Abstract
Objective
Remote ischemic conditioning (RIC) has been demonstrated to be safe and feasible for patients with acute ischemic stroke (AIS), as well as for those receiving intravenous thrombolysis. We assessed the safety and feasibility of RIC for AIS patients undergoing endovascular treatment (ET).
Methods
We conducted a pilot study with patients with AIS who were suspected of having an emergent large‐vessel occlusion in the anterior circulation and who were scheduled for ET within 6 hours of ictus. Four cycles of RIC were performed before recanalization, immediately following recanalization, and once daily for the subsequent 7 days. The primary outcome was any serious RIC‐related adverse events.
Results
Twenty subjects, aged 66.1 ± 12.1 years, were recruited. No subject experienced serious RIC‐related adverse events. The intracranial pressure, cranial perfusion pressure, mean arterial pressure, heart rate, middle cerebral artery peak systolic flow velocity, and pulsatility index did not change significantly before, during, or after the limb ischemia (P > 0.1 for all). Of 80 cycles, 71 (89%) were completed before recanalization and 80 (100%) were completed immediately after recanalization; 444 of 560 cycles (78%) were completed within 7 days posttreatment. No patients had to stop RIC because it affected routine clinical managements. Six subjects (30%) experienced intracerebral hemorrhage, which was symptomatic in one case (5%). At the 3‐month follow‐up, 11 subjects (55%) had achieved functional independence, and two subjects (10%) died.
Interpretation
RIC appears to be safe and feasible for patients with AIS undergoing ET. Investigations are urgently needed to determine the efficacy of RIC in this patient population.
Introduction
Several clinical trials have found evidence for the superiority of endovascular treatment (ET) for the treatment of acute ischemic stroke (AIS) caused by an emergent large‐vessel occlusion.1, 2 However, only a small portion of AIS patients with an emergent large‐vessel occlusion actually received ET under the current emergency medical services system (approximately 13% in the USA) because of the rigid therapeutic time window (less than 6 hours).3 Recently, two large‐scale clinical trials have determined the superiority of ET for treating AIS patients with a mismatch between deficit or hypoperfusion and infarct if selected by perfusion imaging within 6–24 h of ictus.4, 5 The extended therapeutic time window would benefit more AIS patients. As the mismatch between the hypoperfusion and the infarct decay over time,6 there is an urgent need to explore strategies that could slow down – if not altogether prevent – the decay of the mismatch in clinical practice. Additionally, approximately 80% of occluded arteries can be recanalized by ET.1, 7 However, less than 50% of patients achieved functional independence and over 15% died 90 days posttreatment.8 Although the mismatch of successful recanalization with poor prognosis can be attributed to many factors, the reperfusion injury may be among the most important.9, 10 Effective neuroprotective strategies to reduce reperfusion injury after ET are therefore urgently needed.
Remote ischemic conditioning (RIC) is a noninvasive strategy in which one or more cycles of brief and transient limb ischemia confers protection against prolonged and severe ischemia in distant organs.11 In the embolic stroke model, a combination of RIC and intravenous thrombolysis was shown to reduce infarct size and improve neurological outcome more than either therapy did when used alone.12 In addition, a clinical study has also found that the prehospital application of RIC reduces the risk of brain tissue infarction in AIS patients receiving intravenous thrombolysis.13 Furthermore, in the transient focal cerebral ischemia‐reperfusion model, the application of RIC before reperfusion or both before and after reperfusion reduces reperfusion injuries and the final infarct size.14, 15
Because patients with AIS who are treated with ET can achieve high rate of recanalization after focal ischemia, this patient population is akin to the model of transient focal cerebral ischemia‐reperfusion and embolic stroke treated with intravenous thrombolysis. Therefore, it may be reasonable to speculate that RIC could also benefit patients with AIS who were treated with ET. To date, RIC has been investigated in patients with AIS within 24 h of ictus, as well as in those receiving intravenous thromblysis.13, 16 However, no studies have yet recruited subjects treated with ET nor determined whether RIC is safe and feasible for such patients.
In this study, we aimed to investigate the safety and feasibility of RIC for patients with AIS undergoing ET and planned for a future phase II study that investigates the efficacy of RIC.
Methods
Study design and subjects
This study was a single‐armed, open‐label, safety, and feasibility trial registered on clinicaltrials.gov (REVISE‐1, NCT03210051). The protocol was approved by the Ethic Committee of Xuanwu Hospital, Capital Medical University.
Patients with AIS who were suspected of having an emergent large‐vessel occlusion in the anterior circulation and who were eligible for ET within 6 h of ictus were recruited. The inclusion criteria for this study included the following: (1) age between 18 and 80 years; (2) initial National Institute of Health Stroke Scale (NIHSS) score ≥6; (3) baseline Cincinnati Prehospital Stroke Severity Scale (CPSSS) score ≥2; (4) could complete groin puncture within 6 h after symptom onset; (5) no remarkable prestroke functional disability (modified Rankin scale [mRS] score ≤1); (6) informed consent obtained from subjects or their legally authorized representative. The exclusion criteria included the following: (1) hypodensity on pretreatment noncontrast CT scans amounting to an Alberta Stroke Program Early CT score (ASPECTS) of less than 6; (2) significant mass effect with midline shift on CT or MRI scans; (3) subjects participating in other ongoing study; (4) unlikely to be available for 90‐day follow‐up; (5) woman of childbearing potential who is was known to be pregnant or lactating; (6) contraindicating for remote ischemic conditioning: severe soft tissue injury, fracture, dysmelia, and other known peripheral vascular disease in the upper limbs.
RIC intervention
After recruiting participants for this study, RIC was performed before recanalization of the occluded artery, immediately following successful recanalization, and once daily for the subsequent 7 days. The RIC procedure consisted of four cycles of unilateral arm ischemia for 5 minutes, which was followed by reperfusion for another 5 minutes. The procedure was performed with an electric, autocontrol device with a cuff that inflated to a pressure of 200 mmHg during the ischemia period. If four cycles were not completed after arriving to the catheter laboratory, the procedure was discontinued if it affected the preparations for ET. RIC was also discontinued if the subject died or was discharged in under 7 days.
Assessment of intracranial pressure and cerebral perfusion pressure
The intracranial pressure and cerebral perfusion pressure were measured on the first day post‐ET, when RIC was performed. Intracranial pressure was measured using a noninvasive intracranial pressure monitoring system (Chongqing Hiwelcom Iatrical Apparatus Co. Ltd, Chongqing, China) that was based on flash visual evoked potentials17 at the following three time points in each cycle: before the initiation of limb ischemia, during limb ischemia, and after limb ischemia. Cerebral perfusion pressure was calculated from the intracranial pressure and corresponding blood pressure with the following formula: cerebral perfusion pressure = (Systolic blood pressure+2*Diastolic blood pressure)/3‐intracranial pressure.
Assessment of cerebral hemodynamics
Cerebral hemodynamics were measured on the first day post‐ET, when RIC was performed. Transcranial Doppler, which was performed by an experienced technician, was used to continuously monitor the middle cerebral artery peak systolic blood flow velocity and pulsatility index. The values were documented at the following three time points in each cycle: before the initiation of limb ischemia, during limb ischemia, and after limb ischemia.
Assessment of vital signs
Systolic blood pressure, diastolic blood pressure, and heart rate were continuously monitored with a multiparameter electrocardiac monitor. Blood pressure (with cuff placed on the contralateral arm) and heart rate were documented at the following three time points in each cycle: before the initiation of limb ischemia, during limb ischemia, and after limb ischemia. Mean arterial pressure was calculated with the formula: mean arterial pressure = (Systolic blood pressure+2*Diastolic blood pressure)/3.
Assessment of imaging
Pretreatment head CT was performed for all patients immediately after their admission to our institution. Posttreatment head CT was generally performed 12–24 h post‐ET, and reperformed seven to nine days post‐ET or before discharge (whichever came earlier), and whenever an intracranial hemorrhage was indicated by clinical evidence. The ASPECTS was evaluated using the pretreatment CT. All images (including the angiograms, the pre‐ET CT, and the post‐ET CT) were analyzed separately by a neurologist and a neuroradiologist. Disagreements were resolved by a consensus reached between the two reviewers, or, if no consensus could be reached, a third reader had the final decision.
Assessment of clinical outcomes and adverse events
All clinical outcomes (including NIHSS score, mRS score, and symptomatic intracranial hemorrhage) and adverse events were evaluated separately by two investigators. Any disagreements were resolved by a consensus reached between them. If the disagreement persisted, a third investigator made the final decision.
Endpoints assessment
Safety assessment
The primary safety outcome was any serious RIC‐related adverse events. Other safety outcomes included any other RIC‐related adverse events as well as any significant changes in intracranial pressure, cerebral perfusion pressure, cerebral hemodynamics, mean arterial pressure, and heart rate before, during, or after limb ischemia.
Feasibility assessment
Feasibility was assessed by the percentage of patients who completed the RIC procedures before and after reperfusion, and the percentage of those whom the doctors or nurses requested to cease the RIC procedure on account of the influence of other treatments.
Other endpoints assessment
Other endpoints included any post‐ET intracranial hemorrhage, symptomatic intracranial hemorrhage according to the European Cooperative Acute Stroke Study III definition18 during the study period, and functional outcomes assessed by mRS at the 3‐month follow‐up.
Statistical analysis
For continuous data, either means ± standard deviation or medians (interquartile range, IQR) were used to summarize data. For binary data, frequencies and percentages were used. The values for intracranial pressure, cerebral perfusion pressure, cerebral hemodynamics, mean arterial pressure, and heart rates measured before, during, and after limb ischemia were self‐compared using repeated‐measures ANOVA. All data were analyzed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA) with p values less than 0.05 (two sides) indicating significance.
Results
In total, 87 patients who were suspected to have AIS were screened in Xuanwu Hospital between July 2017 and September 2017. Twenty of them who were suspected of having an emergent large‐vessel occlusion in the anterior circulation were enrolled in this study.
Baseline characteristics
The demographic characteristics of the subjects are summarized in Table 1. The average age at onset was 66.1 ± 12.1 years. Thirteen subjects (65%) were male and seven subjects (35%) had received intravenous thrombolysis. The median baseline NIHSS score was 16 (IQR: 12–18), the median baseline ASPECTS was 10 (IQR: 8–10), and the median times from onset to groin puncture and recanalization were 325 min (IQR: 296–371) and 408 min (IQR: 367–471), respectively. Seventeen subjects (85%) achieved good or excellent reperfusion (Thrombolysis in Cerebral infarction ≥ 2b), while one subject (5%) failed to achieve any recanalization (Thrombolysis in Cerebral infarction = 0). The vascular risk factors, etiology of stroke, and operational details are shown in Table 1.
Table 1.
Demographic and clinical characteristics
| Characteristics | Value, n = 20 |
|---|---|
| Age | 66.1 ± 12.1 |
| Male | 13 (65) |
| NIHSS | 16 (12–18) |
| ASPECTS | 10 (8–10) |
| Treatment with intravenous alteplase | 7 (35) |
| Onset to groin puncture time | 325 (296–371) |
| Onset to recanalization time | 408 (367–471) |
| Vascular risk factors | |
| Hypertension | 14 (70) |
| Diabetes mellitus | 9 (45) |
| Atrial fibrillation | 5 (25) |
| Smoking | 11 (55) |
| Etiology of stroke | |
| Large artery atherosclerosis | 12 (60) |
| Cardioembolism | 6 (30) |
| Other | 2 (10) |
| Occluded vessel | |
| Internal carotid artery | 7 (35) |
| Middle cerebral artery | 13 (65) |
| Operational details | |
| General anesthesia | 10 (50) |
| Permanent stenting | 5 (25) |
| Intracranial stenting | 3 (15) |
| Extracranial stenting | 2 (10) |
| Balloon angioplasty | 1 (5) |
| TICI=2b/3 | 17 (85) |
| TICI=0 | 1 (5) |
Data are mean ± standard deviation, n (%) or median (interquartile range). NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT score; TICI, Thrombolysis in Cerebral Infarction.
Safety
No serious RIC‐related adverse events occurred. One subject experienced arm skin petechiae without discomfort, and RIC was performed continually.
Values of intracranial pressure, cerebral perfusion pressure, mean arterial pressure, and heart rate measured before, during, and after limb ischemia did not change significantly (P > 0.1 for all) (Figure 1 and Table 2). The peak systolic blood flow velocity and pulsatility index of the affected side and nonaffected side of the middle cerebral artery did not significantly change either (P > 0.1 for all) (Figure 1 and Table 2).
Figure 1.
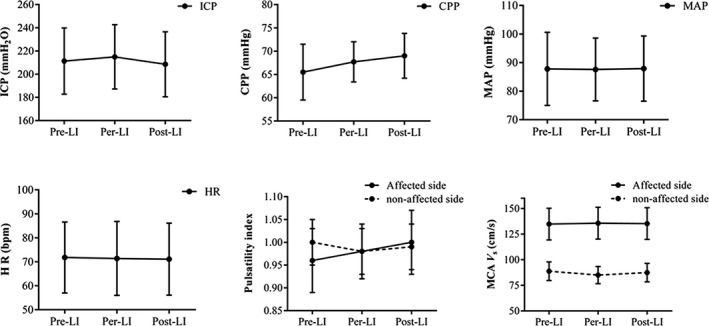
The distribution of ICP, CPP, MAP, HR, and cerebral hemodynamics before, during, and after limb ischemia. Data presented are mean and standard deviation. LI: limb ischemia; ICP, intracranial pressure; CPP, cranial perfusion pressure; MAP, mean arterial pressure; HR, hear rate; MCA V s, middle cerebral artery peak systolic blood flow velocity. Repeated‐measures ANOVA was used for statistical test, no significant difference was found (P > 0.1 each).
Table 2.
Changes of ICP, CPP, MAP, heart rate, and cerebral hemodynamics
| Variable | Pre‐LI | Per‐ LI | Post‐ LI | P value |
|---|---|---|---|---|
| ICP, mmH2O | 211.3 ± 28.5 | 214.9 ± 27.7 | 208.5 ± 28.0 | 0.429 |
| CPP, mmHg | 65.5 ± 6.0 | 67.7 ± 4.3 | 69.0 ± 4.8 | 0.291 |
| MAP, mmHg | 87.8 ± 12.8 | 87.6 ± 11.0 | 87.9 ± 11.4 | 0.824 |
| Heart rate, bpm | 71.8 ± 14.8 | 71.4 ± 15.4 | 71.1 ± 15.0 | 0.784 |
| Affected MCA peak V s (cm/sec) | 134.8 ± 15.5 | 135.7 ± 15.5 | 135.3 ± 15.5 | 0.961 |
| Healthy MCA peak V s (cm/sec) | 88.8 ± 9.1 | 85.0 ± 8.4 | 87.4 ± 9.0 | 0.161 |
| Affected MCA pulsatility index | 0.96 ± 0.07 | 0.98 ± 0.06 | 1.00 ± 0.07 | 0.441 |
| Healthy MCA pulsatility index | 1.00 ± 0.05 | 0.98 ± 0.05 | 0.99 ± 0.05 | 0.807 |
Data are mean ± standard deviation. RIC, remote ischemic conditioning; ICP, intracranial pressure; CPP, cranial perfusion pressure; MAP, mean arterial pressure; MCA, middle cerebral artery; V s: systolic blood flow velocity; LI, limb ischemia.
Feasibility
In total, 71 of 80 cycles (89%) were completed before recanalization and 80 cycles (100%) were completed immediately after recanalization; 444 of 560 cycles (78%) were completed within 7 days posttreatment. Six subjects did not complete the four cycles of RIC prerecanalization because the time from their admission to the catheter laboratory was too short. Ten subjects did not complete the 7‐day post‐ET RIC. Eight subjects were discharged early because of their good recovery, while two subjects died before completing the RIC procedure. No patients were asked to cease RIC because of it influencing routine clinical management (e.g., intravenous fluids through arms and rehabilitation).
Clinical outcomes
Six subjects (30%) experienced intracranial hemorrhage, with one case (5%) being symptomatic. At the 3‐month follow‐up, 11 subjects (55%) achieved functional independence (mRS ≤ 2), while two subjects (10%) died (mRS = 6).
Discussion
In this study, we found that RIC was well‐tolerated and had no significant influence on the intracranial pressure, cerebral perfusion pressure, mean arterial pressure, heart rate, or cerebral hemodynamics of AIS patients treated with ET – even of those after receiving intravenous thrombolysis – and it did not influence routine clinical managements. In addition, over half of the subjects achieved functional independence, and 10% of the subjects died by the 3‐month follow‐up.
RIC could be performed before (i.e., perconditioning) and after reperfusion (i.e., postconditioning). Perconditioning has been demonstrated to reduce the risk of brain tissue infarction,12, 13 and strong evidence shows that postconditioning attenuates reperfusion injury in transient focal ischemia‐reperfusion model.11, 19 These observations informed our study, in which we combined perconditioning with postconditioning in these patients whose afflictions resembled the transient focal ischemia‐reperfusion model.
This phase I study was designed to obtain safety and feasibility data for RIC in patients with AIS undergoing ET. Although the safety and feasibility of RIC have been investigated in humans and in patients with acute stroke,11, 20, 21, 22 patients with AIS who were treated with ET have its own specific characteristics. Generally, patients with AIS and emergent large‐vessel occlusion are much more severely affected than the previously studied patients with AIS, and the rates of recanalization and sufficient reperfusion are much higher in patients treated with ET.8, 13, 16 Furthermore, this patient population is vulnerable, and slight hemodynamic changes may lead to disastrous consequences (e.g., ischemic or hemorrhagic stroke).23 Additionally, these patients often need complicated intensive care, and several peripheral venous accesses might be used.2, 24 Therefore, the application of RIC may be more difficult. Consistent with previous studies that investigating the safety and feasibility of RIC in human,11, 20, 21, 22, 25 we found that RIC could also be safely used in AIS patients treated with ET.
The main concern of the use of RIC in this patient population was repeated limb ischemia induced by occlusion of upper limb vessels, as it might potentially elevate blood pressure and cerebral perfusion pressure, as well as impact cerebral hydrodynamics, which may cause cerebral hyperperfusion. Fortunately, self‐comparisons of blood pressure, intracranial pressure, cerebral perfusion pressure, bilateral middle cerebral artery peak systolic blood flow velocity, and pulsatility index before, during, and after limb ischemia were sufficient to determine the influence of RIC on the aforementioned parameters. Therefore, only one group of participants was recruited in this study, and a separate control group may not be needed. Furthermore, this analysis method could avoid any known or unknown biases between the treatment and control groups.
Several subjects completed only two to three cycles of perconditioning because of the time from their admission to the catheter laboratory was too short to perform the four cycles that the investigation required. Therefore, much earlier (e.g., in prehospital scenarios) initiation of RIC may be a better choice for future studies; it would not only give enough time for four cycles of RIC but also preserve more brain tissue at risk of infarction.26 In addition, the perconditioning protocol has yet to be defined: two or three cycles of stimuli might have similar protective effects as four cycles. Therefore, patients who did not complete four cycles of stimuli might also have benefited from RIC. Compared with previous studies of endovascular treatment for AIS,7, 8, 27 this study found a slightly higher proportion of patients who had achieved functional independence (mRS ≤ 2) at 3‐month follow‐up.
This study has several limitations. First, this is a single‐armed study, and data were not compared with control group. Instead, the data of before, during, and after the limb ischemia were self‐compared, and the clinical outcomes were compared with those of previous studies. Second, the RIC protocol used in this study was rather pragmatic, and the optimal protocol for this patient population needs further investigations. Finally, the trend of improvement in clinical outcome was not powered to determine the efficacy of RIC in patients with AIS who were treated with ET, and this requires further urgent investigation, which is planned in the REVISE‐2, a phase 2 parallel‐group study (https://clinicaltrials.gov/ct2/show/NCT 03045055).
In conclusion, these results suggest that RIC is safe and feasible for patients with AIS who were treated with ET, but the preliminary clinical benefit was not supported by current data. Further studies are needed to confirm these results and determine the efficacy of RIC in this patient population.
Author Contributions
Wenbo Zhao, Changhong Ren, Ran Meng, and Xunming Ji contributed to the conception and design of study. Wenbo Zhao, Ruiwen Che, Sijie Li, Chuanjie Wu, Chuanhui Li, Hui Lu, Jian Chen, and Jiangang Duan contributed to the acquisition and analysis of data. Wenbo Zhao and Xunming Ji contributed to the drafting and revising of the manuscript.
Conflict of Interest
Dr Xunming Ji is one of the inventors of the electric autocontrol device that has been patented in China (ZL 200820123637.X). The other authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by National Key R&D Program of China (No. 2017YFC1308405), Cheung Kong (Chang jiang) Scholars Program (T2014251), The Capital Health Research and Development of Special (2016‐4‐1032), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201706).
Funding Information
This study was supported by National Key R&D Program of China (No. 2017YFC1308405), Cheung Kong (Chang jiang) Scholars Program (T2014251), The Capital Health Research and Development of Special (2016‐4‐1032), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201706).
Funding Statement
This work was funded by National Key R&D Program of China grant 2017YFC1308405; Cheung Kong (Chang jiang) Scholars Program grant T2014251; The Capital Health Research and Development of Special grant 2016‐4‐1032; Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support grant ZYLX201706.
References
- 1. Campbell BC, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta‐analysis of randomized trials. Stroke 2016;47:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 3. Rai AT, Seldon AE, Boo S, et al. A population‐based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg 2017;9:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 6. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017;48:2621–2627. [DOI] [PubMed] [Google Scholar]
- 7. Zhao W, Shang S, Li C, et al. Long‐term outcomes of acute ischemic stroke patients treated with endovascular thrombectomy: a real‐world experience. J Neurol Sci 2018;390:77–83. [DOI] [PubMed] [Google Scholar]
- 8. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 9. Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol 2015;14:758–767. [DOI] [PubMed] [Google Scholar]
- 10. Xunming J. Forward thinking in stroke treatment: advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circ 2015;1:1–2. [Google Scholar]
- 11. Zhao W, Meng R, Ma C, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof‐of‐concept, randomized controlled trial. Circulation 2017;135:1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoda MN, Siddiqui S, Herberg S, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue‐type plasminogen activator in murine model of embolic stroke. Stroke 2012;43:2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014;45:159–167. [DOI] [PubMed] [Google Scholar]
- 14. Ren C, Gao M, Dornbos D 3rd, et al. Remote ischemic post‐conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res 2011;33:514–519. [DOI] [PubMed] [Google Scholar]
- 15. Ren CH, Wang PC, Wang B, et al. Limb remote ischemic per‐conditioning in combination with post‐conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci 2015;33:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. England TJ, Hedstrom A, O'Sullivan S, et al. Recast (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase ii trial in acute ischemic stroke. Stroke 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 17. Tan G, Zhou J, Yuan D, et al. Formula for use of mannitol in patients with intracerebral haemorrhage and high intracranial pressure. Clin Drug Invest 2008;28:81–87. [DOI] [PubMed] [Google Scholar]
- 18. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 19. Simon R. Post‐conditioning and reperfusion injury in the treatment of stroke. Dose‐Response 2014;12:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez NR, Connolly M, Dusick JR, et al. Phase i clinical trial for the feasibility and safety of remote ischemic conditioning for aneurysmal subarachnoid hemorrhage. Neurosurgery 2014;75:590–598.; discussion 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S, Ma C, Shao G, et al. Safety and feasibility of remote limb ischemic preconditioning in patients with unilateral middle cerebral artery stenosis and healthy volunteers. Cell Transplant 2015;24:1901–1911. [DOI] [PubMed] [Google Scholar]
- 22. Koch S, Katsnelson M, Dong C, et al. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase ib study of safety and feasibility. Stroke 2011;42:1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mistry EA, Mistry AM, Nakawah MO, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc 2017;6:e006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leslie‐Mazwi T, Chen M, Yi J, et al. Post‐thrombectomy management of the elvo patient: guidelines from the society of neurointerventional surgery. J Neurointerv Surg 2017;9:1258–1266. [DOI] [PubMed] [Google Scholar]
- 25. Lin E, Snell GI, Levvey BJ, et al. Safety, feasibility, and effect of remote ischemic conditioning in patients undergoing lung transplantation. J Heart Lung Transplant 2014;33:1139–1148. [DOI] [PubMed] [Google Scholar]
- 26. Savitz SI, Baron JC, Yenari MA, et al. Reconsidering neuroprotection in the reperfusion era. Stroke 2017;48:3413–3419. [DOI] [PubMed] [Google Scholar]
- 27. Zhao W, Che R, Shang S, et al. Low‐dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 2017;48:3289–3294. [DOI] [PubMed] [Google Scholar]


