Abstract
Monocarboxylate transporter-1 (MCT-1) is a transmembrane transporter for monocarboxylates including lactate and pyruvate. Silencing Mct1 by its small interfering RNA (siRNA) suppressed the expression of marker genes for osteoblast differentiation, namely, Tnap, Runx2, and Sp7, induced by BMP-2 in mouse myoblastic C2C12 cells. Mct1 siRNA also suppressed alkaline phosphatase activity, as well as expressions of Tnap and Bglap mRNAs in mouse primary osteoblasts. On the other hand, Mct1 siRNA did not have effects on the Smad1/5 or ERK/JNK pathways in BMP-2-stimulated C2C12 cells, while it up-regulated the mRNA expression of p53 (Trp53) as well as nuclear accumulation of p53 in C2C12 cells in a BMP-2-independent manner. Suppression of osteoblastic differentiation by Mct1 siRNA in C2C12 cells was abolished by co-transfection of Trp53 siRNA. Together, these results suggest that MCT-1 functions as a positive regulator of osteoblast differentiation via suppression of p53.
Introduction
Monocarboxylate transporters (MCTs) are a group of transmembrane transporters for monocarboxylates. Among 14 subtypes identified thus far, inward and outward transportation of H+ and monocarboxylates, such as lactate and pyruvate, have been shown to be mediated by 4 types of MCTs, i.e., MCT-1, -2, -3, and -41. Of those, MCT-1 is ubiquitously expressed, and its major physiological role is believed to facilitate influx and efflux of L-lactate across the plasma membrane depending on the cellular metabolic state. For example, L-lactate absorbed through MCT-1 is used for gluconeogenesis in parenchymal cells of the liver and the proximal convoluted tubule cells of the kidney2. Furthermore, L-lactate and ketone bodies absorbed through MCT-1 are used as fuel for aerobic respiration in cardiac muscle and red skeletal muscle cells3. On the other hand, most cells must release L-lactate to outside of the cell via MCT-1 when glycolysis is predominant under a hypoxic condition. Transportation of H+ together with L-lactate through MCT-1 prevents intracellular acidification, which is preferable for maintaining an intracellular condition suitable for high glycolytic flux3.
In our previous study, we demonstrated that MCT-1 is required for chondrocyte death induced by interleukin-1β (IL-1β), which occurred in a nitric oxide- and reactive oxygen species (ROS)-dependent manner4. Furthermore, MCT-1 knockdown by siRNA strongly suppressed expression of phagocyte-type NADPH oxidase (NOX-2), an enzyme specialized for production of ROS, in mouse chondrocyte-like ATDC5 cells. We also found that an MCT-1-dependent increase in mitochondrial ROS production was required for late phase activation of NF-κB, which led to expression of NOX-2 in ATDC5 cells stimulated by IL-1β. Together, those observations suggested that MCT-1 might have possible hidden functions, in addition to regulation of energy metabolism and intracellular pH. MCT-1 is also known to be ubiquitously expressed in various tissues including bones1,5. Furthermore, we have shown that acetoacetate enhanced and β-hydroxybutyrate suppressed mineralization by mouse osteoblastic MC3T3-E1 cells, in both the presence and absence of bone morphogenetic protein (BMP)-26. These ketone bodies are known to be transported across plasma membranes via MCT-11, while knockdown of Mct1 nullified their effects6.
Those findings led us to explore the role of MCT-1 in osteoblast differentiation in the present study, for which we chose osteoblastic differentiation of mouse myoblastic C2C12 cells induced by BMP-2 as a model of osteoblast differentiation7. BMP-2, a cytokine that belongs to the transforming growth factor-β superfamily, binds to a complex of type I and type II receptors, and induces phosphorylation and activation of Smad-1, -5, and -8, transcription factors that activate the expression of RUNX2, a master transcription factor for osteoblast differentiation, while BMP receptors also activate the MAP-kinase pathway8. Based on the present results, we herein describe a function of MCT-1 in BMP-2-induced osteoblast differentiation, including the Smad and MAP-kinase pathways. In addition, we also examined the role of MCT-1 in activation of p53, a negative regulator of osteoblast differentiation9.
Results
Mct1 knockdown suppressed osteoblast differentiation of C2C12 cells after stimulation by BMP-2
In C2C12 cells, the expression level of Mct1 mRNA was the highest among mRNAs for MCT-1, -2, -3, and -4 (Suppl. Fig. S1a), known transmembrane transporters for monocarboxylate such as lactate and pyruvate1. Hence we silenced Mct1 mRNA expression by introducing Mct1 siRNA into C2C12 cells (Suppl. Fig. S1b). Intracellular lactate in Mct1 siRNA-introduced C2C12 cells was 3 times higher than that in cells introduced with the control siRNA (Suppl. Fig. S1c). Since intracellular lactate is thought to be generated as a result of glycolysis, its elevated concentration is assumed to be the result of suppressed efflux of lactate through MCT-1 in Mct1 siRNA-introduced C2C12 cells.
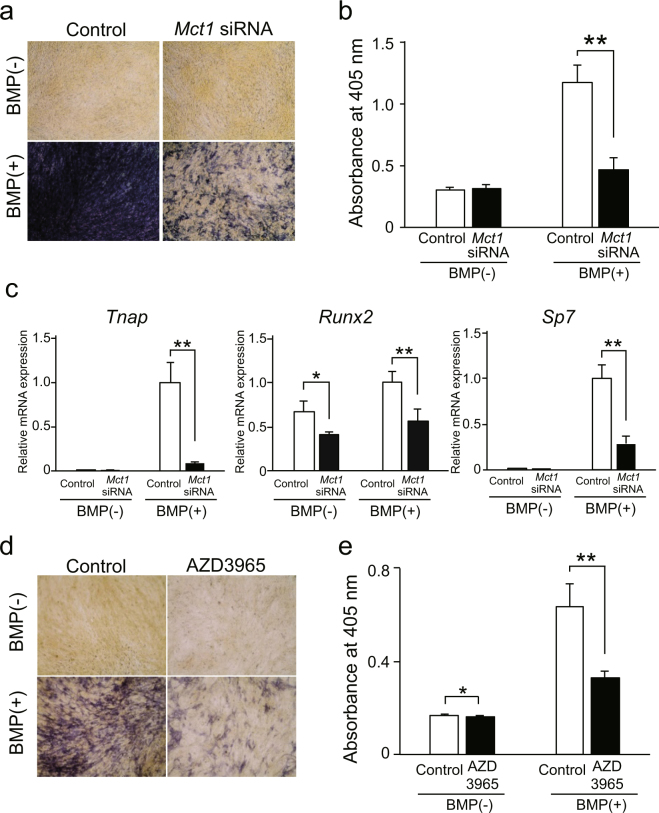
Mct1 knockdown did not have effects on proliferation of C2C12 cells in either the presence or absence of BMP-2 (Suppl. Fig. S1d). It is conceivable that differentiation of C2C12 cells into osteoblast-like cells induced by BMP-2 mimics the differentiation of osteoblasts10. Hence, we analyzed the effect of Mct1 knockdown on osteoblastic differentiation of C2C12 cells stimulated by BMP-2. We found that the increase in number of ALP-positive cells, enhanced ALP activity, and increased expression of tissue-nonspecific ALP gene (Tnap) in C2C12 cells after exposure to BMP-2 were each suppressed by introduction of Mct1 siRNA (Fig. 1a–c). In addition, Mct1 knockdown suppressed mineralization of extracellular matrices of C2C12 cells cultured in the presence of BMP-2 (Suppl. Fig. S2). Furthermore, Mct1 knockdown suppressed BMP-2-induced expression of genes for transcription factors required for osteoblast differentiation, i.e., RUNX2 (Runx2) and Osterix (Sp7), in C2C12 cells following stimulation with BMP-2 (Fig. 1c). Also, down-regulation of ALP activity and Tnap expression were observed in C2C12 cells treated with AZD3965, a specific inhibitor of MCT-1 (Fig. 1d,e). These results suggest that lowered expression of MCT-1 or its inhibition suppresses BMP-2-induced differentiation of C2C12 cells into osteoblast-like cells.
Figure 1.
Suppression of osteoblastic differentiation of C2C12 cells by introduction of Mct1 siRNA or inhibition of MCT-1 activity. C2C12 cells introduced with control or Mct1 siRNA were cultured for 72 (a,b) or 48 (c) hours in the absence (−) or presence (+) of BMP-2 (300 ng/mL). (a) Results of ALP activity staining. (b) ALP activity in lysates of C2C12 cells. (c) Expressions of mRNAs for Tnap, Runx2, and Sp7 were determined using real-time RT-PCR, then normalized against that of Gapdh, with the results shown as relative values. (d) C2C12 cells were cultured for 48 hours in the presence or absence of 1 μmol/L of AZD3965, a specific inhibitor of MCT-1, and with (+) or without (−) BMP-2 (300 ng/mL). ALP activity was detected by staining. (e) ALP activity in lysates of C2C12 cells cultured under the same conditions. (b,c,e) Values are expressed as the mean ± SD (n = 3–5). **Significantly different from control group (p < 0.01).
Mct1 knockdown suppressed expression of ALP in mouse calvarial osteoblasts
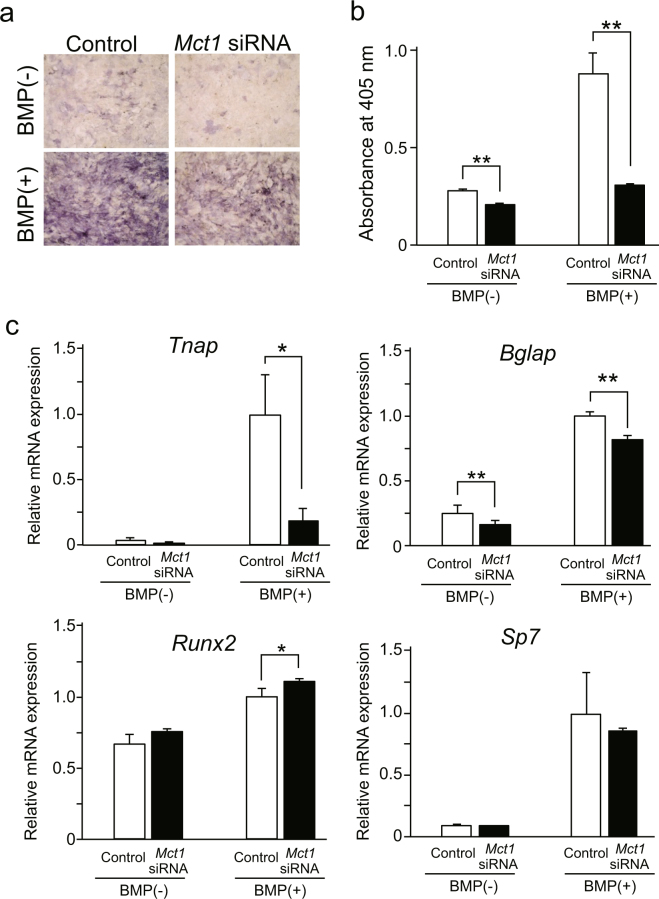
In order to investigate the role of MCT-1 in expression of osteoblast phenotypes by differentiated osteoblasts, we examined the effects of Mct1 knockdown on ALP activity and expression of osteoblast marker genes in mouse primary osteoblasts cultured with or without BMP-2. Mct1 knockdown reduced ALP activity in primary cultures of osteoblasts even in the presence of BMP-2 (Fig. 2a,b), while it also suppressed BMP-2-induced expression of mRNAs for Tnap and Bglap. In contrast, Mct1 siRNA induced a 10% increase in Runx2 mRNA expression. Sp7 mRNA expression was not affected by Mct1 siRNA (Fig. 2c).
Figure 2.
Suppressed expression of osteoblast differentiation markers in primary mouse osteoblasts by introduction of Mct1 siRNA. Control or Mct1 siRNA was introduced into primary osteoblasts isolated from mouse calvaria. Osteoblasts were cultured for 72 (a,b) or 48 (c) hours in the absence (−) or presence (+) of BMP-2 (300 ng/mL). (a) ALP activity, detected by staining. (b) ALP activity in cell lysates. (c) Expression of mRNAs for Tnap, Bglap, Runx2, and Sp7 were analyzed by real-time RT-PCR, then normalized against that of Gapdh, with the results indicated as relative values. (b,c) Data are expressed as the mean ± SD (n = 3–5). *, **Significantly different from control group (*p < 0.05, **p < 0.01).
No effects of Mct1 knockdown on Smad and MAPK pathways in C2C12 cells stimulated by BMP-2
To elucidate the mechanism by which Mct1 knockdown suppresses osteoblast differentiation of C2C12 cells induced by BMP-2, we investigated its effects on the downstream signaling pathways of BMP-211. Initially, we examined the effect of Mct1 knockdown on the Smad pathway. Phosphorylation of Smad1/5 after addition of BMP-2 was not affected by introduction of Mct1 siRNA (Fig. 3a, Suppl. Fig. S3a). Although Id1 is well known as a target gene of Smad1/5/8, introduction of Mct1 siRNA did not have an effect on BMP-2-induced expression of luciferase activity in C2C12 cells transfected with the Id1 promotor-coupled luciferase gene (Fig. 3b) or mRNA expression of intrinsic Id1 induced by BMP-212 (Fig. 4c). Furthermore, while Mct1 knockdown up-regulated the mRNA expression of Dlx2, another target gene of BMP/Smad pathway13, in the absence of the cytokine, it did not have effects on BMP-2-induced expression of Dlx2 (Fig. 3d). These results indicated that reduced Mct1 expression downregulates osteoblastic differentiation via mechanisms other than modulation of the Smad pathway. Next, we examined the effect of Mct1 knockdown on activation of the MAP kinase pathway, which is known to positively regulate osteoblast differentiation14. Introduction of Mct1 siRNA did not have effects on phosphorylation of ERK1/2 or JNK (Fig. 3e, Suppl. Fig. S3b,c). The PI3K/AKT pathway is also known to induce osteoblast differentiation under stimulation by BMP-215. However, Mct1 knockdown only slightly augmented the phosphorylation of AKT, while BMP-2 did not have an effect on activation of AKT in Mct1-silenced C2C12 cells (Suppl. Fig. S4a,b). These effects of Mct1 knockdown on AKT phosphorylation cannot explain the suppressed osteoblastic differentiation of C2C12 cells introduced with Mct1 siRNA. We concluded that the MAP kinase pathway is not a major point of action of MCT-1 for promotion of BMP-2-induced osteoblast differentiation.
Figure 3.
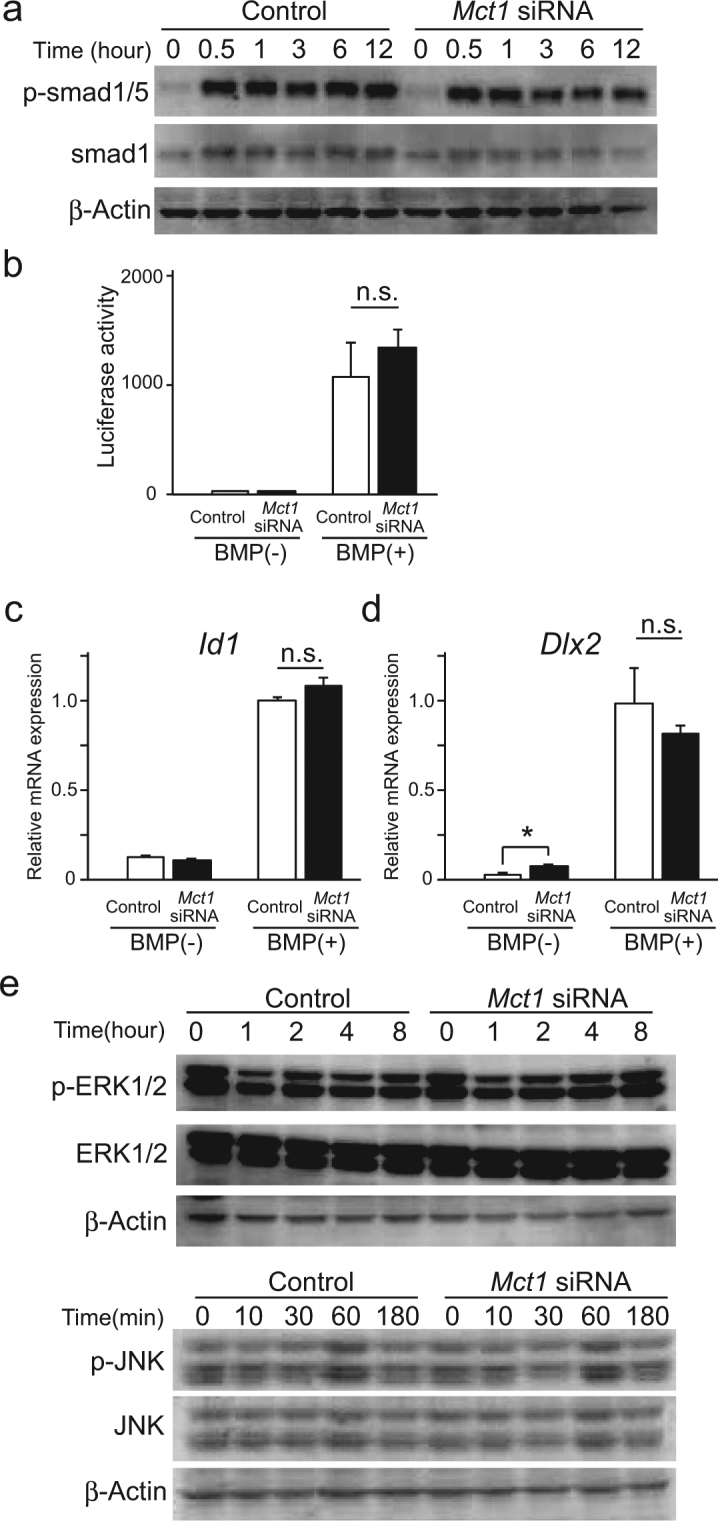
Effects of Mct1 knockdown on Smad and MAP kinase pathways in C2C12 cells after stimulation by BMP-2. (a) Control and Mct1 siRNA-introduced C2C12 cells were exposed to BMP-2 (300 ng/mL) for the indicated periods. Phosphorylation of Smad1/5 and expression of Smad1 at various times after addition of BMP-2 were evaluated by western blotting. (b) C2C12 cells harboring control or Mct1 siRNA were transfected with an Id1.0-luciferase reporter plasmid and TK vector. After incubation for 24 hours with (+) or without (−) BMP-2 (300 ng/mL), light emission intensity was measured. Relative firefly luciferase reaction values were normalized against that of the luminescent reaction of Renilla luciferase from the TK vector. (c,d) C2C12 cells harboring control or Mct1 siRNA were incubated for 24 hours with (+) or without (−) BMP-2 (300 ng/mL). Expressions of Id1 mRNA (c) and Dlx2 mRNA (d) were quantitatively assessed by real-time RT-PCR, then normalized against that of Gapdh, with the results shown as relative values. (b,c) Data are expressed as the mean ± SD (n = 3–5). n.s., not significant. (e) Control and Mct1 siRNA-introduced C2C12 cells were exposed to BMP-2 (300 ng/mL) for the indicated periods, then expressions of ERK1/2 and JNK, as well as their phosphorylation were evaluated by western blotting. (a,e) Representative data from 3 independent experiments are shown. Quantitative evaluations of phosphorylation of Smad1/5, ERK1/2, and JNK are shown in Supplementary Figure S3. Full-length blots are presented in Supplementary Figure S9.
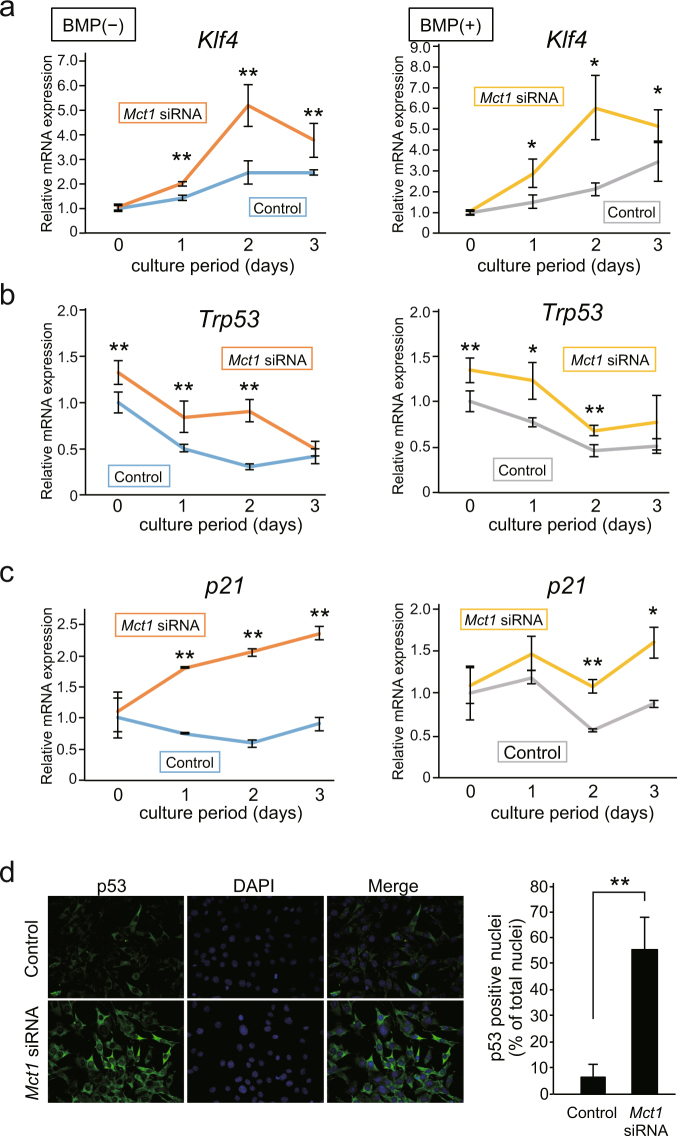
Figure 4.
Induction of expression and activation of p53 in C2C12 cells by Mct1 knockdown. (a–c) Control and Mct1 siRNA-introduced C2C12 cells were cultured for the indicated periods in the absence (−) or presence (+) of BMP-2 (300 ng/mL). Expressions of mRNAs for Klf4 (a), Trp53 (b), and p21 (c) were analyzed by real-time RT-PCR, then normalized against that of Gapdh, with the results indicated as relative values. (a–c) Values are expressed as the mean ± SD (n = 3–5). (d) C2C12 cells were introduced with control or Mct1 siRNA, then p53 protein was detected using an immunocytochemical method. Nuclei were stained with DAPI. (e) Ratios of p53-positive nuclei in cultures of C2C12 cells introduced with control or Mct1 siRNA. (a–c,e) Data are expressed as the mean ± SD (n = 3–5). **Significantly different from control group (*p < 0.05, **p < 0.01).
Mct1 knockdown enhanced expression of Trp53 and Klf4
It has been reported that a reduced concentration of serum in culture medium induces myogenic differentiation of C2C12 cells in the absence of BMP-27. However, the role of MCT-1 in myogenic differentiation has not been described, thus we analyzed the effects of Mct1 knockdown on differentiation of C2C12 cells into myocyte-like cells. The expression of mRNAs for MyoD, a myocyte marker gene16,17, was not significantly affected by introduction of Mct1 siRNA in C2C12 cells (Suppl. Fig. S5). On the other hand, that of Myogenin, another myocyte marker gene, was up-regulated by co-transfection of Mct1 siRNA and Trp53 siRNA. It was previously reported that activation of KLF4 by ERK5 promoted myotube formation in cultures of C2C12 cells18, while the present results showed that the expression of Klf4 mRNA in C2C12 cells following introduction of Mct1 siRNA was increased in both the absence and presence of BMP-2 (Fig. 4a). It has also been shown that KLF4 directly binds to RUNX2 and inhibits its transcriptional activity19. However, Mct1 knockdown suppressed the expression of Runx2 in C2C12 cells (Fig. 1c), indicating that increased Klf4 gene expression is not the sole mechanism for suppression of differentiation of C2C12 cells into osteoblast-like cells. Nevertheless, we noted increased expression of mRNA for the p53 gene Trp53 (Fig. 4b).
In order to clarify the relationship between Trp53 and Klf4 in Mct1 siRNA-introduced C2C12 cells, a time course study of the expression of each gene was performed. An increase in expression of Trp53 in Mct1 siRNA-introduced C2C12 cells was observed prior to induction of differentiation caused by lowering the concentration of serum in both the presence and absence of BMP-2 (Fig. 4b). On the other hand, as shown in Fig. 4a, increased expression of Klf4 mRNA was observed at least 24 hours after induction of both myogenic and osteoblastic differentiation by cultivation of C2C12 cells in the absence and presence of BMP-2. It was previously reported that p53 induces the expression of Klf420, thus it is plausible that Trp53, whose expression was shown to be augmented by Mct1 knockdown, induces Klf4 expression. We confirmed functional increments of p53 and KLF4 in Mct1-silenced C2C12 cells by enhanced expression of p21 (Fig. 4c), one of the target genes of both p53 and KLF420. Furthermore, fluorescent immunostaining of p53 revealed that both p53 protein expression and its accumulation in nuclei were enhanced in Mct1-silenced C2C12 cells (Fig. 4d).
MCT-1 positively regulated osteoblastic differentiation of C2C12 cells via down-regulation of p53
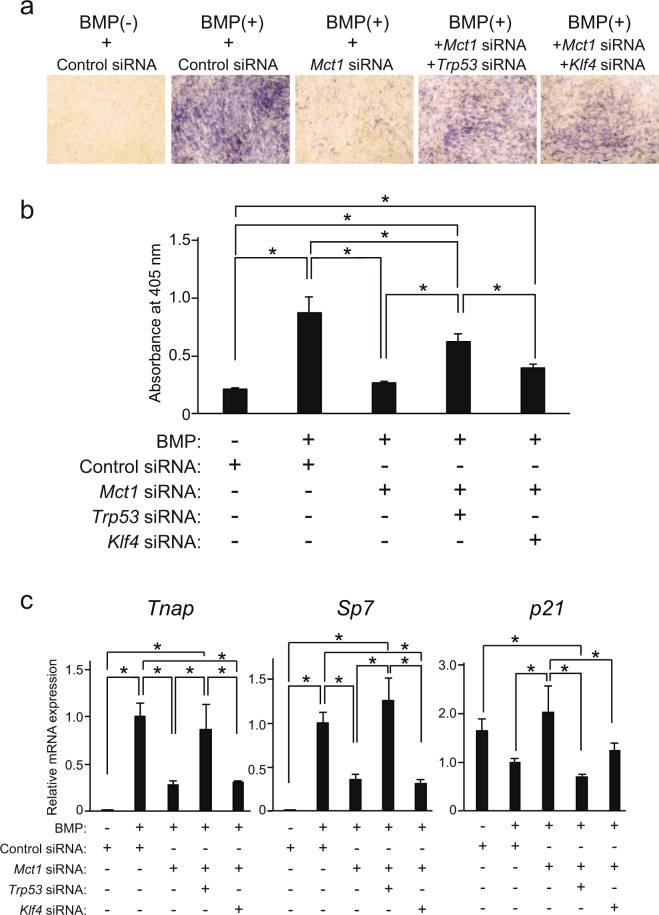
The suppression of BMP-2-induced ALP activity in C2C12 cells by Mct1 siRNA was partially recovered by co-introduction of Trp53 siRNA (Fig. 5a,b), which also led to recovery of suppressed expression of Sp7 mRNA as well as that of Tnap mRNA (Fig. 5c). Together, these results suggested that Mct1 knockdown induces increased expression of p53, leading to suppressed osteoblastic differentiation of C2C12 cells in the presence of BMP-2. On the other hand, Klf4 siRNA did not have significant effects on ALP activity lowered by Mct1 siRNA (Fig. 5a,b) nor the expression of Tnap, Sp7, or p21 in Mct1-silenced C2C12 cells.
Figure 5.
Osteoblastic differentiation suppressed by Mct1 siRNA recovered by introduction of Trp53 siRNA. C2C12 cells were introduced with control or Mct1 siRNA in combination with Trp53 siRNA or Klf4 siRNA, then cultured for 72 (a) or 48 (b) hours in the absence (−) or presence (+) of BMP-2 (300 ng/mL). (a) ALP activity in the cultures was detected by staining. (b) ALP activity in lysates of C2C12 cells. (c) Expression of mRNAs for Tnap and Sp7 were analyzed by real-time RT-PCR, then normalized against that of Gapdh, with the results indicated as relative values. (b,c) Data are expressed as the mean ± SD (n = 3–5). *Significant difference between groups (*p < 0.05).
Discussion
Although it has been widely reported that MCT-1 plays important roles in cellular energy metabolism1–3, other biological roles of this transporter have rarely been examined. In the present study, we found two novel functions, requirement of MCT-1 for osteoblast differentiation, and its activity to suppress expression and activation of p53. Differentiation of myoblastic C2C12 cells into osteoblast-like cells following exposure to BMP-2 is regarded as a model of osteoblast differentiation from cells in a lower stage of differentiation, such as mesenchymal stem cells21. Using this model, we found that Mct1 knockdown suppressed osteoblastic differentiation of C2C12 cells.
We also noted that Mct1 siRNA suppressed the expressions of Tnap and Bglap, as well as ALP activity in not only C2C12 cells but also mouse primary osteoblasts (Fig. 2a–c). Although Mct1 siRNA induced a 10% increase in expression of Runx2 mRNA, that limited escalation of expression did not alter the expression of Sp7 (Fig. 2c). Since Mct1 knockdown suppressed the expression of Tnap and Bglap, late-phase marker genes of osteoblasts, without suppression of Runx2 or Sp7 expression in differentiated osteoblasts, it is possible that MCT-1 promotes expression of these late-phase differentiation markers of osteoblasts by regulating other transcription factors or the signaling system independent of induction of RUNX2 and Sp722.
We also did not find a noticeable effect of Mct1 siRNA on well-known events downstream of stimulation by BMP-2, namely, expression of the Id1 gene or phosphorylation of Smad-1/5, ERK, JNK, and AKT (Fig. 3, Suppl. Figs S3 and S4). These results suggested that Mct1 knockdown suppresses osteoblast differentiation of C2C12 cells in a BMP-2-independent manner. It is interesting that Mct1 siRNA augmented the expressions of Trp53, Klf4, and p21 in C2C12 cells in both the presence and absence of BMP-2 (Fig. 4a–c), indicating that MCT-1 suppresses expression of those genes independent of BMP-2. Our finding of increased expression of p21 mRNA in Mct1-silenced C2C12 cells is consistent with induction of Trp53 expression, since p21 is one of the genes targeted by p5323. It is well known that p53 suppresses cell proliferation by induction of expression of p21, a cyclin-dependent kinase inhibitor, while another study reported that galectin-3 stabilizes p21 protein in prostate cancer cells24. It is possible that p21instability explains why the augmented expression of mRNAs for Trp53 and p21 did not have an effect on proliferation of C2C12 cells harboring Mct1 siRNA.
The well-known tumor suppressor and transcription factor p53 has a diverse array of biological functions, among which DNA repair, regulation of cell cycle and senescence, and induction of apoptosis are regarded as the most important25. In addition, reports have shown a role of p53 in differentiation of various types of cells26, such as osteoblasts, in which p53 suppresses transcription of the RUNX2 gene27. It is also known that glycolysis is very active in tumor cells even in aerobic conditions, resulting in acidification of tumor tissues by enhanced production and excretion of lactate28. Upregulated expression of MCT-1 in p53-null and -mutated tumor cells has also been reported29,30. On the other hand, regulation of p53 expression by MCT-1 has not been previously examined. The present findings showed that Mct1 knockdown enhanced the expression of Trp53 (Fig. 4), indicating that MCT-1 is a negative regulator of p53 expression.
KLF4 is also known as a multifunctional protein and has been shown to be an essential factor for induction of pluripotent stem cells31,32, while another report noted that KLF2 and KLF4 function as positive regulators of myotube formation by C2C12 cells18. In our experimental settings, the effect of Mct1 knockdown on expression of Myogenin, a marker gene of myogenic differentiation, was small and insignificant, and co-transfection of Klf4 siRNA also did not have a significant effect on expression of Myogenin mRNA in Mct1-silenced C2C12 cells, either (Suppl. Fig. S5). Therefore, enhanced expression of Klf4 in Mct1-silenced C2C12 cells (Fig. 4a) does not have an appreciable role in differentiation of C2C12 cells into myocyte-like cells (Suppl. Fig. S5). On the other hand, introduction of Trp53 siRNA significantly enhanced expression of the Myogenin gene in Mct1-silenced C2C12 cells (Suppl. Fig. S5), indicating that p53 has an ability to suppress myogenic differentiation in C2C12 cells.
A recent report described incorporation of lactate through MCT-1-stabilized HIF-1α, which caused promotion of osteoblast differentiation33. It has also been reported that HIF-1α enhanced the expression of Trp5334. To examine if HIF-1α has an important role in induction of Trp53 in our experimental settings, we performed immunostaining of C2C12 cells for HIF-1α with or without introduction of Mct1 siRNA. However, Mct1 siRNA did not have a noticeable effect on either the expression or nuclear accumulation of HIF-1α protein in C2C12 cells following stimulation with BMP-2 (Suppl. Fig. S6b). Thus, it seems unlikely that HIF-1α plays an important role in the induced expression of Trp53 after Mct1 knockdown. On the other hand, in the absence of BMP-2, cytoplasmic HIF-1α expression was increased by Mct1 siRNA (Suppl. Fig. S6a). It is well known that the protein level of HIF-1α is up-regulated by hypoxia, and it has also been shown that transcription of the HIF1A gene is promoted by activation of the PI3K-Akt and MAPK pathways35,36. In our experiments, Mct1 siRNA did not alter nuclear accumulation of HIF-1α, whereas phosphorylation of Akt was augmented in Mct1-silenced C2C12 cells even in the absence of BMP-2 (Suppl. Fig. S4). Therefore, it is possibile that decreased expression of MCT-1 stimulates the Akt pathway and enhances HIF-1α expression.
Another study showed that aerobic glycolysis suppresses p53 activation in cancer cells37. In the present experiments, the amount of intracellular lactate was found to be increased in Mct1-silenced C2C12 cells (Suppl. Fig. S1c), indicating a decrease in excretion of lactate via MCT-1 in those cells. Since lactate dehydrogenase catalyzes both the reduction of pyruvate into lactate and oxidation of lactate into pyruvate, it can be speculated that lactate accumulation in cytosol suppresses the former reaction and facilitates the latter, which might reduce the efficacy of glycolysis by lowering the NAD+/NADH ratio. We observed reduced amounts of incorporated L-glucose in Mct1 siRNA-introduced C2C12 cells when cultured in differentiation-inducing medium with a lower serum concentration (2.5%) (Suppl. Fig. S7). In addition, osteoblastic differentiation of C2C12 cells when cultured in DMEM containing a low level of L-glucose was suppressed (Suppl. Fig. S8). Therefore, suppression of glycolysis may promote p53 activation in and osteoblastic differentiation of Mct1-silenced cells.
In conclusion, we found that MCT-1 facilitates osteoblast differentiation via suppression of p53 expression and activation, thus knockdown of the Mct1 gene resulted in increased p53 activity, which induced the expression of Klf4 and p21. We speculate that the p53 pathway is involved in inhibition of Runx2 and Sp7 expression, as well as expression of osteoblast marker genes, such as Tnap and Bglap.
Methods
Ethical approval statement
All experimental protocols were approved by Showa University Animal Experiment Facility and Showa University Genetic Recombination Laboratory (Approval number: 17053). We confirm that all experiments were performed in accordance with the relevant guidelines and regulations.
Reagents
Recombinant human BMP-2 was obtained from R&D Systems (Minneapolis, MN, USA). AZD3965, a specific MCT-1 inhibitor, was purchased from Cayman Chemical (Ann Arbor, MI, USA). Antibodies against smad1, phospho-smad1/5, ERK1/2, phospho-ERK1/2, ERK5, phospho-ERK5, JNK, phospho-JNK, p38MAPK, phospho-p38MAPK, AKT, and phosphor-AKT were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against myosin heavy chain (MF-20), p53, and HIF-1α were obtained from Developmental Studies Hybridoma Bank (Iowa City, IA, USA), Abcam (Cambridge, UK), and GeneTex, Inc. (Irvine, CA, USA), respectively.
Cell cultures
C2C12 cells were purchased from RIKEN BioResource Center (C2C12 #RCB0987, Tsukuba, Japan) and grown in Dulbecco’s modified Eagle’s medium (DMEM, Wako Pure Chemical Industries, Osaka, Japan) supplemented with 15% fetal bovine serum (FBS). To induce differentiation into osteoblast-like cells, the medium was replaced with DMEM supplemented with 2.5% FBS and 300 ng/mL BMP-2, while myotube formation was induced by replacing the medium with DMEM supplemented with 2.5% FBS. Mouse primary osteoblasts were isolated from calvaria of neonatal ddY mice and grown in αMEM (Wako Pure Chemical Industries) supplemented with 10% FBS. Osteoblast phenotypes were induced by stimulation with 10% FBS and 300 ng/ml BMP-2 in the same medium. StealthTM siRNAs (Invitrogen, Carlsbad, CA, USA) for mouse Mct1, Trp53, and Klf4, and negative control siRNA were introduced into 40–50% confluent cells using LipofecttamineTM RNAiMAX (Invitrogen) by reverse transfection. BMP-2 was added to the cultures at 24 hours after siRNA introduction.
Real-time RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription reactions were performed using ReverTra ACE RT qPCR master Mix (TOYOBO Co. Ltd., Osaka, Japan). Quantitative real-time RT-PCR was performed using a TaqManTM Gene Expression Assay (Applied Biosystems, Carlsbad, CA, USA). The assay IDs of the genes were as follows: Gapdh, Mm99999915_g1; Mct1, Mm01306379_m1; Mct2, Mm00441442_m1; Mct3, Mm00445115_m1; Mct4, Mm00446102_m1; Tnap, Mm00475834_m1; Runx2, Mm00501584_m1; Sp7, Mm04209856_m1; Bglap, Mm03413826_mH; Dlx2, Mm00438427_m1; Trp53, Mm01731290_g1; p21, Mm00432448_m1; Klf4, Mm00516104_m1; and Id1, Mm00775963_g1. Amplification signals from the target genes were normalized against that of Gapdh.
Cell proliferation assay
C2C12 cells were plated at a density of 1 × 104 cells/well in 96-well plates, and cultured in the presence or absence of BMP-2 (300 ng/mL) for various periods up to 72 hours. At the end of each culture period, cells were incubated for 1 hour with solution from a CellTiter 96® Aqueous One Solution cell proliferation assay kit (Promega, Madison, WI, USA), then MTS-formazan was determined by reading absorbance at 490 nm.
Determination of intracellular lactate
C2C12 cells were plated at a density of 5 × 105 cells/well in 6-well plates and cultured for 24 hours, then washed with PBS and homogenized in 1% Nonidet P-40 (200 μL) under sonication on ice. Lactate concentrations in the cell lysates were determined using F-kit D-lactic acid/L-lactic acid (J.K. International, Tokyo, Japan), according to the manufacturer’s instructions.
ALP activity staining
Cells were plated in 96-well plates at a density of 1 × 104 cells/well, and cultured for 72 hours in the presence or absence of BMP-2 (300 ng/mL). Cells were fixed for 30 minutes in 4% paraformaldehyde and washed with PBS, then incubated for 30 minutes at 37 °C with 100 mmol/L Tris-HCl buffer (pH 8.5) containing 270 μmol/L naphthol AS-MX phosphate (Sigma-Aldrich) and 1.4 mmol/L Fast blue BB (Sigma-Aldrich). After washing with tap water, they were observed under a microscope.
Determination of ALP activity
Cells were cultured in the same conditions as described in the ALP activity staining subsection above, then washed with PBS and homogenized with 1% Nonidet P-40 (50 μL) under sonication on ice. Cell lysates (10 μL) were added to 50 μL of 0.2 mol/L Tris–HCl buffer (pH 9.5) containing 1 mmol/L MgCl2 and 12.5 mmol/L disodium p-nitrophenyl phosphate (Wako Pure Chemical Industries). After incubation for 15 minutes at 37 °C, reactions were terminated by addition of 50 μL of 0.5 mol/L NaOH and absorbance of the reaction mixture at 405 nm was read using a micro-plate reader (SH-1000, Corona Electric, Ibaraki, Japan). The increase in absorbance in after 15 minutes was divided by the amount of cellular protein and the obtained value was used to express the specific activity of ALP.
Alizarin red staining
Cells were plated in 96-well plates at a density of 1×104 cells/well, and cultured for 4 days in the medium described above in the presence or absence of BMP-2 (300 ng/mL), followed by additional 3-day culture in DMEM supplemented with 2.5% FBS, ascorbic acid (50 μg/mL), β-glycerophosphate (10 mmol/L), and dexamethasone (10 nmol/L). Cells were washed with PBS, fixed in 95% methanol, stained with 1% alizarin red S (pH 6.3–6.5) (Wako Pure Chemicals) for 5 minutes, and washed with water to remove unbound dye. Calcified nodules visualized by alizarin red S were observed under a microscope. For evaluation of calcium deposition, alizarin red S bound to cell matrices was dissolved in 10% (w/v) cetylpyridinium chloride in water and quantified by measuring absorbance at 570 nm.
Immunostaining of myosin heavy chain protein
C2C12 cells were plated in 96-well plates at a density of 1 × 104 cells/well and cultured for 6 days. Next, they were fixed for 30 minutes in 4% paraformaldehyde, then washed with PBS, permeated with 0.1% Triton X-100 in PBS for 5 minutes, and incubated overnight at 4 °C with 0.5 μg/mL of a mouse anti-αMHC monoclonal antibody in PBS containing 10% rabbit serum. After washing again with PBS, cells were incubated for 30 minutes with Histofine® Simple StainTM MAX PO (MULTI) (Nichirei. Co., Tokyo, Japan). Finally, the reaction products were visualized using a Histofine AEC substrate kit (Nichirei Co., Tokyo, Japan).
Immunostaining of p53 and HIF-1α
C2C12 cells were fixed for 20 minutes in 4% paraformaldehyde at room temperature, then permeated with 0.1% Triton X-100 in PBS for 5 minutes. Next, they were incubated overnight at 4 °C with 0.5 μg/mL of the anti-p53 antibody or anti-HIF-1α in PBS containing 10% rabbit serum, followed by incubation for 45 minutes at room temperature with an Alexafluor-488-conjugated secondary antibody (Invitrogen) diluted in PBS (1:100). Nuclei were stained with 4′,6′-diamidine-2′-phenylindole dihydrochloride (DAPI) and fluorescence was observed using a BZ-9000 microscope (KEYENCE, Osaka, Japan).
Western blot analysis
C2C12 cells were lysed in 10 mmol/L Tris-HCl (pH 7.8) with 1% Nonidet P-40, 0.15 mol/L NaCl, and a protease inhibitor mixture containing EDTA (Roche Applied Science, Penzberg, Germany). Cell lysates (5 μg of protein) were subjected to SDS-PAGE (10% polyacrylamide gel) under a reducing condition. Following electrophoresis, proteins were transferred onto PVDF membranes and incubated overnight at 4 °C with the primary antibodies against smad1, phospho-smad1/5, ERK1/2, phospho-ERK1/2, ERK5, phospho-ERK5, JNK, phospho-JNK, p38 MAPK, phospho-p38 MAPK, AKT, and phosphor-AKT, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (GE Healthcare, Little Chalfont, UK). Immunoreactive bands were visualized by an enhanced chemiluminescence reaction with an ECL Prime Western Blot Detection System (GE Healthcare). Intensity of the chemiluminescent bands was quantitatively analyzed using Versa Doc 5000 MP (Bio-Rad Laboratories, Hercules, CA, USA). The ratio for intensity of a band for phosphorylated protein to that for total protein was calculated.
Luciferase assay
C2C12 cells were plated in 24-well plates at a density of 1 × 104 cells/well and transfected with an Id1.0-luc reporter plasmid and TK vector (Promega, Madison, WI, USA). After 24 hours of incubation in the presence or absence of BMP-2 (300 ng/ml), luminescence was detected using a Dual-Luciferase Reporter Assay System (Promega). The relative activity of firefly luciferase was obtained after normalization against the luminescent reaction of Renilla luciferase encoded by the TK vector. A pGL4 [luc2/Neo] vector was used as the negative control.
Statistical analysis
Values are expressed as the mean ± SD. Student’s t-test was used to compare results between 2 groups. One-way ANOVA with post-hoc Tukey test was performed for comparing results from 3 or more groups. A p-value less than 0.05 was considered to indicate statistical significance.
Data availability statement
The datasets generated and analyzed for the current study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Author Contributions
K.Y., Y.M. and R.K. wrote the main manuscript. K.S., A.Y., and D.S. performed the experiments. H.I. and M.Y. performed the experiments shown in Supplementary Figures S1 and S3. K.N. and K.M. performed the experiments shown in Supplementary Figures S4. K.Y. directed the project. All authors reviewed the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28605-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 2.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 1999;343:281–299. doi: 10.1042/bj3430281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 1993;264:C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura K, et al. Monocarboxylate transporter-1 is required for cell death in mouse chondrocytic ATDC5 cells exposed to interleukin-1β via late phase activation of nuclear factor κB and expression of phagocyte-type NADPH oxidase. J. Biol. Chem. 2011;286:14744–14752. doi: 10.1074/jbc.M111.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takebe K, Takahashi-Iwanaga H, Iwanaga T. Intensified expressions of a monocarboxylate transporter in consistently renewing tissues of the mouse. Biomed. Res. 2011;32:293–301. doi: 10.2220/biomedres.32.293. [DOI] [PubMed] [Google Scholar]
- 6.Saito A, et al. Enhanced and suppressed mineralization by acetoacetate and β-hydroxybutyrate in osteoblast cultures. Biochem. Biophys. Res. Commun. 2016;473:537–544. doi: 10.1016/j.bbrc.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 7.Katagiri T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006;21:637–646. doi: 10.1359/jbmr.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2006;172:115–125. doi: 10.1083/jcb.200507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nojima J, et al. Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J. Biol. Chem. 2010;285:15577–15586. doi: 10.1074/jbc.M109.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;14:15005. doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katagiri T, et al. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. doi: 10.1046/j.1365-2443.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris SE, Guo D, Harris MA, Krishnaswamy A, Lichtler A. Transcriptional regulation of BMP-2 activated genes in osteoblasts using gene expression microarray analysis: Role of Dlx2 and Dlx5 transcription factors. Front. Biosci. 2003;8:s1249–1265. doi: 10.2741/1170. [DOI] [PubMed] [Google Scholar]
- 14.Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007;176:709–718. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh-Choudhury N, et al. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 16.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 17.Hasty P, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 18.Sunadome K, et al. ERK5 regulates muscle cell fusion through Klf transcription factors. Dev. Cell. 2011;20:192–205. doi: 10.1016/j.devcel.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, et al. Kruppel-like factor 4 attenuates osteoblast formation, function, and cross talk with osteoclasts. J. Cell Biol. 2014;204:1063–1074. doi: 10.1083/jcb.201308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaleb AM, et al. Krüppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K-S, et al. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000;20:8783–8792. doi: 10.1128/MCB.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, et al. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histone H3 and H4. Mol. Endocrinol. 2003;17:743–756. doi: 10.1210/me.2002-0122. [DOI] [PubMed] [Google Scholar]
- 23.El-Deiry WS, et al. WAF1, a potent mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Galectin-3 regulates p21 stability in human prostate cancer cells. Oncogene. 2013;32:5058–5065. doi: 10.1038/onc.2012.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 26.Molchadsky A, Rivlin N, Brosh R, Rotter V, Sarig R. p53 is balancing development, differentiation and de-differentiation to assure cancer prevention. Carcinogenesis. 2010;31:1501–1508. doi: 10.1093/carcin/bgq101. [DOI] [PubMed] [Google Scholar]
- 27.Lengner CJ, et al. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 29.Boidot R, et al. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72:939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Davis KD, Coffman CR. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development. 2008;135:207–216. doi: 10.1242/dev.010389. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, et al. Lactate induces osteoblast differentiation by stabilization of HIF1α. Mol. Cell. Endocrinol. 2017;452:84–92. doi: 10.1016/j.mce.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mottet D, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 36.Sang N, et al. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278:14013–14019. doi: 10.1074/jbc.M209702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masson EF, et al. Aerobic glycolysis suppresses p53 activity to provide selective protection from apoptosis upon loss of growth signals or inhibition of BCR-Abl. Cancer Res. 2010;70:8066–8076. doi: 10.1158/0008-5472.CAN-10-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for the current study are available from the corresponding author upon reasonable request.