfp0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrickson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depression and Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neuroscience and Biobehavioral Reviews. 2013;37:860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]


