Abstract
Stem cell research for treating or curing ischemic heart disease has, till date, culminated in three basic approaches: the use of induced pluripotent stem cell (iPSC) technology; reprogramming cardiac fibroblasts; and cardiovascular progenitor cell regeneration. As each approach has been shown to have its advantages and disadvantages, exploiting the advantages while minimizing the disadvantages has been a challenge. Using human germline pluripotent stem cells (hgPSCs) along with a modified version of a relatively novel cell-expansion culture methodology to induce quick, indefinite expansion of normally slow growing hgPSCs, it was possible to emphasize the advantages of all three approaches. We consistently found that unipotent germline stem cells, when removed from their niche and cultured in the correct medium, expressed endogenously, pluripotency genes, which induced them to become hgPSCs. These cells are then capable of producing cell types from all three germ layers. Upon differentiation into cardiac lineages, our data consistently showed that they not only expressed cardiac genes, but also expressed cardiac-promoting paracrine factors. Taking these data a step further, we found that hgPSC-derived cardiac cells could integrate into cardiac tissue in vivo. Note, while the work presented here was based on testes-derived hgPSCs, data from other laboratories have shown that ovaries contain very similar types of stem cells that can give rise to hgPSCs. As a result, hgPSCs should be considered a viable option for eventual use in patients, male or female, with ischemic heart disease
Keywords: Cardiomyocytes, Paracrine factors, Human germline pluripotent stem cells, Cardiogenesis, Cardiac repair, Neuregulin, Embryonic stem cells, Adult stem cells, Heart disease
Introduction
After a half century of focused research, heart disease remains a leading cause of death in developed nations. Although advancements in research have led to numerous breakthroughs and progress for treating cardiovascular disease, in many respects, we are still only scratching the surface of innovative ideas for reducing cardiac-related deaths. A significant problem remaining to be solved is identifying the parameters and/or inductive signals that are necessary for repairing heart muscle damaged by ischemia. To solve this problem, decades of research have led to three primary areas of investigation: The potential use of induced pluripotent stem cell (iPSCs) technology, direct reprogramming of surviving cardiac fibroblasts, and regeneration of endogenous cardiovascular progenitor cells[1, 2]. The first approach using iPSCs has shown promise in vitro; however, problems remain for their use in vivo such as teratoma formation, genetic instability, and accumulation of mitochondrial DNA mutations in iPSCs from elderly patients, which would most likely be the largest demographic needing iPSC-therapy[3, 4]. In contrast, the latter two regenerative approaches have recently gained interest due to their paracrine-inducing abilities for restoring cardiac function[1, 5-9]; however, the methodologies and protocols for inducing cardiac regeneration continue to vary widely.
Clinical trials that test the efficacy of transplanted adult stem cells (ASCs) within ischemic heart tissue have been common practice for nearly a decade; however, outcomes have not provided definitive clinical applications for patients. One explanation for the investigative ambiguity stems from the many different types of ASCs that have been pursued for transplantation. For example, adult epidermal stem cells are different than adult mesenchymal bone marrow stem cells with respect to gene expression, physiology, and origin. As a result, when introduced into the cardiac niche, the different types of ASCs present unanticipated variations. Consequently, finding a stem cell population that is the most suitable for treating cardiac ischemia remains an important endeavor.
Some of the more successful stem cell trials have been those that utilize both direct and indirect mechanisms to help induce cardiac repair[2, 10]. Stem cells that are differentiated into cardiomyocytes in vitro (or in vivo) can integrate and adhere upon transplantation with endogenous cardiac cells via gap junctions (e.g., connexin 43 gap junction proteins). Cellular adhesion can then exert direct physiological interaction/repair. Some stem cells can also secrete paracrine factors that indirectly affect surrounding tissue to ‘regenerate’ or inhibit apoptosis[5, 9, 11-13]. Debate has arisen, though, concerning which of these approaches is best for clinical use. For example, direct physiological interaction where stem cell-derived cardiomyocytes physically ‘beat’ can result in positive or negative outcomes for patients. If stem cell-derived cardiomyocytes are electrically connected to the heart muscle, ventricular force can be significantly restored[14-16]. However, if that electrical connection is not complete, transplanted cardiomyocytes that beat can cause detrimental arrhythmias and decreased ventricular force. Alternatively, stem cells that do not beat, but do secrete paracrine factors that can effect surrounding healthy cardiac tissue, have become a strong investigative mechanism for repairing ischemic tissue.
Previously we, and others as well, presented evidence that germline stem cells when removed from their niche acquire the ability to differentiate into cell types from all three germ layers (ectoderm, mesoderm, and endoderm)[17-21]. Others have since confirmed this work; however, testing their application within a cardiac setting has not been thoroughly analyzed.
Our hypothesis is straightforward. We postulate that germline stem cells when removed from their niche begin to express factors redefining their stemness from unipotent (able to make sperm or eggs) to pluripotent. These redefined cells, known as germline pluripotent stem cells (hgPSCs), can then be induced to form paracrine effector-yielding cardiac cells. At first, our data provided constant, clear evidence that hgPSCs could be induced to form cardiomyocytes; however, we encountered a consistent obstacle with our initial approach; hgPSCs grew very slowly. Expansion of cells from dish to dish took months and it was concluded that their growth curve could significantly impede their use in vivo. Thus, to solve this problem, we modified a novel technological cell culture advancement that had been shown to promote certain cell types to acquire or maintain ‘stemness’ while simultaneously pushing them into the cell cycle[22]. This approach had not been reported in the literature for expanding hgPSCs. Our initial observations after subjecting hgPSCs to this novel culturing technology resulted in data leading us to generate a secondary hypothesis; that hgPSCs could be ‘quickly’ and indefinitely expanded to enrich their population, followed by their differentiation into cardiomyocytes. After testing our hypotheses, the data revealed that cardiomyocytes generated from hgPSCs acquired the ability to positively influence cardiac tissue.
Methods and Materials
Gene names and abbreviations
Table 1 provides a reference list of abbreviations for the genes and proteins that were analyzed.
Table 1: Gene names and functions.
| SSC GENE CANDIDATES | Abbreviation | BP Size | Publis hed function | Refs |
| G protein coupled receptor 125 | GPR125 | 136BPS | Canidate marker for human and mouse SSCs. Multiple functions in different tissues. | [32, 61] |
| GDNF family receptor 1 alpha | GFR1a | 163BPS | Canidate marker for human and mouse SSCs. | [32, 61] |
| Stage-specific antibody-4 | SSEA-4 | 359nps | Expressed in specific cells of the seminiferous basal membrane. Canidate marker for human and mouse SSCs. | [47, 48, 51] |
| hESC GENES | Abbreviation | BP Size | Published function | Refs |
| SRY (Sex Determining Region Y)-Box 2 | SOX2 | 153bps | Transcriptional regulator of somatic cell reprogramming; helps maintain pluripotency. | [21, 62-64] |
| Octamer-Binding Transcription Factor 3/4 | OCT3/4 | 117bps | Plays the central role in pluripotency. | [21, 65, 66] |
| LIN28 Homolog A | LIN 28A | 128bps | Regulates sternness and self renewal capacity in human and mouse pluripotent stem cells. | [21, 67, 68] |
| Homeobox Transcription Factor Nanog | NANOG | 200bps | Required for final states of pluripotency. | [69, 70] |
| Kruppel-like factor 4 | KLF-4 | 143 bps | Essential for ESC maintenance and self-renewal capacity. | [21, 71, 72] |
| Cluster of differentiation 73 | CD73 | 219bps | Essential stemness marker for human somatic cells. | [34] |
| EARLY CARDIAC GENES | Abbreviation | BP Size | Published function | Refs |
| Activated Leukocyte Cell Adhesion Molecule | ALCAM | 388bps | Surface marker for early cardiomyocytes | [73] |
| Cardiac Helicase Activated MEF2C protein | CHAMP | 200bps | Cardiac transcription factor expressed specifically in postnatal and embryonic cardiomyocytes. | [40] |
| T-Box Transcription Factor 18 | TBX18 | 112bps | It is critical for early sino atrial node (SAN) specification (pacemaker cells). | [74] |
| DIFFERENTIATED CARDIAC GENES | Abbreviation | BP Size | Published function | Refs |
| Atrial Natriuretic Peptide | ANP | 204bps | Cardiac hormone that regulates blood pressure, vasodilation, natriuresis, and diuresis. | [75] |
| NK2 homeobox 5 | NKX2.5 | 215bps | Cardiac transcription factor responsible for heart formation and development. | [76] |
| Cardiac Troponin-I | CTNI | 335bps | Key regulatory protein associated with the thin filament, inhibits actomyosin interactions at diastolic levels of intracellular Ca2+. | [77] |
| Cardiac Troponin-T | CTNT | 217bps | Fixation of troponin complex on the actin filament and also participitates in muscle contraction | [78] |
| Myosin heavy chain | MHC | 542bps | Molecular motor of the heart that generates motion by coupling its ATPase activity to its cyclic interaction with actin. | [26, 79] |
| Myosin light chain 2A | MLC2A | 270bps | Atrial marker expressed during devlopment and adulthood. Also regulates heart contraction along with MLC 2V. | [80, 81] |
| Myosin light chain 2V | MLC2V | 380bps | Ventricular marker during human heart development and in adulthood | [80, 81] |
| Myocyte Enhancer Factor 2C | ISL-1 | 127bps | a LIM homeodomain transcription factor expressed in majority of cells in both right ventricle and atria of the heart. ISL1 is also responsible for survival, proliferation, and migration of cardiac progenitor cells. | [82-84] |
| PARACRINE FACTORS | Abbreviation | BP Size | Published function | Refs |
| Vascular Endothelial growth factor-A | VEGFA | 280bps | Promotes vasculo-and angiogenesis in myocardium and cardiomyocyte proliferation. Also helps regenerates mycoardium. | [1, 11, 39, 85, 86] |
| Insulin-like growth factor | IGF-1 | 372bps | Switch macrophages from pro-inflammatory to anti-inflammatory phenotype both in vitro and vivo. Also promotes resident stem mobilization and cardiac lineage commitment. | [1, 11, 39, 85, 86] |
| Stromal derived factor-1 | SDF-1 | 250bps | Promotes repair and regeneration by recruiting circulating progenitor cells to the injured site. Also secreted by cardiac stem cells. | [1, 11, 39, 85, 86] |
| Connective tiddue growth factor | CTGF | 237bps | Acts as a cofactor for other growth factor that promotes fibrosis and wound healing by enhancing ECM protein synthesis. | [1, 11, 39, 85, 86] |
| Endothelin-1 | END-1 | 270bps | Vasoactive peptide secreted by the endothelium required for cardiomyocyte survival. It decreases susceptibility to TNF-mediated apoptosis; secreted from cardiomyocytes under mechanical stress. | [1, 11, 39, 85, 86] |
| Angio-associated migratory protein | AAMP | 283bps | Associated with angiogenesis, endothelial tube formation, and migration of endothelial cells. It may also regulate smooth muscle cell migration via the RhoA pathway. | [1, 11, 39, 85, 86] |
| Neuregulin-1 | NRG-1 | 203bps | Activates ErbB2 receptor present on differentiated cardiomyocytes and promotes cardiomyocyte proliferation. | [1, 11, 39, 85, 86] |
| Indoleamine 2,3-dioxygenase | IDO | 222 bps | Inhibits T-cell and NK cell proliferation, cytotoxicity, cytokine production; also mediates T-cell apoptosis. | [1, 11, 39, 85, 86] |
| TERATOMA GENES | Abbreviation | BP Size | Published Function | Refs |
| Dead end gene | DND1 | 271 bps | It is an RNA-binding protein that suppresses teratoma growth | [38] |
| P18 Cyclin dependent kinase Inhibitor | p18 INK4C CDKI | 270 bps | It is responsible for growth of EBs and is known as a suppressor of teratoma formation. Also a negative regulator of cell cycle. | [37,38] |
| P19 Cyclin dependent kinase Inhibitor | P19 CDKI | 441 bps | Negative regulator of cell cycle and a suppressor of teratoma formation | [37,38] |
Isolation and culture of SSCs from human testes
Testes were acquired from the Washington Regional Transplant Community (WRTC) with permission from the next of kin. The tunica albica was removed and the seminiferous tubules were cut into 1g tissue samples and either stored in liquid nitrogen or used fresh[18].
Frozen tissue samples were transferred to a 120ml container with 40ml ice-cold DMEM/F12 (Life Technologies Cat #11320082) + Antibiotic- Antimycotic (Life Technologies Cat #15240062), and washed twice. After washing in the medium, 2-3ml of the medium was left in the 120ml container (on ice) where the sample tissue is sliced by sterile scissors. The tissue was transferred into a 50ml tube with an additional 40ml ice-cold DMEM/F12 + Antibiotic- Antimycotic. The tissue was allowed to sediment for 2-5 minutes and supernatant is removed. A 10ml enzyme solution of 1x Hank’s Balanced Salt Solution (HBSS) (Life Technologies Cat #14025076) was prepared with 2.5 mg/ml Collagenase Type IV (Life Technologies Cat #17104019), 1.25 mg/ml Dispase (Life Technologies Cat #17105041), vortex and filter through 0.22μm syringe filter (MidSci Cat #TP99722). The solution is added to the tissue sediment. The enzyme solution along with the tissue sediment was incubated 30mins in a 37 °C water bath with 100rpm shaking. Afterwards, the enzyme was removed and re-suspended in 10ml human embryonic stem cell (hESC) medium (500ml DMEM/F12, 20% knockout serum replacement (Life Technologies Cat #A3181502), 0.1mM beta-mercaptoethanol (Life Technologies Cat #21985023), 5ml Non-essential amino acids (100X) (Life Technologies Cat #11140050), L-glutamine (100X) (Life Technologies Cat #25030081), and Antibiotic-Antimycotic. A 40μm mesh filter (Sigma Aldrich Cat #22363547) was placed atop of a 50ml tube and the supernatant and sample were slowly filtered through it to extract spermatogonial cells.
The filtered tissue sample was then centrifuged (1000rpm/5min). The supernatant was removed, and re-suspended in fresh 6ml hESC medium. The medium and sample were then seeded into a 6-well tissue culture plate along with 3.5μl of 10ng/ml Recombinant human GDNF (Life Technologies Cat #PHC7045) and the plate was placed into a 34 °C and 5% CO2 incubator and cultured for 4 days.
Originally, we coated plates with gelatin (Sigma Aldrich Cat #Z707910); however, we later found that hgPSCs could grow virtually the same in uncoated TPP tissue culture plates (Sigma Inc. #92006).
Production of hESLCs from SSCs
After isolation, SSCs were cultured in hESC medium along with 3.5μl of GDNF for 4 days to stimulate growth and colony formation. They were incubated at 37 °C and 5% CO2. Media was changed every other day. After the 4th day of incubation, the hESC medium plus GDNF was replaced with hESC medium supplemented with 10 ng/ml fibroblast growth factor (bFGF) (Peprotech Cat #100-18C-10UG). Colonies must be cultured for at least 10 days to form hgPSCs[18].
hgPSC culture in modified GE médium
hgPSCs were expanded in germline expansion medium (GEM: Complete DMEM high glucose (500ml) supplemented with 10% human serum (Sigma Aldrich Cat #H4522) and 100X Pen/Strep (Life Technologies Cat #11995040), (100X) Ham’s F12 nutrient mixture (Life Technologies Cat #1165054), 0.13μg/ml Hydrocortisone (Sigma Aldrich Cat #H0888-10G), EGF (Life Technologies Cat #PHG0313), 5mg/ml Insulin (Sigma Aldrich Cat #91077C-1G), 11.7μM Cholera Toxin (Sigma Aldrich Cat #C8052-5MG), 10mg/ml Gentamicin (Life Technologies Cat #15710072)) containing 5mM ROCK inhibitor (Y-27632) (Axxora Inc Cat #ALX-270-333-M005) for 7-10 days[22]. Note: We did not use the J2 cell component found in the protocol by Suprynowicz et al[22]. We also replaced fetal bovine serum with human serum to remove all animal products. The ROCK inhibitor assists in the increased proliferation of hgPSCs to enhance colony population for later differentiation into cardiomyocytes[23].
Reestablishing hgPSCs from expansion in GEM
After expansion, GEM was replaced with hESC medium containing bFGF. This medium was replaced every other day. Colonies spontaneously and consistently formed within 5-10 days.
Differentiation of hgPSCs into cardiac lineages
Un-expanded or previously expanded hgPSCs were cultured in hESC medium and 0.25μM Cardiogenol-C Hydrochloride (Sigma Aldrich Cat #C4866-5MG) for 10 days. After differentiation, the media was replaced into complete DMEM medium supplemented with 20% human serum. This medium was considered post- differentiation medium where the cardiac clusters could be cultured for up to 30 days.
Confocal analysis
Colonies were isolated using Dumont #5 forceps and fixed in 4% paraformaldehyde for 1 hour, followed by permeabilization with 1% Triton-X-100 for 30 minutes. After two washes in PBS, areas were blocked with 2% BSA in PBS and 1% Tween 20. Primary (1:100) and secondary (1:800) antibodies were diluted in blocking solution, and colonies were incubated with each antibody for 1 hour at 37˚ C or overnight at 4oC. In the final step, colonies were moved directly from secondary antibody into a DAPI solution at 2μg/mL. Colonies were then mounted onto slides with anti-fade and sealed with coverslips. Confocal images of colonies were taken on an Olympus Fluoview 500 Laser Scanning Microscope (Olympus America Inc., Melville, NY) using the accompanying Fluoview image acquisition and analysis software (version 4.3). Cells within areas were imaged using a 1.3 numerical aperture, 40X Olympus objective. Primary Antibodies: Sox2 [488nm] (Goat; Santacruz SC #17320), Nanog [594nm] (Mouse; Abcam ab 62734), Oct 4 [647nm] (Mouse; Santacruz SC 5279) and Lin28 [647nm] (Rabbit; Cell Signaling A177 Cat #3978S). Secondary Antibodies: Donkey-anti-mouse Alexa fluor 647 nm (Abcam Cat # ab150107), Donkey-anti-rabbit Alexa fluor 594 nm (Abcam Cat #ab150076) and Donkey-anti-goat Alexa fluor 488 nm (Abcam Cat #ab150129)
Embryo and heart tube procurement and cell fusion
Female FVB mice were gonadotropin primed. E9.5 Embryos were collected from the uterine horns using Dumont #5 forceps to tease away fetal membranes; a modified version of previously described protocols[24, 25]. Embryos were transferred to organ culture dishes (No. 3037, Falcon Inc., Lincoln Park, NJ). Beating hearts were isolated using two Dumont #5 forceps, one to spread the head away from the tail of the embryo, the other forceps was used to snip the heart from the body at the most caudal and rostral ends of the heart. Fetal hearts were then placed into a well of a 96 well plate and incubated at 37 °C and 5% CO2. Hearts could beat for 72-96 hrs without distortion of morphology.
Mature Cardiomyocyte colonies were transiently transfected with uncut 5ul of cMHC-GFP DNA for 24 hours in 6-well dishes using Lipofectamine transfection kit (Life Technologies Cat #L3000001). cMHC-GFP (a kind gift from Dr. Eugene Kolossov[26]) transfected colonies were picked using a 10μl pipette tip and placed into a holding well containing differentiation medium (above). They were then seeded into crevasses of fetal hearts using hand-drawn glass needle attached to a mouth pipette. Excess GFP-positive colonies were laid on top of beating fetal hearts. The 96 well plate was then incubated at 37 °C and 5% CO2 for 48hrs.
Fetal hearts and colonies were analyzed first using a Leica stereoscope equipped with a fluorescent light source to globally identify areas of GFP within heart tissue. Those that appeared positive were fixed in 4% paraformaldehyde (Electron Microscopy Sciences Cat# 15714-S) for 2 hrs and then quenched in 1% glycine/PBS for 30min. Fetal hearts were then permeabilized with 1% Triton-X-100 for 2hrs. After three 30min washes in PBS, hearts were blocked with 2% BSA in PBS and 1% Tween 20. Primary CNX43 antibody (Rabbit Cell Signaling Cat #3512) and secondary antibodies (Peroxidase Affinipure Donkey Anti-Rabbit- Cat #711-035-152, Jackson Immunoresearch Labs) were diluted in blocking solution 1:100 and 1:800 respectively, and hearts were incubated with each antibody overnight 4˚ C. In the final step, hearts were moved directly from secondary antibody and incubated in a DAPI (Cat #D1306, ThermoFisher Scientific) solution at 2μg/mL. Hearts were then mounted onto slides with anti-fade and sealed with coverslips. Confocal images of colonies were taken on an Olympus Fluoview 500 Laser Scanning Microscope (See above).
Western Blot analysis
Isolated undifferentiated or differentiated colonies (each comprised of ~1 x 103 cells) were immediately placed into sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS w/v, 1mM ü-mercaptoethanol, 10% glycerol)[27]. Samples were placed into a 95 degrees heat block for 5 min and then loaded onto 4-20% gradient polyacrylamide gels (BioRad Inc. Cat #4561094) with molecular weight markers (Biorad Inc Cat #1610374) and separated by SDS-PAGE. Proteins were transferred to PVDF membrane and blocked with blocking reagent (5% dry milk [Bio Rad Cat #170-6404] in PBS, pH 7.4, with 0.1% Tween 20) at room temperature. The blots were challenged with primary antibody at dilutions of 1/500-1/1000 in block overnight at 4 °C, followed by washing 3 times with PBST (1x PBS + 0.1% Tween 20) at room temperature and challenge with appropriate secondary antibody (1/5,000-1/10,000) conjugated to horseradish peroxidase (Jackson Laboratories) for 2 hours followed by washing 3 times with 1X PBST at room temperature. The blot was then visualized under chemiluminescence in an ECL Imager (Thermo Scientific) using Clarity Western ECL Substrate (Bio Rad Cat #170- 5061). Primary Antibody- Desmin (Rabbit) (Cat #5332P, Cell Signaling). Secondary Antibody- Peroxidase Affinipure Donkey Anti-Rabbit (Cat #711-035-152, Jackson Immunoresearch Labs)
RT-PCR analyses
For each stage of stem cell development, colonies were picked and mRNA was extracted using the miRNeasy Mini Kit (QIAGEN® cat. No. 217004). cDNA was generated via the iScript cDNA synthesis kit (BioRad Cat #1708890) using a 20μl mixture (5x Supermix (4μl), 5x iScript reverse transcriptase (1μl), Nuclease-free water (5μl), and RNA template (10μl). RT-PCR analysis was performed by using a 22μl mixture (MyTaq Supermix (BIOLINE PCR Kit®) 12μl, Forward (2μl) and Reverse (2μl) primers, (2μl) Nuclease-free water, (4ul) cDNA template). These experiments were conducted at a melting temperature of 56 °C and for 40 PCR cycles. No RT control was performed using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Cat #K1651). Primer pairs for each gene are listed in Table 2.
Table 2: Primer Sequences.
| CARDIOMYOCYTE PRIMER SEQUENCES (5'-3') | |
| SSC GENE CANDIDATES | Abbreviation |
| F- AAAGCTTGGCGCAGATGTGA R- TTGCCACGGCATTGGTAAGA |
GPR125 |
| F- TTTACCAACTGCCAGCCAGA R- TGTTGCTGCAGTCACACCAT |
GFR1a |
| F- GAGAAGCTGTTCCAGATAGTGC R- CTCAGGGTACATGAAATGGTGG |
SSEA-4 |
| hESC GENES | Abbreviation |
| F- ATGTACAACATGATGGAGACGG R- CCACACCATGAAGGCATTCA |
SOX2 |
| F- TTTGCCAAGCTCCTGAAGCA R- AAAGCGGCAGATGGTCGTTT |
OCT3/4 |
| F- GAGCATGCAGAAGCGCAGATCAAA R- TATGGCTGATGCTCTGGCAGAAGT |
LIN 28A |
| F- TCAGAGACAGAAATACCTCAGC R- AGGAAGAGTAAAGGCTGGGG |
NANOG |
| F- TTCAACCTGGCGGACATCAA R- TTCAGCACGAACTTGCCCAT |
KLF-4 |
| F- GTATTGCCCTTTGGAGGCAC R- AGGGTCATAACTGGGCACTC |
CD73 |
| EARLY CARDIAC GENES | Abbreviation |
| F- TTCCAGAACACGATGAGGCA R- ACCTGTGACAGCTTGGTAGA |
ALCAM |
| F- AAGGTGTCTAGTAAGACAGCAG R- ATCATTTTGCCTAGCCCACC |
CHAMP |
| F- ATGCATTCTGGCGACCATCA R- ACGCCATTCCCAGTACCTTG |
TBX18 |
| DIFFERENTIATED CARDIAC GENES | Abbreviation |
| F- AGTGGATTGCTCCTTGACGA R- GGGCACGACCTCATCTTCTA |
ANP |
| F- ACCCTAGAGCCGAAAAGAAAG R- GCCGCACAGTAATGGTAAGG |
NKX2.5 |
| F- AAGATCTCCGCCTCGAGAAA R- GCAGAGATCCTCACTCTCCG |
CTNI |
| F- CTTTGATGAGAGACGTCGGG R-CTTCCCACTTTTCCGCTCTG |
CTNT |
| F- GGGGACAGTGGTAAAAGCAA R- TCCCTGCGTTCCACTATCTT |
MHC |
| F- GAGTTCAAAGAAGCCTTCAGC R- ATCCTTGTTCACCACCCCTT |
MLC2A |
| F- GGTGCTGAAGGCTGATTACG R- TTGGAACATGGCCTCTGGAT |
MLC2V |
| F-CTGTGGGCTGTTCACCAACT R- GCCGCAACCAACACATAGG |
ISL-1 |
| PARACRINE FACTORS | Abbreviation |
| F- GGGCAGAATCATCACGAAGT R- TGTTGTGCTGTAGGAAGCTC |
VEGFA |
| F- GAGCCTGCGCAATGGAATAA R- ATACCCTGTGGGCTTGTTGA |
IGF-1 |
| F- TCAACACTCCAAACTGTGCC R- AGCAAGTGAACTGTGGTCCAT |
SDF-1 |
| F- CGACTGGAAGACACGTTTGG R- TTTGGGAGTACGGATGCACT |
CTGF |
| F- CACAACAGAGCCAACAGAGTC R- TCCAGGTGGCAGAAGTAGAC |
END-1 |
| F- AGAGTGAGTCCAACTCGGTG R- AGGGCAAAGTCCAGGATCTC |
AAMP |
| F- GGAGCATATGTGTCTTCAGCTAC R- AAGCTGGCCATTACGTAGTTTTG |
NRG-1 |
| TERATOMA GENES | Abbreviation |
| F- AAGCGGGATTGTGAGCTGTG R- TGAAGGTCATCATCAGGCGG |
DND 1 |
| F- AAAATGGGGGCGGGTTTTTC R-CGCTCCCAGTGACAGTTTCT |
P18 CDKI |
| F- CGGCGAGGAGGAGGGAG R- GTCCCTGCGATGGAGATCAG |
P19 CDKI |
| GENES EXPRESSED BY ALL EMBRYONIC GERM LAYERS | Abbreviation |
| F- TGCCCAGTTTGTTC R- ACATCTCCTCTGCAACAGTGCTCA |
AFP |
| F- AGCCATTCCGTAGTGCCATC R- CAGAAGTGTCGCCTCGAAGT |
BMP4 |
| F- CAGGAGAAACAGGGCCTACAG R- GCACAGGTGTCTCAAGGGTA |
NES |
Detection of secreted proteins
Paracrine factors secreted into the medium were detected by two methods. First, ~150 cardiac differentiated colonies were grown in 1ml of differentiation medium (containing serum replacement instead of human serum). Media was then isolated at 12hrs, 24hrs, and 48hrs and frozen. Antibodies to specific paracrine factors were then added to the thawed medium along with 10ml of protease inhibitor cocktail (Sigma Aldrich Cat #P8340) overnight at 4˚ C with rocking. The next day, magnetic beads (10μl) coated with g-protein (Life Technologies Cat #10003D) were added directly to the medium containing the primary antibodies. The slurry mixed for 2 hrs at 4˚ C. Using a magnet, the beads were washed 5x with PBS. Sample buffer was added to the beads, which were then heated at 95oC for 5 min. 15μl of each time-point sample was added to a lane of a 4-20% polyacrylamide gel and the subjected to SDS-PAGE. The resultant gel was silver-stained (Bio Rad Cat #1610449) to observe the bands.
The second method, ethanol precipitation of proteins [modified from[28-30]], was used to analyze proteins that were either too close to the molecular weight of the antibody heavy chain or was not compatible with Immunoprecipitation (I.P). In this case, 1ml samples of media taken at 12, 24, and 48hrs were subjected to four volumes of 100% ethanol and precipitated overnight at -80oC. The next day the samples were centrifuged at 14,000rpm for 5 min and the supernatant was discarded. The remaining pellet was allowed to dry for 30min-1hr after which sample buffer was added and heated to 95oC for 5 min. 15μl of each time-point sample was added to a lane of a 4-20% polyacrylamide gel and the subjected to SDS-PAGE. The gel was then used for Western Blot analysis to detect the paracrine factors.
ELISA
TGFB secreted into the medium was detected by Human TGF beta 1 Platinum ELISA kit (Cat # BMS249-4, Life Technologies, Inc). Medium cultured in differentiated cardiomyocytes at 0h, 12h, 24h and 48h were tested along with TGFB standards (31pg/ml- 2000pg/ml) at 450nm. A standard curve for TGF beta was plotted using OD values from standards. Concentration of circulating TGFB of each time point was calculated from the standard curve and then multiplied by a dilution factor of 30 (samples were diluted 1:30 prior to experiment). The negative controls in this experiment were ES media cultured at 0h. Concentration of TGFB was normalized to 0h ES media. Final concentrations in media cultured in cardiomyocytes at the different time points were plotted against normalized values to 0h ES using a bar graph.
Statistical analyses.
Standard error of the means were calculated from N > 3, which were then analyzed by student t tests. A p < 0.05 or better was considered significant.
Results
Identifying the source cell from testis
Recent investigations have demonstrated that when isolated and cultured in the proper medium, germline stem cells can be induced to form cell/tissue from all three germ layers (i.e., ectoderm, mesoderm, and endoderm)[18-20, 31].
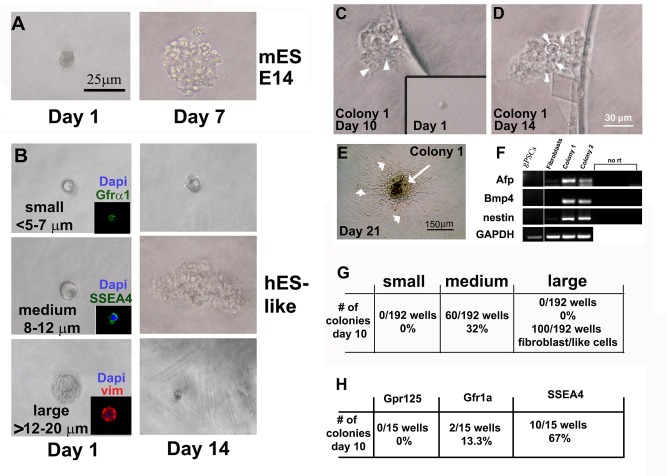
Identifying the stem cells within the human testes that give rise to hgPSCs has been somewhat elusive. To begin identifying this stem cell population, we first generated single cell suspensions assaying them for clonal expansion, which is accepted as a primary characteristic of stem cells. We used this approach first because, particularly in humans, a molecular or cellular target for identifying the actual stem cell population within the testes remains a debated topic in the literature. Consequently, we initially used cell size as a marker for isolating and identifying this population of stem cells. Figure 1 shows typical examples of isolated single cells, while Figure 2A shows the milieu of different sizes of cells enzymatically isolated from human testes tissue[18]. Using a hand-drawn glass pipette connected to a plastic filtered mouth suctioning tip, small (<5-7μm), medium (~8-12μm), and large (>12-20μm) cells were placed one by one into wells of a 96 well plate (Figure 1). As a reference, a typical, single mouse ESC cell expanded into a colony very quickly after plating (Figure 1A). Different cells isolated from the human testis (Figure 1B) did not all expand in vitro. After 10-14 days of culture, only medium-sized cells grew into colonies capable of being differentiated and expressing markers from the three embryonic germ layers (Figure 1 C-F). Although the table in figure 1G reports that only 32% of medium-sized cells actually grew into distinctive colonies identical to that seen in Figure 1E, we concluded that this methodology was useful for confirming that a specific sub-population of cells within the SSC--enriched fraction could grow clonally and generate cells from all three germ layers.
Figure 1:
Identifying the stem cell within testes that gives rise to clonal hgPSCs. A) Acting as a positive control, a single mouse E14 strain embryonic stem cell gives rise to a colony within 7 days of culture. B) After enzymatic digestion and filtration of human testes tissue, distinctive size differences among cells were clearly evident. Small (<5-7μm), medium (~8-12μm), and large (>12-20μm) cells were isolated using a mouth pipette and placed into a well of a 96 well plate. After 14 days of incubation wells were assessed for clonal growth. Only medium size cells produced colonies. C-F) Removing bFGF from the hESC medium to induce differentiation by 10 and 14 days, respectively. Arrowheads in C and D point to individual cells within a colony, while the white circle in D outlines one cell within the colony. By ~21 days of differentiation, RT-PCR showed expression of genes from all three germ layers; Alpha fetal protein (AFP-Endoderm), Bone morphogenic protein 4 (Bmp4-mesoderm), and Nestin (neuroectoderm). However, hgPSCs did not express any of the genes from all three germ layers. GAPDH was our RT-PCR control gene. E) A central mass of differentiated cells (Arrow) are surrounded by fibroblasts, which do not show expression of these three specific genes (arrowheads in E and fibroblasts in F). G) Table quantifies colony formation from the three sizes of cells. F and insets in B) Using antibodies directed against previously explored SSC markers, SSEA4 but not Gpr125 or Gfr1α identified the cells that produced colonies of hgPSCs. SSEA4 positive cells are 8-12μm in diameter, while Gfr1α are much smaller. The large cells were mostly vimentin positive most likely representing Sertoli cells.
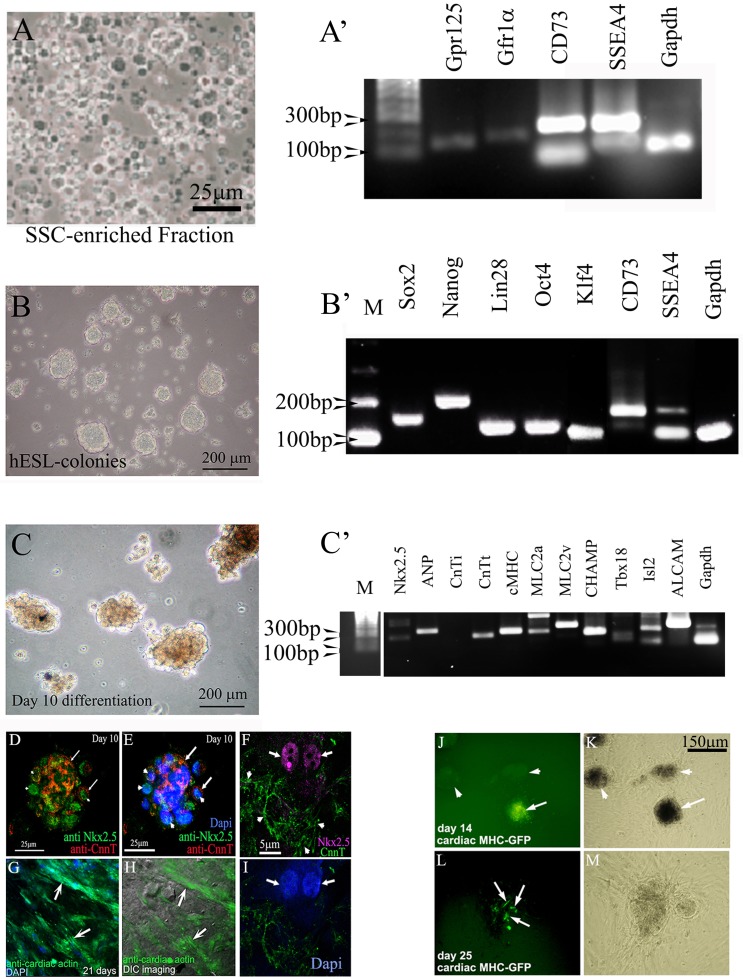
Figure 2.
Cardiac lineages can be produced from hgPSCs. A). Cells of the SSC-enriched fraction are cultured in medium containing GDNF for four days. The fraction is full of cells positive for various SSC markers including SSEA4. B) After four days, the medium is switched to a basic hESC medium containing bFGF and serum replacement. Cells are incubated for at least 10 days, after which rt-PCR shows evidence of all four Yamanaka factors plus nanog and CD73. C) Switching hESC medium for cardiac differentiation medium results in growth and morphologically darker looking colonies. RT-PCR shows expression of 9 out of 10 cardiac genes within 10 days of differentiation. D-I) Confocal analyses show protein expression of specific cardiac genes including nuclear staining of Nkx2.5 (F arrows). Arrows in D and E point to areas positive for cardiac troponin (cTNT), while arrowheads point to nuclear Nkx2.5 staining. Dapi staining in E identifies the nuclei. Arrows in G and H point to distinct filaments of cardiac actin. The DIC image in H reveals the actin fibers within healthy cells. J-M) Transfection of colonies with a cMHC-GFP further confirms cardiac gene expression. Arrow in J points to a GFP positive colony. Arrowheads point to untransfected colonies. These untransfected colonies serve as an internal control ruling out autofluorescence. L) Arrows point to cells within the colony expressing cMHC-GFP. K and M) Phase contrast views show healthy colonies.
To apply a more rigorous approach for identifying which cell type could grow clonally into colonies of hgPSCs, we relied on data from many independent laboratories that had previously analyzed candidate markers for SSCs (publications found in[32]). Different laboratories had previously analyzed candidate genes as markers for the testicular stem cells pinpointing SSEA4, GFR1α and GPR125 as putative SSC markers [publications found in[32]]. To confirm or refute clonal growth of cells expressing each marker, we isolated immunofluorescently tagged cells from a testicular isolate using the same mouth pipette procedure as above, again placing each cell into its own well in a 96 well plate. Cells were labeled with primary antibodies directed against SSEA4, GFR1α, GPR125, or vimentin[32], followed by fluorescently labeled secondary antibodies. The insets in Figure 1 show examples of fluorescent single cells within each well. Interestingly, SSEA4+ cells not only formed colonies, they were the same size as the medium cells previously seen to form colonies. Conversely, Gfrα1+ cells were much smaller and did not form colonies while the larger cells were only able to divide a few times in culture and resembled fibroblasts. Most of the larger cells were vimentin positive suggesting they were Sertoli cells or cells from the lamina propria. As a result, these data confirmed that SSEA4+ cells are most likely the hgPSCs that acquire the ability to not only grow clonally when isolated from the testes and grown in a specified hESC medium, but also form cells/tissues from all three germ layers.
hgPSC colony-derived cardiac-lineage cells express cardio-protective paracrine factors
One approach that is gaining momentum for improving cardiac function after ischemic injury is the delivery of paracrine factors that can reprogram or induce repair of existing cardiac tissue. To identify if hgPSCs could be induced to form cardiac cells that secrete cardio-protective paracrine factors, we subjected them to a protocol used for human ESCs documented by[33], followed by assaying by gene expression analysis using RT-PCR.
The procedure begins with 1g pieces of human testes tissue. Individual tissue samples are thawed and the SSC-enriched fraction is isolated using the protocol from[18]. Each sample yields ~500 hgPSC colonies. SSCs undergo de-differentiation to form hgPSCs when cultured in hESC medium for 10 days. Distinct hgPSC colonies begin to form after 2-3 days and each Yamanaka factor (Sox2, Oct4, Lin28, Klf4) as well as other stemness factors, nanog and CD73, are expressed (Figure 2B). CD73 represents a marker for newly described endogenous plastic somatic cells (ePSCs; [34] Figure 2B).
All are detected in colonies after 10-14 days in culture. It is important to note that unlike iPSCs, which need exogenous expression of Yamanaka factors, these same factors are endogenously expressed and regulated in hgPSCs. Upon differentiation of hgPSC colonies, expression of cardiac genes is usually detected ~10 days of culture (Figure 2C). Confocal microscopy verified distinct cardiac protein expression (Figure 2D-I), while transient transfection with a cardiac MHC-promoter reporter construct confirmed induction of the cardiac lineage (Figure 2J-L).
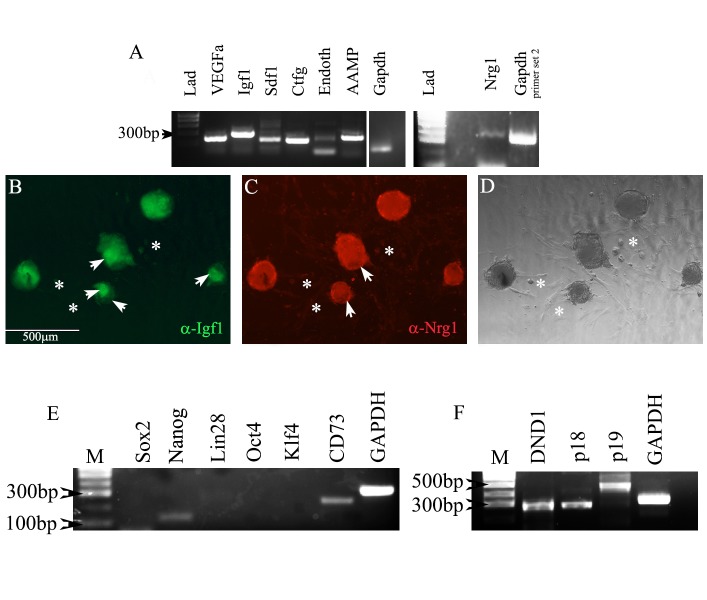
To test the ability of hgPSC-derived cardiac cells to express cardio-protective paracrine factors, we utilized the cMHC-GFP construct to pick cardiac-lineage colonies from the surrounding fibroblast-like cells. We then assayed for cardio-protective paracrine factor expression[1, 6]. Strong gene expression of each paracrine factor was detected (Figure 3A), while protein expression for two paracrine proteins in particular, IGF-1 and NRG1, was also very strong (Figure 3B-D). Expression of IGF-1 and NRG-1 has recently been shown to be important for inducing surrounding healthy cardiac tissue to both proliferate and differentiate into the ischemic region[1, 6]. NRG-1 is of special interest because of its potential for translation into the clinic for treating infarcts[1]. These data reveal that expression of paracrine factors is relatively robust within hgPSC-derived cardiac lineage cells after 21 days of differentiation. To our knowledge, this is the first evidence that hgPSC-induced cardiac lineage cells express these factors.
Figure 3.
Differentiation of cardiac colonies beyond day10 results in colonies that express pro-cardiac regenerative paracrine factors. A) Rt-PCR shows expression of seven pro-cardiac regenerative paracrine factors. B-D) Immunofluorescent and DIC analyses using antibodies directed against IGF-1 and NRG-1 show colonies staining positive for both paracrine factors. Arrowheads in B and C point to regions of variable staining within colonies. Asterisks highlight fibroblasts that can emanate from colonies. They show no fluorescent staining. E) Expression of most pluripotency genes is below the level of detection in differentiated colonies. Nanog and CD73 are expressed; however, nanog expression has been reported at low levels by [35] and CD73 expression has been reported as necessary for cardiac development[37]. F) Genes known to inhibit teratoma formation are expressed in hgPSC-derived cardiomyocytes. They are also expressed in hGPSCs (data not shown).
Differentiated hGPSCs show loss of pluripotency and teratoma formation
For quality-control issues, it was important to confirm that hGPSCs lost their ability to form teratomas. First, using RT-PCR, we verified that virtually all of the pluripotency genes were down regulated (Figure 3E); however, there was weak expression of Nanog and CD73 in cardiomyocytes. Through literature search, we found that Nanog can be expressed in the myocardium of rats[35] and CD73 is needed for successful differentiation into cardiomyocytes[36] (Figure 3E). Equally as important to identifying loss of pluripotency was identifying molecularly, the ability (or loss of) of these cells to form teratomas. The RT-PCR data in Figure 3F clearly show expression of three genes, DND1 and p19 CDKI and p18ink4C in hGPSCs-derived cardiomycoytes[37]. The expression of these genes are consistent with the loss of teratoma formation in hGPSCs and cardiomyocytes[37, 38]. As a result, cardiomyocytes differentiated from hGPSCs are molecularly incapable of forming teratomas. We also performed a no- RT control on p18INK4C primers because they sit on one exon and observed that there was no genomic DNA contamination in both hgPSCs and cardiomyocytes (data not shown).
Quick, efficient expansion of hgPSCs using hgPSC expansion medium (GEM)
The data preceding figure 4 were acquired from primary hgPSCs and cardiac cells differentiated directly from primary hgPSC colonies. However, we found that while hgPSC-derived cardiac cells showed much promise for eventual cardiac repair, it was somewhat painstaking to accumulate the amount of cells that would be needed for injection into a patient. Months were needed to generate enough biological material from 1g biopsy-sized pieces of testes. As a result, we modified a cellular reprogramming technique by[22] and applied it to hgPSCs to determine if we could expand the hgPSC population more quickly (and using no animal components) when compared to conventional expansion protocols.
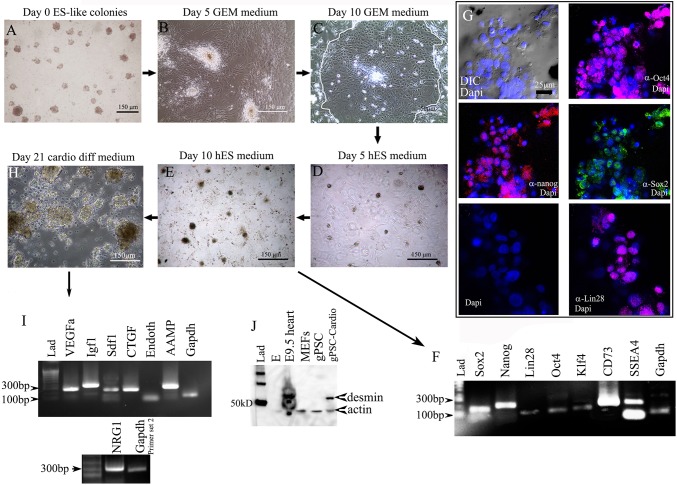
Figure 4.
Culturing hgPSC colonies in GEM allows for their rapid expansion without the loss of stemness. A) hgPSC colonies grown in hESC medium lose their colony structure beginning ~5days post switching to GEM (B). By day 10, most colonies become individual layers of cobble-stone shaped cells (C-white outline shows region of cobblestone pattern of cells), which can be expanded indefinitely. Switching GEM for hESC medium, colonies begin to re-form within 5 days (D), and by day 10 hgPSC colonies fully return (E). F-G) Rt-PCR shows that these colonies express all the same stem cell factors prior to expansion, which is confirmed by confocal microscopy. Nuclear staining of Oct4 (647 nm- Far red), Nanog (594 nm/ Rhodamine- Red), Sox2 (488nm/FITC- Green), and Lin28 (647 nm- Far red) is prevalent (G). H) 21 days post differentiation, large dark colonies form, which are all positive for paracrine factor gene expression (I). J) Western Blot analysis of the colonies shows they are positive for the cardiac intermediate filament desmin similar to the mouse heart. Undifferentiated gPSCs are negative for desmin as are mouse embryonic fibroblasts (MEFs).
Figure 4 illustrates the process of germ cell expansion and subsequent re-establishment of hgPSCs, followed by their differentiation into cardiac lineages. By day 10 in GEM, the cells within colonies took on a cobble-stone appearance typical of previous reports[22]. While in GEM, these cells could be expanded indefinitely and quickly; that is, one colony placed in a 96 well plate would typically be split into two wells of a 96 well plate within 7 days. Comparing expansion rates of primary hgPSCs to expanded hgPSCs grown in GEM from two patients, we found that upon re-establishment in hESC medium, ~4X more colonies were obtained by the fourth passage when compared to primary hgPSCs grown in conventional culture medium. More importantly, it took 30 days less time to expand hgPSCs in GEM compared to primary hgPSCs grown in conventional medium (Figure 5; Table 3). We have expanded hgPSCs in GEM at least 20X. Re-establishing hgPSC colonies is accomplished by replacing GEM with hESC medium. Usually within ten days of culture, gene expression patterns match primary hgPSCs including the expression of all Yamanaka factors, nano([34]; Figure 4F). Confocal microscopy of colonies provided further evidence that these cells retained pluripotent markers (Figure 4G).
Figure 5.
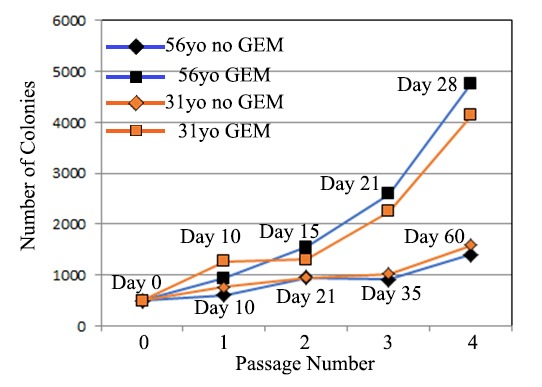
500 hgPSC colonies were expanded by traditional conditions (i.e., by trypsinization and passaging 1:2) or they were expanded 1:2 in GEM. Comparing two different patients, no marked difference was observed until the second and third passages where GEM-grown hgPSCs grew ~2X faster than conventional growth. By the fourth passage, GEM-grown colonies re-generated close to 4X more colonies when compared to conventional growth and passaging. More importantly, those ~4x more colonies were obtained in almost half the time.
Table 3: Comparing expansion rates of primary hgPSCs to expanded hgPSCs grown in GEM.
| Passage # | 56 yo Patient 1 Number of gPSC colonies NO Conditional Reprogramming Medium (No GEM) |
Time | |||||||||||||||
| P0 | 500 | Day 0 | |||||||||||||||
| P1 | 380 | 320 | Day 10 | ||||||||||||||
| P2 | 280 | 220 | 235 | 215 | Day 21 | ||||||||||||
| P3 | 113 | 163 | 89 | 108 | 123 | 93 | 105 | 113 | Day 35 | ||||||||
| P4 | 85 | 94 | 101 | 96 | 58 | 73 | 80 | 93 | 103 | 94 | 76 | 89 | 88 | 91 | 87 | 92 | Day 60 |
| Total # of colonies |
1,400 | ||||||||||||||||
| Passage # | 56yo Patient 1 Number of gPSC colonies Expanded in Conditional Reprogramming Medium (+ GEM) |
Time | |||||||||||||||
| P0 | 500 | Day 0 | |||||||||||||||
| P1 | 455 | 476 | Day 10 | ||||||||||||||
| P2 | 387 | 358 | 396 | 403 | Day 15 | ||||||||||||
| P3 | 308 | 310 | 303 | 317 | 302 | 287 | 363 | 397 | Day 21 | ||||||||
| P4 | 302 | 275 | 280 | 285 | 295 | 283 | 301 | 275 | 267 | 258 | 275 | 285 | 355 | 310 | 365 | 352 | Day 28 |
| Total # of colonies |
4,763 | ||||||||||||||||
| Passage # | 31yo Patient 2 Number of gPSC colonies NO Conditional Reprogramming Medium (No GEM) |
Time | |||||||||||||||
| P0 | 500 | Day 0 | |||||||||||||||
| P1 | 385 | 380 | Day 10 | ||||||||||||||
| P2 | 286 | 222 | 205 | 235 | Day 21 | ||||||||||||
| P3 | 133 | 114 | 113 | 124 | 143 | 127 | 118 | 147 | Day 35 | ||||||||
| P4 | 101 | 102 | 88 | 98 | 84 | 88 | 104 | 102 | 110 | 113 | 95 | 101 | 95 | 87 | 115 | 103 | Day 60 |
| Total # of colonies |
1,587 | ||||||||||||||||
| Passage # | 31yo Patient 2 Number of gPSC colonies Expanded in Conditional Reprogramming Medium (+ GEM) |
Time | |||||||||||||||
| P0 | 500 | Day 0 | |||||||||||||||
| P1 | 489 | 478 | Day 10 | ||||||||||||||
| P2 | 287 | 318 | 337 | 306 | Day 15 | ||||||||||||
| P3 | 221 | 247 | 276 | 285 | 307 | 315 | 287 | 305 | Day 21 | ||||||||
| P4 | 205 | 210 | 222 | 230 | 245 | 237 | 275 | 245 | 301 | 275 | 303 | 289 | 275 | 263 | 300 | 268 | Day 28 |
| Total # of colonies |
4,143 | ||||||||||||||||
These re-established hgPSC colonies could then be differentiated down the cardiac pathway resulting in an expression pattern of paracrine factors similar to that observed in cardiac cells differentiated from primary hgPSCs (Figure 4H, I). As a result, we conclude that re-established cardiac colonies are virtually identical to cardiac colonies generated from fresh primary hgPSCs.
Paracrine factors are secreted by re-established cardiac colonies
While it was important to show that cardiomyocytes derived from hgPSCs expressed cardiac and paracrine factor genes (see Table 1 for paracrine factor list and functions), it was further necessary to identify their physiological ability to secrete those paracrine factors. A consensus of at least six paracrine ‘effects’ categories has been endorsed within the literature[1, 11, 39, 40] (see Table 1). These categories include (Survival, proliferation, immune cells, remodeling, vascularization, and CPC activation). Secretion of paracrine factors would mean they can affect surrounding tissue.
To detect secretion, two different methods were employed. First, after culturing day 21-differentiated cardiomyocytes for 0hrs, 12hrs, 24hrs and 48hrs, the entire compliment of medium (1.0 ml) from each time point was directly tested by immunoprecipitation (IP) using antibodies to specific paracrine factors, followed by antibody isolation using magnetic protein G-coated beads. The pull-down products were subjected to SDS-PAGE, followed by silver stain of the gels. This highly sensitive approach revealed VEGFA, CTGF, IGF-1, and TGFü all increasing in concentration over a 48hr period of incubation (Figure 6).
Figure 6.
Immunoprecipitation (I.P.) and Western Blot analysis of culture medium shows that paracrine factors are secreted from hgPSC-derived cardiac colonies. A) Silver stained SDS-PAGE gel detected IGF-1 by IP after 12hrs of culture, increasing in intensity through 48hrs of culture. B). Silver staining shows TGFb secretion within 12hrs becoming more intense after 48hrs. C) VEGF is detectable by about 24hrs of culture. D) Western analysis of CTGF secretion is detected within 24hrs while NRG-1 secretion (E-F) is detected after 48hrs of culture. Cardiac differentiation of hgPSCs from two patients are shown for Nrg1. All IP experiments were run with a lane containing IgG alone to identify the heavy and light chain bands. 2μl from all samples were analyzed using a nano-drop ND-8000 (Thermo Fisher Inc.) to normalize protein concentrations. G) ELISA bar graph data shows quantitative amounts of TGFü secretion detected within 12-48 hours of culture. ELISA data confirm the silver stained SDS PAGE gels, which detected a similar secretion pattern as found in the ELISA.
The second method we used to detect secreted proteins involved precipitation of proteins from the medium using 4 volumes of ice cold ethanol (as published in[28-30]), then separating them by SDS-PAGE, and probing by Western blot using antibodies directed against each paracrine factor (Figure 6E and F). This Western blot approach was employed primarily where the antibodies had not been tested for applications such as I.P. Here, we found that CTGF and NRG-1 were secreted into the medium by 24-48hrs. When combined, these data provided strong evidence that hgPSC-derived cardiomyocytes secrete paracrine factors known to be proangiogenic and procardiogeneic[1, 6, 41].
It is difficult to quantify these data because secreted “housekeeping” genes are not well characterized for these cells; however, we do know that the lower level of protein detection for silver stained gels is in the ~ 1-10ng range. In an attempt to confirm and quantify what we observed from our silver stain data, we analyzed media by ELISA (Figure 6G). Since, sensitivity of both ELISA and silver stain are similar (0.1-10ng of protein), the ELISA data confirmed what we observed in the silver stains; TGFü is secreted within the 1-10ng/ml range within 48 hrs of culture. More specifically, our silver stain results showed that from 10μl of sample media loaded into each well, ~5.0 x 105 cells secrete paracrine factors in the 1-10ng/ml range by 48 hrs of culture. Figure 6G illustrates ELISA data showing a gradual increase of TGFü from 0h to 24h, with very little change in secretion between 24h and 48h of incubation. According to Beck et al[42], the active form of TGFü can last for over a day after which it is subjected to various types of degradation. This observation by Beck et al[42]may be the reason for the slight decrease we see at 48 hours compared to 24 hours as some of the TGFü might have started to degrade by 48 hours. Most importantly, however, is that these TGFü ELISA data confirm the silver stain results. The concentration of each paracrine factor is well within range for effecting of surrounding tissues (Figure 6). These data are the first to show that hgPSC-derived cardiac cells not only express, but also secrete cardio-protective paracrine effectors.
hgPSC-derived cardiomyocytes can integrate to beating cardiac tissue in vivo
We next wanted to determine if hgPSC-derived cardiomyocytes could fuse with beating heart tissue. Many different animal models have been utilized, with rodent hearts being the most prevalently used model system, to demonstrate infiltration and integration-ability of various types of stem cells within cardiac tissue[14, 43]. One problem with rodent model systems for human stem cell research, however, is that the normal beat-rate for mouse hearts can vary between 400-600 beats/minute. The dramatic difference between human cardiac cells and rodent hearts has been reported to create incompatible physiological variances that can skew results[14]. As a result, we chose the mouse fetal heart as a ‘better’, more physiologically compatible model system for transplanting cardiac-specific, GFP-labeled, hgPSC-derived cardiomyocytes (Figure 2 and 7). This model system is better than adult cardiac model systems because the mouse fetal heart beats at ~60-70 beats/min, which is very similar physiologically to the human heart.
Figure 7.
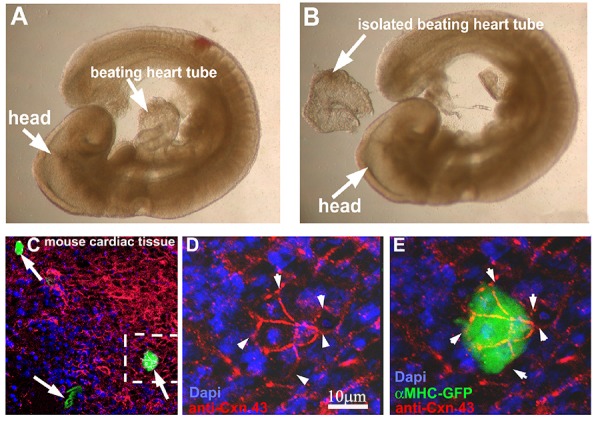
hgPSC-derived cardiac colonies can fuse with beating cardiac tissue. A-B) E9.5 fetal hearts were isolated from mouse embryos using Dumont #5 forceps. 10-15 fetal hearts were placed in one well of a 96 well plate and cMHC-GFP positive colonies were mouth pipetted into crevasses within the beating heart or simply overlaid onto the hearts. 24 hours later, hearts were analyzed live using a Leica stereoscope equipped with fluorescence. Hearts containing green areas were then fixed, stained for CNX43, and Dapi and visualized by confocal microscopy. C) Multiple GFP-positive regions were evident (arrows). D-E) Higher magnification clearly showed GFP positive cells fused to cardiac tissue via gap junctions (Arrowheads) on the same focal plane as surround heart tissue.
cMHC-GFP-positive hgPSC-derived cardiac colonies were physically isolated by mouth pipette using a Leica Fluorescent stereoscope. GFP+ Colonies were then pipetted into tight crevices within the heart tube so that they could not fall away from the beating heart tube. Each well of the 96 well plate contained 10-15 fetal hearts, which forced virtually all loose cardiac colonies to remain in close contact with cardiac tissue. After 48hrs of incubation, fetal hearts were observed live using the same fluorescent stereoscope to identify GFP(+) areas. Upon detection, fetal hearts were fixed in 3.0% formaldehyde for 2hrs, followed by processing for immunofluorescence using an antibody for the cardiac gap junction protein Connexin43 (CNX43). Multiple areas of GFP labeled cells were visibly co-stained with the gap junction protein CNX43 providing evidence that the hgPSC-derived cardiac cells directly fused with the mouse cardiac tissue. Fusion of GFP labeled cells was observed in 8 out of the 10 fetal hearts.
Discussion
Although optimism remains for investigating stem cells for treating or curing cardiovascular disease, much disparity remains among the various published approaches and results. Here, we show three novel outcomes that could lead to the production and delivery of paracrine factors known to induce and improve cardiac function in ischemically injured heart muscle[1, 8, 9, 13]. First, we show that hgPSC-derived cardiac cells express and secrete pro-cardiac regenerative paracrine factors. Secondly, the expansion of hgPSCs can be markedly amplified when cultured in GEM using human serum (no animal products); and thirdly, the data provide strong evidence that hgPSC-derived cardiomyocytes can physically incorporate into the cardiac niche.
Since the initial findings that a germ-line stem cell could ‘revert’ to a state that resembled hESCs/hiPSCs both genotypically and phenotypically, understanding their biology and therapeutic potentials has been consistently investigated by various laboratories[1, 18, 20, 31, 44]. Defining the ‘stemness’ of germ-line stem cells has been debated[45]; however, the most recent study from the Skutella laboratory has provided the best evidence yet as to their true identity[31]. They reported that hgPSCs are adult stem cells, but they share a gene expression profile that is related to, but not identical to true pluripotent stem cells. Perhaps the most important finding in [31] was the confirmation of hgPSC colony plasticity as they were shown to attain the ability to differentiate into cells of all three germ layers[17, 18, 31, 32, 46]. From a swath of previously identified ‘potential’ SSC markers, the elegant studies from two independent laboratories pinpointed SSEA4 as a strong candidate marker for the testicular stem cells that might be giving rise to hgPSCs. Interestingly, these data somewhat contradicted data from other laboratories who had previously suggested Gfr1α and Gpr125 as candidate SSC markers [publications found in[32]]. In light of this controversy, research on human testes using various methodologies including magnetic-activated cell sorting [MACS[47], indirect immunofluorescence[48], and FACS[49]] provided the evidence that SSEA4+ cells were most likely the best candidates that, when cultured in hESC medium, could progressively expressed pluripotent factors and subsequently generate cells from all three germ layers. Closer inspection of those studied showed that they did not clearly identify the ability of SSEA4+ cells to grow clonally into hgPSC colonies.
Here, we confirmed that hgPSCs do endogenously express all of the pluripotent Yamanaka factors plus newly identified others (Nanog and CD73) known to be important for stemness (This study [18]) and that they are readily down-regulated once differentiation commences. Thus, once differentiated, hgPSCs show virtually no risk of teratoma formation in vivo. In fact, closer inspection of reports where teratoma formation was specifically analyzed after injection of naked hgPSCs into nude mice revealed that tens of millions of hgPSCs over a month were needed to generate a tiny nodule containing multiple cell types[18]. This result was in stark contrast to the teratomas that formed within three weeks from mere thousands of ESCs injected into the opposing hind flank of the same nude mice. It is these types of observations, when taken together, that keep hgPSC research relevant.
Upon differentiation of hgPSCs, similar to hESC and hiPSC-derived cardiac cells[15, 50, 51], we found that hgPSC-derived cardiac cells varied with respect to atrial/ventricle gene expression representing a mixed population of cardiac cells; however, one aspect that was not shared was spontaneous beating. Cardiac cells derived from human hESCs and hiPSCs consistently show rhythmic beating; however, hgPSC-derived cardiac cells did not. Initially, we were discouraged by this non-beating phenotype; however, recent revelations within the literature led us to pursue the alternative, paracrine effector, and regenerative pathway.
For years, numerous investigations have tapped into the ‘beating’ phenotype for potentially repairing ischemically damaged heart muscle. In fact, some adult stem cell (ASC) work has shown promise as ejection fractions slightly improve after beating ASC-derived cardiomyocytes are injected into infarcted heart muscle. However, one major detriment with many ASC-cardiac repair studies is the potential for arrhythmias as stem cell-derived cardiomyocytes, in many cases, can beat at their own pace. As a result, instead of matching stem cell/cardiac tissue electrophysiology, a paradigm shift towards cardiac tissue regeneration mediated either directly or indirectly by paracrine factors has been proposed as more beneficial. We originally showed that hgPSCs could express a small cohort of cardiac genes representing differentiation[18]; however, in depth analyses of these cells for potential cardiac repair was not assessed in[18]. There have been hundreds of studies investigating if and how ASCs as well as ESCs and iPSCs function to repair ischemic cardiac tissue (reviewed by[2, 7]). Some of those studies have resulted in clinical trials; however, the varying outcomes of those trials have not led to their routine clinical use because the best mechanism for translating their potential in vivo remains debated. On one hand, generating cardiomyocytes with the ability to electrically couple within the ischemic region of the heart has resulted in some, albeit good, evidence showing attenuation of left ventricular (LV) remodeling and improved LV systolic function[40, 52, 53]. In addition, data from non-human primates and large mammals have also shown neovascularization upon stem cell injection[50, 54]. On the other hand, much of the data where matching stem cell/heart electrophysiology was a focus of the study, stem cells were found to generate a diverse set of mature and immature atrial, ventricle, and even sinusoidal cells periodically resulting in arrhythmic islands of beating cells[50, 54]. As a result, although the electrophysiology avenue of research remains ardently investigated among many different types of stem cells, it was exciting to find that the differentiation potential of hgPSC-derived cardiomyocytes included expression and secretion of key paracrine factors known to be pro-growth and pro-differentiation [1, 9].
A consensus of at least six paracrine ‘effects’ categories has been endorsed within the literature[1, 11, 39, 40]. Analyzing the SSEA+, medium-size hgPSC-derived cardiomyocytes revealed that they expressed and secreted paracrine factors from all six paracrine ‘effects’ categories. Genes including VEGFA (representing vascularization and remodeling), NRG1 (proliferation and vascularization), IGF1 (survival, proliferation, and suppression of immune response), TGFü (Immune regulation, remodeling), and SDF1 (vascularization and CPC activation) all were not only expressed, but also secreted into the media at or near nanogram/ml levels. As a result, the data presented here provide evidence that hgPSC-derived cardiomyocytes can either directly or indirectly influence cardiac tissue via paracrine effects.
Although identifying expression and secretion of paracrine factors was an important step in determining the ability of hgPSC-derived cardiac cells for potential use in vivo, it was also important to identify their ability to integrate into cardiac tissue. Without integration into cardiac tissue, delivery of paracrine effectors would be impaired or highly unlikely. The model system we employed to identify integration took into consideration both successes and failures identified by many investigations[11, 39, 51, 55]. For example, injecting undifferentiated adult stem cells or differentiated hESCs/hiPSCs into infarcted rodent hearts has resulted in widely varying reports from improved cardiac function to little or no attenuation[7, 15, 55]. Efficacy of integration into the cardiac niche was one characteristic dictating their successes or failures. Shared gap junctions characteristically represent good integration[56, 57]; however, even if gap junctions are readily observed between endogenous and exogenous cells, problems can still arise including arrhythmias. Arrhythmic beating can result from injected cells ‘beating’ on their own and/or because they cannot match the beat-rate of the injected heart[7]. Rodent hearts can beat >400 beats/min. A human heart beating that fast would be considered undergoing tachycardia. On the contrary, because embryonic hearts beat at ~70 beats/min, we determined they would serve as a better model system for identifying the integration ability of hgPSC-derived cardiac cells into a cardiac niche. hgPSC-derived cardiac cells expressing a cardiac promoter-driven GFP reporter gene along with immunofluorescent staining of the endogenously expressed cardiomyocyte gap junction protein CNX43 clearly showed multiple areas of integration. As a result, these data provide good evidence justifying further, more detailed investigations of hgPSC-derived cardiac cells for use in cardiac repair, perhaps within a non-human primate model.
None of these data would have been possible, though, if we had not had enough hgPSC-derived cardiac cells to work with. Soon after we began work on the data presented here, a novel technology was published for growing primary epithelial cells indefinitely[22]. That work astonishingly, yet elegantly showed that a small tracheal biopsy could be grown indefinitely in a specifically defined medium (>40 passages), followed by complete re-establishment of all cell types within the trachea when transferred back to a media promoting tracheal development. Their medium was composed of a DMEM/Hams F12 mix (1:1) containing a ROCK inhibitor and 25% J2 cell conditioned medium. We found that hgPSCs grew very quickly in the medium defined by[22]. About 100 colonies would grow to confluency in a 6 well dish within ~5 days and could then be passaged indefinitely; however, we did encounter a problem in about 50% of our experiments. The cells would fill with small, dark vacuoles that would eventually kill the cells. As a result, we tried growing cells in medium using various combinations of the components specified in[22]. We found that growing hgPSCs in medium containing a ROCK inhibitor without the J2 component did not alter their ability to expand indefinitely. We also substituted animal serum with human serum to remove all animal products. Consequently, these two changes were substantial enough to warrant renaming our medium to ‘hgPSC expansion medium’ (GEM) to quell any confusion. So far we have reached ~20 passages of hgPSCs using GEM while not losing the ability to re-establish colonies positive for all Yamanaka factors, CD73 and Nanog.
Conclusion
With this technique in hand, generating cells for experimental analysis has become much more efficient. We also believe this technique could speed up the time needed for treating patients from months to weeks. The resulting re-established hgPSCs also re-attained their differentiation potential as they not only expressed the same cardiac marker set tested in primary hgPSC-derived cardiac cells, but also the same set of paracrine factors. We believe the data presented here could eventually, truly help patients with heart disease. An important fact to note is that the work presented here is not male-centric.
Evidence from the Tilly laboratory has revealed the existence of gPSCs within the ovary[58, 59]. Patients suffering from ischemic heart injury could be subjected to a testes/ovary biopsy (could be done in an IVF clinic). The biopsy is then given to the scientist for isolating SSCs/OSCs (isolated from the biopsy) and subsequent de-differentiation into hgPSCs. Indefinite expansion of hgPSCs in GEM would be followed by re-establishment into hgPSCs, and then differentiation into paracrine factor yielding cardiomyocytes. These cells would be sent back to the medical doctor who would then inject them back into the patient. Some of the expanded hgPSCs in GEM could be frozen down for future treatment (in case the same patient comes back for treatment again).
While we are planning to expand this work into the ovary to identify the potential of female hgPSCs for treating or curing heart disease in women, we also do not want to rule out the research being pursued on very small embryonic-like stem cells (VSELs). It was recently suggested that VSELs might share similarities with gPSCs with respect to their capacity to restore spermatogenesis in mice [60]. Whether VSELs and gPSCs are one in the same remains to be determined. If they are based on the data presented here, both should be pursued with vigor to help treat or cure heart disease.
Acknowledgments
We would like to acknowledge Vaughn E. Gallicano for repeating key I.P. experiments. VEG is in the 8th grade at Mark Twain Middle school. His interest in this project partially fulfilled Fairfax County School District mandatory volunteer requirements.
Glossary
Abbreviations
- GPR125
G protein coupled receptor 125
- GFR1a
GDNF family receptor 1 alpha
- SSEA-4
Stage-specific antibody-4
- SOX2
SRY (Sex Determining Region Y)- Box 2
- OCT3/4
Octamer-Binding Transcription Factor %
- LIN 28A
LIN28 Homolog A
- KLF-4
Kruppel-like factor 4s
- CD73
Cluster of differentiation 73
- ALCAM
Activated Leukocyte Cell Adhesion Molecule
- CHAMP
Cardiac Helicase Activated MEF2C protein
- TBX18
T-Box Transcription Factor 18
- ANP
Atrial Natriuretic Peptide
- NKX2.5
NK2 homeobox 5
- CTNI
Cardiac Troponin I
- CTNT
Cardiac Troponin T
- MHC
Myosin heavy chain
- MLC2A
Myosin light chain 2A
- MLC2V
Myosin light chain 2V
- ISL-1
Myocyte Enhancer Factor 2C
- VEGFA
Vascular Endothelial
- IGF-1
Insulin-lace growth factor
- SDF-1
Stromal derived factor-1
- CTGF
Connective tiddue growth factor
- END-1
Endothelin-1
- AAMP
Angio-associated migratory protein
- NRG-1
Neuregulin-1
- IDO
Indoleamine 2,3-dioxygenase
- AFP
Alpha Fetal Protein
- BMP4
Bone Morphogenic protein
- NES
Nestin
Potential Conflicts of Interests
None
Sponsors/grants
Sponsored in part by The Appleby Foundation and the Partners in Research, Georgetown University Medical Center
References
- 1.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res. 2016;118(1):95–107. doi: 10.1161/CIRCRESAHA.115.305373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi P, Tray N, Nguyen T, Nigam N, Gallicano GI. Mesenchymal stem cell therapy for treatment of cardiovascular disease: helping people sooner or later. Stem Cells Dev. 2010;19(7):1109–20. doi: 10.1089/scd.2009.0465. [DOI] [PubMed] [Google Scholar]
- 3.Kang E, Wang X, Tippner-Hedges R, Ma H, Folmes CD, Gutierrez NM, Lee Y, Van Dyken C, Ahmed R, Li Y, Koski A, Hayama T, Luo S, Harding CO, Amato P, Jensen J, Battaglia D, Lee D, Wu D, Terzic A, Wolf DP, Huang T, Mitalipov S. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell Stem Cell. 2016 May 5;18(5):625–36. doi: 10.1016/j.stem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, Platero-Luengo A, Martinez-Redondo P, Ma H, Lee Y, Hayama T, Van Dyken C, Wang X, Luo S, Ahmed R, Li Y, Ji D, Kayali R, Cinnioglu C, Olson S, Jensen J, Battaglia D, Lee D, Wu D, Huang T, Wolf DP, Temiakov D, Belmonte JC, Amato P, Mitalipov S. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016 Dec 8;540(7632):270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- 5.Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012;367(22):2150–3. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K, Landmesser U, Mummery CL, Janssens S, Willerson J, Eschenhagen T, Ferdinandy P, Sluijter JP. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J. 2016;37(23):1789–98. doi: 10.1093/eurheartj/ehw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 9.Yi BA, Mummery CL, Chien KR. Direct cardiomyocyte reprogramming: a new direction for cardiovascular regenerative medicine. Cold Spring Harb Perspect Med. 2013;3(9):a014050. doi: 10.1101/cshperspect.a014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–5. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun T, Dimmeler S. Breaking the silence: stimulating proliferation of adult cardiomyocytes. Dev Cell. 2009 Aug;17(2):151–3. doi: 10.1016/j.devcel.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115(3):572–83. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 14.Garbern JC, Mummery CL, Lee RT. Model systems for cardiovascular regenerative biology. Cold Spring Harb Perspect Med. 2013;3(4):a014019. doi: 10.1101/cshperspect.a014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102(9):1008–10. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 16.Veerman CC, Kosmidis G, Mummery CL, Casini S, Verkerk AO, Bellin M. Immaturity of human stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem? Stem Cells Dev. 2015;24(9):1035–52. doi: 10.1089/scd.2014.0533. [DOI] [PubMed] [Google Scholar]
- 17.Cooke PS, Simon L, Nanjappa MK, Medrano TI, Berry SE. Plasticity of spermatogonial stem cells. Asian J Androl. 2015;17(3):355–9. doi: 10.4103/1008-682X.148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18(8):1115–26. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27(1):138–49. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, de la Rosette JJ, Knegt AC, Belmonte JC, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25(1):158–67. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC, Jr, Kamonjoh CM, Randell SH, Schlegel R. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci U S A. 2012 Dec 4;109(49):20035–40. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Krawczyk E, Suprynowicz FA, Palechor-Ceron N, Yuan H, Dakic A, Simic V, Zheng YL, Sripadhan P, Chen C, Lu J, Hou TW, Choudhury S, Kallakury B, Tang DG, Darling T, Thangapazham R, Timofeeva O, Dritschilo A, Randell SH, Albanese C, Agarwal S, Schlegel R. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc. 2017;12(2):439–451. doi: 10.1038/nprot.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 1998;143(7):2009–22. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128(6):929–41. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 26.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203(10):2315–27. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Capco DG, Gallicano GI, McGaughey RW, Downing KH, Larabell CA. Cytoskeletal sheets of mammalian eggs and embryos: a latticelike network of intermediate filaments. Cell Motil Cytoskeleton. 1993;24(2):85–99. doi: 10.1002/cm.970240202. [DOI] [PubMed] [Google Scholar]
- 29.Gallicano GI, McGaughey RW, Capco DG. Cytoskeletal sheets appear as universal components of mammalian eggs. J Exp Zool. 1992;263(2):194–203. doi: 10.1002/jez.1402630209. [DOI] [PubMed] [Google Scholar]
- 30.Gallicano GI, McGaughey RW, Capco DG. Cytoskeleton of the mouse egg and embryo: reorganization of planar elements. Cell Motil Cytoskeleton. 1991;18(2):143–54. doi: 10.1002/cm.970180209. [DOI] [PubMed] [Google Scholar]
- 31.Conrad S, Azizi H, Hatami M, Kubista M, Bonin M, Hennenlotter J, Sievert KD, Skutella T. Expression of Genes Related to Germ Cell Lineage and Pluripotency in Single Cells and Colonies of Human Adult Germ Stem Cells. Stem Cells Int. 2016;2016:8582526. doi: 10.1155/2016/8582526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87(1):27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc. 2004;126(6):1590–1. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 34.Pan D, Roy S, Gascard P, Zhao J, Chen-Tanyolac C, Tlsty TD. SOX2, OCT3/4 and NANOG expression and cellular plasticity in rare human somatic cells requires CD73. Cell Signal. 2016;28(12):1923–1932. doi: 10.1016/j.cellsig.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Land SC, Walker DJ, Du Q, Jovanovic A. Cardioprotective SUR2A promotes stem cell properties of cardiomyocytes. Int J Cardiol. 2013;168(5):5090–2. doi: 10.1016/j.ijcard.2013.07.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Qi LJ, Guo ZK, Li H, Zuo HB, Li NN. CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol. 2013;44(4):411–22. doi: 10.1007/s10735-013-9492-9. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Pal R, Sung LY, Feng H, Miao W, Cheng SY, Tian C, Cheng T. An opposite effect of the CDK inhibitor, p18(INK4c) on embryonic stem cells compared with tumor and adult stem cells. PLoS One. 2012;7(9):e45212. doi: 10.1371/journal.pone.0045212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takashima S, Hirose M, Ogonuki N, Ebisuya M, Inoue K, Kanatsu-Shinohara M, Tanaka T, Nishida E, Ogura A, Shinohara T. Regulation of pluripotency in male germline stem cells by Dmrt1. Genes Dev. 2013;27(18):1949–58. doi: 10.1101/gad.220194.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Liu ZP, Nakagawa O, Nakagawa M, Yanagisawa H, Passier R, Richardson JA, Srivastava D, Olson EN. CHAMP, a novel cardiac-specific helicase regulated by MEF2C. Dev Biol. 2001;234(2):497–509. doi: 10.1006/dbio.2001.0277. [DOI] [PubMed] [Google Scholar]
- 41.Zacchigna S, Giacca M. Extra- and intracellular factors regulating cardiomyocyte proliferation in postnatal life. Cardiovasc Res. 2014;102(2):312–20. doi: 10.1093/cvr/cvu057. [DOI] [PubMed] [Google Scholar]
- 42.Beck LS, DeGuzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92(6):2841–9. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306(5694):247–52. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada M, De Chiara L, Seandel M. Spermatogonial Stem Cells: Implications for Genetic Disorders and Prevention. Stem Cells Dev. 2016. [DOI] [PMC free article] [PubMed]
- 45.Ko K, Reinhardt P, Tapia N, Schneider RK, Arauzo-Bravo MJ, Han DW, Greber B, Kim J, Kliesch S, Zenke M, Schöler HR. Brief report: evaluating the potential of putative pluripotent cells derived from human testis. Stem Cells. 2011;29(8):1304–9. doi: 10.1002/stem.671. [DOI] [PubMed] [Google Scholar]
- 46.Kanatsu-Shinohara M, Takashima S, Ishii K, Shinohara T. Dynamic changes in EPCAM expression during spermatogonial stem cell differentiation in the mouse testis. PLoS One. 2011;6(8):e23663. doi: 10.1371/journal.pone.0023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term Culture of Human SSEA-4 Positive Spermatogonial Stem Cells (SSCs) J Stem Cell Res Ther. 2011;2(2):pii: 2488. doi: 10.4172/2157-7633.S2-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman E, Yango P, Moustafa R, Smith JF, Klatsky PC, Tran ND. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction. 2014;148(4):417–27. doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith JF, Yango P, Altman E, Choudhry S, Poelzl A, Zamah AM, Rosen M, Klatsky PC, Tran ND. Testicular niche required for human spermatogonial stem cell expansion. Stem Cells Transl Med. 2014;3(9):1043–54. doi: 10.5966/sctm.2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong JJ, Murry CE. Cardiac regeneration using pluripotent stem cells--progression to large animal models. Stem Cell Res. 2014;13(3 Pt B):654–65. doi: 10.1016/j.scr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luan NT, Sharma N, Kim SW, Ha PT, Hong YH, Oh SJ, Jeong DK. Characterization and cardiac differentiation of chicken spermatogonial stem cells. Anim Reprod Sci. 2014 Dec 30;151(3)(4):244–55. doi: 10.1016/j.anireprosci.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Cai J, Yi FF, Yang XC, Lin GS, Jiang H, Wang T, Xia Z. Transplantation of embryonic stem cell-derived cardiomyocytes improves cardiac function in infarcted rat hearts. Cytotherapy. 2007;9(3):283–91. doi: 10.1080/14653240701247838. [DOI] [PubMed] [Google Scholar]
- 53.Caspi O, Huber, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50(6):1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 54.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stemcell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellison GM, Vicinanza C, Smith AJ, Aquila, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 56.Lundy SD, Murphy SA, Dupras SK, Dai J, Murry CE, Laflamme MA, Regnier M. Cell-based delivery of dATP via gap junctions enhances cardiac contractility. J Mol Cell Cardiol. 2014;72:350–9. doi: 10.1016/j.yjmcc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014;35(9):2798–808. doi: 10.1016/j.biomaterials.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 58.Navaroli DM, Tilly JL, Woods DC. Isolation of Mammalian Oogonial Stem Cells by Antibody-Based Fluorescence-Activated Cell Sorting. Methods Mol Biol. 2016;1457:253–68. doi: 10.1007/978-1-4939-3795-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand S, Patel H, Bhartiya D. Chemoablated mouse seminiferous tubular cells enriched for very small embryonic-like stem cells undergo spontaneous spermatogenesis in vitro. Reprod Biol Endocrinol. 2015 Apr 18;13:33. doi: 10.1186/s12958-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82(2):363–72. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Bou G, Sun R, Guo S, Xue B, Wei R, Cooney AJ, Liu Z. Sox2 is the faithful marker for pluripotency in pig: evidence from embryonic studies. Dev Dyn. 2015;244(4):619–27. doi: 10.1002/dvdy.24248. [DOI] [PubMed] [Google Scholar]
- 63.White MD, Angiolini JF, Alvarez YD, Kaur G, Zhao ZW, Mocskos E, Bruno L, Bissiere S, Levi V, Plachta N. Long-Lived Binding of Sox2 to DNA Predicts Cell Fate in the Four-Cell Mouse Embryo. Cell. 2016;165(1):75–87. doi: 10.1016/j.cell.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 64.Foshay KM, Gallicano GI. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev. 2008;17(2):269–78. doi: 10.1089/scd.2007.0098. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Chen X, Li M, Liu X, Gao Y, Kou X, Zhao Y, Zheng W, Zhang X, Huo Y, Chen C, Wu Y, Wang H, Jiang C, Gao S. Hierarchical Oct4 Binding in Concert with Primed Epigenetic Rearrangements during Somatic Cell Reprogramming. Cell Rep. 2016;14(6):1540–1554. doi: 10.1016/j.celrep.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Goolam M, Scialdone A, Graham SJL, Macaulay IC, Jedrusik A, Hupalowska A, Voet T, Marioni JC, Zernicka-Goetz M. Heterogeneity in Oct4 and Sox2 Targets Biases Cell Fate in 4-Cell Mouse Embryos. Cell. 2016;165(1):61–74. doi: 10.1016/j.cell.2016.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12(4):395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155(4):778–92. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138(4):722–37. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Em S, Kataria M, Shah F, Yadav PS. A comparative study on efficiency of adult fibroblasts and amniotic fluid-derived stem cells as donor cells for production of hand-made cloned buffalo (Bubalus bubalis) embryos. Cytotechnology. 2016;68(4):593–608. doi: 10.1007/s10616-014-9805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang L, Zhang J, Zhang H, Yang X, Jin X, Zhang L, Skalnik DG, Jin Y, Zhang Y, Huang X, Li J, Wong J. H3K4 Methyltransferase Set1a Is A Key Oct4 Coactivator Essential for Generation of Oct4 Positive Inner Cell Mass. Stem Cells. 2016;34(3):565–80. doi: 10.1002/stem.2250. [DOI] [PubMed] [Google Scholar]
- 72.Zhang P, Andrianakos R, Yang Y, Liu C, Lu W. Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem. 2010;285(12):9180–9. doi: 10.1074/jbc.M109.077958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirata H, Murakami Y, Miyamoto Y, Tosaka M, Inoue K, Nagahashi A, Jakt LM, Asahara T, Iwata H, Sawa Y, Kawamata S. ALCAM (CD166) is a surface marker for early murine cardiomyocytes. Cells Tissues Organs. 2006;184(3)(4):172–80. doi: 10.1159/000099624. [DOI] [PubMed] [Google Scholar]
- 74.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31(1):54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Temsah R, Nemer M. GATA factors and transcriptional regulation of cardiac natriuretic peptide genes. Regul Pept. 2005;128(3):177–85. doi: 10.1016/j.regpep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 76.Kodama H, Inoue T, Watanabe R, Yasuoka H, Kawakami Y, Ogawa S, Ikeda Y, Mikoshiba K, Kuwana M. Cardiomyogenic potential of mesenchymal progenitors derived from human circulating CD14+ monocytes. Stem Cells Dev. 2005;14(6):676–86. doi: 10.1089/scd.2005.14.676. [DOI] [PubMed] [Google Scholar]
- 77.Saito T, Kuang JQ, Lin CC, Chiu RC. Transcoronary implantation of bone marrow stromal cells ameliorates cardiac function after myocardial infarction. J Thorac Cardiovasc Surg. 2003;126(1):114–23. doi: 10.1016/s0022-5223(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 78.Bin Z, Sheng LG, Gang ZC, Hong J, Jun C, Bo Y, Hui S. Efficient cardiomyocyte differentiation of embryonic stem cells by bone morphogenetic protein-2 combined with visceral endoderm-like cells. Cell Biol Int. 2006;30(10):769–76. doi: 10.1016/j.cellbi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, Alpert NR, Warshaw DM, Perryman MB, Helmke SM, Robbins J. Analysis of myosin heavy chain functionality in the heart. J Biol Chem. 2003;278(19):17466–74. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 80.Mobley S, Shookhof JM, Foshay K, Park M, Gallicano GI. PKG and PKC Are Down-Regulated during Cardiomyocyte Differentiation from Embryonic Stem Cells: Manipulation of These Pathways Enhances Cardiomyocyte Production. Stem Cells Int. 2010;2010:701212. doi: 10.4061/2010/701212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79(5):528–35. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 82.Barnes RM, Harris IS, Jaehnig EJ, Sauls K, Sinha T, Rojas A, Schachterle W, McCulley DJ, Norris RA, Black BL. MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development. 2016;143(5):774–9. doi: 10.1242/dev.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palazzolo G, Quattrocelli M, Toelen J, Dominici R, Anastasia L, Tettamenti G, Barthelemy, Blot S, Gijsbers R, Cassano M, Sampaolesi M. Cardiac Niche Influences the Direct Reprogramming of Canine Fibroblasts into Cardiomyocyte-Like Cells. Stem Cells Int. 2016;2016:4969430. doi: 10.1155/2016/4969430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lui KO, Zangi L, Chien KR. Cardiovascular regenerative therapeutics via synthetic paracrine factor modified mRNA. Stem Cell Res. 2014;13(3 PtB):693–704. doi: 10.1016/j.scr.2014.06.007. [DOI] [PubMed] [Google Scholar]