Abstract
Introduction
Centella asiatica is a plant used for centuries to enhance memory. We have previously shown that a water extract of Centella asiatica (CAW) attenuates age‐related spatial memory deficits in mice and improves neuronal health. Yet the effect of CAW on other cognitive domains remains unexplored as does its mechanism of improving age‐related cognitive impairment. This study investigates the effects of CAW on a variety of cognitive tasks as well as on synaptic density and mitochondrial and antioxidant pathways.
Methods
Twenty‐month‐old CB6F1 mice were treated with CAW (2 mg/ml) in their drinking water for 2 weeks prior to behavioral testing. Learning, memory, and executive function were assessed using the novel object recognition task (NORT), object location memory task (OLM), and odor discrimination reversal learning (ODRL) test. Tissue was collected for Golgi analysis of spine density as well as assessment of mitochondrial, antioxidant, and synaptic proteins.
Results
CAW improved performance in all behavioral tests suggesting effects on hippocampal and cortical dependent memory as well as on prefrontal cortex mediated executive function. There was also an increase in synaptic density in the treated animals, which was accompanied by increased expression of the antioxidant response gene NRF2 as well as the mitochondrial marker porin.
Conclusions
These data show that CAW can increase synaptic density as well as antioxidant and mitochondrial proteins and improve multiple facets of age‐related cognitive impairment. Because mitochondrial dysfunction and oxidative stress also accompany cognitive impairment in many pathological conditions this suggests a broad therapeutic utility of CAW.
Keywords: aging, antioxidant, memory
1. INTRODUCTION
As the aging population in the United States continues to grow, so does the need for treating age‐related declines in health and cognitive function. The majority of elderly individuals experience some form of memory loss that affects their activities of daily life including episodic and source memory deficits (Cansino, 2009; Johnson et al., 2016; Park et al., 2002), decreased sensitivity to novelty (Fandakova, Lindenberger, & Shing, 2014) and impairments in executive function tasks, including attention, planning, inhibitory control, and cognitive flexibility (Buckner, 2004; Methqal et al., 2017; Park et al., 2002). Similar deficits are observed in aged rodents (Burke, Wallace, Nematollahi, Uprety, & Barnes, 2010; Dalley, Cardinal, & Robbins, 2004; Gilbert et al., 2009; McQuail & Nicolle, 2015; Shimizu, Oki, Mitani, Tsuchiya, & Nabeshima, 2016; Young, Powell, Geyer, Jeste, & Risbrough, 2010).
The healthy aging brain displays mitochondrial abnormalities, like decreased mitochondrial content and reduced electron transport chain (ETC.) activity along with increased levels of reactive oxygen species (ROS) and markers of oxidative damage (Haider et al., 2014; Liu, Atamna, Kuratsune, & Ames, 2002). Studies have also demonstrated a relationship between mitochondrial function, antioxidant capacity, and memory (Forster et al., 1996; Masiero & Sandri, 2010; Olsen, Johnson, Zuloaga, Limoli, & Raber, 2013; Perrig, Perrig, & Stähelin, 1997) sparking an interest in identifying agents that target mitochondria and antioxidant pathways for the improvement of cognitive function.
The plant Centella asiatica (L) Urban, (Apiaceae), known in the United States as Gotu Kola, is used in traditional Chinese and Ayurvedic medicine to improve cognitive function (Kapoor, 1990; Shinomol, Muralidhara, & Bharath, 2011). Extracts of the plant have widely reported neuronal antioxidant and mitoprotective effects in vitro and in vivo (Gray, Harris, Quinn, & Soumyanath, 2016; Gray, Zweig, Matthews, et al., 2017; Gray, Zweig, Murchison, et al., 2017; Gupta & Flora, 2006; Haleagrahara & Ponnusamy, 2010; Ponnusamy, Mohan, & Nagaraja, 2008; Prakash, 2013; Shinomol & Muralidhara, 2008).
The cognitive enhancing effects of the plant have been supported by a handful of small clinical trials in healthy middle aged and older adults (Dev, Hambali, & Samah, 2009; Wattanathorn et al., 2008). A number of preclinical studies have also demonstrated similar cognitive enhancing effects of Centella asiatica in multiple rodent models of pathological cognitive impairment (Gupta & Srivastava, 2003; Kumar & Gupta, 2002; Soumyanath et al., 2012; Veerendra Kumar & Gupta, 2003). Our laboratory has previously shown that a water extract of Centella asiatica (CAW) added to the drinking water can attenuate spatial memory impairments in healthy aged mice (Gray et al., 2016).
Yet the effects of CAW on cognitive domains beyond spatial memory remains relatively unexplored as does its mechanism of improving age‐related cognitive impairment. Here we explore the effects of CAW on multiple cognitive domains beyond spatial memory, including recognition memory and executive function in healthy aged mice. We also examine the effects of the extract on synaptic density as well as mitochondrial and antioxidant protein expression in the brains of treated animals.
2. MATERIALS AND METHODS
2.1. Aqueous extract of Centella asiatica
Dried leaves of Centella asiatica was purchased (Oregon's Wild Harvest, GOT‐03193c‐OHQ01) and its identity was confirmed by comparing its thin layer chromatographic profile with that reported in the literature (Günther & Wagner, 1996) and the Centella asiatica samples used in our previous studies (Gray et al., 2014; Gray et al., 2016; Soumyanath et al., 2012). CAW was prepared by refluxing Centella asiatica (160 g) with water (2,000 ml) for 2 hr, filtering the solution and freeze drying to yield a powder (~16–21 g).
2.2. Animals
Twenty‐month‐old male and female CB6F1 mice were obtained from the NIH National Institute on Aging (NIA) aged rodent colony. Mice were maintained in a climate‐controlled environment with a 12‐hr light/12‐hr dark cycle, and fed AIN‐93M Purified Rodent Diet (Dyets Inc., Bethlehem, PA). Diet and water were supplied ad libitum. Mice were exposed to CAW in their drinking water (2 g/L) for 2 weeks prior to the beginning of behavioral testing. Control animals were given normal, unsupplemented drinking water. Thirty‐six mice (18 male, 18 female) were randomly assigned to treatment groups. Water consumption was monitored throughout the experiment to ensure the addition of CAW did not affect overall water intake. Following 3 weeks of behavioral testing, animals were sacrificed and tissue harvested as outlined in the timeline below (Figure 1). All mice completed behavioral testing and thus none were excluded from analysis. Based on pilot experiments we expected to see changes of 20%–25% in the behavioral tests after CAW treatment (standard deviation ~5%). Based on these estimates, we calculated 5–8 animals per condition to obtain adequate power with the Odor Discrimination Reversal Learning (ODRL) task requiring the most animals due to more subtle changes observed in our pilot experiments. Because of somewhat inconsistent availability of the CB6F1 mice from the NIA colony animals were run in two cohorts of 4–5 animals of each gender per treatment condition in each cohort. Each cohort was tested in Odor Discrimination reversal learning and either Novel Object Recognition or Object Location Memory. All animals were evaluated for protein expression changes and a subset of animals (3 of each gender per treatment condition) was also assessed by Golgi analysis. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Portland VA Medical Center.
Figure 1.
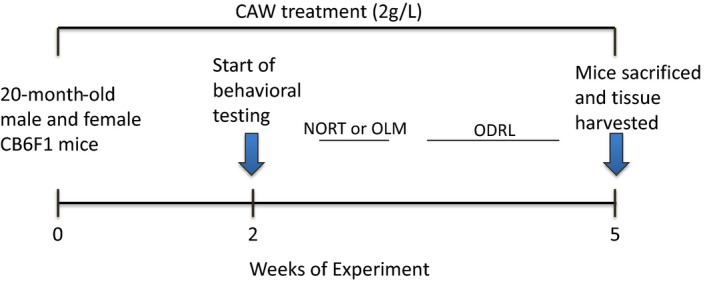
Timeline of CAW treatment and behavioral assessment. Mice were treated with CAW 2 weeks prior to the beginning of behavioral testing and treatment continued throughout the experiment. After testing, animals were sacrificed and tissue was harvested. CAW treatment lasted a total of 5 weeks
2.3. Behavioral testing
Novel Object Recognition Task (NORT): This test takes advantage of the exploratory nature of mice and evaluates recognition memory. The experimental apparatus consisted of a white rectangular open field (39 cm x 39 cm x 39 cm). Habituation took place by exposing the animal to the experimental apparatus for 5 min in the absence of objects one time, on the day before training. During the training phase, mice were placed in the experimental apparatus in the presence of two identical objects and allowed to explore for three 10 min sessions. After 2 hr and 24 hr, mice were placed again in the apparatus, where this time one of the objects was replaced by a novel one (distinct at each time point). Mice were allowed to explore for 5 min. Preference for the novel object was expressed as the percent time spent exploring the novel object relative to the total time spent exploring both objects. The objects were a glass cylindrical votive, a metal cylindrical jar, a plastic rectangular box, and a plastic trapezoidal prism, all with approximately the same height. The identity of the objects—which one was novel or familiar—was balanced between groups. No preference was observed in this study for any object over the others.
Object Location Memory task (OLM): The OLM also capitalizes on the exploratory nature of mice and evaluates location memory. The experimental apparatus, habituation, and training for this task were identical as described above for the NORT. For the testing phases after 2 hr or 24 hr, mice were placed back in the apparatus but one of the objects was displaced to a novel spatial location (a third location was used at 24 hr). Mice were again allowed to explore the environment for 5 min Time spent exploring the displaced and nondisplaced objects was measured. Exploration was analyzed during both the training and testing phases.
In both the NORT and the OLM, objects were cleaned between trials with chlorhexidine (Nolvasan) to eliminate odor cues. All testing and training sessions were videotaped and analyzed by an experimenter blind to the treatment of the animals. It was considered exploration of the objects when mice were facing and sniffing the objects within very close proximity and/or touching. Mouse behavior was recorded with a video camera positioned over the behavioral apparatus and the collected videos were analyzed with the ANY‐MAZE software (Stoelting Co., Wood Dale, IL, USA).
Odor Discrimination Reversal Learning test (ODRL): This task, also called attention set‐shifting task, evaluates executive function. The test is comprised of four phases: shaping, learning, acquisition, and shift. Mice can be readily trained to dig in small bowls to retrieve food rewards (Bissonette et al., 2008; Young, Sharkey, & Finlayson, 2009; Young et al., 2007). Plastic cups (4.5 × 3 cm) were used for digging bowls and filled with a digging material of home cage bedding, dried black beans, or alder wood chips. The digging material was scented with lavender, mint or vanilla all commercially available (Fred Myers brand). The food reward was a part of a Froot Loop for each correct trial. The test apparatus was a gray rigid PVC enclosure (12″ × 8″7″) with a removable divider in the center. The digging bowls were placed on one side of the divider and the mouse on the other. At the beginning of each trial, the divider was removed.
In the shaping phase, mice were introduced to the testing chamber and trained to dig for a food reward in lavender scented bedding material. Mice were presented with a single bowl containing the food reward that was progressively filled with bedding in five stages, 0%, 25%, 50%, 75%, and 100% filled. The mouse advanced to the subsequent training step when it had successfully retrieved the food reward 5 times in a row.
The acquisition phase began after mice had completed the shaping phase. In this phase, mice were presented with two cups one containing dried beans and the other wood chips. In every trial, one digging material had the vanilla odor and the other the mint odor and the odor and material pairings were randomly alternated between trials but balanced over the acquisition phase so that each mouse was exposed to roughly equal combinations of each odor and digging material. Whether the baited cup was presented on the right or left side of the apparatus was also balanced throughout testing. In the acquisition phase, the mint‐scented bowl was always baited regardless of digging material. Example trials are found in Table 1. Each trial was initiated by raising the divider and allowing access to both bowls. Mice were required to make eight correct digs in any bout of 10 in order to reach criteria. Trials to criteria and latency to retrieve the reward were recorded.
Table 1.
Example of test pairings for Odor Discrimination Reversal Learning (ODRL) test
| Right position | Left position | |
|---|---|---|
| Acquisition phase | D1 + O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 | |
| Shift phase | D1 + O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 |
Representative combinations of odor and digging material pairings during each phase of the ODRL.
D1: dried bean; D2: wood chips; O1: vanilla; O2 = mint.
Italicized indicates correct trial.
After a mouse reached criteria in the acquisition phase, they immediately proceeded to the shift phase. As in the previous phase in the shift phase were presented with two cups one containing dried beans and the other wood chips. In every trial, one digging material had the vanilla odor and the other the mint odor and again the odor + digging material pairings were balanced throughout the trial as was right/left location of the baited cup. In the shift phase, however, the cup with the dried beans was always baited regardless of odor. Again, criteria were defined as eight correct trials in any bout of 10 and trials to criteria as well as latency to retrieve the reward was recorded. Mice were food restricted the night before each phase of the ODRL in order to motivate the animals.
2.4. Golgi
The FD Rapid GolgiStain™ Kit (FD Neurotechnologies) was used as per the manufacturer's instructions. In brief, one hemisphere of the brain was fixed for 9 days, sectioned coronally into 200um slices on a vibratome and mounted on gel‐coated slides. After drying, slides were stained and coverslipped using Permount (Fisher Scientific). Images were acquired using an Axio Imager M2 with an Apotome™ attachment and two cameras; an Axiocam 512 color and an AxioCam 506 mono. The system is driven by Zen 2™ software. Images taken with an EC Plan‐Neofluar40×/1.3 DIC WD = 0.21 objective in 5 phases and processed with a weak‐medium filter. Large area images were acquired with the Tiles module in a 2 × 2 array and stitched in the software. This system is part of the Advanced Light Microscopy Core at the Jungers Center, OHSU Portland, Oregon. Dendritic spines on pyramidal neurons from the CA1 region of the hippocampus were counted using Image J software (http://rsbweb.nih.gov/ij). Between three and six neurons were analyzed per animal and at least 50uM of clearly visible dendritic length was quantified per cell as previously described (Del Valle et al., 2012). To avoid bias, images were acquired and analyzed by two separate individuals both of whom were blinded to treatment conditions of the samples were analyzed per animal and at least 50uM of clearly visible dendritic length was quantified per image.
2.5. Western blot
Tissue (whole hippocampus) was homogenized and boiled in Laemmli buffer. Samples were separated electrophoretically on an SDS gel, transferred onto nitrocellulose membranes and immunoblotted using antibodies for NRF2 (Nuclear factor (erythroid‐derived 2)‐like 2, also called NFE2L2, Abcam Cat# ab62352, http://scicrunch.org/resolver/AB_944418), phosphorylated NRF2 (S40) (Abcam Cat# ab76026, http://scicrunch.org/resolver/AB_1524049), synaptophysin (Abcam Cat# ab14692, http://scicrunch.org/resolver/AB_301417), porin (also known as VDAC1, Abcam Cat# ab15895, http://scicrunch.org/resolver/AB_2214787), and GAPDH (Thermo Fisher Scientific Cat# MA5‐15738, http://scicrunch.org/resolver/AB_10977387). The optical density of the bands was quantified using Image J software and normalized to GAPDH.
2.6. Graphs and statistics
All bar graphs have error bars reflecting standard error of the mean. Statistical significance was determined using one‐ or two‐way analysis of variance or with appropriate t tests. Bonferroni post hoc tests were also conducted. Significance was defined as p ≤ 0.05. Analyses were performed using Excel or GraphPad Prism 6.
3. RESULTS
3.1. CAW improves location memory in aged mice
We previously demonstrated the CAW improves spatial memory in aged C57BL6 mice (Gray et al., 2016). To validate this finding in the CB6F1 mouse line we used the OLM test. Aged CB6F1 mice (20 months) were treated with CAW in their drinking water (2 g/L) for 2 weeks prior to behavioral testing, and exposure to CAW continued throughout testing (Figure 1). CAW treatment improved performance in the OLM in both genders (Figure 2a). At 2 hr post‐training, a slight preference for the novel location was evident in the control mice but this preference was dramatically increased in the CAW‐treated male and female animals to males spending 68% and females 78% of their 5‐min test time with the new location (Figure 2b). Similar results were seen 24 hr post‐training. At this time point while control animals spent equal time exploring both locations, CAW‐treated mice spent far more time exploring the object in the new location, 68% of the time for both genders (Figure 2c).
Figure 2.
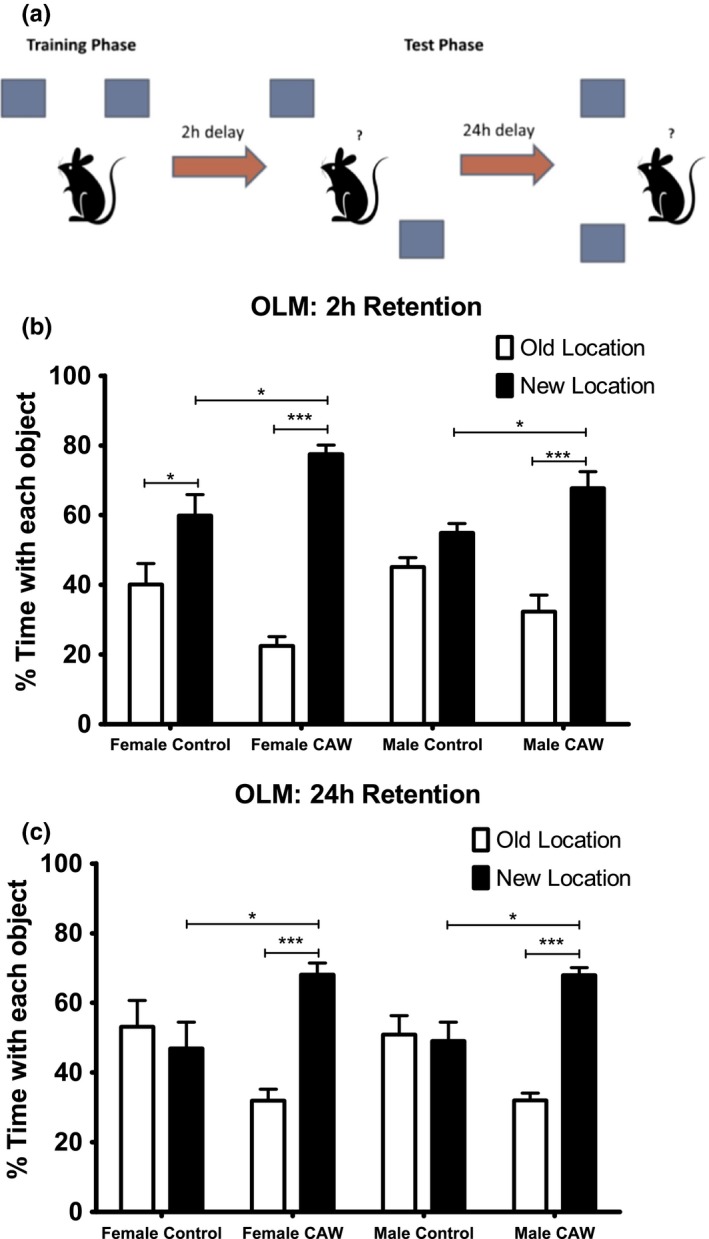
CAW treatment improves object location memory retention in aged mice. (a) Schematic of the Object Location Memory task (OLM) set up. (b) At the 2 hr retention time point CAW‐treated male and female mice had an enhanced preference for the novel location (F = 14.59 and 26.82 respectively). (c) A similar enhancement was also apparent in both genders at the 24 hr time point (F = 12.86 males, F = 5.801 females). n = 5 in each group, *p < 0.05, ***p < 0.001
3.2. CAW improves recognition memory in aged mice
CAW treatment also improved performance in the NORT in both male and female mice (Figure 3a). While control animals did not display a preference for the novel object at either 2 hr or 24 hr post‐training, CAW‐treated animals spent significantly more time exploring the novel object than the familiar object at both time points, with males and females spending 63% and 58% of the 5 min test time, respectively, at 2 hr and 65% and 59% of their time, respectively, after 24 hr (Figure 3b,c).
Figure 3.
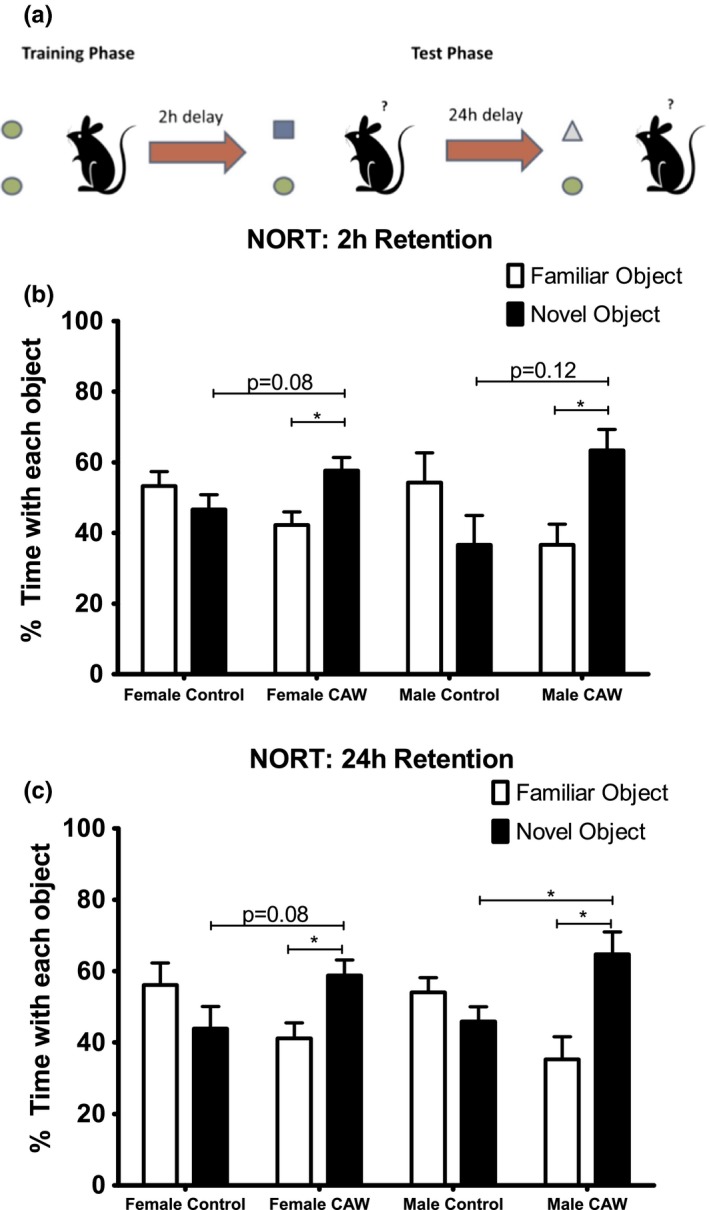
CAW treatment improves recognition memory retention in aged mice. (a) Schematic of the Novel Object Recognition Task (NORT) set up. (b) Control treated animals had no preference for the novel object at the 2 hr time point while there was a significant preference for the novel object in CAW‐treated male and female mice (F = 2.487 and 3.098, respectively). (c) The novel object preference was also observable after 24 hr in the CAW‐treated mice (F = 5.497 males, F = 2.703 females). n = 5 in each group, *p < 0.05
3.3. CAW improves learning and executive function in aged mice
The ODRL, also called attention set‐shifting task, evaluates executive function. Executive function includes behaviors like attentional selection, behavioral inhibition, cognitive flexibility, task switching, planning, and decision‐making (Buckner, 2004). The two test components of the OD RL are the acquisition and shift phases. While the acquisition phase assesses learning, the shift phase probes executive function, specifically cognitive flexibility. In the acquisition phase of the ODRL male CAW‐treated mice took significantly fewer trials than controls to reach criteria (Figure 4a). Female CAW‐treated mice showed a similar trend toward improved performance in the acquisition phase but it did not achieve significance. In contrast, in the shift phase, CAW‐treated female mice needed significantly fewer trials to reach criteria than their control counterparts. In the male mice, CAW treatment reduced the number of trials necessary as well but not significantly (Figure 4a). Interestingly in male mice, CAW treatment also significantly increased the latency retrieve the reward in both the acquisition and shift phases. Female CAW‐treated mice showed a similar trend toward increased latency but it was not significant in either test phase (Figure 4b).
Figure 4.
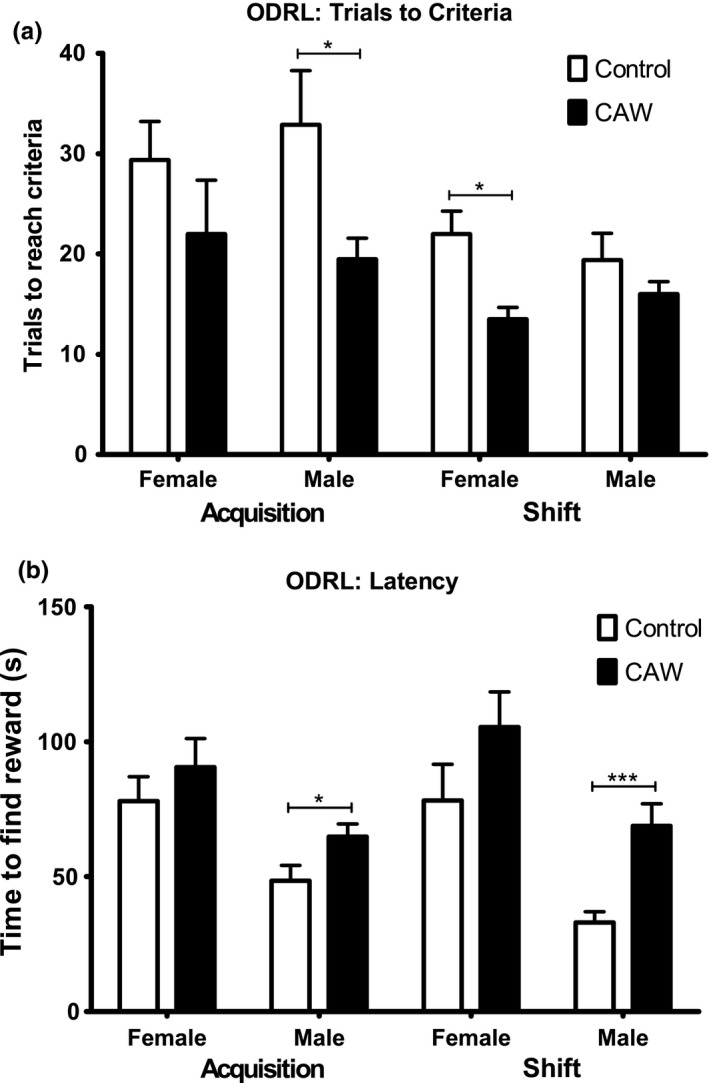
CAW treatment improves executive function in aged mice. (a) In the acquisition phase of the ODRL CAW reduced the number of trials to reach criteria in male aged mice. In the shift phase of the test, there was also a reduction in the number of trials to reach criteria in the CAW‐treated animals although this only reached significance in the female mice (F = 4.618 males, F = 3.369 females). (b) The time to find the reward was significantly increased by CAW treatment in the male animals in both the acquisition and shift phases (F = 3.750). n = 8 in each group, *p < 0.05, ***p < 0.001
3.4. CAW increases synaptic density in the hippocampus of aged mice
CAW treatment resulted in a significant increase in dendritic spine density in the CA1 region of hippocampus in male mice. There was a comparable increase in hippocampal spine density in CAW‐treated female mice (Figure 5a,b). CAW also increased the hippocampal expression of the presynaptic protein synaptophysin in female mice when normalized to GAPDH. A similar trend was observed in CAW‐treated male mice although it did not reach significance (Figure 6a–c).
Figure 5.
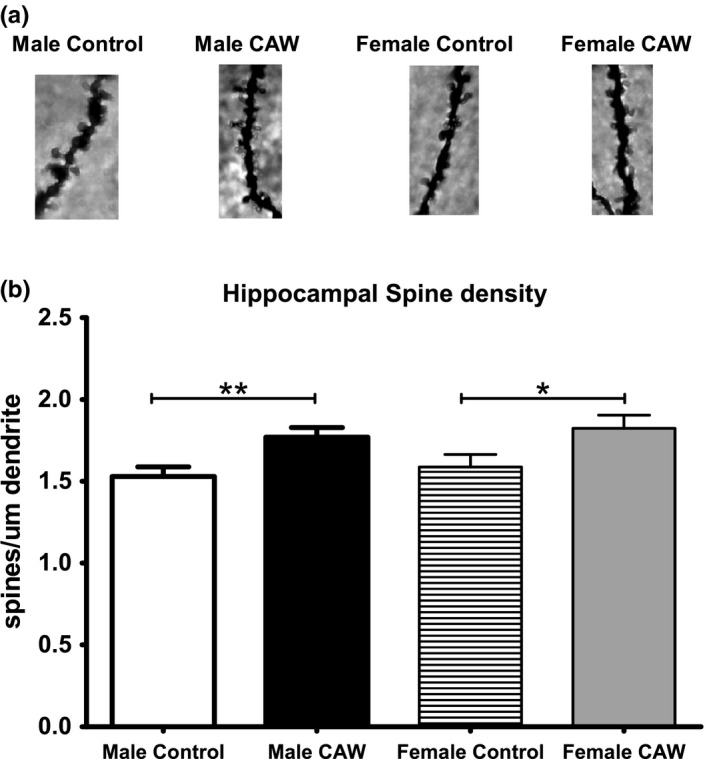
CAW increases spine density in the hippocampus of aged mice. (a) Example images of dendritic spines on hippocampal neurons from aged animals. (b) Quantification of spine density. Three animals were evaluated per treatment condition with 3–6 images quantified per animal. *p < 0.05, **p < 0.01
Figure 6.
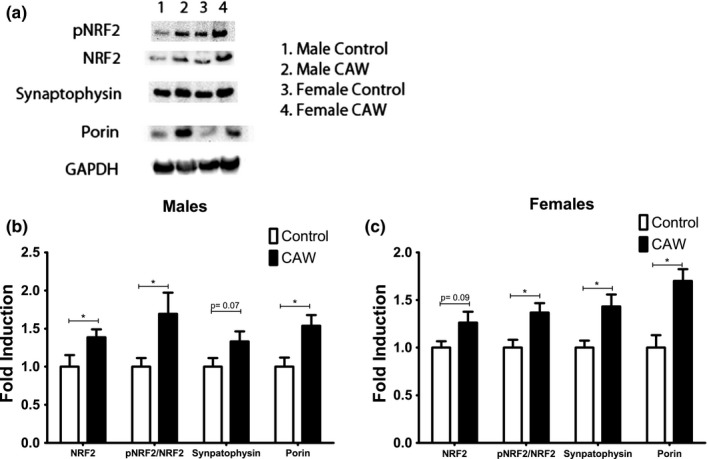
CAW increases antioxidant, mitochondrial, and synaptic proteins in the hippocampus of aged mice. (a) Representative western blot from aged animals. (b) Quantification of multiple blots. CAW increased the expression of NRF2 protein as well as the ratio of phosphorylated NRF2 to total NRF2 in the hippocampus of aged male mice. CAW treatment also significantly increased expression of the mitochondrial protein porin (F = 41.28). (c) Quantification of multiple blots. CAW increased the ratio of phosphorylated NRF2 to total NRF2 in the brains of aged female mice. The expression of porin and synaptophysin were similarly increased in these animals (F = 31.03). n = 8–9 in each group, *p < 0.05
3.5. CAW increases hippocampal expression of antioxidant and mitochondrial proteins in aged mice
CAW increased the expression of the antioxidant regulatory protein NRF2 in the hippocampus of male and female mice (Figure 6a–c). The ratio of phosphorylated NRF2 to total NRF2 was also increased both genders. The mitochondrial protein porin (also called VDAC1) was robustly increased in the brains of the CAW‐treated animals.
4. DISCUSSION
The beneficial effects of CAW on neuronal health and cognitive function have been well‐documented both in vitro and in vitro (Gupta & Flora, 2006; Gupta & Srivastava, 2003; Kumar & Gupta, 2002; Shinomol & Muralidhara, 2008; Soumyanath et al., 2005; Veerendra Kumar & Gupta, 2003). Our laboratory has previously reported that CAW improves performance in the Morris Water Maze (MWM) in mice exposed to Aβ as well as healthy older mice (Gray et al., 2016; Soumyanath et al., 2012). In this study, we further explore the effects of CAW on age‐related cognitive impairment using a battery of behavioral tests assessing learning, memory, and executive function.
We found that treatment with CAW for 2 weeks prior to the beginning of, and continuing throughout behavioral testing (Figure 1), improved the performance of both male and female 20‐month‐old CB6F1 mice in the OLM test. The OLM is a hippocampal‐dependent test of location memory (Assini, Duzzioni, & Takahashi, 2009; Cipolotti, 2006) which is known to be impaired with aging in both humans and rodents (Arias‐Cavieres, Adasme, Sánchez, Muñoz, & Hidalgo, 2017; Sapkota, van der Linde, Lamichhane, Upadhyaya, & Pardhan, 2017; Wimmer, Hernandez, Blackwell, & Abel, 2012). The improvement in the OLM seen in this study is consistent with previous studies showing that asiatic acid, a major triterpene component of Centella asiatica (Siddiqui, Aslam, Ali, Khan, & Begum, 2007), improves performance in the same task in healthy as well as cognitively impaired rodents (Chaisawang et al., 2017; Sirichoat et al., 2015; Umka Welbat et al., 2016). It is also in line with reports that OLM performance by aged mice is improved by treatment with polyphenols (Carey, Gomes, & Shukitt‐Hale, 2014; Matsui et al., 2009), a class of compounds which both Centella asiatica in general (Siddiqui et al., 2007; Subban, Veerakumar, Manimaran, Hashim, & Balachandran, 2007) and CAW specifically are rich in (Gray, Zweig, Matthews, et al., 2017; Gray, Zweig, Murchison, et al., 2017; Soumyanath et al., 2012). It is likewise consistent with our laboratory's previous finding that CAW improves MWM performance, another hippocampal‐dependent task, in healthy aged mice (Gray et al., 2016). Interestingly in that task, there was a more pronounced effect of CAW in the male animals while in the OLM no gender differences were observed.
We also observed improvements in the NORT performance following CAW treatment in aged CB6F1 mice. This suggests that the beneficial effects of CAW may not be restricted to the hippocampus as both the hippocampus and cortex are known to play an important role in object recognition memory (Aggleton, Albasser, Aggleton, Poirier, & Pearce, 2010; Buckmaster, Eichenbaum, Amaral, Suzuki, & Rapp, 2004; Clark, Zola, & Squire, 2000; Hammond, Tull, & Stackman, 2004). Age‐related impairments in object recognition are seen in both rodents and humans (Diaz et al., 2017; Kaviani et al., 2017; Li et al., 2015; Merriman, Ondřej, Roudaia, O'Sullivan, & Newell, 2016; Singh & Thakur, 2014). While to our knowledge this is the first report of Centella asiatica affecting recognition memory in aged rodents, it has been demonstrated that performance in the NORT is improved following administration of other polyphenol‐containing plant extracts (Carey et al., 2014; Matias et al., 2017; Nam et al., 2013; Yu et al., 2013).
This study is also the first report, to our knowledge, of effects of Centella asiatica on executive function. Executive function includes elements like impulse control, attention, planning, cognitive flexibility, and problem solving. It is mediated by the prefrontal cortex and is very sensitive to age‐related decline (Buckner, 2004; Raz & Rodrigue, 2006). The Wisconsin Card Sorting Test (WCST) is one of a number of widely used tests to assess executive function in humans. In this task subjects are required to adapt behavioral responses to choose the “correct” stimulus array based on sudden rule changes across multiple modalities (Eling, Derckx, & Maes, 2008). Performance in this task declines with age (Ashendorf & McCaffrey, 2008). The ODRL, also called attention set‐shifting task, is a parallel test that has been developed for rats and, more recently, mice (Birrell & Brown, 2000; Garner, Thogerson, Würbel, Murray, & Mench, 2006). Like the WCST the ODRL requires paying attention to relevant stimuli while ignoring irrelevant stimuli and subsequently shifting the attention, either within dimensions or between dimensions of the test stimuli (Birrell & Brown, 2000). Also like the WCST performance in the ODRL declines with age (Barense, Fox, & Baxter, 2002; Beas, Setlow, & Bizon, 2013; Young et al., 2010).
We found that CAW treatment improved performance in both the acquisition and shift phases of this test. The acquisition phase of the ODRL assesses classical learning and the improvement observed following CAW treatment is in line with our previous report of enhanced performance of CAW‐treated aged mice in the hidden platform phase of the MWM. The shift phase of ODRL is the metric of cognitive flexibility. Here we also observed an improvement following CAW treatment.
Interestingly while the number of trials to reach criteria in both ODRL phases decreased with CAW treatment, the latency to find the reward appeared to increase in treated animals, especially the treated males. This combination of effects could suggest decreased impulsivity in the CAW‐treated animals or an improved accuracy trade‐off strategy with a shift toward more goal‐directed action instead of habitual action. The selection of goal‐directed actions is governed by associations between the value of the consequences and is sensitive to changes in the causal relationship between the action and those consequences whereas habituation actions are controlled through stimulus‐response associations without the association with the value of the outcome (Griffiths, Morris, & Balleine, 2014). Imbalances between goal‐directed and habitual action are observed in many neurological disorders including Parkinson's disease, Tourette Syndrome and obsessive‐compulsive disorder (Gillan & Robbins, 2014; Pappas, Leventhal, Albin, & Dauer, 2014; Redgrave et al., 2010) so these results may indicate a broad therapeutic benefit of CAW beyond age‐related cognitive impairment. It would be interesting in future studies to see if similar effects are seen in young animals.
In this study, we also observed increased synaptic density in the CAW‐treated animals. We have previously demonstrated that CAW can increase spine density in primary hippocampal neurons in culture (Gray, Zweig, Matthews, et al., 2017; Gray, Zweig, Murchison, et al., 2017) but here we show oral administration of the extract exerts the same effects in vivo. In addition, the CAW‐induced an increase in the expression of synaptophysin is consistent with our previous report of increased gene expression of synaptophysin and postsynaptic density protein 95 the brains of aged CAW‐treated mice (Gray et al., 2016). As increased synaptic density is known to correlate with improved cognitive function (Terry et al., 1991) this is likely the physiological underpinning of the improvement in hippocampal‐dependent tests seen in this study. The fact that we also saw improvements in executive function suggests that these structural changes are likely occurring in other brain regions as well, specifically the prefrontal cortex. In fact, the changes in synaptic gene expression that we reported previously (Gray et al., 2016) were found to occur in multiple brain regions further supporting this idea.
The observed changes in NRF2 and porin are in accordance with our previous gene expression data as well (Gray et al., 2016). These increases suggest an activation of the antioxidant pathway and an increase in mitochondrial content respectively. It remains to be seen what contribution each of these play in the memory enhancing properties of CAW, but in mice cognitive decline is associated with dysfunctional mitochondria (Masiero & Sandri, 2010) and increased oxidative damage (Forster et al., 1996). Moreover, both over‐expressing antioxidant enzymes and increasing mitochondrial content has been shown to improve memory in rodents (Cao et al., 2010; Chen, Na, & Ran, 2014; Olsen et al., 2013). Experiments are underway in the laboratory to evaluate the effects of the extract on NRF2KO mice to determine if activation of the NRF2 pathway is required for the cognitive enhancing effects of CAW.
Our findings here further demonstrate the cognitive enhancing effects of CAW. Relatively short treatment with extract improved several different domains of cognitive performance in aged animals and enhanced synaptic density as well as mitochondrial and antioxidant response pathways in vivo. While the exact relationship between these effects remains to be elucidated, the fact that synaptic dysfunction and cognitive impairment accompany oxidative stress and mitochondrial dysfunction in many pathological conditions (Emerit & Bricaire, 2004; Lin & Beal, 2006) suggests the potential utility of CAW is far broader than for aging alone.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This work was funded by NIH‐NCCIH grant R00AT008831 (Gray), NIH‐NCCIH grant R01AT008099 (Soumyanath), and a Department of Veterans Affairs Merit Review grant awarded to J. Quinn. The authors acknowledge Dr. Matthew Lattal and Dr. Gregory Peters for their assistance with the behavioral assays.
Gray NE, Zweig JA, Caruso M, et al. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav. 2018;8:e01024 10.1002/brb3.1024
REFERENCES
- Aggleton, J. , Albasser, M. M. , Aggleton, D. J. , Poirier, G. L. , & Pearce, J. M. (2010). Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behavioral Neuroscience, 124, 55–68. 10.1037/a0018320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias‐Cavieres, A. , Adasme, T. , Sánchez, G. , Muñoz, P. , & Hidalgo, C. (2017). Aging Impairs hippocampal‐ dependent recognition memory and LTP and prevents the associated RyR up‐regulation. Frontiers in Aging Neuroscience, 9, 111 10.3389/fnagi.2017.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf, L. , & McCaffrey, R. J. (2008). Exploring age‐related decline on the Wisconsin Card Sorting Test. The Clinical Neuropsychologist, 22(2), 262–272. 10.1080/13854040701218436 [DOI] [PubMed] [Google Scholar]
- Assini, F. , Duzzioni, M. , & Takahashi, R. N. (2009). Object location memory in mice: Pharmacological validation and further evidence of hippocampal CA1 participation. Behavioral Brain Research, 204(1), 206–211. 10.1016/j.bbr.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Barense, M. , Fox, M. T. , & Baxter, M. G. (2002). Aged rats are impaired on an attentional set‐shifting task sensitive to medial frontal cortex damage in young rats. Learning & Memory, 9(4), 191–201. 10.1101/lm.48602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas, B. , Setlow, B. , & Bizon, J. L. (2013). Distinct manifestations of executive dysfunction in aged rats. Neurobiology of Aging, 34(9), 2164–2174. 10.1016/j.neurobiolaging.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell, J. , & Brown, V. J. (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience, 20(11), 4320–4324. 10.1523/JNEUROSCI.20-11-04320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette, G. , Martins, G. J. , Franz, T. M. , Harper, E. S. , Schoenbaum, G. , & Powell, E. M. (2008). Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. Journal of Neuroscience, 28(44), 11124–11130. 10.1523/JNEUROSCI.2820-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster, C. , Eichenbaum, H. , Amaral, D. G. , Suzuki, W. A. , & Rapp, P. R. (2004). Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. Journal of Neuroscience, 24, 9811–9825. 10.1523/JNEUROSCI.1532-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208. 10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Burke, S. , Wallace, J. L. , Nematollahi, S. , Uprety, A. R. , & Barnes, C. A. (2010). Pattern separation deficits may contribute to age‐associated recognition impairments. Behavioral Neuroscience, 124(5), 559–573. 10.1037/a0020893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino, S. (2009). Episodic memory decay along the adult lifespan: A review of behavioral and neurophysiological evidence. International Journal of Psychophysiology, 71(1), 64–69. 10.1016/j.ijpsycho.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Cao, X. , Xiao, H. , Zhang, Y. , Zou, L. , Chu, Y. , & Chu, X. (2010). 1, 5‐Dicaffeoylquinic acid‐mediated glutathione synthesis through activation of Nrf2protects against OGD/reperfusion‐induced oxidative stress in astrocytes. Brain Research, 1347, 142–148. 10.1016/j.brainres.2010.05.072 [DOI] [PubMed] [Google Scholar]
- Carey, A. , Gomes, S. M. , & Shukitt‐Hale, B. (2014). Blueberry supplementation improves memory in middle‐aged mice fed a high‐fat diet. Journal of Agriculture and Food Chemistry, 62(18), 3972–3978. 10.1021/jf404565s [DOI] [PubMed] [Google Scholar]
- Chaisawang, P. , Sirichoat, A. , Chaijaroonkhanarak, W. , Pannangrong, W. , Sripanidkulchai, B. , Wigmore, P. , & Welbat, J. U. (2017). Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5‐fluorouracil chemotherapy. PLoS One, 12(7), e0180650 10.1371/journal.pone.0180650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Na, R. , & Ran, Q. (2014). Enhanced defense against mitochondrial hydrogen peroxide attenuates age‐associated cognition decline. Neurobiology of Aging, 35(11), 2552–2561. 10.1016/j.neurobiolaging.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Cipolotti, L. B. C. (2006). Amnesia and the hippocampus. Current Opinion in Neurology, 19(6), 593–598. 10.1097/01.wco.0000247608.42320.f9 [DOI] [PubMed] [Google Scholar]
- Clark, R. , Zola, S. M. , & Squire, L. R. (2000). Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience, 20, 8853–8860. 10.1523/JNEUROSCI.20-23-08853.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J. , Cardinal, R. N. , & Robbins, T. W. (2004). Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews, 28(7), 771–784. 10.1016/j.neubiorev.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Del Valle, J. B. S. , Camins, A. , Beas‐Zárate, C. , Velázquez‐Zamora, D. A. , González‐Burgos, I. , & Pallàs, M. (2012). Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons underlying memory deficits in the SAMP8 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 32(1), 233–240. 10.3233/JAD-2012-120718 [DOI] [PubMed] [Google Scholar]
- Dev, R. D. O. M. S. , Hambali, Z. , & Samah, B. A. (2009). Comparison on cognitive effects of Centella asiatica in healthy middle aged female and male volunteers. European Journal of Scientific Research, 31, 553–565. [Google Scholar]
- Diaz, A. , Treviño, S. , Vázquez‐Roque, R. , Venegas, B. , Espinosa, B. , Flores, G. , … Guevara, J. (2017). The aminoestrogen prolame increases recognition memory and hippocampal neuronal spine density in aged mice. Synapse (New York, NY), 71, e21987. [DOI] [PubMed] [Google Scholar]
- Eling, P. , Derckx, K. , & Maes, R. (2008). On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain and Cognition, 67(3), 247–253. 10.1016/j.bandc.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Emerit, J. E. M. , & Bricaire, F. (2004). Neurodegenerative diseases and oxidative stress. Biomedicine & Pharmacotherapy, 58(1), 39–46. 10.1016/j.biopha.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Fandakova, Y. , Lindenberger, U. , & Shing, Y. L. (2014). Deficits in process‐specific prefrontal and hippocampal activations contribute to adult age differences in episodic memory interference. Cerebral Cortex, 24(7), 1832–1844. 10.1093/cercor/bht034 [DOI] [PubMed] [Google Scholar]
- Forster, M. , Dubey, A. , Dawson, K. M. , Stutts, W. A. , Lal, H. , & Sohal, R. S. (1996). Age‐related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. PNAS, 93(10), 4765–4769. 10.1073/pnas.93.10.4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner, J. , Thogerson, C. M. , Würbel, H. , Murray, J. D. , & Mench, J. A. (2006). Animal neuropsychology: Validation of the Intra‐Dimensional Extra‐Dimensional set shifting task for mice. Behavioral Brain Research, 173(1), 53–61. 10.1016/j.bbr.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Gilbert, P. , Pirogovsky, E. , Brushfield, A. M. , Luu, T. T. , Tolentino, J. C. , & Renteria, A. F. (2009). Age‐related changes in associative learning for olfactory and visual stimuli in rodents. Annals of the New York Academy of Sciences, 1170, 718–724. 10.1111/j.1749-6632.2009.03929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan, C. , & Robbins, T. W. (2014). Goal‐directed learning and obsessive–compulsive disorder. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369(1655), 20130475 10.1098/rstb.2013.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, N. , Harris, C. J. , Quinn, J. F. , & Soumyanath, A. (2016). Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. Journal of Ethnopharmacology, 180, 78–86. 10.1016/j.jep.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, N. E. M. J. , Kelley, J. , Maier, C. S. , Stevens, J. F. , Quinn, J. F. , & Soumyanath, A. (2014). Caffeoylquinic acids in centella asiatica protect against amyloid‐β toxicity. Journal of Alzheimer's Disease, 40, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, N. , Zweig, J. A. , Matthews, D. G. , Caruso, M. , Quinn, J. F. , & Soumyanath, A. (2017). Centella asiatica attenuates mitochondrial dysfunction and oxidative stress in Aβ‐exposed hippocampal neurons. Oxidative Medicine and Cellular Longevity, 2017, 7023091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, N. , Zweig, J. A. , Murchison, C. , Caruso, M. , Matthews, D. G. , Kawamoto, C. , … Soumyanath, A. (2017). Centella asiatica attenuates Aβ‐induced neurodegenerative spine loss and dendritic simplification. Neuroscience Letters, 646, 24–29. 10.1016/j.neulet.2017.02.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, K. , Morris, R. W. , & Balleine, B. W. (2014). Translational studies of goal‐directed action as a framework for classifying deficits across psychiatric disorders. Frontiers in Systems Neuroscience, 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther, B. , & Wagner, H. (1996). Quantitative determination of triterpenes in extracts and phytopreparations of Centella asiatica (L.) urban. Phytomedicine, 3(1), 59–65. 10.1016/S0944-7113(96)80011-0 [DOI] [PubMed] [Google Scholar]
- Gupta, R. , & Flora, S. J. (2006). Effect of Centella asiatica on arsenic induced oxidative stress and mental distribution in rats. Journal of Applied Toxicology, 26, 21322. [DOI] [PubMed] [Google Scholar]
- Gupta, Y. K. V. K. M. , & Srivastava, A. K. (2003). Effect of Centella asiatica on pentylenetetrazole‐induced kindling, cognition and oxidative stress in rats. Pharmacology Biochemistry and Behavior, 74(3), 579–585. 10.1016/S0091-3057(02)01044-4 [DOI] [PubMed] [Google Scholar]
- Haider, S. , Saleem, S. , Perveen, T. , Tabassum, S. , Batool, Z. , Sadir, S. , … Madiha, S. (2014). Age‐related learning and memory deficits in rats: Role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age, 36(3), 9653 10.1007/s11357-014-9653-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleagrahara, N. , & Ponnusamy, K. (2010). Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague Dawley rats. Journal of Toxicological Sciences, 35(1), 41–47. 10.2131/jts.35.41 [DOI] [PubMed] [Google Scholar]
- Hammond, R. , Tull, L. E. , & Stackman, R. W. (2004). On the delay dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory, 82, 26–34. 10.1016/j.nlm.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Johnson, S. , Sacks, P. K. , Turner, S. M. , Gaynor, L. S. , Ormerod, B. K. , Maurer, A. P. , … Burke, S. N. (2016). Discrimination performance in aging is vulnerable to interference and dissociable from spatial memory. Learning & Memory, 23(7), 339–348. 10.1101/lm.042069.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, L. (1990). Handbook of Ayurvedic medicinal plants. Boca Raton: CRC Press. [Google Scholar]
- Kaviani, E. , Rahmani, M. , Kaeidi, A. , Shamsizadeh, A. , Allahtavakoli, M. , Mozafari, N. , & Fatemi, I. (2017). Protective effect of atorvastatin on d‐galactose‐induced aging model in mice. Behavioral Brain Research, 335, 55–60. 10.1016/j.bbr.2017.07.029 [DOI] [PubMed] [Google Scholar]
- Kumar, M. , & Gupta, Y. K. (2002). Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. Journal of Ethnopharmacology, 79(2), 253–260. 10.1016/S0378-8741(01)00394-4 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Abdourahman, A. , Tamm, J. A. , Pehrson, A. L. , Sánchez, C. , & Gulinello, M. (2015). Reversal of age‐associated cognitive deficits is accompanied by increased plasticity‐related gene expression after chronic antidepressant administration in middle‐aged mice. Pharmacology, Biochemistry and Behavior, 135, 70–82. 10.1016/j.pbb.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Lin, M. , & Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443(7113), 787–795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Atamna, H. , Kuratsune, H. , & Ames, B. N. (2002). Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Annals of the New York Academy of Sciences, 959, 133–166. 10.1111/j.1749-6632.2002.tb02090.x [DOI] [PubMed] [Google Scholar]
- Masiero, E. , & Sandri, M. (2010). Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy, 6(2), 307–309. 10.4161/auto.6.2.11137 [DOI] [PubMed] [Google Scholar]
- Matias, I. , Diniz, L. P. , Buosi, A. , Neves, G. , Stipursky, J. , & Gomes, F. C. A. (2017). Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF‐β1. Frontiers in Aging Neuroscience, 9, 184 10.3389/fnagi.2017.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, N. , Takahashi, K. , Takeichi, M. , Kuroshita, T. , Noguchi, K. , Yamazaki, K. , … Akagi, M. (2009). Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Research, 1305, 108–117. 10.1016/j.brainres.2009.09.107 [DOI] [PubMed] [Google Scholar]
- McQuail, J. , & Nicolle, M. M. (2015). Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiology of Aging, 36(1), 323–333. 10.1016/j.neurobiolaging.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman, N. , Ondřej, J. , Roudaia, E. , O'Sullivan, C. , & Newell, F. N. (2016). Familiar environments enhance object and spatial memory in both younger and older adults. Experimental Brain Research, 234(6), 1555–1574. 10.1007/s00221-016-4557-0 [DOI] [PubMed] [Google Scholar]
- Methqal, I. , Provost, J. S. , Wilson, M. A. , Monchi, O. , Amiri, M. , Pinsard, B. , … Joanette, Y. (2017). Age‐related shift in neuro‐activation during a word‐matching task. Frontiers in Aging Neuroscience, 9, 265 10.3389/fnagi.2017.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, S. , Choi, J. H. , Yoo, D. Y. , Kim, W. , Jung, H. Y. , Kim, J. W. , … Hwang, I. K. (2013). Valeriana officinalis extract and its main component, valerenic acid, ameliorate D‐galactose‐induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Experimental Gerontology, 48(11), 1369–1377. 10.1016/j.exger.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Olsen, R. , Johnson, L. A. , Zuloaga, D. G. , Limoli, C. L. , & Raber, J. (2013). Enhanced hippocampus‐dependent memory and reduced anxiety in mice over‐expressing human catalase in mitochondria. Journal of Neurochemistry, 25(2), 303–313. 10.1111/jnc.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, S. , Leventhal, D. K. , Albin, R. L. , & Dauer, W. T. (2014). Mouse models of neurodevelopmental disease of the basal ganglia and associated circuits. Current Topics in Developmental Biology, 109, 97–169. 10.1016/B978-0-12-397920-9.00001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. , Lautenschlager, G. , Hedden, T. , Davidson, N. S. , Smith, A. D. , & Smith, P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. 10.1037/0882-7974.17.2.299 [DOI] [PubMed] [Google Scholar]
- Perrig, W. , Perrig, P. , & Stähelin, H. B. (1997). The relation between antioxidants and memory performance in the old and very old. Journal of the American Geriatrics Society, 1997(45), 6. [DOI] [PubMed] [Google Scholar]
- Ponnusamy, K. , Mohan, M. , & Nagaraja, H. S. (2008). Protective antioxidant effect of Centella asiatica bioflavonoids on lead acetate induced neurotoxicity. Medical Journal of Malaysia, 63(Suppl A), 102. [PubMed] [Google Scholar]
- Prakash, A. K. A. (2013). Mitoprotective effect of Centella asiatica against aluminum‐induced neurotoxicity in rats: Possible relevance to its anti‐oxidant and anti‐apoptosis mechanism. Neurological Sciences, 34(8), 1403–1409. 10.1007/s10072-012-1252-1 [DOI] [PubMed] [Google Scholar]
- Raz, N. , & Rodrigue, K. M. (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews, 30(6), 730–748. 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave, P. , Rodriguez, M. , Smith, Y. , Rodriguez‐Oroz, M. C. , Lehericy, S. , Bergman, H. , … Obeso, J. A. (2010). Goal‐directed and habitual control in the basal ganglia: Implications for Parkinson's disease. Nature Reviews Neuroscience, 11(11), 760–772. 10.1038/nrn2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota, R. , van der Linde, I. , Lamichhane, N. , Upadhyaya, T. , & Pardhan, S. (2017). Patients with mild cognitive impairment show lower visual short‐term memory performance in feature binding tasks. Dementia and Geriatric Cognitive Disorders Extra, 7(1), 74–86. 10.1159/000455831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, C. , Oki, Y. , Mitani, Y. , Tsuchiya, Y. , & Nabeshima, T. (2016). Moderate‐dose regular lifelong alcohol intake changes the intestinal flora, protects against aging, and keeps spatial memory in the senescence‐accelerated mouse prone 8 (SAMP8) model. Journal of Pharmacy and Pharmaceutical Sciences, 19(4), 430–447. 10.18433/J3990V [DOI] [PubMed] [Google Scholar]
- Shinomol, G. K. , & Muralidhara. (2008). Prophylactic neuroprotective property of Centella asiatica against 3‐nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology, 29(6), 948–957. 10.1016/j.neuro.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Shinomol, G. , Muralidhara, & Bharath, M. M. (2011). Exploring the role of “Brahmi” (Bocopa monnieri and Centella asiatica) in brain function and therapy. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery, 5(1), 33–49. [DOI] [PubMed] [Google Scholar]
- Siddiqui, B. S. , Aslam, H. , Ali, S. T. , Khan, S. , & Begum, S. (2007). Chemical constituents of Centella asiatica. Journal of Asian Natural Products Research, 9(3–5), 407–414. 10.1080/10286020600782454 [DOI] [PubMed] [Google Scholar]
- Singh, P. , & Thakur, M. K. (2014). Reduced recognition memory is correlated with decrease in DNA methyltransferase1 and increase in histone deacetylase2 protein expression in old male mice. Biogerontology, 15(4), 339–346. 10.1007/s10522-014-9504-5 [DOI] [PubMed] [Google Scholar]
- Sirichoat, A. , Chaijaroonkhanarak, W. , Prachaney, P. , Pannangrong, W. , Leksomboon, R. , Chaichun, A. , … Welbat, J. U. (2015). Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients, 7(10), 8413–8423. 10.3390/nu7105401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath, A. , Zhang, Y. , Henson, E. , Wadsworth, T. , Bishop, J. , Gold, B. G. , & Quinn, J. F. (2012). Centella asiatica extract improves behavioral deficits in a mouse model of alzheimer's disease: Investigation of a possible mechanism of action. International Journal of Alzheimer's Disease, 2012, 381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath, A. , Zhong, Y. P. , Gold, S. A. , Yu, X. , Koop, D. R. , Bourdette, D. , & Gold, B. G. (2005). Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro. Journal of Pharmacy & Pharmacology, 57(9), 1221–1229. 10.1211/jpp.57.9.0018 [DOI] [PubMed] [Google Scholar]
- Subban, R. , Veerakumar, A. , Manimaran, R. , Hashim, K. M. , & Balachandran, I. (2007). Two new flavonoids from Centella asiatica (Linn.). Journal of Natural Medicines, 62(3), 369–373. [DOI] [PubMed] [Google Scholar]
- Terry, R. , Masliah, E. , Salmon, D. P. , Butters, N. , DeTeresa, R. , Hill, R. , … Katzman, R. (1991). Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology, 30(4), 572–580. 10.1002/(ISSN)1531-8249 [DOI] [PubMed] [Google Scholar]
- Umka Welbat, J. , Sirichoat, A. , Chaijaroonkhanarak, W. , Prachaney, P. , Pannangrong, W. , Pakdeechote, P. , … Wigmore, P. (2016). Asiatic acid prevents the deleterious effects of valproic acid on cognition and hippocampal cell proliferation and survival. Nutrients, 8(5), e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerendra Kumar, M. H. , & Gupta, Y. K. (2003). Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer's disease in rats. Clinical and Experimental Pharmacology and Physiology, 30(5–6), 336–342. 10.1046/j.1440-1681.2003.03842.x [DOI] [PubMed] [Google Scholar]
- Wattanathorn, J. M. L. , Muchimapura, S. , Tongun, T. , Pasuriwong, O. , Piyawatkul, N. , Yimtae, K. , … Singkhoraard, J. (2008). Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. Neurotoxicology, 29(6), 948–957. [DOI] [PubMed] [Google Scholar]
- Wimmer, M. , Hernandez, P. J. , Blackwell, J. , & Abel, T. (2012). Aging impairs hippocampus‐dependent long‐term memory for object location in mice. Neurobiology of Aging, 33(9), 2220–2224. 10.1016/j.neurobiolaging.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. , Crawford, N. , Kelly, J. S. , Kerr, L. E. , Marston, H. M. , Spratt, C. , … Sharkey, J. (2007). Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. European Neuropsychopharmacology, 17(2), 145–155. 10.1016/j.euroneuro.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Young, J. , Powell, S. B. , Geyer, M. A. , Jeste, D. V. , & Risbrough, V. B. (2010). The mouse attentional set‐shifting task: A method for assaying successful cognitive aging? Cognitive, Affective & Behavioral Neuroscience, 10(2), 243–251. 10.3758/CABN.10.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. , Sharkey, J. , & Finlayson, K. (2009). Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiology of Aging, 30(9), 1430–1443. 10.1016/j.neurobiolaging.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Yu, S. , Zhang, M. , Luo, J. , Zhang, L. , Shao, Y. , & Li, G. (2013). Curcumin ameliorates memory deficits via neuronal nitric oxide synthase in aged mice. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 45, 47–53. 10.1016/j.pnpbp.2013.05.001 [DOI] [PubMed] [Google Scholar]


