Abstract
223Radium (223Ra) is the first alpha-emitting therapy proven effective in human cancer. Prospective randomized trials indicate that 223Ra, which concentrates after intravenous injection in areas of osteoblastic metastatic disease, can prolong survival in bone-dominant castrate resistant prostate cancer patients. Though radium isotopic therapy is conceptually critical to demonstrate that alpha emitters can be safe and effective, 223Ra has inherent limitations given its restriction to bone metastatic disease. To overcome this limitation, targeted alpha therapy (TAT) is now being actively evaluated in prostate cancer, and other neoplasms. Key to TAT in prostate tumors in current studies is the overexpression of prostate specific membrane antigen (PSMA), a folate hydrolase expressed on the cell surface of malignant adenocarcinomas of the prostate. Using PSMA targeting (small molecules or antibodies), alpha emitting agents such as 225Actinium (225Ac) or 213Bismuth (213Bi) can be delivered to PSMA expressing tumors regardless of their metastatic location. Initial results from TAT in prostate cancer are highly promising and rapid development of these agents is anticipated in the years ahead assuming adequacy of isotope availability and appropriate clinical trial design. TAT may be develop as an independent approach, or synergize with a variety of other approaches including external beam radiation, hormonal therapies, chemotherapies, various radiation sensitizers, DNA repair inhibitors, and/or immune modulators. Clinical investigation opportunities in this field will rapidly increase in the years ahead.
Keywords: Radium, alpha emitter, actinium, prostate specific membrane antigen (PSMA), prostate cancer, radionuclides
Introduction to alpha emitters
Alpha emitters have unstable nuclei and emit a helium nucleus upon decay (2 protons/2 neutrons). The characteristics of alpha particles and their effects have been well defined in biological systems (1). Upon emission, most alphas travel initially at a speed about 5% of the speed of light. The combination of heavy particles and high speed make for highly energetic particles capable of substantial tissue damage. Alpha particles have short tissue penetration, usually in the range of 40–90 µm. Deposition of energy for alphas, and other heavy particles, is non-linear; most energy of a particle is released in a “Bragg peak” which occurs just prior to the particle coming to rest.
The limited penetration of alphas in biologic systems represents both an opportunity and challenge. If alphas can be delivered precisely to cancerous cells, and the cancer microenvironment, the risk of adverse side effects can be mitigated. However, the successful delivery of alphas clearly depends on precise tumor targeting. The linear energy transfer (LET) of alpha particles, approximately 25–230 kEv/µm, can be 100–1,000 times fold higher than the LET of beta particles, translating into high rates of biologic damage (2). Tissue damage, and cellular kill, is predominantly in the form of DNA strand breaks, with a propensity for the alpha-induced breaks to be double stranded and lethal. In general double strand breaks are difficult to repair via normal DNA repair mechanisms (3). The potential role of immunologic factors in radiation induced cell kill has been hypothesized as well (4).
Some alpha particles such as 223-radium (223Ra) and 224Ra can be used as naked molecules given their bone-targeting abilities. Most alphas however require targeting to the proper site once injected into a cancer patient. Thus, targeted alpha therapies (TATs) require a guiding molecule that delivers the isotope to the region of interest. Most TATs are comprised of a component that binds regions of biologic interest, a chelate to hold the radionuclide in place, and the active radioactive particle (5,6).
A variety of molecules have been used to guide the radiopharmaceutical to the location of interest. Antibodies and their derivatives (minibodies, diabodies, various fragments, etc.) are of course logical compounds that can deliver chelates precisely to cancerous locations and these have been used in a number of clinical studies. Small molecules can also be used and most prostate focused therapies to date have focused on molecules capable of binding a molecule called prostate specific membrane antigen (PSMA) which is over-expressed on a number of prostate cancer cells (vide infra). Imaging with PSMA ligands indicates the specificity of the interaction (7). For other tumors, alphas can be directed to tumor tissue by a variety of targeting agents such as substance P, somatostatin analogues, various other peptides, small molecules, nanoparticles, and polymers (5,6,8,9).
Alpha emitting isotopes used in cancer focused clinical studies to date include 223Ra, 225-actinium (225Ac), and 213-bismuth (213Bi), 211-astatine (211At), 212-lead (212Pb), and 227-thorium (227Th). In addition to targeting mechanisms, half-life considerations, daughters, chelation, and production in adequate amounts necessary for clinical studies are all considerations when selecting an alpha emitter for a targeted cancer therapy (9). The decay patters of 227Th, 223Ra, and 225Ac are shown in Figures 1 and 2. Of note, 227Th decays to 223Ra so these decay chains are near identical. Notably, 213Bi, 212Pb, and 211At, each have a single alpha emission whereas 223Ra, 227Th and 225Ac have multiple [4–5] alpha decays before they reach stability. Though multiple alpha emissions provide greater opportunities for tumor kill, the diffusion of daughters also provide opportunities for untargeted cellular damage.
Figure 1.
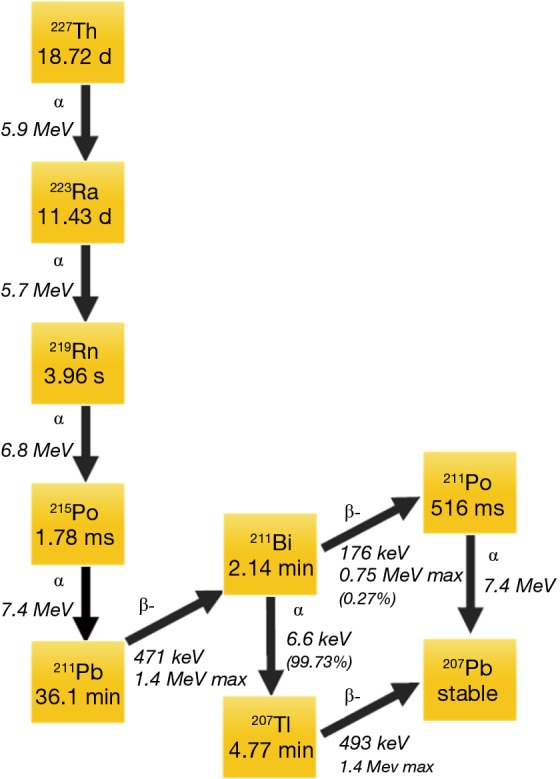
Decay chain for 227Th and 223Ra.
Figure 2.
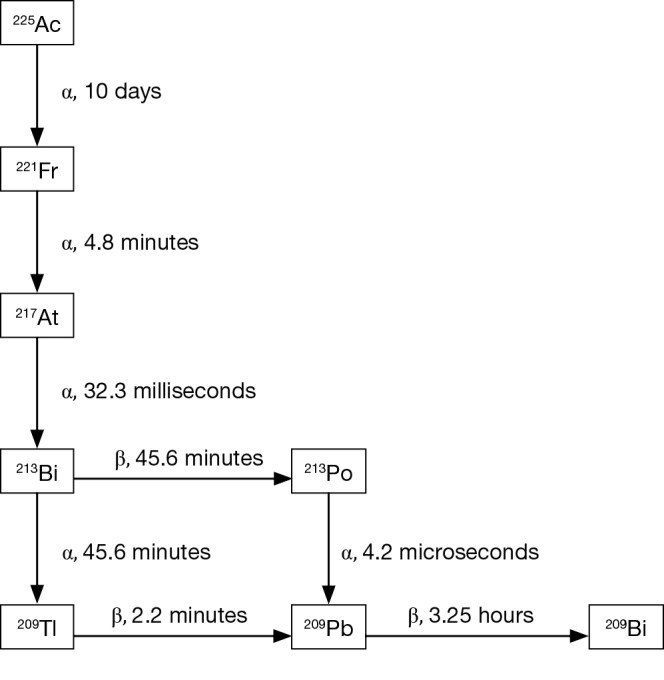
Decay chain for 225Ac.
223Ra is FDA approved in prostate cancer and is being investigated in multiple USA based clinical trials and will be discussed in detail below. 227Th is conjugated to a CD22 directed antibody and is currently in a first-in-man USA-based phase I (NCT02581878). 225Ac is in an active clinical trial conjugated to an antibody to CD33 (lintuzumab) in older patients with acute myeloid leukemia (AML) (NCT02575963) and in a randomized trial for AML patients using a 225Ac conjugated anti-CD45 antibody prior to stem cell transplant (NCT02665065). Not all ex-USA based trials are registered thus it is difficult to know with certainty where active alpha-emitter clinical trials are ongoing outside of the USA. Investigators in New York, USA; Heidelberg, Germany; and Pretoria, South Africa have reported very recent experiences in prostate cancer patients using alpha emitters.
Upon alpha emission, there is a substantial recoil and the alpha particle derived nucleus (100–200 KeV), is disengaged from the binding any chelate designed to date (and likely higher than any chelate designed in the future) (10). The recoil energy post-alpha emission is simply higher than the chemical bonds that radionuclide in place. Recoil places the daughter molecules outside the chelate where they can readily diffuse. Thus in truth, targeted molecules and chelates can only hope to deliver the initial isotope to the site of interest. For nuclides with a single alpha emission this is less problematic as compared to those nuclides such as 223Ra, 225Ac, and 227Th that have multiple alpha emissions. Diffusion of daughters is dependent on both the half-life and the chemical properties of the remaining elements.
Introduction to 223Ra
223Ra is the only alpha emitter approved by regulatory agencies for routine human use. Survival was prolonged in prospective randomized studies in selected prostate cancer patients as discussed below. Herein we review initial preclinical studies with radium and then cover in more detail the 223Ra clinical trials reported to date. The field is evolutionary given the constant design and execution of new clinical studies. 223Ra is an alkali earth metal in the periodic chart and targets bone by binding to hydroxyapatite, similar to other alkali earth metals (such as 89Sr). No additional targeting mechanism is required for 223Ra, thus no chelate is required (11). In fact, attempts to create stable chelates with radium isotopes to date has been unsuccessful.
223Ra has a half-life of approximately 11.4 days then decays with a total of 3 additional alpha emissions (11). After the initial decay, a series of short-lived alpha-emitting daughters are created. A total of four alpha particles and two beta particles are emitted until stability is reached with 207Pb. The alpha particles emitted in the 223Ra decay schema range from 5.8 to 7.6 MeV in energy. The first daughter in the 223Ra decay pathway 219-radon (219Rn), then to 215-polonium (215Po), 211Pb, 211Pb, then to either 211Po or 207-thallium (207Tl), before reaching stable 207Pb. The half-lives and energies of the various 223Ra daughters are depicted in Figure 2.
Pre-clinical 223Ra studies
Initial rodent studies compared the relative bio-distribution and dosimetric analysis of (223Ra) and 89-strontium (89Sr), two calcium-mimetic radionuclides, in soft tissue and bone (12). After intravenous injection of 223Ra chloride, femur uptake in normal bone reached maximum at 1 hour and persisted during the 14 days of study. Approximately 40% of injected dose/gm of femur was seen at 24 hours post-injection. Whereas the soft tissue uptake was considerably lower and decreased with time, bone radioactivity levels did not reduce significantly over 14 days indicating tight binding and little reversibility of the bone binding. Although the bio-distribution pattern of 89Sr was similar, the femur uptake (18% and 21% in femur injected dose/g at 1 h and 14 d, respectively) was lower than that for 223Ra. Only approximately 2% of the 223Ra daughter radionuclides redistributed from the site of radium decay in the bone to soft tissues. It was found that the absorbed dose received by the marrow, a critical organ for potentially limiting normal tissue toxicity, would be considerably lower than that from 89Sr due to short range of the alpha particle from 223Ra compared to the longer range of the high energy beta from 89Sr.
In a companion rodent study (13), it was demonstrated that 223Ra has a significant antitumor effect in a bone metastases model. A significantly higher symptom-free survival was seen in the bone-tumor bearing rats (MT-1 breast cancer cell) administered 6 and 11 kBq of 223Ra, with 36% of the animals treated with 11 kBq having improved symptom-free survival as compared to control rats (that developed tumor-induced paralysis within 20–30 days). In another treatment regimen, animals were administered with a bisphosphonate (pamidronate) after injecting 0, 5, 10 or 30 kBq of 223Ra. The group receiving pamidronate alone has no survivors beyond 21 days, suggesting no therapeutic effect from this bone resorption inhibitor. Two out 5 animals from the 10 and 30 kBq groups survived for more than 50 days. No bone marrow toxicity or body weight loss was seen in the treated group. These results suggested that bone metastases could be treated by the intravenous administration of 223Ra in rodents without undue toxicity to bone marrow. Another pre-clinical study to study the acute toxicity after iv administration of potentially toxic amounts of 223Ra in Balb/c mice was then conducted (14). Results suggested that high amounts 223Ra did not completely inactivate the blood-producing cell, which could be due to the distant location of red bone marrow cells from the bone surface (thus not affected by the short-range alpha-particles), and also the lost blood-producing cells being compensated by the recruitment of blood forming progenitor cells.
A bio-distribution of 223Ra in canines had shown affinity for and stability within calcified tissues (15). Elimination of radioactivity was mainly via the intestine, as it is in humans. 223Ra resided in transit within the gut content with minimal activity in intestinal walls. The highest concentration of 223Ra as determined by alpha-track micro-autoradiography, was found on the surfaces of trabecular bone, with concentration in osteoblastic bone matrix.
Clinical trials for 223Ra
Encouraged by the positive pre-clinical results, a phase I study was initiated to find the safety and tolerability of 223Ra in breast and prostate cancer patients with bone metastases and to evaluate pain palliation (16). The study enrolled 25 patients; 15 patients with prostate cancer and 10 patients with breast carcinomas, who were subdivided into groups of 5 patients and received a single injection of 46, 93, 163, 213, or 250 kBq/kg of 223Ra followed for 8 weeks. Ten out of 25 patients suffered from mild and transient diarrhea, 1 patient had a grade 1 thrombocytopenia, grade 3 neutropenia and leucopenia occurred in 2 and 3 patients respectively. Nausea and vomiting occurred in 4/5 patients at the highest dosage cohort. Another phase I study (17) involving 6 prostate cancer patients demonstrated the safety profile of repeated 223Ra injections at two fixed dosage levels, 2×125 or 5×50 kBq/kg of 223Ra, administered respectively with 6- or 3-week intervals between injections. No significant adverse effects from repeated treatment were observed; the toxic effects experienced by patients that were given 5×50 kBq/kg were very similar to those experienced by patients from the previous study wherein the radioactivity was administered in a single lot. Uptake of 223Ra was preferentially located in the osteoblastic lesions demonstrable on bone scans. Excretion was via the gut, a process still not fully understood.
Following these results, a randomized, multicenter, double-blind, placebo-controlled, phase II study was conducted with 64 patients with castrate-resistant prostate cancer (CRPC) and bone pain requiring external beam radiotherapy (18). Patients were treated with four 50 kBq/kg of 223Ra (i) at 4-week intervals, whereas the control group received saline. The median overall survival in the 223Ra group was 65.3 weeks compared with 46.4 weeks in the placebo group. Three 223Ra treated patients had an irreversible grade 2+ neutropenia which was not seen in control group, while thrombocytopenia was observed in one patient receiving placebo. Only minimal hematological toxicity was observed. The median relative change in prostate-specific antigen (PSA) during the same time interval was −23.8% (range, −98.6% to 545.6%) in the radionuclide group and +44.9% (range, −91.3% to 563.5%) in the placebo group (P=0.003).
Based on the phase I and phase II clinical trials, a multi-center phase III trial, ALSYMPCA (Alpharadin in Symptomatic Prostate Cancer Patients), was designed and accrued (19). Eligibility criteria include bone metastatic CRPC patients who had received, were not eligible to receive, or declined chemotherapy. Patients with visceral disease or nodal metastases more than 3 cm (short axis) were excluded. Patients were randomized in 2:1 fashion to receive best standard of care (SOC) + 6 injections of 223Ra (50 kBq per kilogram of body weight intravenously), or matching placebo, with injections every 4 weeks. Best standard of care included virtually any hormonal therapy used at that time (bicalutamide, estrogens, dexamethasone, prednisone, ketoconazole, etc.), bisphosphonates, or external beam radiation. Excluded concomitant therapies included chemotherapies and experimental therapies. A total of 921 patients were enrolled and stratified according to prior use (yes or no), baseline alkaline phosphatase level (<220 U per liter vs. ≥220), and current use or no use of bisphosphonate. The study endpoints were defined as: overall survival (primary endpoint), time to first symptomatic skeletal event (SSE), time to total alkaline phosphatase (ALP) progression, total ALP response, total ALP normalization, time to PSA progression, safety and quality of life (secondary endpoints). The majority of patients were with advanced disease (a large number of metastatic localizations). The trial was stopped at interim by the data monitoring committee for pre-defined overall survival (OS) benefit. Those randomized to SOC + 223Ra had a median OS of 14 vs. 11.2 months with standard of care + placebo; hazard ratio of 0.70, 95% CI, 0.58 to 0.83; P<0.001). Assessment of all main secondary efficacy points also showed a benefit of 223Ra. The final analysis further confirmed the results of interim analysis with OS 14.9 vs. 11.3 months), longer time to SREs (median 15.6 months with SOC + 223Ra vs. 9.8 with SOC + placebo, hazard ratio, 0.66; 95% CI, 0.52 to 0.83; P<0.001), lower incidence of SREs in 223Ra group, the time to an increase in the total alkaline phosphatase level (hazard ratio, 0.17; 95% CI, 0.13 to 0.22; P<0.001), and the time to an increase in the PSA level [hazard ratio (HR), 0.64; 95% CI, 0.54 to 0.77; P<0.001]. The 223Ra treatment was well tolerated, with low incidence of grade 3/4 neutropenia (1.8% vs. 0.8%) and thrombocytopenia (4% vs. 2%). Adverse events included vomiting 18% of 223Ra versus 14% in placebo, and diarrhea 25% vs. 15% (higher in those with radionuclide therapy). Based on these pivotal data (19), 223Ra therapy was approved by regulatory agencies around the world.
Analysis of stratified subsets in the ALSYMPCA trial has led to additional insights regarding this agent (20,21). Analysis of symptomatic skeletal events (pathologic fractures, radiation to bone, surgery to bone, and spinal cord compression) suggests that 223Ra alone minimally diminishes rates of SSEs (HR, 0.77 and P=0.07) as compared to 223Ra combined with bisphosphonates (HR, 0.49, P<0.00048). Analysis of subsets in docetaxel naïve and prior docetaxel treated indicated that both subsets of patients had a statistically significant survival improvement (21). Quality of life is improved with 223Ra treatment as well (22).
Many questions with 223Ra remain. Expedient decisions were made with regard to dose and frequency of 223Ra and optimal dose and frequency of administration are unsettled issues. A current clinical trial evaluates both of these questions (with unreported results to date) (NCT 02023697). In this trial standard doses of radium are used for 6 cycles, 12 cycles, or for 6 cycles at a dose of 80 kBq/kg. Another major question concerns the ability of 223Ra to successfully be used to add value to the novel hormonal therapies such as abiraterone and enzalutamide. Thus prospective randomized trials in bone-metastatic CRPC have been launched including abiraterone +/− 6 doses of conventionally dosed 223Ra (ERA-223) or enzalutamide +/− 6 doses of conventionally dosed 223Ra (PEACE III). A recent press release indicates that the ERA-223 trial has been recommended for unblinding by the data monitoring committee due to excess fractures and deaths in the 223Ra arm (23). Further commentary awaits analysis of complete unblended data. It is not known if the fractures or pathologic or non-pathologic in nature. Any protective effects of bisphosphonates or denosumab on fractures are unknown in this setting. Reasons for the excess deaths are not yet known and the death rates from unplanned early interim analyses are problematic from a conceptual perspective. More data on survival will be available after unblinding and longer follow up. These results have significant implications for 223Ra combined with abiraterone/prednisone in men with bone-metastatic CRPC and call into question the wisdom of combining 223Ra with abiraterone until more safety data are available.
PSMA TATs
PSMA is a glutamate carboxypeptidase encoded by the FOLH1 (folate hydrolase 1) (24). The human enzyme contains 750 amino acids with most of the enzyme resides in the extracellular space. PSMA highly expressed in the vast majority of human prostate cancers and a multiplicity of studies indicate that both localized and metastatic adenocarcinomas of the prostate cancers express PSMA (25). Normal tissue expression of PSMA (outside of the prostate) is limited and studies indicate expression is limited to normal prostate tissue, small bowel, proximal renal tubules, salivary glands, and astrocytes (26). Lacrimal glands show uptake on PSMA scans as well. In tumors, PSMA expression has been linked to adverse prognosis and progression in many studies (27) but noteworthy is the fact that only adenocarcinomas express PSMA, small cell cancers do not express PSMA. Cell lines that lack AR do not express PSMA. Small molecule PSMA based imaging show high specificity for prostate cancer lesion and also uptake in the kidney, salivary glands, and lacrimal glands (28,29). Interestingly the PSMA targeted antibodies do not show salivary/lacrimal gland uptake leading to speculation that the uptake seen on small molecule scans may not be fully explained by PSMA expression.
A number of small molecule ligands have been used in PSMA targeted radionuclides. These compounds can be classified into urea-based compounds, glutamate phosphoramidates and 2-(phosphinylmethyl) pentanedioic acids (30). A large number molecules capable of binding tightly to PSMA have been synthesized and patented. Among these compounds, two are particularly in active clinical development (PSMA-617 and DCFPyL) which can serve in both imaging and therapeutic capacities. Thus these molecules are referred to as theragnostic agents (7).
Several PSMA small molecules (PSMA-617, PSMA I&T, MIP-1095) have been used with beta-emitters such as lutetium-177 (177Lu) or iodine-131 (131I) (31). Limited studies are available with PSMA-617 using Ac-225 and Bi-213. These will be covered in detail below. 225Ac represents a production challenge but several sites have now produced 225Ac including the Oak Ridge National Laboratories in the USA, the TRIUMF facility in Canada, the Institute for Transuranium Elements in Germany, and the ROSATOM State Corporation in Russia.
The group in Heidelberg has been the pioneer in 225Ac-PSMA targeted therapy (32). The initial report indicated remarkable responses in two heavily pretreated patients (32). The first patient was treated as a 9th line therapy with 3 cycles of 100 kBq/kg of 225Ac-PSMA-617 at 2-month intervals, then a later fourth cycle consolidation at a lower dose of 6 MBq. The second patient received 100 kBq/kg dosing for 3 cycles given at 2-month intervals. Xerostomia was notable for both patients but no other adverse effects were reported. Hematologic parameters indicated no significant adverse effects despite the clear anti-tumor effects. Responses, as assessed by PSA declines and PSMA imaging were remarkable but no cross sectional or other imaging modalities were utilized thus leaving open the possibility that non-PSMA producing tumors were not assessed. The PSA decline was dramatic despite multiple prior therapies. The second patient had been previously treated and relapsed after multiple therapies including 177Lu-PSMA indicating that TAT can overcome resistance to targeted beta therapy, a concept previously shown in neuroendocrine tumors (33).
In a second 225Ac-PSMA-617 series from Heidelberg with very heavily pretreated CRPC patients using 50 (n=4), 100 (n=4), 150 (n=2), and 200 kBq/kg (n=4) doses (34), a total of 8 of 14 patients received more than one cycle at intervals of 2-4 months. All 4 patients in the 200 kBq/kg group and 1 of 2 patients in the 150 kBq/kg group discontinued therapy or insisted on dose reduction suggesting that these doses were intolerable. The authors considered 100 kBq/kg of body weight (kgBW) the maximum tolerable dose, with xerostomia being dose limiting. Xerostomia was observed starting 2–5 days post-injection. In some cases, xerostomia did not recover. One patient in the 200 kBq/kg group reported dry eyes, likely a consequence of alpha-induced lacrimal toxicity. No renal or liver toxicity was noted by routine laboratory evaluation in short term follow-up. This is important as renal toxicity might be anticipated. Responses as measured by PSA decline of >50% were seen in 4/9 evaluable patients. Overall survival was a median of 8 months in this very advanced group of patients. The authors suggested a dose of 100 kBq/kg administered q 2 months to be a potential dose/schedule for further evaluation of 225Ac-PSMA-617.
A single patient has been reported from South Africa (35) after treatment with 213Bi-PSMA-617 in a CRPC patient progressive after unspecified “conventional therapy”. The patient was treated with two cycles of 213Bi-PSMA-617 with a cumulative activity of 592 MBq and demonstrated an excellent response as assessed by PSA decline and PSMA imaging. Not significant toxicities were reported and no cross-sectional imaging was reported. Dosimetry estimates have been reported with 213Bi-PSMA-617 in three patients. A relative biologic effectiveness (RBE) of 5 for the 8.4 MeV alpha 213Bi emission was used in the dosimetry calculations based on a number of assumptions (36). The authors concluded that 213Bi, as compared to 225Ac, is a “second choice” when using PSMA-617 as a ligand.
A single patient has been treated with 225Ac-J591, a monoclonal antibody (J591) specific for an external epitope of the PSMA antibody [Neil Bander, personal communication (December, 2017)]. No data are available from this patient.
Future considerations
The clinical development of 223Ra is proceeding in multiple combination trials using immunotherapies such as PD-1 inhibitors, inhibitors of DNA repair (PARP inhibitors, ATR inhibitors), various hormonal therapies and chemotherapies (including docetaxel) that may serve as radiation sensitizers (see Table 1 for combination trials in progress). There is an intense interest to study DNA damaging agents such as 223Ra in patients with known DNA repair defects (such as BRCA2) as those patients may be especially susceptible to such treatments (37). Though preliminary data on repeat radium are available (38), more data are needed. The increased fracture rate and death rate leading to the ERA-223 unblinding is a serious issue for 223Ra that will need additional careful study given the unanticipated safety signal (23). Though biomarker dynamics after 223Ra have been studied (39), clearly more data are needed to better understand biomarkers associated with response and progression after 223Ra treatments.
Table 1. Ongoing clinical trials with 223Ra in combination therapy.
| Isotope | Combined therapy | NCT | Phase |
|---|---|---|---|
| 223Ra | Niraparib (PARPi) | NCT03076203 | 1B |
| 223Ra | Pembrolizumab (anti-PD1 | NCT03093428 | II |
| 223Ra | Atezolizumab (anti-PDL1 | NCT02814669 | I |
| 223Ra | Enzalutamide | NCT02194842 | III |
| 223Ra | Abiraterone/prednisone | NCT02043678 | III |
| 223Ra | Enzalutamide | NCT02225704 | II |
TAT is ongoing and developing on multiple fronts in prostate cancer with a focus on the PSMA targeting molecules. Both antibodies and small molecules will likely continue to drive TAT progress in multiple diseases (40-42). Both 225Ac and 227Th platforms are going forward in prostate trials. Necessary trials will include conventional endpoints such as PSA declines, tumor shrinkage as measured by soft tissue cross-sectional assessments, progression-free survival, and overall survival. Careful adverse events assessment using conventional common toxicity criteria (CTC) criteria are needed. To prove utility for TAT, such therapies will need comparison to appropriate therapeutic alternatives in randomized trials. Safety issues with TAT are yet to be fully assessed by CTC criteria but PSMA-617 targeted TAT studies indicate that xerostomia, and possibly dry eyes, are the most clinically relevant toxicities to date. Mitigation strategies for these toxicities are ongoing but nothing promising has been published. Longer term data are needed to make definitive conclusions regarding toxicity but given that long term survival of advanced CRPC is problematic, there is limited concern about long term toxicities in patients with poor prognosis.
As TAT develops as a monotherapy, one can readily visualize potential synergistic opportunities developing in a manner similar to 223Ra. Combinations with external beam, hormonal therapies, radiation sensitizers, various DNA repair inhibitors, chemotherapies, and immunotherapies will likely develop in the future.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev 2008;60:1402-6. 10.1016/j.addr.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 2.Aghevlian S, Boyle AJ, Reilly RM. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting alpha-particles or auger electrons. Adv Drug Deliv Rev 2017;109:102-18. 10.1016/j.addr.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Wieder HA, Lassmann M, Allen-Auerbach MS, et al. Clinical use of bone-targeting radiopharmaceuticals with focus on alpha-emitters. World J Radiol 2014;6:480-5. 10.4329/wjr.v6.i7.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014;5:403-16. 10.18632/oncotarget.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted α-particle radiation therapy. Clin Cancer Res 2013;19:530-7. 10.1158/1078-0432.CCR-12-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgqvist J. Targeted alpha therapy: part I. Curr Radiopharm 2011;4:176. 10.2174/1874471011104030176 [DOI] [PubMed] [Google Scholar]
- 7.Lütje S, Heskamp S, Cornelissen AS, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics 2015;5:1388-401. 10.7150/thno.13348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekempeneer Y, Keyaerts M, Krasniqi A, et al. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther 2016;16:1035-47. 10.1080/14712598.2016.1185412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elgqvist J, Frost S, Pouget JP, et al. The potential and hurdles of targeted alpha therapy - clinical trials and beyond. Front Oncol 2014;3:324. 10.3389/fonc.2013.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Kruijff RM, Wolterbeek HT, Denkova AG. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals (Basel) 2015;8:321-36. 10.3390/ph8020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humm JL, Sartor O, Parker C, et al. Radium-223 in the treatment of osteoblastic metastases: a critical clinical review. Int J Radiat Oncol Biol Phys 2015;91:898-906. 10.1016/j.ijrobp.2014.12.061 [DOI] [PubMed] [Google Scholar]
- 12.Henriksen G, Fisher DR, Roeske JC, et al. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003;44:252-9. [PubMed] [Google Scholar]
- 13.Henriksen G, Breistol K, Bruland OS, et al. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002;62:3120-5. [PubMed] [Google Scholar]
- 14.Larsen RH, Saxtorph H, Skydsgaard M, et al. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo 2006;20:325-31. [PubMed] [Google Scholar]
- 15.Bruland OS, Jonasdottir TJ, Fisher DR, et al. Radium-223: From Radiochemical Development to Clinical Applications in Targeted Cancer Therapy. Current Radiopharmaceuticals 2008;1:203-8. 10.2174/1874471010801030203 [DOI] [Google Scholar]
- 16.Nilsson S, Larsen RH, Fossa SD, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451-9. 10.1158/1078-0432.CCR-04-2244 [DOI] [PubMed] [Google Scholar]
- 17.Nilsson S, Balteskard L, Fossa SD, et al. Phase 1 study of alpharadin (223Ra) an alpha-emitting bone-seeking agent in patients with skeletal metastases. Eur J Nucl Med Mol Imaging 2004;31:S290. [Google Scholar]
- 18.Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587-94. 10.1016/S1470-2045(07)70147-X [DOI] [PubMed] [Google Scholar]
- 19.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 20.Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014;15:738-46. 10.1016/S1470-2045(14)70183-4 [DOI] [PubMed] [Google Scholar]
- 21.Hoskin P, Sartor O, O'Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014;15:1397-406. 10.1016/S1470-2045(14)70474-7 [DOI] [PubMed] [Google Scholar]
- 22.Nilsson S, Cislo P, Sartor O, et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol 2016;27:868-74. 10.1093/annonc/mdw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phase III trial of radium Ra 223 dichloride in combination with abiraterone acetate and prednisone/prednisolone for patients with metastatic castration-resistant prostate cancer unblinded early. Available online: https://www.prnewswire.com/news-releases/phase-iii-trial-of-radium-ra-223-dichloride-in-combination-with-abiraterone-acetate-and-prednisoneprednisolone-for-patients-with-metastatic-castration-resistant-prostate-cancer-unblinded-early-300564844.html, accessed 12/10/2017.
- 24.O'Keefe DS, Su SL, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta 1998;1443:113-27. 10.1016/S0167-4781(98)00200-0 [DOI] [PubMed] [Google Scholar]
- 25.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol 2004;6 Suppl 10:S13-8. [PMC free article] [PubMed] [Google Scholar]
- 26.Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81-5. [PubMed] [Google Scholar]
- 27.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 2003;9:6357-62. [PubMed] [Google Scholar]
- 28.Kiess AP, Banerjee SR, Mease RC, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging 2015;59:241-68. [PMC free article] [PubMed] [Google Scholar]
- 29.Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017;44:2117-36. 10.1007/s00259-017-3780-7 [DOI] [PubMed] [Google Scholar]
- 30.Rowe SP, Gorin MA, Allaf ME, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis 2016;19:223-30. 10.1038/pcan.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmett L, Willowson K, Violet J, et al. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci 2017;64:52-60. 10.1002/jmrs.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med 2016;57:1941-4. 10.2967/jnumed.116.178673 [DOI] [PubMed] [Google Scholar]
- 33.Kratochwil C, Giesel FL, Bruchertseifer F, et al. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging 2014;41:2106-19. 10.1007/s00259-014-2857-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med 2017;58:1624-31. 10.2967/jnumed.117.191395 [DOI] [PubMed] [Google Scholar]
- 35.Sathekge M, Knoesen O, Meckel M, et al. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:1099-100. 10.1007/s00259-017-3657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kratochwil C, Schmidt K, Afshar-Oromieh A, et al. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging 2018;45:31-7. 10.1007/s00259-017-3817-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberger AE, Cotogno P, Ledet EM, et al. Exceptional duration of radium-223 in prostate cancer with a BRCA2 mutation. Clin Genitourin Cancer 2017;15:e69-71. 10.1016/j.clgc.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 38.Sartor O, Heinrich D, Mariados N, et al. Re-treatment with radium-223: first experience from an international, open-label, phase I/II study in patients with castration-resistant prostate cancer and bone metastases. Ann Oncol 2017;28:2464-71. 10.1093/annonc/mdx331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol 2017;28:1090-7. 10.1093/annonc/mdx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenblat TL, McDevitt MR, Mulford DA, et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 2010;16:5303-11. 10.1158/1078-0432.CCR-10-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milenic DE, Baidoo KE, Kim YS, et al. Targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease: an investigation of the human anti-EGFR monoclonal antibody, panitumumab. Transl Oncol 2017;10:535-45. 10.1016/j.tranon.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan HS, de Blois E, Morgenstern A, et al. In vitro comparison of 213Bi- and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS One 2017;12:e0181473. 10.1371/journal.pone.0181473 [DOI] [PMC free article] [PubMed] [Google Scholar]


