Abstract
Recurrence is one of the major causes of poor prognosis for patients with hepatocellular carcinoma (HCC), and drug resistance is closely associated with disease recurrence. Histone deacetylase (HDAC) inhibitor scriptaid functions as an anticancer agent in many different types of tumors, but its possible roles in HCC progression have not been explored to date. Herein, we show that HDAC inhibitor scriptaid decreases HCC cell proliferation and induces cell cycle G2/M-phase arrest in a dose-dependent manner. Furthermore, scriptaid triggered HCC cell death via transcriptional activation of p21 and subsequent elevated global H3Ac levels. Importantly, we found that scriptaid showed robust antitumor activity against HCC. Thus, our findings indicate that HDAC inhibitor scriptaid could be an important potential candidate for treatment of HCC patients.
Keywords: apoptosis, hepatocelluar carcinoma, proliferation, Scriptaid
Introduction
Hepatocellular carcinoma (HCC) is one of the most universal cancers around the world [1]. Metastasis and recurrence are the major limiting factors that decrease the average survival rate of 5 years in HCC [2]. Despite great success in diagnosis and therapy in HCC, it is still associated with an adverse prognosis. Therefore, it is urgent to pursue new molecular targetted drugs for effective treatment of HCC and explore the fundamental pathogenesis of HCC.
Histone deacetylases (HDACs) are usually dysregulated in cancers, especially in HCC [3,4]. Recent studies have shown that the expression of both HDAC1 and HDAC2 are positively associated with the mortality of 156 Southeast Asian patients with HCC [5]. Depletion of HDAC1 and HDAC2 increased cell death and inhibited cell viability in hepatocellular cancer cell lines [5]. Additionally, miR-376a regulated the epigenetic modification via targetting HDAC9 in HCC, and HDAC9 inhibited miR-376a by reducing the H3K18Ac modification levels [6]. Down-regulation of HDAC5 also inhibited liver cancer cell proliferation through mediating cell-cycle arrest and apoptosis [7]. Therefore, targetting HDACs is the most efficient approach to explore the association between HCC and the imbalance of histone acetylation and deacetylation.
Currently, several HDAC inhibitors are being used in tumor therapy or fundamental research. Our previous study showed that HDACi NaBut-induced multiple myeloma cell-cycle G2/M-phase arrest and cell apoptosis [8]. Vorinostat treatment led to HCC cell apoptosis via activating caspase-3 [9]. Despite increased numbers of HDAC inhibitors, only resminostat and belinostat have undergone Phase I and II clinical trials for HCCs [10,11]. Novel HDAC inhibitor scriptaid (6-(1,3-dioxo-1H-benzo[de]isoquinolin-2-yl)N-hydroxyhexanamide) was first identified from high-throughput system screening [12], and it has been reported that scriptaid exhibited its antitumor activities against endometrial, ovarian, colon, and lung cancers, as well as glioma and multiple myeloma [13–17]. In 2006, Lai et al. [18] reported that human sulphatase 1 enzyme enhanced scriptaid-mediated proapoptotic properties in HCC. Nevertheless, scriptaid’s specific function in HCC was unclear.
In the present study, we discovered that HDAC inhibitor scriptaid decreased HCC cell-line viabilities in dose- and time-dependent manner. Furthermore, we also found that scriptaid led to HCC cell-cycle G2/M-phase arrest. Scriptaid treatment triggered HCC cell apoptosis via transcriptional activation of p21. Importantly, we found that scriptaid treatment evidently reduced the weights and volumes of HCC cell-line HepG2 primary tumors. These data provide an insight into the therapeutic intervention of advanced HCC.
Materials and methods
Cell culture
HepG2 and Hep3B cell lines were purchased from the American Type Culture Collection (ATCC). HCC cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS at 37°C with 5% CO2.
Western blot
Experiments were carried out as previously reported [19]. The following antibodies were used against β-actin (Santa Cruz Biotechnology): caspase-3 and caspase-9 (Cell Signaling Technology), PARP1, p21, and H3 (Proteintech), and H3Ac and H4Ac (Active Motif).
Cell viability analysis
For cell viability testing, 1 × 104 cells/well were seeded into 96-well cell-culture plates. Then, the cells were treated with different doses of scriptaid for 24 h or treated with 5 μM scriptaid for different times. Cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay as previously described [8].
Cell cycle and apoptosis analysis
The cells were treated using different doses of scriptaid for 24 h. Then, cell cycle distribution was performed as previously reported [8]. The indicated cells treated by scriptaid underwent an apoptosis assay with the Annexin V-FITC/PI Apoptosis Detection kit (KeyGen, China).
Real-time PCR analysis
Total RNA was isolated from the indicated cells treated with scriptaid using TRIzol reagent (TaKaRa, Japan) according to the manufacturer’s protocol. Then, the RNA was reverse transcribed with reverse transcriptase (Promega). Real-time PCR was performed with the SYBR Green reaction mix and a Roche 480 PCR machine.
Luciferase reporter gene assay
The indicated cells were seeded in 24-well plates at approximately 30% density. Then, the p21 luciferase reporter gene was transfected into the indicated cells using PolyJet DNA transfection reagent according to the protocol. After 6 h, scriptaid was added into the corresponding wells for 24 h, and then, the luciferase activity was measured as previously described [8].
Xenograft tumor model
Briefly, BALB/c nude mice were subcutaneously injected with HepG2 cells. Then, DMSO or scriptaid was injected every 2 days, using a dose of 5 mg/kg body weight. After 4 weeks, all mice were killed, and the tumor weights and sizes were measured. The tumor volume was calculated with the formula: V = (length)2 × (width)/2. This animal study was approved by the Animal Care Committee of Xuzhou Medical University, China.
Statistical analysis
All data are presented as the mean ± S.D. of three independent experiments. Student’s t test was used to determine the statistical difference. P<0.05 was considered as statistically significant. All statistical analyses were carried out using GraphPad Prism 5 software.
Results
Scriptaid inhibited HCC cell proliferation
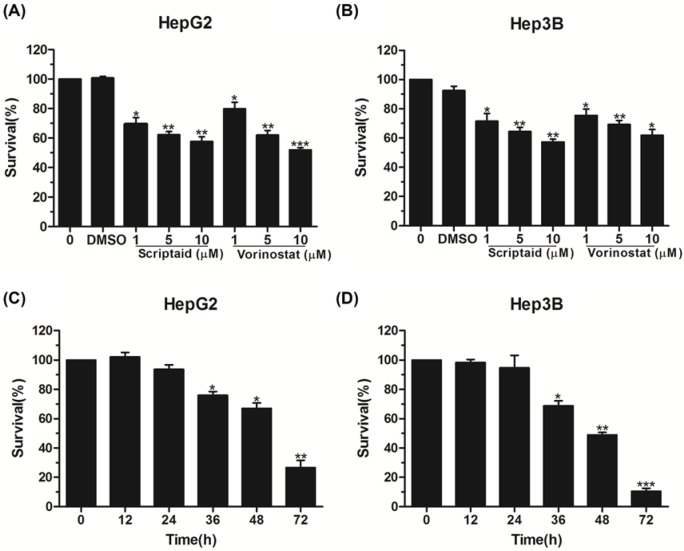
To determine the effect of HDAC inhibitor scriptaid on HCC cell proliferation, HepG2 cells were treated using different concentrations of scriptaid (0, 1, 5, and 10 μM). After 24 h, the CCK-8 assay was conducted, and the results showed that scriptaid inhibited HepG2 cell proliferation in a dose-dependent manner (Figure 1A). Subsequently, we conducted a cell viability assay using another HCC cell line, Hep3B. Carcinoma cell viability was inhibited following treatment with scriptaid in a concentration-dependent manner (Figure 1B). We also compared the antitumor activity between scriptaid and well-known HDAC inhibitor vorinostat, and both of them showed similar antiproliferation activity, implying that scriptaid could be a useful addition to the treatment options for patients with HCC (Figure 1A,B). Additionally, HepG2 and Hep3B cells were also treated with 5 μM scriptaid for different amounts of time, and the results demonstrated that scriptaid visibly decreased multiple HCC cell viabilities in a time-dependent manner (Figure 1C,D). The above data indicate that HDAC inhibitor scriptaid inhibited HCC cell proliferation in a dose- and time-dependent manner.
Figure 1. Scriptaid inhibited HCC cell proliferation.
(A,B) Cell proliferation analysis of HepG2 and Hep3B cells treated by scriptaid and vorinostat at different concentrations (0, 1, 5, and 10 μM), with DMSO as negative control. (C,D) CCK-8 analysis of HepG2 and Hep3B cells exposed to 5 μM scriptaid at different times. The data represent three independent experiments. Error bars: mean + S.D.; *, P<0.05; **, P<0.01; ***, P<0.001.
Scriptaid induced G2/M-phase arrest of HCC cells
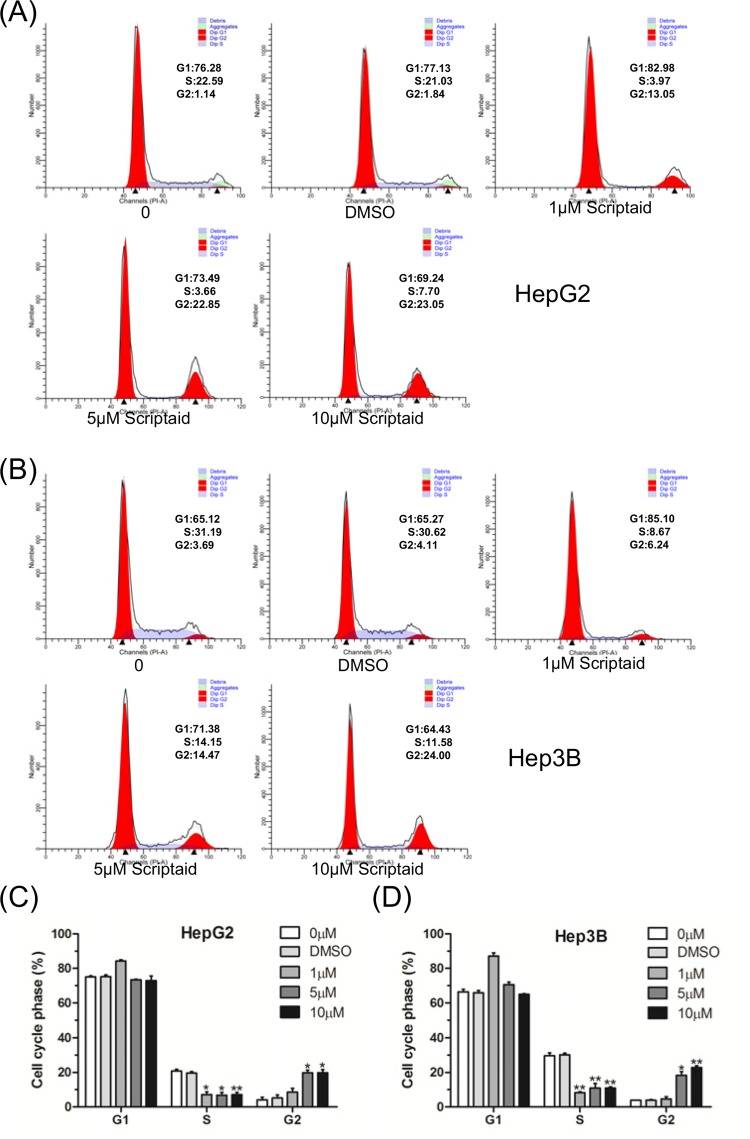
Next, we examined the effect of scriptaid on the cell cycle distribution of HepG2 cells. Following treatment with different concentrations of scriptaid, we found that scriptaid clearly and dose-dependently increased the cell ratio of G2/M-phase compared with the DMSO control group (Figure 2A,C). Furthermore, we also detected the effect of scriptaid on the Hep3B cell cycle, and the results indicated that scriptaid treatment led to Hep3B cell cycle G2/M-phase arrest in liver cancer cells in a dose-dependent manner (Figure 2B,D).
Figure 2. Scriptaid induced G2/M-phase arrest of HCC cells.
(A,B) HepG2 and Hep3B cells were treated with different concentrations of scriptaid for 24 h, and the cell cycle was detected by flow cytometry. (C,D) The distribution of the HepG2 and Hep3B cells’ subpopulations treated with scriptaid was analyzed based on three independent experiments. The experiments were conducted three times. Error bars: mean + S.D.; *, P<0.05; **, P<0.01.
Scriptaid promoted apoptosis in HCC cells
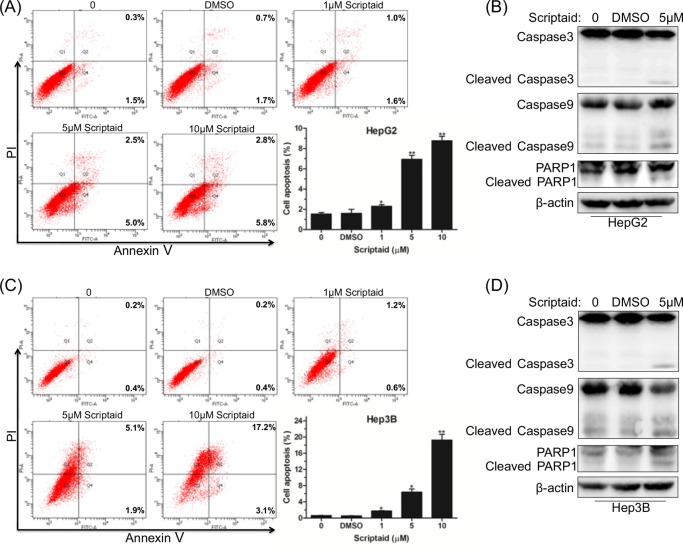
To clarify the mechanism of scriptaid-induced cytotoxicity in HCC cells, we conducted flow cytometry analysis based on Annexin V-FITC/7-AAD staining. As shown in Figure 3A, scriptaid treatment clearly increased the percentage of apoptotic HepG2 cells in a dose-dependent manner. The caspase family plays an important role in the progression of apoptosis, and therefore, we detected the expression level of apoptosis-associated proteins. The results showed that caspase-3, caspase-9, and PARP1 were activated by proteolytic cleavage in response to scriptaid treatment (Figure 3B). Furthermore, we also found that scriptaid dose-dependently induced Hep3B cell apoptosis (Figure 3C). Western blot results showed elevated expression of activated caspase-3, caspase-9, and PARP1 in Hep3B cell lines (Figure 3D). These data indicated that scriptaid promoted HCC cell apoptosis via activation of caspase-3, caspase-9, and PARP1.
Figure 3. Scriptaid promoted apoptosis in HCC cells.
(A) After treatment with scriptaid for 24 h, HepG2 cells were analyzed using an Annexin V-FITC/PI detection kit. (B) Western blot analysis of apoptosis-associated factors caspase-3, caspase-9, PARP1, and their cleaved forms. (C) Flow cytometry analyses of Hep3B cell apoptosis following treatment with different doses of scriptaid for 24 h. (D) Assessment of caspase-3, caspase-9, PARP1, and corresponding cleaved forms in Hep3B cells treated with scriptaid. Experiments were repeated for at least three times. Error bars: mean + S.D. *, P<0.05; **, P<0.01.
Scriptaid-induced cell apoptosis was associated with p21 expression
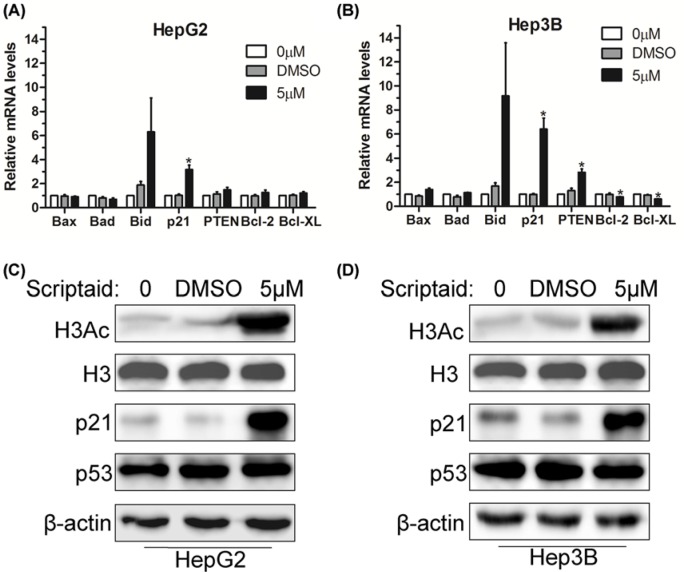
To further elucidate the mechanism by which scriptaid inhibited HCC cell proliferation and induced cell apoptosis, we used Q-PCR analysis to identify possible potential targets of scriptaid. We first investigated the expression of various apoptosis- and proliferation-associated factors, and the results indicated that scriptaid treatment clearly increased p21 expression at mRNA levels in HepG2 cells compared with the DMSO control group (Figure 4A). Then, Hep3B cells were also treated with 5 μM scriptaid for 24 h, and Q-PCR analysis showed that it augmented the expression of pro-apoptotic factors p21 and PTEN, with a concomitant decrease in pro-survival factors Bcl-2 and Bcl-XL at mRNA levels (Figure 4B). Therefore, we speculated that p21 could be a key regulator of scriptaid-mediated cell death. However, we found that scriptaid treatment led to a significant decrease in p53 at mRNA levels (data not shown). Specifically, HepG2 and Hep3B cells treated with scriptaid were characterized by a significant increase in p21 expression, but basically no significant change in p53 at protein levels (Figure 4C,D). As an HDAC inhibitor, gene transcription regulation by scriptaid may rely on the changes in H3Ac and H4Ac modification levels. Western blot showed that scriptaid treatment led to a significant increase in the global H3Ac modification levels in both HepG2 and Hep3B cells (Figure 4C,D). Our data confirmed that scriptaid-induced HCC cell apoptosis was associated with p21 expression.
Figure 4. Scriptaid-induced cell apoptosis was associated with p21 expression.
(A,B) HepG2 and Hep3B cells were treated with 5 μM scriptaid for 24 h. Expression levels of apoptosis-related factors were determined by Q-PCR. *, P<0.05. (C,D) Western blot analysis of H3Ac, p21, and p53 in HepG2 and Hep3B cells treated with scriptaid. H3 and β-actin were used as internal controls.
Antitumor activity of scriptaid in an HCC xenograft model
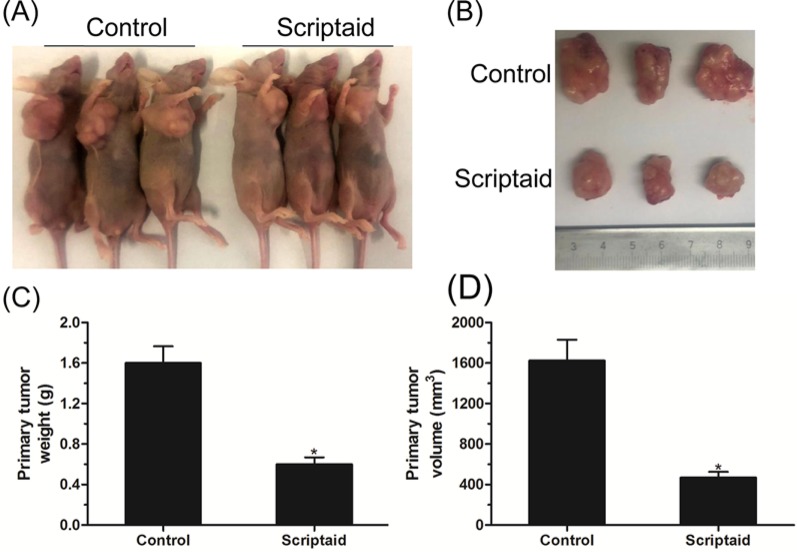
To explore whether scriptaid had an impact on tumorigenesis in vivo, we assessed its ability to repress tumor growth in vivo by using a subcutaneous HepG2 murine xenograft model. As shown in Figure 5A,B, scriptaid treatment evidently reduced the tumor growth compared with the untreated group. After 4 weeks, the mice were killed, and the tumor weight and volume were recorded. We detected a marked decrease in the primary tumor weight and volume in mice treated with scriptaid (Figure 5C,D). Collectively, the above data provide evidence for the possibility of clinical trials and treating HCC patients with the HDAC inhibitor scriptaid.
Figure 5. Antitumor activity of scriptaid in an HCC xenograft model.
(A,B) Representative image of xenograft tumors from BALB/c nude mice subcutaneously injected with HepG2 cells and treated with PBS or scriptaid twice a week. (C,D) Primary tumor weights and volumes in BALB/c nude mice that received scriptaid treatment. Error bars: mean + S.D. (n=6). *, P<0.05.
Discussion
HCC is one of the most common malignancies of primary liver cancer, which leads to a lower patient survival rate because of its metastasis and recurrence. Drug resistance is a major cause for recurrence, and therefore, it is urgent to develop new molecular-targetted therapeutic drugs. Epigenetic regulation is closely associated with HCC progression [20]. Amongst them, histone acetylation and deacetylation are dynamic changes, which require histone acetyltransferase (HAT) and HDAC to mediate gene activation or repression [21]. The imbalance between HAT and HDAC is associated with malignant disease and tumors [22].
HDAC inhibitors can be applied in tumor therapy for various cancers by altering the HDAC expression or disrupting acetylation homeostasis. In recent years, an increasing number of HDAC inhibitors have appeared and served as potential drugs for patients with HCC, such as resminostat, quisinostat, entinostat, and valproic acid [10,23–25]. However, only resminostat underwent a Phase II clinical trial for HCC patients. Therefore, it is still urgent to explore novel HDAC inhibitors and their mechanism of antitumor activities for HCC. In the present study, we found that the novel HDAC inhibitor scriptaid inhibited multiple HCC cell proliferation in a dose- and time-dependent manner. Further study confirmed that scriptaid led to liver cancer cell cycle G2/M phase arrest and triggered cell apoptosis.
In terms of the mechanism, we found that scriptaid promoted p21 gene transcription in liver cancer cells, indicating that p21 could be a key regulator of scriptaid-mediated cell apoptosis. It has been reported that p21 interacts with p53 [26]. Surprisingly, tumor suppressor p53 was down-regulated in a manner that corresponded with scriptaid treatment (data not shown). However, the p53 protein levels remained basically unchanged (Figure 4). Therefore, we speculated that scriptaid-induced HCC cell apoptosis was associated with p21 expression, and p21 participated in the scriptaid-mediated antitumor activity independent of p53.
In conclusion, our results proved that HDAC inhibitor scriptaid decreased HCC cell survival and induced cell cycle G2/M-phase arrest. p21 could be an important mediator of scriptaid-induced HCC cell death and antitumor activity. Therefore, our study highlights scriptaid’s therapeutic potential.
Abbreviations
- 7-AAD
7-amino actinomycin D
- Bcl-2
B cell lymphoma 2
- Bcl-xL
B cell lymphoma-extra large
- CCK-8
cell counting kit-8
- HAT
histone acetyltransferase
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- H3Ac
histone H3 acetylation
- H3K18Ac
histone H3 lysine 18 acetylation
- PARP1
poly (ADP-ribose) polymerase 1
- PTEN
phosphatase and tensin homolog
- Q-PCR
quantitative polymerase chain reaction
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81600173]; the Natural Science Foundation of Jiangsu Province [grant number BK20160230]; the Postdoctoral Science Foundation of China [grant number 2016M601895]; the Postdoctoral Science Foundation of Jiangsu Province [grant number 1601092B]; and the Science and Technology Project of Xuzhou City [grant numbers KC16SY149, KC16SY154].
Author contribution
L.L. and R.Y. designed the present study. L.L., X.S., Y.X., Y.Z., and R.Y. carried out the experiments and performed the statistical analysis. R.Y. and K.X. wrote the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J. and Jemal A. (2015) Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Nault J.C. (2017) The end of almost 10 years of negative RCTs in advanced hepatocellular carcinoma. Lancet 389, 4–6 10.1016/S0140-6736(16)32480-1 [DOI] [PubMed] [Google Scholar]
- 3.Witt O., Deubzer H.E., Milde T. and Oehme I. (2009) HDAC family: what are the cancer relevant targets? Cancer Lett. 277, 8–21 10.1016/j.canlet.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 4.Bai X., Wu L., Liang T., Liu Z., Li J., Li D. et al. (2008) Overexpression of myocyte enhancer factor 2 and histone hyperacetylation in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 134, 83–91 10.1007/s00432-007-0252-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ler S.Y., Leung C.H., Khin L.W., Lu G.D., Salto-Tellez M., Hartman M. et al. (2015) HDAC1 and HDAC2 independently predict mortality in hepatocellular carcinoma by a competing risk regression model in a Southeast Asian population. Oncol. Rep. 34, 2238–2250 10.3892/or.2015.4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y., Chen H., Yin M., Ye X., Chen G., Zhou X. et al. (2015) MiR-376a and histone deacetylation 9 form a regulatory circuitry in hepatocellular carcinoma. Cell. Physiol. Biochem. 35, 729–739 10.1159/000369733 [DOI] [PubMed] [Google Scholar]
- 7.Fan J., Lou B., Chen W., Zhang J., Lin S., Lv F.F. et al. (2014) Down-regulation of HDAC5 inhibits growth of human hepatocellular carcinoma by induction of apoptosis and cell cycle arrest. Tumour Biol. 35, 11523–11532 10.1007/s13277-014-2358-2 [DOI] [PubMed] [Google Scholar]
- 8.Yao R., Han D., Sun X., Fu C., Wu Q., Yao Y. et al. (2017) Histone deacetylase inhibitor NaBut suppresses cell proliferation and induces apoptosis by targeting p21 in multiple myeloma. Am. J. Transl. Res. 9, 4994–5002 [PMC free article] [PubMed] [Google Scholar]
- 9.Carlisi D., Lauricella M., D’Anneo A., Emanuele S., Angileri L., Di Fazio P. et al. (2009) The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur. J. Cancer 45, 2425–2438 10.1016/j.ejca.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 10.Zhao J. and Lawless M.W. (2016) Resminostat: opening the door to epigenetic treatments for liver cancer. Hepatology 63, 668–669 10.1002/hep.27853 [DOI] [PubMed] [Google Scholar]
- 11.Yeo W., Chung H.C., Chan S.L., Wang L.Z., Lim R., Picus J. et al. (2012) Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter Phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J. Clin. Oncol. 30, 3361–3367 10.1200/JCO.2011.41.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su G.H., Sohn T.A., Ryu B. and Kern S.E. (2000) A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res. 60, 3137–3142 [PubMed] [Google Scholar]
- 13.Takai N., Ueda T., Nishida M., Nasu K. and Narahara H. (2006) A novel histone deacetylase inhibitor, scriptaid, induces growth inhibition, cell-cycle arrest and apoptosis in human endometrial cancer and ovarian cancer cells. Int. J. Mol. Med. 17, 323–329 [PubMed] [Google Scholar]
- 14.Sharma V., Koul N., Joseph C., Dixit D., Ghosh S. and Sen E. (2010) HDAC inhibitor, scriptaid, induces glioma cell apoptosis through JNK activation and inhibits telomerase activity. J. Cell. Mol. Med. 14, 2151–2161 10.1111/j.1582-4934.2009.00844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E.J., Lee B.B., Kim S.J., Park Y.D., Park J. and Kim D.H. (2008) Histone deacetylase inhibitor scriptaid induces cell cycle arrest and epigenetic change in colon cancer cells. Int. J. Oncol. 33, 767–776 [PubMed] [Google Scholar]
- 16.Brazelle W., Kreahling J.M., Gemmer J., Ma Y., Cress W.D., Haura E. et al. (2010) Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. PLoS ONE 5, e14335 10.1371/journal.pone.0014335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao R., Han D., Sun X., Xie Y., Wu Q., Fu C. et al. (2018) Scriptaid inhibits cell survival, cell cycle, and promotes apoptosis in multiple myeloma via epigenetic regulation of p21. Exp. Hematol. 10.1016/j.exphem.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Lai J.P., Yu C., Moser C.D., Aderca I., Han T., Garvey T.D. et al. (2006) SULF1 inhibits tumor growth and potentiates the effects of histone deacetylase inhibitors in hepatocellular carcinoma. Gastroenterology 130, 2130–2144 10.1053/j.gastro.2006.02.056 [DOI] [PubMed] [Google Scholar]
- 19.Yao R., Jiang H., Ma Y., Wang L., Wang L., Du J. et al. (2014) PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 74, 5656–5667 10.1158/0008-5472.CAN-14-0800 [DOI] [PubMed] [Google Scholar]
- 20.Liu K.Y., Wang L.T. and Hsu S.H. (2018) Modification of epigenetic histone acetylation in hepatocellular carcinoma. Cancers (Basel) 10, 10.3390/cancers10010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannister A.J. and Kouzarides T. (2011) Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haberland M., Montgomery R.L. and Olson E.N. (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 10.1038/nrg2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F., Wang T., Wang Z., Chen X. and Liu R. (2017) Histone deacetylase inhibitor quisinostat activates caspase signaling and upregulates p53 acetylation to inhibit the proliferation of HepG2 cells. Mol. Med. Rep. 16, 6094–6101 10.3892/mmr.2017.7355 [DOI] [PubMed] [Google Scholar]
- 24.Gahr S., Peter G., Wissniowski T.T., Hahn E.G., Herold C. and Ocker M. (2008) The histone-deacetylase inhibitor MS-275 and the CDK-inhibitor CYC-202 promote antitumor effects in hepatoma cell lines. Oncol. Rep. 20, 1249–1256 [PubMed] [Google Scholar]
- 25.Pathil A., Armeanu S., Venturelli S., Mascagni P., Weiss T.S., Gregor M. et al. (2006) HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology 43, 425–434 10.1002/hep.21054 [DOI] [PubMed] [Google Scholar]
- 26.Antognelli C., Ferri I., Bellezza G., Siccu P., Love H.D., Talesa V.N. et al. (2017) Glyoxalase 2 drives tumorigenesis in human prostate cells in a mechanism involving androgen receptor and p53-p21 axis. Mol. Carcinog. 10.1002/mc.22668 [DOI] [PubMed] [Google Scholar]