Abstract
Objective
Gut health improvements were monitored with respect to growth performance, diarrhea incidence, fecal bacterial population and intestinal morphology of suckling pigs orally supplemented with live Lactobacillus salivarius (L. salivarius) oral suspensions and challenged with F4+ enterotoxigenic Escherichia coli (ETEC).
Methods
Two groups of newborn pigs from 18 multiparous sows were randomly designated as non-supplemented (control: n = 114 piglets) and L. salivarius supplemented groups (treatment: n = 87 piglets). Treatment pigs were orally administered with 2 mL of 109 colony-forming unit (CFU)/mL L. salivarius on days 1 to 3, then they were orally administered with 5 mL of 109 CFU/mL L. salivarius on days 4 to 10, while those in control group received an equal amount of phosphate buffered saline solution. On day 24 (2 weeks post supplementation), one pig per replicate of both groups was orally administered with 108 CFU/mL F4+ ETEC, then they were euthanized on day 29 of experiment.
Results
Results revealed that pigs in treatment group had a statistically significant increase in average daily gain, body weight and weight gain, and tended to lower diarrhea throughout the study. Numbers of Lactobacillus population in feces of treatment pigs were higher than control pigs, especially on day 10 of study. Numbers of total bacteria in intestinal contents of control pigs were also increased, but not Coliform and Lactobacillus populations. Histological examination revealed statistically significant improvements of villous height and villous/crypt ratio of duodenum, proximal jejunum and distal jejunum parts of treatment pigs compared with controls. Duodenal pH of treatment group was significantly decreased.
Conclusion
Oral supplementation of live L. salivarius during the first 10 days of suckling pig promoted growth performance and gut health, reduced diarrhea incidence, increased fecal Lactobacillus populations and improved intestinal morphology.
Keywords: Enterotoxigenic Escherichia coli F4, Fecal Bacterial Population, Growth Performance, Intestinal Morphology, Lactobacillus salivarius, Suckling Pigs
INTRODUCTION
The first month of piglet life is the most critical period, because the immune system remains not fully competent. Newborn piglets are often infected with pathogenic bacteria transmitted from either sows or environment. Neonatal diarrhea of piglets is caused by enterotoxigenic Escherichia coli (ETEC) [1]. Severity of diarrhea can be up to 50% in suckling pigs [2]. ETEC, especially fimbriae type 4 (F4) harboring strain is the most important bacterial pathogen during the first week of a pigs life. It causes severe watery diarrhea in newborn and also early weaned piglets. ETEC produces toxins including heat-stable enterotoxins (STa and STb) and heat-labile enterotoxin (LT), which potentially inhibit the absorption of sodium and chloride ions from intestinal lumen and stimulate the secretion of intestinal fluid, resulting in water and electrolyte losses [3]. In general, antimicrobials are often used to treat the bacterial infection. Among the side effects of intensive antimicrobial usage to fight the pathogen; some adverse effects such as destroying the beneficial bacteria, residues in pork products, and possibly increasing antimicrobial resistance are cause concern [4]. Therefore, non-antibiotic substances are considered to be alternatives to avoid such deleterious effect. Nowadays, prebiotics, probiotics, organic acids, enzymes, herbs and other non-antibiotic products/compound are widely used in pig production.
Supplementation of probiotics (live microorganisms) is aimed to balance intestinal microflora, increase number of beneficial microorganisms and stimulate the pig immune system. In previous study, improvement of growth performance of probiotic supplemented pigs was demonstrated [5]. Lactobacillus salivarius (L. salivarius) is a member of gram-positive bacteria. It grows in anaerobic and acidic conditions, with the optimal pH being 5.5 to 6.5 [6]. L. salivarius produces lactic acid and short chain fatty acids from glucose fermentation in glycolysis pathway [7]. It also inhibits bacterial pathogen growth [8]. From this circumstance, use of L. salivarius as probiotic would appear to offer benefits for pig gut health. In the present study, an isolate of L. salivarius was obtained from a Thai commercial pig producing farm and considered as a potential candidate for use as probiotic. The objective of this experiment was to investigate the effects of L. salivarius on growth performance, diarrhea incidence, fecal and intestinal microorganism, and intestinal morphology of suckling pigs challenged with Escherichia coli (E. coli) F4.
MATERIALS AND METHODS
Animal care
This research investigation was conducted according to protocol approved by Chulalongkorn University Animal Care and Use Committee (Animal Use Protocol No.1431071).
Experimental designs
The experiment was performed at a commercial pig producing farm in Ratchaburi Province, Western part of Thailand. Standard farm management system was routinely performed by farm staff. Eighteen crossbred newborn pig (Duroc×Yorkshire×Landrace) litters were randomly divided into two groups (10 to 12 piglets/L): control group (n = 114 piglets, from 10 sows) and treatment group (n = 87 piglets, from 8 sows). No cross-fostering was implemented in any experiment litters throughout the study. Each litter was individually housed in stainless steel pens with plastic-coated and concrete expanded floors. Piglets had free access to sow milk and water.
During the first 3 days of study, each piglet in treatment group was orally administered with 2 mL of L. salivarius 109 colony-forming unit (CFU)/mL/d. An increased volume of inoculum up to 5 mL of L. salivarius 109 CFU/mL/d was orally administered during days 4 to 10. All control piglets were given the equal volumes of phosphate buffered saline solution (PBS). On days 10 to 29 of experiment, creep feed was offered ad libitum.
In challenge experiment, one piglet per litter from each group was randomly chosen (10 control and 8 treatment pigs), and orally administered with 5 mL of 108 CFU/mL E. coli F4. Clinical observations of diarrhea sign were monitored and recorded daily. On days 5, 10, 17, and 24, fecal samples from all pigs were collected by rectal swab technique (sterile cotton swab) and stored at 4°C until analysis within each collection day. On day 29, all challenged pigs were humanely euthanized with sodium pentobarbital 50 mg/kg intravenous injection. Standard necropsy procedure was carefully performed to inspect pathological lesions. Samples and luminal contents were collected from duodenum, proximal jejunum, distal jejunum, and ileum for histology and bacterial cultivation, respectively. The pH of intestinal contents from such locations was measured using digital pH meter.
Five hundred grams of creep feed were randomly collected for proximate analysis as follows: dry matter and moisture were analyzed with air oven method, ash (muffle furnace method), crude protein (Kjeldahl method), crude fat (Soxhlet method), crude fiber, calcium and phosphorus (UV-VIS spectrophotometer) and gross energy analyzed with bomb calorimeter [9].
Microbiological laboratory
Preparation of L. salivarius and E. coli
L. salivarius was freshly prepared by culture on DE MAN, ROGOSA and SHARPE (MRS agar, Lab M Ltd, Heywood, England). Colonies of L. salivarius were inoculated to 5 mL MRS broth (pH 5.5) and incubated at 37°C in carbon dioxide incubator for 24 hours, transferred into a total volume 1 L, and incubated at 37°C for 24 hours in carbon dioxide incubator. After that, L. salivarius in MRS broth was adjusted to 109 CFU/mL.
Stock of E. coli F4 strain was cultured on MacConkey agar and incubated at 37°C for 18 hours. Colonies of overnight grown E. coli F4 were then transferred to 100 mL tryptic soy broth (TSB, Merck Co., Ltd, Darmstadt, Germany) and incubated at 37°C for 24 hours and a count made of the number of colonies. Then, E. coli F4 in TSB was adjusted to 108 CFU/mL using 0.5 McFarland as standard and then cultivated on plate count agar (PCA, Merck Co., Ltd, Germany) for determination of bacterial concentration.
Laboratory analyses for determination of fecal/intestinal bacterial population
One gram of samples (fresh feces or intestinal contents) was transferred into 9 mL of sterile peptone PBS (0.1%) and mixed thoroughly. One mL of fecal suspension was transferred into another tube containing 9 mL sterile PBS. A serial of 10-fold dilution was made to 10−3 to 10−8. Then, one mL of each dilution was duplicated and transferred to sterile agar plate and topped up with freshly made sterile agar and spread plate. The culture media for total bacteria, coliform and lactobacillus counts, including culture conditions were PCA incubated at 37°C for 48 hours; violet red bile agar (VRB, Merck Co., Ltd, Germany) incubated at 37°C for 24 hours; and MRS agar incubated in carbon dioxide incubator at 37°C for 72 hours, respectively. The dilution plates with colony numbers range of 15 to 150 colonies were recorded [10]. Finally, an average of duplicate plates was calculated and expressed as log10 CFU/mL.
Clinical evaluations
Diarrhea incidence and fecal score
Number of pigs showing clinical signs of diarrhea and consistency of feces were daily monitored by the same investigator. Fecal consistency representing severity of diarrhea was evaluated and categorized as 4 scores: score 0 as normal solid feces; score 1as semi-solid feces; score 2 as water feces with some solid material; and score 3 as profuse watery feces with little or no solid fecal content [11]. Fecal scores and diarrhea incidence (DI) of each group were calculated according to the formula below [12,13].
Pig performance
All animals were individual weighed on days 1, 10, 17, 24, and 29. This was used to calculate average body weight, average daily gain (ADG) and weight gain.
Intestinal morphology
Intestinal tissues from middle part of duodenum, proximal jejunum, distal jejunum, and ileum were cut approximately 2 cm length. The intestinal segment was fixed with pins on a foam and kept in 10% formalin buffered solution. The sample was embedded in paraffin and cut with microtome and stained with hematoxylin and eosin staining (H&E) [14]. Five villi and crypt areas were systemically chosen per sample for villous height and crypt depth measurement. Villous height was measured from tip of villi to villi-crypt junction. Crypt depth was measured from base of villi to the lowest point of crypt. Villous height and crypt depth were measured using an Isolution lite software version 10.1.
Statistical analyses
All data were expressed as mean±standard error of the mean. The statistical analyses were analyzed using unpaired t-test for growth performance, diarrhea incidence, fecal and intestinal bacterial counts. The weight of piglets before and after challenged with E. coli F4 was analyzed by paired t-test. Differences of p<0.05 were considered as statistical significant difference using MIXED procedure of SAS 2002 [15].
RESULTS
Oral supplementation of live L. salivarius suspension in suckling pigs was investigated following an E. coli F4 challenge, with respect to growth performance, diarrhea incidence, fecal bacterial population, and intestinal morphology. The pig production and health situations during experiment remained within an acceptable level of Thai farm standard. Porcine Reproductive and Respiratory Syndrome (PRRS) antibody was detected in suckling period from sows and remained positive in nursery period until 11 to 12 weeks of age. No unstable PRRS was identified in sow and nursery population. The result of feed analysis is shown in Table 1.
Table 1.
The nutrients composition analysis of creep feed used in this study
| Nutrients | % |
|---|---|
| Gross energy1) (kJ/kg) | 16,616.15 |
| Dry matter | 92.46 |
| Moisture | 7.54 |
| Protein | 19.89 |
| Crude fat | 5.50 |
| Crude fiber | 0.88 |
| Ash | 6.33 |
| Calcium | 0.73 |
| Phosphorus | 0.50 |
Gross energy was analyzed using Bomb Calorimeter.
Overall effects of L. salivarius supplementation on pig growth performances were determined. In Table 2, average pig weight of both groups was 1.59 kg at the beginning of experiment. On days 10, 17, and 24 pigs in treatment group appeared to be heavier than control, but the only statistically significant difference was found on day 24. During days 1 to 24, weight gain of treatment group was higher than control group for 0.68 kg (4.58 and 3.90 kg/pig) (p<0.05). Also, the ADG of treatment group was better than control for 28.62 g/d (191.20 and 162.58 g/d) during the same period (p<0.05). Following E. coli F4 challenge, weight loss of control piglets was greater than in the treatment group for 0.12 kg/pig (0.45 and 0.33 kg, respectively) (p<0.01).
Table 2.
Effect of Lactobacillus salivarius on pig weight (kg) of piglets before and after being challenged with Escherichia coli F4
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Weight on day 1 | 1.59 | 1.59 | 0.01 | 0.911 |
| Weight on day 10 | 3.17 | 3.29 | 0.08 | 0.511 |
| Weight on day 17 | 4.39 | 4.74 | 0.13 | 0.203 |
| Weight on day 24 | 5.49 | 6.18* | 0.16 | 0.033 |
| ADG (g/d) | 162.58 | 191.20* | 6.85 | 0.033 |
| Weight gain | 3.90 | 4.58* | 0.16 | 0.033 |
| Before challenged (day 24) | 6.09a | 6.08 | - | - |
| After challenged (day 29) | 5.64b | 5.75 | - | - |
| SEM | 0.17 | 0.23 | - | - |
| p-value | 0.005 | 0.057 | - | - |
SEM, standard error of the mean; ADG, average daily gain.
Means in the same row are significant different (p<0.05).
Means in the same column (pair T-test) are significant different (p<0.01).
Improvement of pig performance is theoretically linked to pig gut health. Therefore, clinical data of pig diarrhea was collected to evaluate impact of L. salivarius supplementation on pig gut health. Diarrhea incidence, number of diarrhea days and fecal scores of treatment group were less severe than control group. Treatment group had overall average diarrhea incidence 9.16%, whereas control group was 13.26%. Similar circumstances to diarrhea score, treatment group appeared to be lower than control group during pre- and post-inoculation periods, but did not show a statistical significant difference. Only average diarrhea pig number between two groups (4.70 and 6.80 pigs/d) was statistically significant different during pre-inoculation period. Furthermore, fecal scores of piglets in treatment group seemed to decrease compared with control group (Table 3).
Table 3.
Effect of Lactobacillus salivarius on diarrhea incidence of piglets challenged with Escherichia coli F4
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Number of piglets (n) | 114 | 87 | - | - |
| Diarrhea incidence (%) | 13.26 | 9.16 | 1.30 | 0.122 |
| Fecal score1) | 0.50 | 0.39 | 0.04 | 0.194 |
| Fecal score2) | 1.40 | 1.05 | 0.09 | 0.065 |
| Average number of diarrhea day (days 1 to 24) | 4.80 | 4.25 | 0.245 | 0.278 |
| Average number of diarrhea day (days 25 to 29) | 3.10 | 2.80 | 0.198 | 0.588 |
| Average number of piglets diarrhea on days 1 to 24 | 6.80 | 4.70* | 0.488 | 0.033 |
SEM, standard error of the mean.
Fecal score of piglets before challenge (experimental days 1–24).
Fecal score of piglets after challenge (experimental days 25–29).
Means in the same row are significant different (p<0.05).
Following improvement of clinical data in L. salivarius supplementation, gut environmental conditions were determined to explain whether L. salivarius supplementation could modulate intestinal pH, microbial population and gut morphology and subsequently supported pig gut health or not. The intestinal content pH was measured regarding to various parts of intestine. A pH range of 6.08 to 6.88 was observable. Intestinal content pH values of treatment and control groups were not dissimilar in proximal and distal jejunum or ileum. Only average pH of duodenal content in treatment group (6.08) was statistically significantly reduced, compared to control group (6.45) (Table 4).
Table 4.
Effect of Lactobacillus salivarius on pH of intestinal contents of piglets challenged with Escherichia coli F4
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Duodenum | 6.45 | 6.08* | 0.07 | 0.012 |
| Proximal Jejunum | 6.23 | 6.30 | 0.10 | 0.740 |
| Distal Jejunum | 6.79 | 6.71 | 0.07 | 0.622 |
| Ileum | 6.88 | 6.84 | 0.04 | 0.669 |
SEM, standard error of the mean.
Means in the same row are significant different (p<0.05).
Effect of L. salivarius supplementation on microbial population of pig gut was further investigated. Overall fecal bacterial population of treatment groups tended to be higher than control, including coliform bacterial and Lactobacillus species (Table 5). The average total bacteria in feces of control and treatment groups was log108.41 and 8.71 CFU/mL, respectively. The average total coliform bacteria was log107.87 and 8.01 CFU/mL, whereas average total Lactobacillus species was log107.56 and 8.25 CFU/mL, respectively. Only on day 10 of experiment, total fecal lactobacillus count of treatment group was increased compared with the control group (p<0.01). Moreover, the ratio of total lactobacillus count and total bacteria count ratio (TLC:TBC ratio), total coliform and total bacterial count (TCC:TBC) and the total lactobacillus count and total coliform count ratio (TLC:TCC ratio), were not significantly different (data not shown). Challenge experiment using E. coli F4 strain was further conducted by examination of bacterial populations. Piglets in treatment group had a higher average total bacterial count in duodenum, proximal jejunum, distal jejunum and ileum (log106.32, 6.99, 7.69, and 7.79 CFU/mL) compared with control group (log105.13, 5.69, 6.51, and 6.23 CFU/mL) (p<0.05) (Table 6). When focused on each location, total bacteria in treatment group was significantly higher than control (p<0.05), but total coliform, total lactobacillus counts and TLC:TBC, TCC:TBC, and TLC:TCC ratios were not different (data not shown).
Table 5.
Effect of Lactobacillus salivarius on fecal bacterial population of piglets challenged with Escherichia coli F4 (Log10 cfu/mL)
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Total bacteria | ||||
| Day 5 | 8.70 | 9.01 | 0.10 | 0.155 |
| Day 10 | 8.64 | 8.93 | 0.10 | 0.177 |
| Day 17 | 8.26 | 8.50 | 0.14 | 0.423 |
| Day 24 | 8.05 | 8.39 | 0.14 | 0.306 |
| Average | 8.41 | 8.71 | - | - |
| Total coliform | ||||
| Day 5 | 8.16 | 8.23 | 0.10 | 0.724 |
| Day 10 | 8.07 | 8.15 | 0.16 | 0.838 |
| Day 17 | 7.62 | 8.03 | 0.15 | 0.202 |
| Day 24 | 7.63 | 7.63 | 0.14 | 0.988 |
| Average | 7.87 | 8.01 | ||
| Total lactobacillus | ||||
| Day 5 | 7.83 | 8.07 | 0.14 | 0.420 |
| Day 10 | 7.04 | 8.51* | 0.17 | 0.001 |
| Day 17 | 7.73 | 8.17 | 0.14 | 0.118 |
| Day 24 | 7.67 | 8.23 | 0.15 | 0.078 |
| Average | 7.56 | 8.25 | - | - |
SEM, standard error of the mean.
Means in the same row are significant different (p<0.01).
Table 6.
Effect of Lactobacillus salivarius on intestinal bacterial population of piglets challenged with Escherichia coli F4 (Log10 cfu/mL)
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Total bacteria | ||||
| Duodenum | 5.13 | 6.32* | 0.28 | 0.032 |
| Proximal Jejunum | 5.69 | 6.99* | 0.29 | 0.021 |
| Distal Jejunum | 6.51 | 7.69* | 0.27 | 0.027 |
| Ileum | 6.23 | 7.79* | 0.33 | 0.014 |
| Total coliform | ||||
| Duodenum | 5.14 | 5.95 | 0.51 | 0.452 |
| Proximal Jejunum | 5.68 | 6.71 | 0.60 | 0.374 |
| Distal Jejunum | 5.31 | 6.16 | 0.52 | 0.434 |
| Ileum | 5.02 | 6.06 | 0.45 | 0.268 |
| Total lactobacillus | ||||
| Duodenum | 5.41 | 6.73 | 0.41 | 0.116 |
| Proximal Jejunum | 5.79 | 6.93 | 0.39 | 0.124 |
| Distal Jejunum | 6.43 | 6.88 | 0.34 | 0.532 |
| Ileum | 5.54 | 6.71 | 0.40 | 0.149 |
SEM, standard error of the mean.
Means in the same row are significant different (p<0.05).
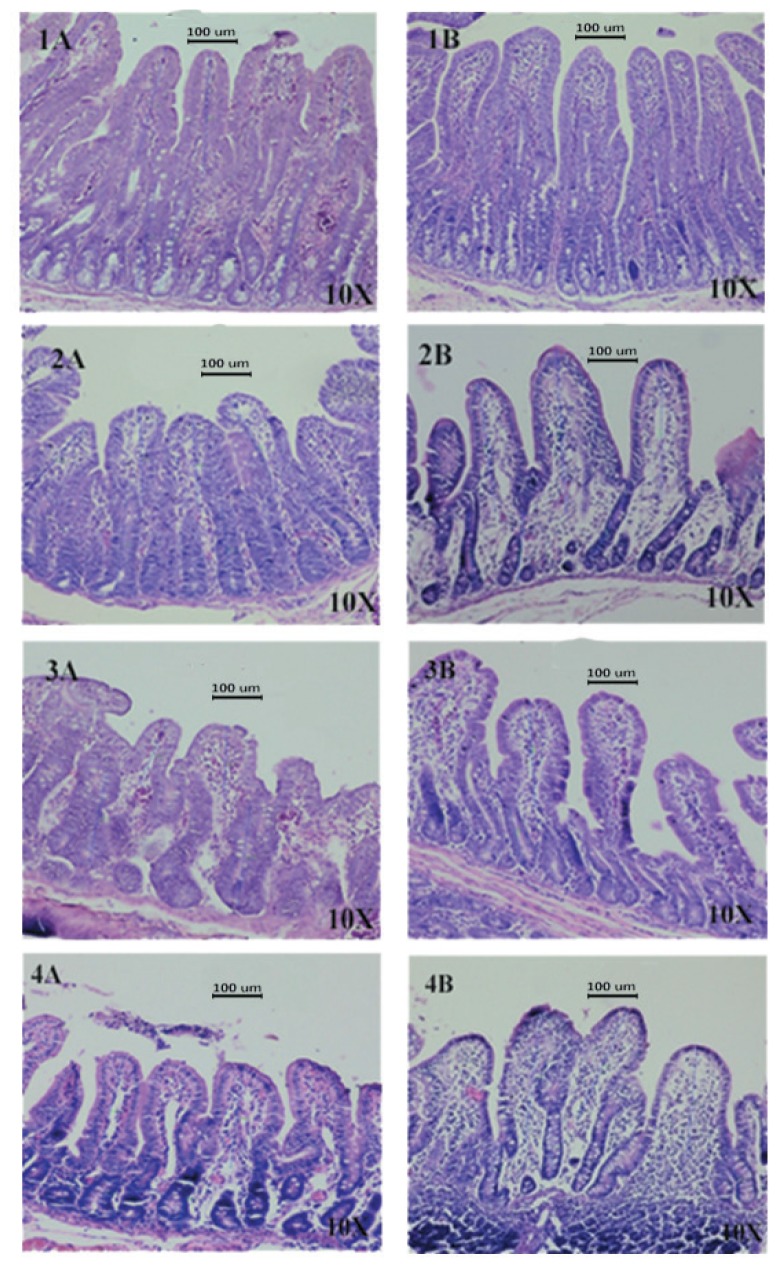
Intestinal morphology of piglets was evaluated following challenge. Treatment group had better villi height in duodenum, proximal jejunum, and distal jejunum parts, compared to those of control group (Figure 1, p<0.01). The crypt depth did not differ between groups. However, the villous height and crypt depth ratio (VH:CD) in duodenum in treatment group was significantly increased (p<0.05) (Table 7).
Figure 1.
Effect of Lactobacillus salivarius (L. salivarius) on intestinal microscopic morphology (H&E staining) of piglets challenged with Escherichia coli F4. Villus height and crypt depth (μm) were measured using an Isolution lite software version 10.1. (A) control group, (B) L. salivarius group, (1) duodenum, (2) proximal jejunum, (3) distal jejunum, and (4) ileum.
Table 7.
Effect of Lactobacillus salivarius on intestinal morphology of piglets challenged with Escherichia coli F4
| Items | Control | L. salivarius | SEM | p-value |
|---|---|---|---|---|
| Villous height (VH, μm) | ||||
| Duodenum | 251.13 | 297.65** | 9.169 | 0.007 |
| Proximal Jejunum | 253.85 | 290.41** | 6.871 | 0.004 |
| Distal Jejunum | 258.36 | 281.82** | 4.416 | 0.004 |
| Ileum | 158.01 | 175.10 | 6.377 | 0.199 |
| Crypt depth (CD, μm) | ||||
| Duodenum | 135.85 | 142.83 | 5.059 | 0.509 |
| Proximal Jejunum | 123.53 | 132.16 | 3.296 | 0.254 |
| Distal Jejunum | 129.83 | 130.68 | 3.353 | 0.904 |
| Ileum | 107.55 | 105.88 | 4.054 | 0.849 |
| Villi height:crypt depth ratio (VH:CD ratio) | ||||
| Duodenum | 1.91 | 2.12* | 0.055 | 0.048 |
| Proximal Jejunum | 2.11 | 2.26 | 0.046 | 0.116 |
| Distal Jejunum | 2.02 | 2.25 | 0.064 | 0.068 |
| Ileum | 1.48 | 1.75 | 0.075 | 0.077 |
SEM, standard error of the mean.
Means in the same row are significant different (p<0.05 and p<0.01, respectively).
DISCUSSION
This study was conducted to investigate the effects of oral live L. salivarius suspension supplementation in suckling pigs following with E. coli F4 challenged, with respect to growth performance, diarrhea incidence, fecal bacterial population, and intestinal morphology. Overall effects revealed that L. salivarius supplementation improved ADG and weight gain of piglets. This is similar to previous studies that reported L. salivarius B1 [16] or L. brevis 1E-1 [17] supplementation increased daily weight gain of piglets. This advantageous effect of L. salivarius supplementation on growth performance may relate to improve villous height of piglets as demonstrated in this study. L. salivarius may also modulate intestinal environment and/or growth of intestinal microflora, resulting in limitation of diarrhea [18]. Other previous studies suggested that Lactobacillus species supplementation may stimulate the secretion of mucus, then promote growth of intestinal microflora [4,19]. In general, probiotics are intended to maintain the intestinal ecosystem and improve animal health [7]. Probiotic bacteria produce several anti-microorganism substances such as bacteriocin, hydrogen peroxide, carbon dioxide, and acetic acid [20], which support gut health. For example, bacteriocin allows inhibition of peptidoglycan of pathogenic bacteria and interference the function of cell membranes, resulting in inhibition of bacteria pathogens [21]. Enhancement of epithelial barrier [22], and concomitant inhibition of pathogen adhesion [23] by L. salivarius may also prevent intestinal damage, thus gut health and growth performances are increased [20]. However, this study did not investigate such mentioned effects.
Body weight loss is considered as a deleterious consequence following challenge. This is due to diarrhea following E. coli F4 exposure. ETEC, a common cause of diarrhea in newborn piglets [5], usually adheres to epithelium in duodenum, jejunum, and ileum. The toxins produced by E. coli F4 causes increase of Cl− secretion from crypt cells and impair of Na+ and Cl− absorption by villus cells. Excess amount of electrolytes in intestinal lumen causes water lose and subsequent diarrhea [5]. In the present study, treatment group had a benefit of reduced diarrhea severity. This may be because L. salivarius produce lactic acid and other organic acids, which destroy electrochemical proton gradient of pathogenic cells. Therefore, pathogenic bacteria are degenerated [12,20].
Earlier studies suggest that L. salivarius supplementation increases number of total Lactobacillus species in intestines, adheres to intestinal wall, and promotes excretion of pathogenic bacteria in feces [24,25]. Therefore, the present study was designed to supplement L. salivarius to newborn piglets for a consecutive 10-day period; this might be possible on farm using L. salivarius in form of oral suspension. We anticipated the modulation of L. salivarius in the pig gut and might prevent or reduce of diarrhea following E. coli challenge. As expected, numbers of intestinal Lactobacillus species were only enhanced during period of supplementation. This signifies that L. salivarius may not last in intestine for long period. Therefore, a longer supplementation to producea higher Lactobacillus population may be an option for better outcome. Another concern in the present study was that total bacterial population in L. salivarius supplemented group increased and differed from control, whereas total lactobacillus and the total coliform bacteria did not differ between groups. Similar study [26] reported that Lactobacilli supplementation increased the number of total bacteria in jejunum. The explanation may be due to a limited period of L. salivarius supplementation, causing no prolonged growth of Lactobacillus in gut, and perhaps, due to the modulation of other bacterial communities in pig gut, resulting in higher total bacteria.
Intestinal morphology is an important indicator for probiotic supplementation. Better villous height presumes an improvment in the absorptive ability of small intestine. Lactobacillus spp. can produce short chain fatty acids to stimulate epithelial cells and enterocytes, and increase the villous height [27]. In the present study, increase of villous height of L. salivarius supplemented group was similar to an earlier result [22] who reported that, piglets in L. plantarum CGMCC had enhanced villi height and VH:CD ratio of duodenum and jejunum. Similar extent following supplementation with Lactobacilli was also revealed [28].
In conclusion, live L. salivarius oral supplementation during the first 10 days of suckling pig promoted growth performance and gut health, lessen diarrhea incidence and severity, and periodical enhanced fecal Lactobacillus populations, and improved intestinal morphology.
ACKNOWLEDGMENTS
This study was supported, in part, by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Chulalongkorn University, Thailand.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Chan G, Farzan A, DeLay J, et al. A retrospective study on the etiological diagnoses of diarrhea in neonatal piglets in Ontario, Canada, between 2001 and 2010. Can J Vet Res. 2013;77:254–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Begum S, Patra MK, Ngullie L, et al. Antimicrobial resistance pattern in swine colibacillosis under farm and field condition in Nagaland. Int J Livest Res. 2014;4:81–8. [Google Scholar]
- 3.Chandler D, Mynott T. Bromelain protects piglets from diarrhoea caused by oral challenge with K88 positive enterotoxigenic Escherichia coli. Gut. 1998;43:196–202. doi: 10.1136/gut.43.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estienne M, Hartsock T, Harper A. Effects of antibiotics and probiotics on suckling pig and weaned pig performance. Intern J Appl Res Vet Med. 2005;3:303–8. [Google Scholar]
- 5.Moxley RA, Duhamel GE. Comparative pathology of bacterial enteric disease of swine. Adv Exp Med Biol. 1999;473:83–101. doi: 10.1007/978-1-4615-4143-1_7. [DOI] [PubMed] [Google Scholar]
- 6.Salih N, Hutari A, Gaseem W, et al. Maximization of growth and storage of locally isolated Lactobacillus salivarius subsp Salivarius with high stability and functionality. Proceedings of Southern Biomedical Engineering 2009; 2009 May 15–17; Miami, FL, USA: Springer-Verlag Berlin heidelberg; 2009. pp. 175–8. [Google Scholar]
- 7.Meng QW, Yan L, Ao X, et al. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J Anim Sci. 2010;88:3320–6. doi: 10.2527/jas.2009-2308. [DOI] [PubMed] [Google Scholar]
- 8.Vasala A, Panula J, Neubauer P. Efficient lactic acid production from high salt containing dairy by-products by Lactobacillus salivarius ssp. salicinius with pre-treatment by proteolytic microorganisms. J Biotechnol. 2005;117:421–31. doi: 10.1016/j.jbiotec.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Latimer GW, AOAC International . Official methods of analysis of AOAC International. 19th ed. Gaithersburg, MD, USA: AOAC International; 2012. [Google Scholar]
- 10.Bacteriological analytical manual online [Internet] Food and Drug Administration; 2001. [cited 2014 Mar 18]. Available from: https://www.epa.gov/sites/production/files/2015-07/documents/fda-bam-appendix1.pdf. [Google Scholar]
- 11.Robb EJ, Bryson WL, Chester ST, et al. Efficacy of ceftiofur sodium for the control of mortality in neonatal pigs orally inoculated with K88 (F4) enterotoxigenic Escherichia coli. J Swine Health Prod. 2003;11:7–11. [Google Scholar]
- 12.Giang HH, Viet TQ, Ogle B, et al. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest Sci. 2010;129:95–103. [Google Scholar]
- 13.Liu P, Piao X, Thacker P, et al. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with K88. J Anim Sci. 2010;88:3871–9. doi: 10.2527/jas.2009-2771. [DOI] [PubMed] [Google Scholar]
- 14.Tsirtsikos P, Fegeros K, Kominakis A, et al. Modulation of intestinal mucin composition and mucosal morphology by dietary phytogenic inclusion level in broilers. Animal. 2012;6:1049–57. doi: 10.1017/S1751731111002680. [DOI] [PubMed] [Google Scholar]
- 15.SAS . SAS/SAT guide for personal computers. 9.00ed. Carry, NC, USA: SAS Inst., Inc.; 2002. [Google Scholar]
- 16.Zhang J, Deng J, Wang C, et al. Modulatory effects of Lactobacillus salivarius on intestinal mucosal immunity of piglets. Curr Microbiol. 2011;62:1623–31. doi: 10.1007/s00284-011-9906-4. [DOI] [PubMed] [Google Scholar]
- 17.Banach S, Rehberger T, Parrott T, et al. Influence of Lactobacillus brevis strain 1E-1 on the gastrointestinal microflora of pre-weaning and weaning pigs. American Society of Animal Science Meeting 2002; 2002 Mach 18–20; Iowa, USA. [Google Scholar]
- 18.Rondon AJ, Ojito Y, Arteaga FG, et al. Probiotic effect of Lactobacillus salivaruis C 65 on productive and health indicators of lactating piglets. Cuban J Agric Sci. 2013;47:401–7. [Google Scholar]
- 19.Yu H, Wang A, Li X, et al. Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. J Anim Feed Sci. 2008;17:61–9. [Google Scholar]
- 20.Ammor S, Tauveron G, Dufour E, et al. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility: 1—Screening and characterization of the antibacterial compounds. Food Control. 2006;17:454–61. [Google Scholar]
- 21.Huang C, Qiao S, Li D, et al. Effects of lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian-Australas J Anim Sci. 2004;17:401–9. [Google Scholar]
- 22.Yang KM, Jiang ZY, Zheng CT, et al. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. 2014;92:1496–503. doi: 10.2527/jas.2013-6619. [DOI] [PubMed] [Google Scholar]
- 23.Miriam BB, Julio PD, Sergio MQ, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–74. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 24.Lähteinen T, Malinen E, Koort JM, et al. Probiotic properties of Lactobacillus isolates originating from porcine intestine and feces. Anaerobe. 2010;16:293–300. doi: 10.1016/j.anaerobe.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Xu YQ, Liu HY, et al. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol. 2010;141:142–8. doi: 10.1016/j.vetmic.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Lähteinen T, Rinttilä T, Koort JM, et al. Effect of a multispecies lactobacillus formulation as a feeding supplement on the performance and immune function of piglets. Livest Sci. 2015;180:164–71. [Google Scholar]
- 27.Suo C, Yin Y, Wang X, et al. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet Res. 2012;8:89. doi: 10.1186/1746-6148-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maria MR, Deise OS, Ernesto AT, et al. IgA production, coliforms analysis and intestinal mucosa IgA production, coliforms analysis and intestinal mucosa morphology of piglets that received probiotics with viable or morphology of piglets that received probiotics with viable or inactivated cells inactivated cells. Pesq Vet Bras. 2007;27:241–5. [Google Scholar]