Abstract
Retinal nerve fiber layer (RNFL) loss in diabetic patients is especially common regardless of diabetic retinopathy (DR). The correlations between nonglaucomatous RNFL loss and systemic characteristics in diabetic patients have aroused interests in many aspects. 167 subjects with type 2 diabetes who underwent evaluation for arterial stiffness and cardiovascular autonomic function using heart rate variability (HRV) were included in this study. Arterial stiffness was measured using cardio-ankle vascular index (CAVI) and ankle-brachial index (ABI). Multivariate regression analysis was performed to determine factors influencing the presence of RNFL loss according to age. Factors determining the superior location of diabetic RNFL loss were also investigated. CAVI were worse in patients with RNFL loss, especially in those with old age (≥50 yrs) (p = 0.037). Influential factor of RNFL defect in old group was ABI (p = 0.007). However, in young group (<50 yrs), HRV parameter (low-frequency/high-frequency ratio) determined the presence of RNFL loss (p = 0.040). Significant determinants of superior RNFL defect in old subjects were CAVI and ABI (p = 0.032 and p = 0.024). For young diabetic patients, autonomic dysfunction may have relationship with RNFL loss, but as patients get older, arterial stiffness could aggravate vascular autoregulation and diabetic RNFL loss. RNFL loss in diabetes may be correlated with systemic vascular conditions.
Introduction
Retinal nerve fiber layer (RNFL) defect refers to the loss of retinal neuronal cell1. It is typically observed in glaucoma patients. However, it is also seen in nonglaucomatous retina of diabetic patients. RNFL loss in diabetic patients is especially common regardless of diabetic retinopathy (DR)2. This suggests that RNFL loss without DR can be a type of optic neuropathy in diabetic patients3.
Diabetic RNFL loss is influenced by multiple factors, including oxidative stress3, advanced glycation end products4, and blocked retrograde axonal flow of retinal ganglion cells5. Among these factors, oxidative stress could be influenced by systemic vascular conditions. Retinal vascular circulation is controlled through autonomic nervous system6, and it also could be impaired by arterial stiffness through narrowing vascular caliber7.
Several studies have evaluated the stiffness of vessel in diabetic patients and found that systemic vascular incompetence commonly occurs, wielding an influence on diabetic complications8,9. Shim et al.10 have reported that increased arterial stiffness is associated with existence of open-angle glaucoma in diabetic patients using brachial-ankle pulse wave velocity (baPWV) index. In addition, increased arterial stiffness can be affected by old age itself. Increased trend of arterial stiffness is even shown in healthy subjects with increasing age11. So, it is natural that the older the diabetic patient is, the more affected by the vascular stiffness.
Cardiovascular autonomic function could be another factor affecting systemic circulation in diabetic patients. Indeed, cardiovascular autonomic dysfunction has been widely reported using multiple indices12,13. Worse heart rate variability (HRV) parameters could contribute to cardiac functional damage in diabetic patients, so we hypothesised that peripheral circulation might be influenced by compromised cardiac function, especially in retina of diabetes.
Our purpose of this study is to look into how systemic vascular changes of diabetes can affect retina, and to investigate the correlations between nonglaucomatous RNFL loss and systemic vascular characteristics in patients with type 2 diabetes according to aging.
Results
Of a total of 167 subjects, 105 had RNFL losses while 62 had no RNFL loss as age- and sex- matched controls. Baseline characteristics of both groups are summarized in Table 1. There were no statistically significant differences in mean age, ratio of gender, presence of hypertension, diabetic duration, percentage of diabetic retinopathy, laboratory findings, arterial stiffness indices, and HRV parameters.
Table 1.
Baseline characteristics of T2DM patients without or with RNFL loss.
| Without RNFL Loss (n = 62) | With RNFL Loss (n = 105) | P Value | |
|---|---|---|---|
| Age, years | 57.55 (±13.55) | 55.26 (±13.15) | 0.284* |
| Sex, male:female | 34:27 | 46:60 | 0.102‡ |
| Hypertension, n (%) | 25 (40.3%) | 48 (45.3%) | 0.531‡ |
| DM duration, years | 11.65 (±7.20) | 12.77 (±7.34) | 0.335* |
| Diabetic retinopathy, n (%) | 24 (38.7%) | 53 (48.6%) | 0.156‡ |
| Laboratory findings | |||
| HbA1c | 7.91 (±1.88) | 8.38 (±2.25) | 0.157* |
| eGFR | 92.15 (±30.98) | 89.74 (±25.90) | 0.593* |
| Total cholesterol | 162.52 (±44.42 | 168.12 (±45.65) | 0.443* |
| Triglyceride | 158.06 (±205.70) | 134.26 (±96.76) | 0.395* |
| HDL | 47.53 (±15.67) | 46.86 (±12.53) | 0.764* |
| LDL | 85.96 (±31.12) | 95.39 (±37.48) | 0.114* |
| CAVI | 8.07 (±1.23) | 8.40 (±1.58) | 0.157* |
| ABI | 1.05 (±0.09) | 1.02 (±0.13) | 0.254* |
| Presence of carotid plaque, n (%) | 14 (26.9%) | 35 (33.1%) | 0.239‡ |
| SDNN | 69.57 (±295.42) | 29.47 (±22.88) | 0.338* |
| LF/HF | 3.66 (±4.06) | 3.20 (±5.46) | 0.605* |
*Student t-test.
‡Chi-square test.
HbA1c: glycosylated hemoglobin; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; CAVI: cardio-ankle vascular index; ABI: ankle-brachial index.
Data are mean (±SD) or number (%), as appropriate.
Subdividing subjects according to age
In consideration of the effect of aging on vessel wall, arterial stiffness indices were compared after subdividing patients into old and young groups. According to American Diabetes Association (ADA) 2016 guidelines14, there was different criteria of considering antiplatelet agents to prevent atherosclerotic cardiovascular disease (ASCVD) based on age 50. So we determined the cutoff point of grouping subjects as age of 50. In young age group, there were no significant differences in mean value of CAVI, ABI, and ratio of patients with abnormal arterial stiffness index (Table 2).
Table 2.
Comparison of arterial stiffness index according to age.
| Without RNFL Loss (n = 62) | With RNFL Loss (n = 105) | P Value | |
|---|---|---|---|
| Old (≥50 yrs) | |||
| N | 48 | 69 | |
| CAVI | 8.38 (±1.05) | 8.92 (±1.53) | 0.037* |
| ABI | 1.06 (±0.10) | 1.01 (±0.14) | 0.051* |
| Abnormal CAVI, n (%) | 16 (33.3%) | 35 (50.7%) | 0.062‡ |
| Abnormal ABI, n(%) | 3 (6.6%) | 13 (18.8%) | 0.051‡ |
| Presence of carotid plaque, n (%) | 13 (34.2%) | 30 (48.4%) | 0.165‡ |
| Young (<50 yrs) | |||
| N | 14 | 36 | |
| CAVI | 6.98 (±1.22) | 7.43 (±1.19) | 0.237* |
| ABI | 1.00 (±0.08) | 1.04 (±0.10) | 0.226* |
| Abnormal CAVI, n (%) | 1 (7.1%) | 3 (8.3%) | 0.889‡ |
| Abnormal ABI, n(%) | 2 (14.3%) | 2 (5.6%) | 0.307‡ |
| Presence of carotid plaque, n (%) | 1 (7.1%) | 5 (14.7%) | 0.471‡ |
*Student t-test.
‡Chi-square test.
CAVI: cardio-ankle vascular index; ABI = ankle-brachial index.
On the other hand, in the old age group, there were statistically significant or considerable differences. In old patients who had RNFL loss, mean arterial stiffness indices were worse (CAVI: 8.38 vs. 8.92; ABI: 1.06 vs. 1.01) and ratios of subjects who had abnormal CAVI or ABI results were also higher compared to subjects without RNFL loss (CAVI: 50.7% vs. 33.3%; ABI: 18.8% vs. 6.6%, Table 2). The P value was significant in CAVI value, but mean ABI and ratio of subjects with abnormal ABI showed marginal P values (0.051 and 0.051).
Multivariate regression analysis including HRV parameters and arterial stiffness indexs was performed for subgroup analysis. Table 3 shows the results of statistically significant multivariate logistic regression analysis model of each group. In the old age group, only ABI had significant effect on RNFL loss (β = −11.057, p = 0.007). Values of autonomic functional evaluation, such as SDNN (standard deviation of the NN intervals) and LF/HF (low-frequency/high-frequency), did not show any significant or meaningful effect. LF/HF ratio were statistically significant in multivariate model analysis in the young age group (β = −0.170, p = 0.040). In this group, HbA1c level was also meaningful for diabetic RNFL loss (β = 0.391, p = 0.033).
Table 3.
Multivariate regression analysis of RNFL loss in old and young age groups including autonomic nerve function indices (SDNN and LF/HF), arterial stiffness indices (CAVI and ABI) and laboratory findings.
| Old (≥50 yrs) | Young (<50 yrs) | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| Duration of DM | −0.010 | 0.891 to 1.100 | 0.857 | 0.128 | 0.990 to 1.305 | 0.069 |
| SDNN | 0.052 | 0.997 to 1.114 | 0.062 | −0.026 | 0.931 to 1.019 | 0.255 |
| LF/HF | 0.033 | 0.818 to 1.306 | 0.783 | −0.170 | 0.717 to 0.992 | 0.040 |
| HbA1c | 0.075 | 0.592 to 1.963 | 0.806 | 0.391 | 1.031 to 2.119 | 0.033 |
| eGFR | 0.007 | 0.969 to 1.047 | 0.723 | −0.001 | 0.969 to 1.030 | 0.967 |
| Total cholesterol | −0.134 | 0.720 to 1.064 | 0.180 | 0.039 | 0.959 to 1.127 | 0.345 |
| TG | 0.032 | 0.990 to 1.078 | 0.139 | −0.009 | 0.977 to 1.005 | 0.195 |
| HDL | 0.227 | 0.981 to 1.605 | 0.071 | −0.090 | 0.832 to 1.003 | 0.059 |
| LDL | 0.147 | 0.952 to 1.409 | 0.142 | −0.035 | 0.886 to 1.052 | 0.423 |
| CAVI | 0.222 | 0.550 to 2.835 | 0.596 | −0.061 | 0.541 to 1.636 | 0.828 |
| ABI | −11.057 | 0.000 to 0.046 | 0.007 | 4.258 | 0.191 to 26166.7 | 0.158 |
Enter mode was used for this logistic regression analysis.
CI: confidence interval.
SDNN: standard deviation of normal to normal intervals in heart rate variability; LF/HF: ratio of low-frequency power to high-frequency power in heart rate variability; HbA1c: glycosylated hemoglobin; eGFR: estimated glomerular filtration rate; TG: triglyceride; HDL: high-density lipoprotein; LDL: low-density lipoprotein; CAVI: cardio-ankle vascular index; ABI: ankle-brachial index.
Discrimination of RNFL location
Among patients with RNFL loss, the location of RNFL defect was also classified. Results on their correlations with arterial stiffness indices are summarized in Table 4. In the young age group, no significant difference in arterial stiffness indices was found between superior and inferior RNFL loss subjects. In the old age group, the ratio of patients with abnormal CAVI values was higher (60.5 vs. 34.6%) in subjects with superior RNFL loss compared to that in subjects with inferior RNFL loss. Mean CAVI was worse (9.20 vs. 8.46) in superior RNFL loss subjects, but the p value was marginally insignificant (p = 0.054). ABI value or ratio of abnormal ABI patients was not significantly different between superior and inferior RNFL loss groups.
Table 4.
Comparison of arterial stiffness index among patients with RNFL loss according to RNFL loss location (superior or inferior).
| Superior RNFL Loss | Inferior RNFL Loss | P Value | |
|---|---|---|---|
| Old (≥50 yrs) | |||
| N | 43 | 26 | |
| CAVI | 9.20 (±1.59) | 8.46 (±1.32) | 0.054* |
| ABI | 1.03 (±0.12) | 0.98 (±0.17) | 0.212* |
| Abnormal CAVI, n (%) | 26 (60.5%) | 9 (34.6%) | 0.037‡ |
| Abnormal ABI, n(%) | 7 (16.3%) | 6 (23.1%) | 0.484‡ |
| Presence of carotid plaque, n (%) | 17 (44.7%) | 13 (54.2%) | 0.469‡ |
| Young (<50 yrs) | |||
| N | 29 | 7 | |
| CAVI | 7.45 (±1.23) | 7.35 (±1.09) | 0.843* |
| ABI | 1.04 (±0.11) | 1.04 (±0.08) | 0.981* |
| Abnormal CAVI, n (%) | 3 (10.3%) | 0 (0.0%) | 0.374‡ |
| Abnormal ABI, n(%) | 2 (6.9%) | 0 (0.0%) | 0.475‡ |
| Presence of carotid plaque, n (%) | 4 (14.3%) | 1 (16.7%) | 0.881‡ |
*Student t-test.
‡Chi-square test.
CAVI: cardio-ankle vascular index; ABI: ankle-brachial index.
As shown in Table 5, logistic regression analysis was done to discriminate meaningful factor affecting the existence of superior RNFL loss in old group. The CAVI and ABI were associated significantly (p = 0.032 and 0.024, respectively) in multivariate logistic regression analysis, but autonomic functional indices such as SDNN and LF/HF did not show significant effect on superior RNFL loss in old group.
Table 5.
Univariate and multivariate regression analysis of superior RNFL loss in old age group (≥50 yrs).
| Univariate Analysis | Multivariate Analysis* | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| Duration of DM | 0.026 | 0.962 to 1.094 | 0.435 | |||
| SDNN | 0.004 | 0.984 to 1.024 | 0.709 | |||
| LF/HF | 0.016 | 0.939 to 1.101 | 0.686 | |||
| HbA1c | 0.131 | 0.913 to 1.424 | 0.247 | 0.122 | 0.802 to 1.592 | 0.486 |
| eGFR | −0.014 | 0.954 to 1.020 | 0.431 | |||
| Total cholesterol | −0.003 | 0.985 to 1.009 | 0.619 | |||
| TG | 0.004 | 0.995 to 1.013 | 0.418 | |||
| HDL | −0.019 | 0.944 to 1.020 | 0.330 | |||
| LDL | −0.002 | 0.985 to 1.012 | 0.809 | |||
| CAVI | −0.356 | 0.483 to 1.015 | 0.060 | −0.492 | 0.390 to 0.959 | 0.032 |
| ABI | −2.163 | 0.004 to 3.500 | 0.215 | −4.668 | 0.000 to 0.545 | 0.024 |
*Variables with P value less than 0.3 in univariate analysis were included.
CI: confidence interval.
SDNN: standard deviation of normal to normal intervals in heart rate variability; LF/HF: ratio of low-frequency power to high-frequency power in heart rate variability; HbA1c: glycosylated hemoglobin; eGFR: estimated glomerular filtration rate; TG: triglyceride; HDL: high-density lipoprotein; LDL: low-density lipoprotein; CAVI: cardio-ankle vascular index; ABI: ankle-brachial index.
Discussion
Diabetic retinopathy and nephropathy can be caused by microvascular impairment due to oxidative stress with advanced glycation end products (AGEs)15. In the kidney, oxidative stress can cause endothelial injury, podocyte loss, albuminuria, and glomerular failure in sequence16. Similar microvascular endothelial structural impairment also occurs in retinal vessels. Therefore, this structural problem of vessel is a crucial factor that determines autoregulation of retinal circulation.
Autonomic neuropathy in diabetes is also important complication. It can affect cardiovascular function and increase the risk of morbidity and mortality. Dysfunction of axon and dendrite of sympathetic ganglion has been identified by multiple experiments17. Activation of the AGE, protein kinase C (PKC), polyol, poly(ADP-ribose) polymerase, and hexosamine pathways can contribute to metabolic imbalance in hyperglycemic environment18. Because arteriovenous anastomoses of capillaries are usually under sympathetic control, compromised autonomic function in diabetic patients could affect microvascular circulation.
We have been focusing on the suggestion that retinal vascular homeostasis in diabetic patient is maintained through both autoregulation and autonomic regulation of vessel. To evaluate vascular autoregulation that is associated with structural changes of vessel, arterial stiffness indices such as CAVI and ABI were selected in this study. HRV parameters were also included to assess the autonomic functional status of vessels in diabetic subjects.
CAVI has been recently proposed as an indicator of arterial stiffness and arteriosclerosis independent of arterial blood pressure19. Kim et al. have suggested that CAVI was related to smooth muscle contraction status of vessel20. In addition, ABI has been widely used to estimate vascular status as a simple and useful method21. ABI was known as a good index of atherosclerosis and lower ABI has been reported to be useful in the diagnosis of peripheral arterial disease22,23. Also, higher ABI was reported to represent medial artery calcification and to be related with the risk of cardiovascular event24,25.
Autoregulation of retinal vessel can be attained by modulating vascular tone which could be achieved through multiple mechanisms affecting vascular structures including smooth muscle cells26. Changes of vascular structures in old age could affect retinal vascular autoregulation. Indeed, it has been reported that smooth muscle cells of arterioles have fibrosis, degradation and decreased cross-sectional area due to aging27,28.
Several studies have reported that diabetic RNFL loss is more frequently found at the superior hemisphere than that at the inferior hemisphere3,29,30. Compromised vascular responsiveness upon metabolic imbalance31 in diabetic patients could explain why microaneurysm, acellular capillary, and nerve fiber loss are common in superior retinal region. Under diabetic condition, impaired vascular response could bring about deficient homeostasis of blood circulation at superior retina which may have decreased blood volume due to gravitational effect3,32. In our study, when RNFL loss location was classified as superior and inferior in old age group, the subjects with superior RNFL loss presented worse CAVI values (Table 4), and, statistically significant determinants of superior RNFL loss were CAVI and ABI values (Table 5). Those are at the same line of thought that the impaired autoregulation in retinal arteriole impairs retinal circulation, and it exert influence on retinal neuronal loss more at superior hemisphere.
However, in spite of relatively better vascular tone, RNFL loss can still occurs in younger diabetic patients. Our results presented that cardiovascular autonomic functional indices were only associated with RNFL loss in young subjects (Table 3). It has been reported that low SDNN of diabetic patient was associated with subclinical cardiovascular problem such as left ventricular hypertrophy33. Canani et al. suggested that diabetic patients with low HRV parameters including LF/HF ratio had more peripheral arterial disease and it could reflect dysfunction of vascular modulation through autonomic system. As above, diabetic patients are known to have increased risk of cardiovascular autonomic dysfunction in many aspects and autonomic dysfunction of vessels could explain the RNFL loss in young patients with relatively good vascular autoregulation. When meaningful factors of diabetic RNFL loss were compared between subgroups according to age, this explanation is still applicable.
Systemic vascular conditions and associated cardiovascular disease are strong predictors of mortality and prognosis in diabetic patients34,35. Our results implied that diabetic RNFL loss could be used as a surrogate of systemic vascular characteristics. RNFL loss in diabetic retina might represent blood flow impairment and precise optic disc evaluation in each diabetic patient may be helpful for improving the prognosis of this chronic and multisystemic disease.
Materials and Methods
Study design and Population
This retrospective and cross-sectional study was performed according to the tenets of the Declaration of Helsinki. It was approved by Institutional Review and Ethics Boards of Seoul St. Mary’s Hospital, South Korea. The need for written informed consent was waived by our Review Board.
A total of 167 subjects with type 2 diabetes who underwent evaluation for arterial stiffness and cardiovascular autonomic function from January 2008 to March 2015 at Seoul St. Mary’s Hospital were included in this study. If both eyes had diabetic RNFL losses, the right eye was selected. All eyes had no signs of glaucomatous optic disc morphology such as narrowing or disappearance of neuroretinal rim, disc hemorrhage, or cup-to-disc ratio asymmetry >0.2. All subjects included in this study had open angle on gonioscopy and intraocular pressure of lower than 21 mmHg without history of periorbital trauma. Excluded patients were one of following cases: type 1 diabetes, active ocular disease such as uveitis, history of retinal disease, or history of ocular surgery other than simple cataract extraction.
All subjects underwent complete ophthalmic examination including visual acuity, Goldmann applanation tonometry, slit-lamp examination, gonioscopy, red-free RNFL photography (CF-60UD; Canon, Tokyo, Japan). Best corrected visual acuity was 20/40 or better and spherical equivalent was within ± 5.0 diopters in all subjects.
Eyes without diabetic retinopathy (DR) or mild nonproliferative diabetic retinopathy (NPDR) were included. Stage of DR was evaluated by a retinal specialist (J.H.L) through fundus photography based on Early Treatment Diabetic Retinopathy Study (ETDRS) classification. Eyes with clinically significant macular edema were also excluded36.
Only well centered retinal photographs were used to evaluate the existence of RNFL loss. These photographs were obtained using digital fundus camera at 60° view (Fig. 1). Two glaucoma specialists (S.J.J. and H.Y.P.) evaluated the fundus photograph of each patient in a blind manner. If there was disagreement between the two observers, the eye was excluded from our study. Localized diabetic RNFL loss was defined as followings: (1) width larger than a major retinal vessel at a distance of 1-disc diameter from the edge of the disc, (2) diverged in an arcuate or wedge shape, (3) reaching the edge of the disc with clear margin. Photographs containing multiple RNFL defects were excluded in order to compare difference in vascular index according to the location of RNFL loss: superior RNFL loss and inferior RNFL loss.
Figure 1.
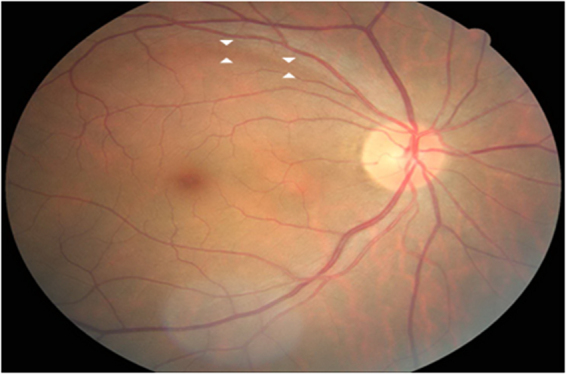
Representative case of diabetic retinal nerve fiber layer (RNFL) loss A 46-year-old male with type 2 diabetes had RNFL defect in superior portion of retina. The morphology of optic disc was not glaucomatous. White arrows demarcate the border of RNFL loss.
Systemic examinations
Patients were diagnosed as diabetes if fasting plasma glucose level was more than 126 mg/dl, or symptoms of diabetes appeared with random plasma glucose level of more than 200 mg/dl, or HbA1c was more than 6.5%37. Hypertension was defined when systolic blood pressure was ≥140 mmHg and diastolic blood pressure was ≥90 mmHg, or any antihypertensive medication was used38.
After patients had fasted for 12 hours, blood samples were collected and blood glucose was measured using an automated enzymatic method. Glycated hemoglobin (HbA1c) level was measured to evaluate the controlled state of diabetes.
Lipid profiles (total cholesterol, triglycerides, low density lipoprotein, and high density lipoprotein) were measured and glomerular filtration rate was estimated using the Modification of Diet in Renal Disease Study equation39.
Measurement of arterial stiffness indices
The arterial stiffness was measured using cardio-ankle vascular index (CAVI) and ankle-brachial index (ABI). CAVI and ABI values were obtained with a VaSera VS-1000 device (Fukuda Denshi, Tokyo, Japan).
CAVI was calculated using the distance from the level of aortic valve to the measured area and delayed time from closing of aortic valve to arterial pressure change at measure point40. ABI was calculated at each leg, dividing the higher pressure of posterior tibial or dorsalis pedis artery by systolic pressure at brachial artery41. The presence of plaque in carotid artery was distinguished using carotid sonography (HP 5500, Hewlett-Packard, USA).
Evaluation of cardiovascular autonomic function
Cardiovascular autonomic nervous function was evaluated with parameters of heart rate variability, including standard deviation of normal to normal intervals (SDNN) and ratio of low-frequency power to high-frequency power (LF/HF). HRV evaluation was done as follows42. Echocardiography signals of all patients were documented after 30 minutes of rest. They were then transferred to Medicore Heart Rate Analyzer, Model SA-3000P (Medicore, Seoul, Korea).
Time domain methodologic evaluation was done through SDNN, the standard deviation of the normal rate-to-rate intervals mainly reflecting parasympathetic status. Frequency domain methodologic evaluation included LF/HF ratio. LF reflects both parasympathetic and sympathetic function while HF reflects parasympathetic dysfunction using vagal nervous compartment. Therefore, the ratio of LF/HF primarily reflects the imbalance of autonomic nervous system43,44.
Statistical analysis
All statistical analyses were performed with SPSS version 19.0 (SPSS Inc., Chicago, IL). Student t tests and Chi-square tests were used to evaluate clinical characteristics between RNFL loss group and the group without showing RNFL loss. Comparison was also done dividing subjects into age-specific groups. Patients with RNFL loss were also grouped according to the location of RNFL defect (superior and inferior). Univariate and multivariate logistic regression analyses were performed to determine significant factors influencing diabetic RNFL defect. In all analysis, P < 0.05 was considered statistically significant.
Acknowledgements
None of the authors has any proprietary interests in any device or drug mentioned in the article.
Author Contributions
Soo Ji Jeon wrote the main manuscript text. Soo Ji Jeon, Hae-Young Lopilly Park, and Jae Hyung Lee performed the data review and analysis. The critical revision of the manuscript was done by Chan Kee Park. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tatham AJ, et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol. 2013;156:578–587 e571. doi: 10.1016/j.ajo.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dijk HW, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86:725–728. doi: 10.1136/bjo.86.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amano S, et al. Advanced glycation end products in human optic nerve head. Br J Ophthalmol. 2001;85:52–55. doi: 10.1136/bjo.85.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Inoue M, Dong K, Yamamoto M. Alterations in retrograde axonal transport in optic nerve of type I and type II diabetic rats. Kobe J Med Sci. 1998;44:205–215. [PubMed] [Google Scholar]
- 6.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triantafyllou A, et al. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens. 2014;27:1472–1478. doi: 10.1093/ajh/hpu074. [DOI] [PubMed] [Google Scholar]
- 8.Ando A, et al. Cardio-Ankle Vascular Index and Indices of Diabetic Polyneuropathy in Patients with Type 2 Diabetes. J Diabetes Res. 2017;2017:2810914. doi: 10.1155/2017/2810914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AS, Urbina EM. Vascular and Endothelial Function in Youth with Type 2 Diabetes Mellitus. Curr Diab Rep. 2017;17:36. doi: 10.1007/s11892-017-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shim SH, et al. The Role of Systemic Arterial Stiffness in Open-Angle Glaucoma with Diabetes Mellitus. Biomed Res Int. 2015;2015:425835. doi: 10.1155/2015/425835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen W, et al. Age-related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis. 2015;238:147–152. doi: 10.1016/j.atherosclerosis.2014.10.089. [DOI] [PubMed] [Google Scholar]
- 12.Jin J, et al. Cardiovascular Autonomic Neuropathy Is an Independent Risk Factor for Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. Biomed Res Int. 2017;2017:3270617. doi: 10.1155/2017/3270617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva AKFD, Christofaro DGD, Bernardo AFB, Vanderlei FM, Vanderlei LCM. Sensitivity, Specificity and Predictive Value of Heart Rate Variability Indices in Type 1 Diabetes Mellitus. Arq Bras Cardiol. 2017;108:255–262. doi: 10.5935/abc.20170024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes A. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des. 2008;14:962–968. doi: 10.2174/138161208784139729. [DOI] [PubMed] [Google Scholar]
- 16.Qi H, et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes. 2017;66:763–778. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol. 2002;50:257–292. doi: 10.1016/S0074-7742(02)50080-5. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Nave KA, Jensen TS, Bennett DL. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun CK. Cardio-ankle vascular index (CAVI) as an indicator of arterial stiffness. Integr Blood Press Control. 2013;6:27–38. doi: 10.2147/IBPC.S34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim B, Takada K, Oka S, Misaki T. Influence of blood pressure on cardio-ankle vascular index (CAVI) examined based on percentage change during general anesthesia. Hypertens Res. 2011;34:779–783. doi: 10.1038/hr.2011.31. [DOI] [PubMed] [Google Scholar]
- 21.Yan BP, et al. Borderline ankle-brachial index is associated with increased prevalence of micro- and macrovascular complications in type 2 diabetes: A cross-sectional analysis of 12,772 patients from the Joint Asia Diabetes Evaluation Program. Diab Vasc Dis Res. 2015;12:334–341. doi: 10.1177/1479164115590559. [DOI] [PubMed] [Google Scholar]
- 22.Fishbane S, Youn S, Flaster E, Adam G, Maesaka JK. Ankle-arm blood pressure index as a predictor of mortality in hemodialysis patients. Am J Kidney Dis. 1996;27:668–672. doi: 10.1016/S0272-6386(96)90101-8. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc. 1997;45:1472–1478. doi: 10.1111/j.1532-5415.1997.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 24.Resnick HE, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 25.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 26.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res. 1990;66:1747–1754. doi: 10.1161/01.RES.66.6.1747. [DOI] [PubMed] [Google Scholar]
- 28.Knox CA, Yates RD, Chen I, Klara PM. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathol. 1980;51:1–13. doi: 10.1007/BF00688844. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M, et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 30.Ozdek S, Lonneville YH, Onol M, Yetkin I, Hasanreisoglu BB. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye (Lond) 2002;16:761–765. doi: 10.1038/sj.eye.6700207. [DOI] [PubMed] [Google Scholar]
- 31.Chung HS, et al. Regional differences in retinal vascular reactivity. Invest Ophthalmol Vis Sci. 1999;40:2448–2453. [PubMed] [Google Scholar]
- 32.Tang J, Mohr S, Du YD, Kern TS. Non-uniform distribution of lesions and biochemical abnormalities within the retina of diabetic humans. Curr Eye Res. 2003;27:7–13. doi: 10.1076/ceyr.27.2.7.15455. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso CR, Moraes RA, Leite NC, Salles GF. Relationships between reduced heart rate variability and pre-clinical cardiovascular disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2014;106:110–117. doi: 10.1016/j.diabres.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370–379. doi: 10.1016/j.atherosclerosis.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Wadwa RP. Cardiovascular disease risk in youth with diabetes mellitus. Rev Endocr Metab Disord. 2006;7:197–204. doi: 10.1007/s11154-006-9016-y. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson CP, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 37.Kerner W, Brückel J, German D. Association. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:384–386. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC). Vital signs: awareness and treatment of uncontrolled hypertension among adults–United States, 2003–2010. MMWR Morb Mortal Wkly Rep 61, 703–709 (2012). [PubMed]
- 39.Levey AS, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Yambe T, et al. Brachio-ankle pulse wave velocity and cardio-ankle vascular index (CAVI) Biomed Pharmacother. 2004;58(Suppl 1):S95–98. doi: 10.1016/S0753-3322(04)80015-5. [DOI] [PubMed] [Google Scholar]
- 41.Aboyans V, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 42.Malik M, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 43.Kim YH, Jung KI, Song CH. Effects of serum calcium and magnesium on heart rate variability in adult women. Biol Trace Elem Res. 2012;150:116–122. doi: 10.1007/s12011-012-9518-2. [DOI] [PubMed] [Google Scholar]
- 44.Lee NY, Park HY, Na KS, Park SH, Park CK. Association between heart rate variability and systemic endothelin-1 concentration in normal-tension glaucoma. Curr Eye Res. 2013;38:516–519. doi: 10.3109/02713683.2012.745881. [DOI] [PubMed] [Google Scholar]


