STRUCTURED ABSTRACT
Objectives
To evaluate the utility of intra-atrial dyssynchrony as a marker of underlying LA remodeling to predict recurrence after the first atrial fibrillation (AF) ablation.
Background
Catheter ablation of AF remains far from curative with relatively high recurrence rates. One of the causes of recurrence is poor patient selection out of a diverse patient population with different degrees of LA remodeling.
Methods
We included 208 patients with a history of AF (59.4±10 years, 26.0% nonparoxysmal AF) referred for catheter ablation of AF who underwent pre-ablation cardiac magnetic resonance (CMR) in sinus rhythm. Clinical follow-up was 20 ± 6 months. Using tissue-tracking CMR, we measured the LA longitudinal strain in each of 12 equal-length segments in two- and four-chamber views. We defined Intra-atrial dyssynchrony as the standard deviation of the time to the peak longitudinal strain corrected by the cycle length (SD-TPS, %).
Results
The patients with AF recurrence after ablation (n=101) had significantly higher SD-TPS than those without (n=107; 3.9 vs. 2.2 %, p<0.001). Multivariable cox analysis showed SD-TPS was associated with recurrence after adjusting for clinical risk factors, AF type, LA structure/function and fibrosis (p<0.001). Furthermore, receiver-operator characteristics analysis showed SD-TPS improved prediction of recurrence better than clinical risk factors, LA structure/function and fibrosis.
Conclusions
Intra-atrial dyssynchrony during sinus rhythm is an independent predictor of recurrence after the first catheter ablation of paroxysmal or persistent AF. Assessment of intra-atrial dyssynchrony may improve ablation outcomes by refining patient selection.
Keywords: Pulmonary vein isolation, left atrial, left atrial function, cardiac MRI, atrial fibrillation
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia in human beings, with the incidence increasing with the aging population (1). Catheter ablation involving pulmonary vein isolation (PVI) is the cornerstone treatment for drug-refractory AF (2), but it is associated with a relatively high recurrence rate (2). One of the major causes of recurrence after ablation is poor patient selection out of a diverse patient population with different degrees of left atrial (LA) remodeling (2). Previous studies reported that indices associated with LA remodeling can predict AF recurrence after successful ablation, including the minimum LA volume, LA emptying fraction (3), LA peak longitudinal strain, LA strain rate (3), and LA fibrosis(4). Recently, LA intra-atrial dyssynchrony assessed by three-dimensional (3-D) echocardiogram was also reported to predict AF recurrence in patients with paroxysmal AF (5). It is possible that intra-atrial dyssynchrony reflects the underlying LA remodeling and LA fibrosis better than the other indices regardless of AF type. In this study, we aimed to investigate the role of LA intra-atrial dyssynchrony during sinus rhythm in predicting the outcomes of ablation for both paroxysmal and persistent AF. We hypothesized that LA intra-atrial dyssynchrony of quantified by tissue-tracking cardiac magnetic resonance (CMR) prior to catheter ablation predicts AF recurrence after the first AF ablation, independent of other indices of LA remodeling.
METHODS
Study population
Consecutive patients with symptomatic, drug-refractory AF referred for catheter ablation of AF at the Johns Hopkins Hospital between June 2010 and December 2015 who underwent pre-procedural CMR were included. All patients undergoing AF ablation had pre-ablation imaging, either CMR or cardiac CT. Approximately 50–70% of patients undergo CMR and the remaining 30–50% cardiac CT. The decision to choose CMR over CT is affected by several factors, including patient preference (e.g. claustrophobia), presence of pacemakers/defibrillators, physician preference, and scheduling logistics. The patients who had prior AF ablation or surgical procedure in the LA were excluded. The patients who were in AF at the time of CMR were also excluded (n=37). We also randomly divided our cohort into two groups: Training group (n=103) and test group (n=105). The protocol was approved by the Institutional Review Board and all the patients provided written informed consent.
CMR protocol
CMR was performed with a 1.5-Tesla scanner (Avanto; Siemens Medical Systems, Erlangen, Germany), a 6-channel phased array body coil in combination with a 6-channel spine matrix coil. An electrocardiogram (ECG)-gated, breath-holding cine CMR images were acquired in the long axis two- and four-chamber views by True Fast Imaging with Steady-State Precession (TrueFISP) sequence with the following parameters: TE/TR 3.0/1.5 ms; flip angle 78°; in-plane pixel size 1.5×1.5 mm2; slice thickness 8 mm; slice spacing 2 mm; 30 frames per ECG R-R interval with a temporal resolution of 20–40 ms. A fraction of patients also underwent respiratory-navigated, ECG-gated late gadolinium enhancement (LGE) to quantify LA fibrosis. LGE images were acquired within 15–25 minutes following the injection of gadopentetate dimeglumine (0.2 mmol/kg, Bayer Healthcare Pharmaceuticals, Montville, NJ) using a fat-saturated 3-D inversion recovery-prepared fast spoiled gradient-recalled echo sequence with the following parameters: TE/TR 1.52/3.8 ms; flip angle 10°; in-plane pixel size 1.3×1.3 mm2; slice thickness 2.0 mm. The trigger time for 3-D LGE images was optimized to acquire imaging data during LA diastole as determined by the cine CMR images. The optimal inversion time was determined by an inversion time scout scan (median 270 ms; range 240–290 ms) to maximize nulling of the LA myocardium. The endocardial and epicardial contours were manually draw around the LA myocardium (Figure 1). The image intensity ratio (IIR) (6) was measured to quantify LA fibrosis using QMass MR (version 7.2; Leiden University Medical Center, Leiden, The Netherlands) on axial images from 3-D axial image data (Figure 1). The IIR threshold of 1.22 that corresponds to bipolar voltage 0.3 mV on intracardiac electrogram was used to define LA fibrosis (7).
Figure 1. Left atrial late gadolinium enhancement CMR.
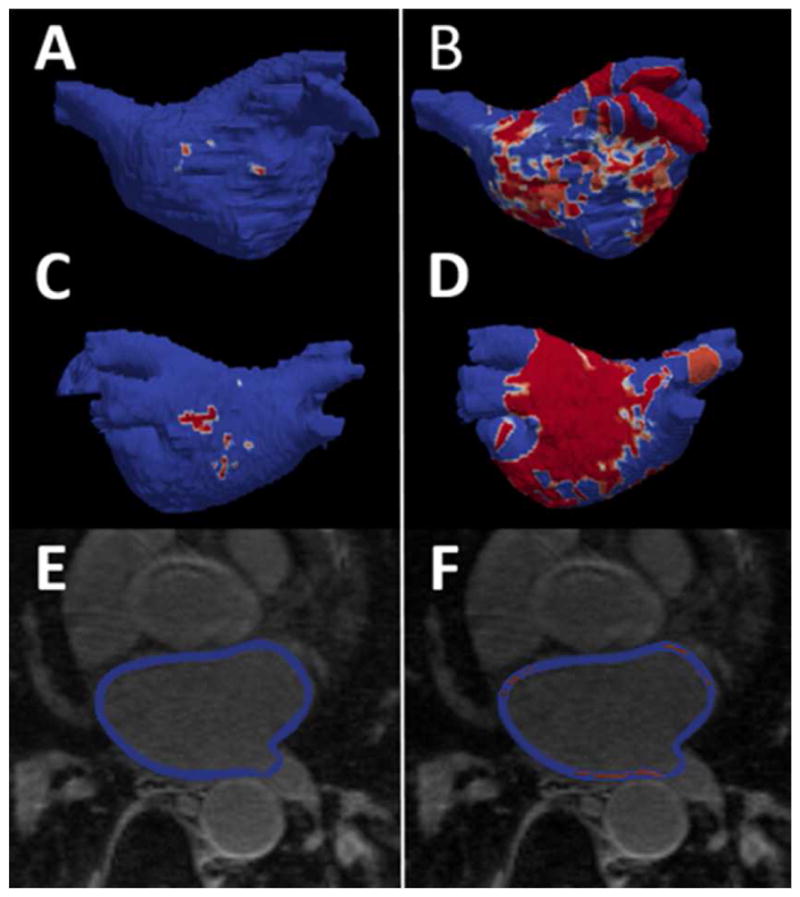
A – B, anterior LA shell view with areas of enhancement (red). C – D, posterior LA shell view with areas of enhancement (red). E – F quantification of LA enhancement by CMR using IIR, areas of enhancement (red). Left side (A, C, and E) individual with low enhancement – right side (B, D and F) individual with high enhancement. CMR, cardiac magnetic resonance; IIR, image intensity ratio.
Ablation
PVI catheter ablation of AF was performed using an electroanatomic mapping system with an image integration module (CARTO and CartoMerge®, Biosense Webster) to merge pre-procedural CMR (8). The electrical isolation of the pulmonary veins were confirmed by a circular multipolar electrode mapping catheter (Lasso, Biosense Webster). In cases of persistent AF, the ablation procedure usually included additional ablation lesions (8). Ablation was performed with either an open-irrigated radiofrequency (RF) ablation catheter with or without force sensing, or a cryoballoon ablation catheter.
Clinical follow-up
AF recurrence was defined as any episodes of AF, atrial flutter, or atrial tachycardia lasting more than 30 seconds after a three-month blanking period (9). All medications, including antiarrhythmic and anticoagulation drugs, were continued in all patients during the blanking period. Patients were seen in clinic 10 to 12 weeks following ablation. At the clinic visit, antiarrhythmic and anticoagulation drugs were discontinued at the discretion of the physician according to patient’s stroke risk. Then the patients were followed by the referring physician on a regular basis. In addition, the patients received direct telephone interviews or emails with standardized questionnaire to report AF symptoms, if any. The patients were excluded if they were not followed for at least 6 months after ablation. When recurrence was suspected, the patients underwent a 24-hour Holter monitor or a 30-day event monitor depending on the symptom frequency.
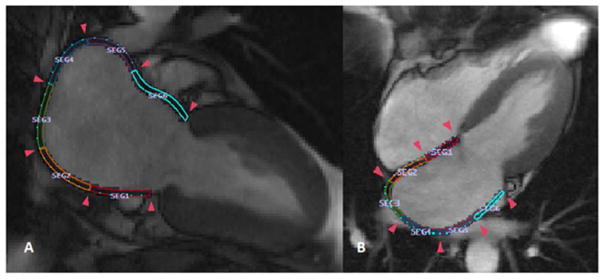
LA dyssynchrony analysis
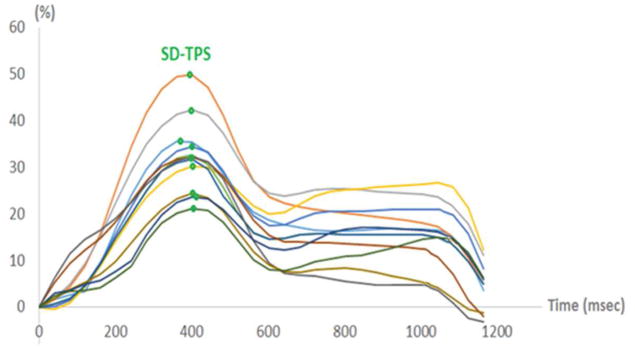
On cine CMR images, the LA endocardial and epicardial borders at the LA end diastole were defined by an operator with more than 3 years of experience in performing LA measurements by CMR, who was also blinded to the group assignment (Figure 2). The multimodality tissue tracking (MTT) software (version 6.1, Toshiba, Japan) automatically divides the LA in six equi-length segments in each of the two- and four-chamber views, creating a total of 12 segments (Figure 2). Longitudinal strain was calculated within each of the 12 segments (Figure 3). Based on those time series we defined the standard deviation (SD) of the time to the peak longitudinal strain (TPS). We then corrected SD-TPS by the cycle length to derive SD-TPS as a percentage of the cycle length ( ). SD-TPS (%) quantifies intra-atrial dyssynchrony of the LA reservoir function, and higher values reflect greater degrees of intra-atrial dyssynchrony.
Figure 2. Quantification of left atrial regional function using cine cardiac magnetic resonance.
The figures show a total of 12 color-coded 12 segments within the left atrium. A, Two-chamber view with six equi-length segments; B, Four-chamber view with six equi-length segments.
Figure 3. Strain curves for all 12 segments.
Green dots indicate the maximum left atrial (LA) longitudinal strain for each segment. SD-TPS is calculated as the standard deviation of the time to reach the maximum LA longitudinal strain of all segments.
Statistical analysis
Baseline patient demographics are presented as mean±SD or percentage, and are compared using Student’s t-test, X2 and, Fisher exact test as appropriate. Multivariable cox proportional-harzards models were used to evaluate the effects of LA dyssynchrony on AF recurrence after ablation, and predictors that had a p-value < 0.25 in univariable analysis were included in multivariable models. Four models are presented: Model 1: unadjusted; Model 2: Adjusted for clinical characteristics (age, sex, type of AF, body mass index (BMI), history of heart failure, hypertension, and obstructive sleep apnea [OSA]); Model 3: Model 2+ minimum LA volume (Vmin) and maximum longitudinal LA strain (Smax). Model 4: Model 3 + %LA fibrosis. Correlation between intra-atrial dyssynchrony (SD-TPS), maximum LA longitudinal strain (Smax) and %LA fibrosis was defined by the Pearson correlation coefficient. Time-to-first recurrence is presented using Kaplan-Meier curves. A log-rank test was conducted to detect differences in the survival distributions for two curves, the two curves were generated based on receiver operating characteristic (ROC) best cut point using the Liu’s criteria on the training group. ROC curves were also generated to evaluate the additional value of intra-atrial dyssynchrony over LA structure and function in predicting AF recurrence. Comparison between the values of c-statistics was based on the method of DeLong et al. (10) In a subset of randomly selected patients (n =15) intra-and inter-observer reproducibility was performed, and the intra-class correlation coefficient (ICC) with a two-way random model (ICC, <0.40, poor; ICC >0.40–0.75, fair to good; and ICC >0.75, excellent agreement) was evaluated. The significance level of 0.05 was used for all hypothesis tests, and all t-tests were 2-sided. The statistical computations were performed using the STATA (version 12.0, StataCorp).
RESULTS
Patient demographics
A total of 208 patients were included in the final analysis. Table 1 summarizes the patients’ clinical characteristics. There were 61 (29.3%) female patients, and the average age was 59±10 years (interquartile range [IQR], 51 – 66). A total of 101 patients (48.6%; recurrence group) developed recurrence after ablation. Compared with the AF-free group, the patients in the recurrence group were older (mean 61.1 vs. 57.1, p=0.023), had higher BMI (30.1 vs. 27.7, p=0.002) and higher CHA2DS2 -VASC scores (1.9 vs. 1.3, p = 0.006) prior to ablation. The patients in the recurrence group were also more likely to have history of hypertension, heart failure, OSA and persistent AF than those in the AF-free group. Seventy percent of the patients (n=146) included in the final analysis underwent respiratory-navigated, ECG-gated LGE to quantify LA fibrosis. Except for hypertension and Ca-channel blockers use, which were higher in the training group, other clinical characteristics were similar between the training and test groups. Four of 101 patients (3.9%) in the recurrence group and two of 107 patients (1.9%) in the AF-free group underwent cardioversion within 3–4 weeks prior to CMR (p=0.49). Acute PVI was achieved in all patients.
Table 1.
Baseline characteristics.
| Recurrence, n = 101 | AF-free, n = 107 | p | |
|---|---|---|---|
| Clinical | |||
| Age, years | 61.1 ± 9.5 | 57.1 ± 10.9 | 0.023 |
| Body mass index, Kg/m2 | 30.1 ± 5.7 | 27.7 ± 5.6 | 0.002 |
| Male, n (%) | 78.0 (77.2) | 67.0 (62.7) | 0.044 |
| Persistent AF, n (%) | 34.0 (33.6) | 20.0 (18.7) | 0.014 |
| Heart failure, n (%) | 17.0 (16.8) | 7.0 (6.5) | 0.020 |
| Coronary artery disease/vascular disease, n (%) | 14.0 (13.8) | 6.0 (5.6) | 0.044 |
| Diabetes, n (%) | 11.0 (10.9) | 11.0 (10.2) | 0.886 |
| Hypertension, n (%) | 55.0 (54.4) | 40.0 (37.4) | 0.013 |
| History of Stroke/TIA, n (%) | 10.0 (9.9) | 4.0 (3.7) | 0.076 |
| CHA2DS2 -VASC | 1.9 ± 1.5 | 1.3 ± 1.4 | 0.006 |
| Obstructive sleep apnea, n (%) | 23.0 (22.7) | 9.0 (8.4) | 0.004 |
| Ablation strategy (Crioablation), n (%) | 17.0 (16.8) | 22.0 (20.1) | 0.491 |
| LVEF, % | 54.7 | 59.5 | 0.098 |
| Medication | |||
| ACEI/ARBS, n (%) | 35.0 (34.7) | 26.0 (24.3) | 0.055 |
| Beta-Blockers, n (%) | 50.0 (49.5) | 55.0 (51.4) | 0.628 |
| Ca-channel blockers, n (%) | 24.0 (23.8) | 23.0 (21.5) | 0.777 |
| Anticoagulant use, total (%) | 94.0 (93.1) | 92.0 (86.0) | 0.375 |
| Number of antiarrhythmic drugs | 1.4.0 ± 0.8 | 1.2.0 ± 0.8 | 0.134 |
Data are presented as mean ± standard deviation, n (%), or median. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers.
Intra-atrial dyssynchrony, recurrence and LA fibrosis
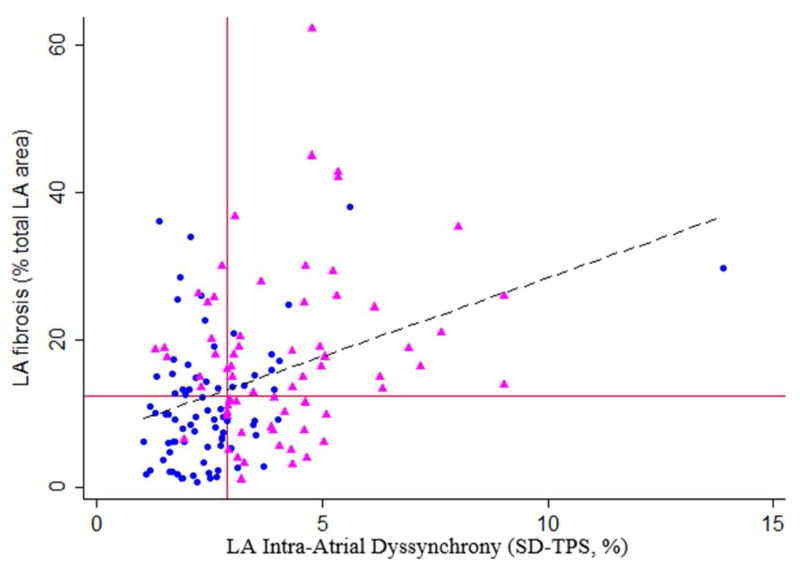
Out of 2,496 LA segments analyzed in a total of 208 patients, a total of 29 segments (1.2%) were excluded from the final analysis because they lacked well-defined peaks in the strain curves. Our results showed an excellent intra-reader and inter-reader reproducibility, with ICC of 0.86 for both indices, with respective mean difference of 0 and −0.05. The patients in the recurrence group had higher maximum and minimum LA volumes, lower total, passive and active LAEF, and lower strain/strain rates (Table 2). In addition, SD-TPS was significantly higher in the recurrence group than that of the AF-free group (3.9 vs. 2.2 %, p <0.001) (Table 2). The LA dyssynchrony analysis was performed in a consistent manner in all cases and took approximately 9 min per case. Figure 4 shows the relationship between intra-atrial dyssynchrony (SD-TPS [%], x-axis) and LA fibrosis (%total LA area). The correlation coefficient (r) between intra-atrial dyssynchrony and LA fibrosis was 0.34 (p <0.001). The correlation coefficient between the maximum LA longitudinal strain and LA fibrosis was −0.21 (p =0.004). All LA structure/function indices were similar between the training and test groups. The LA fibrosis analysis was also performed in a consistent manner in all cases and took approximately 30 min per case (4).
Table 2.
Indices of LA structure and function by groups.
| Recurrence, n = 101 | AF-free, n = 107 | ||||
|---|---|---|---|---|---|
| LA Structure/Function | Mean | CI | Mean | CI | p |
| Minimum LA volume, mm3/m2 | 23.9 ± 9.2 | 22.1 – 25.7 | 18.9 ± 7.3 | 17.5 – 20.3 | <0.001 |
| Maximum LA volume, mm3/m2 | 41.3 ± 10.8 | 39.2 – 43.4 | 38.0 ± 10.4 | 36.0 – 40.0 | 0.02 |
| Total LAEF, % | 43.4 ± 11.0 | 41.2 – 45.6 | 51.2 ± 9.3 | 49.4 – 53.0 | <0.001 |
| Passive LAEF, % | 19.6 ± 7.2 | 18.2 – 21.0 | 24.5 ± 6.3 | 23.3 – 25.8 | <0.001 |
| Active LAEF, % | 29.8 ± 11.3 | 27.5 – 32.0 | 35.3 ± 11.0 | 33.2 – 37.4 | 0.005 |
| Smax, % | 22.6 ± 6.7 | 21.3 – 23.9 | 32.4 ± 9.2 | 30.6 – 34.1 | <0.001 |
| SpreA, % | 12.0 ± 4.8 | 11.1 – 13.0 | 15.5 ± 6.5 | 14.2 – 16.7 | <0.001 |
| SRmax | 0.93 ± 0.33 | 0.87 – 1.00 | 1.3 ± 0.47 | 1.2 – 1.39 | <0.001 |
| SRe | −0.76 ± 0.07 | − (0.83 – 0.69) | −1.2 ± 0.48 | − (1.30 – 1.11) | <0.001 |
| SRa | −1.05 ± 0.53 | − (1.16 – 0.95) | −1.5 ± 0.53 | − (1.58 – 1.38) | <0.001 |
| Dyssynchrony | Median | IQR | Median | IQR | P |
| SD-TPS, % | 3.9 | 2.9 – 5.1 | 2.2 | 1.8 – 2.8 | <0.001 |
| mean-TPS, ms | 396.6 | 368.6 – 427.7 | 403.7 | 379.2 – 425.1 | 0.308 |
| Recurrence, n = 68 | AF-free, n = 78 | ||||
| Median | IQR | Median | IQR | P | |
| LA fibrosis, % total LA area | 15.5 | 10.2 – 22.7 | 9.1 | 9.4 – 14.7 | <0.001 |
Data are presented as median (IQR - interquartile range), or mean ± standard deviation (SD). CI – Confidence interval, LA, left atrium; LAEF, LA emptying fraction; Smax, maximum longitudinal LA strain; SRmax, peak longitudinal strain rate; Sre, early diastolic strain rate; Sra, late diastolic strain rate; TPS, time to peak strain; SpreA, pre-atrial contraction strain; LGE, late gadolinium enhancement.
Figure 4. Correlation between intra-atrial dyssynchrony and left atrial fibrosis.
Red lines, the median for SD-TPS and left atrial (LA) fibrosis; Blue circles, patients in the AF-free group; Pink triangle, patients in the recurrence group. Broken black line, linear regression line.
Multivariable analyses
In Model 1, a univariable (unadjusted) analysis identified age, BMI, AF type, history of heart failure, OSA, minimum LA volume, Smax, and SD-TPS as a contributors of AF recurrence (Table 3). The patients with SD-TPS <2.86 % had protective hazards for recurrence compared with SD-TPS ≥ 2.86 % (HR 3.90, p <0.001). After adjusting for age, sex, BMI, AF type, history of heart failure, OSA, hypertension, Vmin and Smax, SD-TPS remained significantly associated with higher hazards of recurrence (HR 1.21, p<0.001; MODEL 4 in Table 3).
Table 3.
Multivariable analyses.
| Model 1 Unadjusted |
Model 2 Clinical variables |
Model 3 Model 2 + Vmin+ Smax |
Model 4 Model 3 + LA fibrosis |
|||||
|---|---|---|---|---|---|---|---|---|
| Total cohort, n = 208 | HR | p | HR | p | HR | p | HR | p |
| Clinical variables | ||||||||
| Age | 1.02 | 0.036 | ||||||
| Sex (Male) | 0.79 | 0.306 | ||||||
| Body mass index, Kg/m2 | 1.07 | <0.001 | ||||||
| AF type (Persistent) | 1.65 | 0.017 | ||||||
| Hypertension | 1.42 | 0.079 | ||||||
| History of heart failure | 2.64 | <0.001 | ||||||
| History of DM | 1.18 | 0.597 | ||||||
| OSA | 1.64 | 0.037 | ||||||
| Ablation modality (Cryoablation) | 1.04 | 0.884 | ||||||
| MRI variables | ||||||||
| Vmin | 1.04 | <0.001 | 1.04 | 0.001 | ||||
| Smax, % | 0.91 | <0.001 | 0.93 | <0.001 | ||||
| SD-TPS, % | 1.25 | <0.001 | 1.27 | <0.001 | 1.21 | <0.001 | ||
| SD-TPS ≥ 2.8 % vs < 2.8 % |
4.71 | <0.001 | 5.07 | <0.001 | 3.90 | <0.001 | ||
| Subset with available LA-LGE, n= 146 | ||||||||
| LA fibrosis (%total LA area) | 1.61 | 0.002 | ||||||
| SD-TPS, % | 1.24 | <0.001 | 1.38 | <0.001 | 1.21 | 0.003 | 1.23 | 0.003 |
| SD-TPS ≥ 2.8 % vs < 2.8 % |
2.68 | <0.001 | 3.08 | <0.001 | 2.10 | 0.022 | 4.70 | <0.001 |
HR, Hazard ratio; CI, confidence interval; Vmin, minimum LA volume. Abbreviations as in Table 2. Model 1, unadjusted. Model 2, adjusted for age, sex, type of atrial fibrillation, body mass index, history of heart failure, hypertension, obstructive sleep apnea. Model 3, co-variables included in model 2 in addition to minimum LA volume, and maximum LA longitudinal strain. Model 4, co-variables included in model 3 in addition to LA fibrosis.
Intra-atrial dyssynchrony predicts AF recurrence after ablation
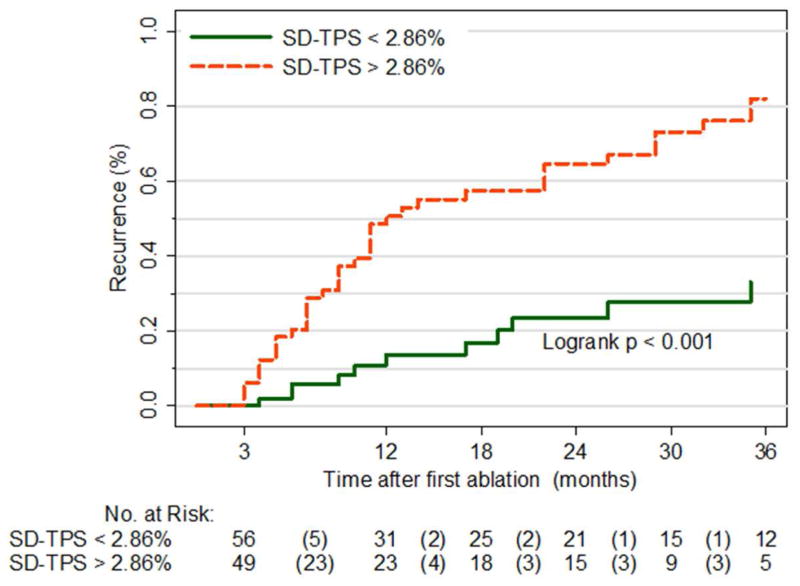
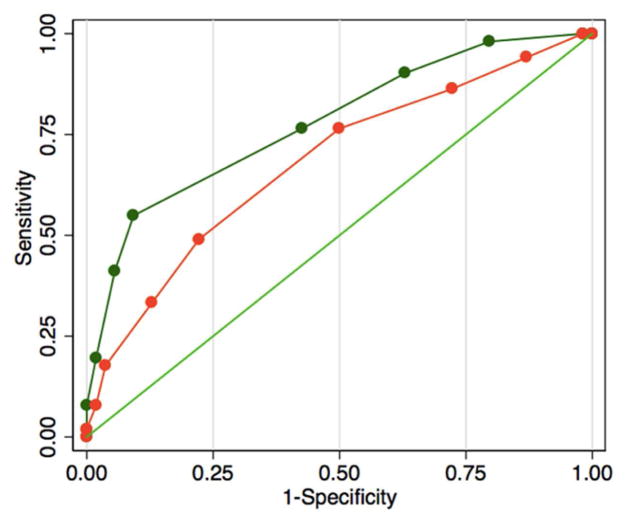
We used the training group to define a cutoff of SD-TPS (<2.86 % vs. >2.86 %) based on the ROC curve, and applied the cutoff to the test group. We found that AF recurrence was significantly higher in patients with higher SD-TPS (p<0.001; Figure 5). Based on this result, we developed a modified CAAP-AF risk score (11) to incorporate SD-TPS in predicting recurrence after AF ablation: Coronary artery disease (+1 point), A [Dyssynchrony (> = 2.86: +2 point) or Volume (> = 37.6: +2 point)], Age (< 50: 1, 50 – 60: +1 point, 60 – 70: +2 point, >=70: +3 points), Persistent or longstanding (+2 point), Antiarrhythmic failed (none: 0, 1 – 2: +1 point, > 2: +2 points), Sex category (Female: +1 point). The modified CAAP-AF risk score showed a higher OR (1.97 vs. 1.44) and a higher C-statistics (0.77 vs. 0.68, p= 0.024) compared with the original CAAP-AF risk score (Figure 6). Similarly, the specificity and positive predictive value of intra-atrial dyssynchrony were higher compared to other indices of LA structure/function (Table 4).
Figure 5. Time to atrial fibrillation (AF) recurrence – Test group.
Orange line, SD-TPS ≥ 2.86%; Green line, SD-TPS < 2.86%.
Figure 6. Receiver operating characteristics (ROC).
Thick green line, risk score including dyssynchrony; Thick orange line, risk score including maximum volume; Thin green line, reference.
Table 4.
Area under the receiver operator characteristic curves, sensitivity, PPV and NPV among each Structural and functional parameter on the test group.
| AUC | P* | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| Minimum LA volume, mm3/m2 | 0.64 | 0.498 | 47 | 81 | 71 | 62 |
| Maximum LA volume, mm3/m2 | 0.61 | 0.091 | 65 | 57 | 59 | 63 |
| Total LAEF, % | 0.70 | 0.889 | 77 | 63 | 66 | 74 |
| Smax, % | 0.72 | 0.053 | 88 | 56 | 65 | 83 |
| SD-TPS, % | 0.79 | 0.030 | 76 | 81 | 79 | 78 |
| LA fibrosis, % total LA area (log) | 0.63 | - | 80 | 46 | 58 | 71 |
NPV, negative predictive value; PPV, positive predictive value.
vs. LA fibrosis (%total LA area).
DISCUSSION
We found that intra-atrial dyssynchrony in pre-ablation CMR is an independent predictor of recurrence in patients referred for the first catheter ablation of AF, either paroxysmal or persistent.
Intra-atrial dyssynchrony as a marker of left atrial remodeling
Several lines of evidence suggest that intra-atrial dyssynchrony by echocardiography contributes indispensably to the mechanism of AF. For example, intra-atrial dyssynchrony is a strong predictor of new-onset AF in patients with heart failure (12). In addition, inter-atrial dyssynchrony predicts progression from paroxysmal to persistent AF (13). Furthermore, intra-atrial dyssynchrony predicts recurrence after catheter ablation of paroxysmal AF (5,14,15). To our knowledge, this is the first study to demonstrate intra-atrial dyssynchrony using CMR. The validation and reproducibility of LA tissue tracking CMR have been established (16). Compared with echocardiography, CMR is associated with higher in-plane spatial resolution and signal-to-noise ratio, which allows a better evaluation of thin LA walls (2–4 mm), particularly of the LA posterior wall where most of the fibrosis is localized.
Our findings suggest that intra-atrial dyssynchrony reflects the underlying LA remodeling independent of LA volume, similar to the extent of LA fibrosis detected by LGE (4) and LA function (3). In fact, our results suggest that intra-atrial dyssynchrony is a more specific marker of myocardial tissue scarring than LA fibrosis or LA function (Table 4). For example, while intra-atrial dyssynchrony was significantly associated with LA fibrosis (Figure 4), the AUC of intra-atrial dyssynchrony was significantly higher than that of LA fibrosis, whereas the AUC of LA function was not (Table 4). LA dyssynchrony also had a higher specificity. A possible explanation to account for these results is that intra-atrial dyssynchrony likely reflects subtle changes in atrial architecture that could generate AF but is not captured by LGE. Of note, our LA strains in patients without recurrence were higher than those of our previous report (3). This likely reflects the current clinical practice to refer patients to catheter ablation in early stages of AF and LA remodeling. The possibility that cardioversion-induced atrial stunning could have confounded our findings is low because: 1) Cardioversion was performed in only a minority of patients in both groups; 2) There was no significant difference in the fraction of patients who underwent cardioversion between both groups; and 3) CMR was performed on average 10 weeks after cardioversion, while cardioversion-induced atrial stunning usually recovers within 4 weeks.(17)
Clinical implications
Catheter ablation is superior to antiarrhythmic drugs (18) in maintaining sinus rhythm, but remains far from curative with recurrence rates up to 40%.(19) Intra-atrial dyssynchrony is a relatively simple method to quantify the degree of underlying LA remodeling to identify candidates who are responsive to catheter ablation. It helps refine patient selection and thus reduce the recurrence by saving candidates with higher degrees of LA remodeling from potentially futile procedures and complications. Intra-atrial dyssynchrony can also be measured by a variety of imaging modalities such as speckle-tracking echocardiography. Incorporation of intra-atrial dyssynchrony into routine clinical practice is relatively straightforward since it does not require extensive post-processing unlike the quantification of LA fibrosis (6). In addition, the assessment of LA mechanical dyssynchrony is less time-consuming than LA-LGE.
Limitations
This study represents a single-center analysis of patients referred for catheter ablation of AF. Therefore, there is a non-negligible chance of selection bias. Our definition of recurrence was strongly influenced by symptoms. Therefore, it is possible that we could have missed recurrence that is completely asymptomatic. For the dyssynchrony analysis, we used only two- and four-chamber cine CMR, which was included in a routine image-acquisition protocol. Therefore, it is possible that our analysis underestimated the degree of dyssynchrony by missing regions that were not covered by those two views. Since the strain was two-dimensional (2-D) and was obtained only in the in-plane direction, SD-TPS may have been underestimated compared with those obtained from 3-D strains. Besides, the CMR temporal resolution may also explain our lower values of dyssynchrony compared to those of 3-D echocardiogram.(20) Despite those potential causes of underestimation, our analysis demonstrated a significant association between intra-atrial dyssynchrony and AF recurrence. Therefore, we believe that the advantage of our approach outweighs the disadvantage of including more views to assess the whole LA deformation, which would increase the scan time and post-processing burden.
Conclusions
Intra-atrial dyssynchrony is an independent predictor of recurrence after catheter ablation of paroxysmal or persistent AF. Intra-atrial dyssynchrony is a relatively simple method to quantify the degree of underlying LA remodeling to identify candidates who are responsive to catheter ablation, and can refine patient selection and thus reduce the recurrence by saving candidates with higher degrees of LA remodeling from potentially futile procedures and complications.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
AF ablation is the indicated treatment for individuals with drug-refractory AF. However, the rate of recurrence after the procedure is relatively high, in part, due to poor patient selection before the intervention. Thus, identifying tools that assist the physician in the selection of adequate candidates is imperative. Our study examined the value of LA intra-atrial dyssynchrony in predicting the success of AF ablation beyond traditional risk factors, and other indices of LA structure and function.
TRANSLATIONAL OUTLOOK
Starting from present observations, further prospective studies are needed to determine the direct implications of this novel index of LA function - intra-atrial dyssynchrony - in the process of selecting the best candidates to be referred to AF ablation.
Acknowledgments
Sources of founding: This work was supported by research grants from NIH/NHLBI R56 HL138429 (to H.A.), W.W. Smith Charitable Trust (to H.A.), Magic That Matters Fund for Cardiovascular Research (to H.A.), Zegar Family Foundation (to H.A.), Johns Hopkins University Institute of Clinical and Translational Research (to H.A.), the Edward St. John Foundation for AF Research (to H.C.), The Roz and Marvin H Weiner and Family Foundation (to H.C.), The Dr. Francis P. Chiaramonte Foundation (to H.C.), The Marilyn and Christian Poindexter Arrhythmia Research Fund (to H.C.), and The Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund (to H.C.).
LIST OF ABBREVIATIONS
- AF
Atrial fibrillation
- CMR
Cardiac magnetic resonance
- LA
Left atrial
- SD
Standard deviation
- TPS
Time to peak strain
Footnotes
Disclosure: No authors have any potential conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Journal of the American Heart Association. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibi M, Lima JA, Gucuk Ipek E, et al. The association of baseline left atrial structure and function measured with cardiac magnetic resonance and pulmonary vein isolation outcome in patients with drug-refractory atrial fibrillation. Heart Rhythm. 2016;13:1037–44. doi: 10.1016/j.hrthm.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Khurram IM, Habibi M, Gucuk Ipek E, et al. Left Atrial LGE and Arrhythmia Recurrence Following Pulmonary Vein Isolation for Paroxysmal and Persistent AF. JACC Cardiovascular imaging. 2016;9:142–8. doi: 10.1016/j.jcmg.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Okura H, Kobayashi Y, et al. Assessment of atrial synchrony in paroxysmal atrial fibrillation and impact of pulmonary vein isolation for atrial dyssynchrony and global strain by three-dimensional strain echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2014;27:1193–9. doi: 10.1016/j.echo.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Khurram IM, Beinart R, Zipunnikov V, et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. 2014;11:85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrispin J, Ipek EG, Habibi M, et al. Clinical predictors of cardiac magnetic resonance late gadolinium enhancement in patients with atrial fibrillation. Europace. 2016 doi: 10.1093/europace/euw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang E, Ipek EG, Balouch M, et al. Factors impacting complication rates for catheter ablation of atrial fibrillation from 2003 to 2015. Europace. 2017;19:241–249. doi: 10.1093/europace/euw178. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Hindricks G, Cappato R, et al. TEMPORARY REMOVAL: 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017 doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 11.Winkle RA, Jarman JW, Mead RH, et al. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Heart Rhythm. 2016;13:2119–2125. doi: 10.1016/j.hrthm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Cho GY, Jo SH, Kim MK, et al. Left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure. Int J Cardiol. 2009;134:336–41. doi: 10.1016/j.ijcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Sakabe K, Fukuda N, Fukuda Y, Morishita S, Shinohara H, Tamura Y. Interatrial dyssynchrony on tissue Doppler imaging predicts progression to chronic atrial fibrillation in patients with non-valvular paroxysmal atrial fibrillation. Heart. 2009;95:988–93. doi: 10.1136/hrt.2008.152561. [DOI] [PubMed] [Google Scholar]
- 14.den Uijl DW, Gawrysiak M, Tops LF, et al. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2011;13:1533–40. doi: 10.1093/europace/eur186. [DOI] [PubMed] [Google Scholar]
- 15.Sarvari SI, Haugaa KH, Stokke TM, et al. Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. European heart journal cardiovascular Imaging. 2016;17:660–7. doi: 10.1093/ehjci/jev185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zareian M, Ciuffo L, Habibi M, et al. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson. 2015;17:52. doi: 10.1186/s12968-015-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan IA. Transient atrial mechanical dysfunction (stunning) after cardioversion of atrial fibrillation and flutter. Am Heart J. 2002;144:11–22. doi: 10.1067/mhj.2002.123113. [DOI] [PubMed] [Google Scholar]
- 18.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 19.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. The New England journal of medicine. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki A, Yuda S, Oi Y, et al. Assessment of left atrial deformation and synchrony by three-dimensional speckle-tracking echocardiography: comparative studies in healthy subjects and patients with atrial fibrillation. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2013;26:165–74. doi: 10.1016/j.echo.2012.10.003. [DOI] [PubMed] [Google Scholar]