Abstract
Objective
Repetitive transcranial magnetic stimulation (rTMS) is currently being tested for suppressing the symptoms of subjective chronic primary tinnitus, although its effect is controversial. The aim of this randomized double‐blinded controlled trial was to determine the effect of rTMS with unique settings for tinnitus treatment.
Methods
Fifty‐three adult patients suffering from chronic subjective unilateral or bilateral nonpulsatile primary tinnitus for at least 6 months were randomly assigned to rTMS (group 1, n = 20), sham stimulation (group 2, n = 12), or medicament therapy only (group 3, n = 21). The dorsolateral prefrontal cortex (frequency 25 Hz, 300 pulses, and 80% resting motor threshold [RMT]) on the left side and primary auditory cortex (1 Hz, 1000 pulses, 110% RMT) were stimulated on both sides in patients in group 1 for 5 consecutive days. The Tinnitus Reaction Questionnaire (TRQ), Tinnitus Handicap Questionnaire (THQ), Tinnitus Handicap Inventory (THI), Beck Depression Inventory (BDI), pure‐tone audiometry with Fowler scoring of hearing loss, and tinnitus analysis were used to evaluate tinnitus in all patients. Data were recorded the day the patient was included in the study and at 1‐ and 6‐month follow‐up.
Results
The study groups were homogenous. No significant effect of rTMS was found at 1 or 6 months based on the BDI, THQ, and TRQ scores or tinnitus masking. There was a significant but clinically irrelevant effect on the THI score after 1 and 6 months.
Interpretation
No significant effect of bilateral low‐frequency rTMS of the primary auditory cortex and high‐frequency stimulation of the left dorsolateral prefrontal cortex was demonstrated.
Introduction
Tinnitus is defined as hearing a noise or sound without any external acoustic stimulation and is a common symptom experienced by approximately 10–15% of the general population, and 4–5% are severely affected by it.1, 2, 3 The perception of tinnitus causes problems with concentration, falling asleep, anxiety, and feelings of depression. Thus, tinnitus can have severe negative implications on the perceived quality of life.4
Concerning etiology, there is a differentiation between objective and subjective tinnitus. Objective tinnitus can be heard by an external observer and is a very rare form of tinnitus that may be caused by a vascular or muscular condition.5 In contrast, subjective tinnitus cannot be heard by an external observer and no acoustic sound source can be identified. The condition is thought to be the result of plastic changes and reorganization processes in the auditory pathway and brain structures, most likely caused by the deprivation of input.6 Tinnitus is considered primary if no cause is revealed or secondary if a cause can be determined, and acute if it lasts less than 6 months or chronic if it lasts longer than 6 months.
Therapy for chronic primary subjective tinnitus is challenging. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive method that can modulate the excitability of the brain cortex and is currently being tested for suppressing the symptoms of tinnitus.7, 8 The use of rTMS in the treatment of tinnitus stems from the development of models of the central generation and maintenance of disabling subjective tinnitus. However, in contrast to the treatment of other brain pathologies, many uncertainties remain regarding the current relevance of the use of rTMS as a treatment for tinnitus, especially in the long term.9 Tinnitus reduction was mainly referred to in earlier studies, which only reached class III (absence of blinded controlled evaluation), and was generally described as partial and temporary with large interindividual variations.9 On the other hand, class I studies recently showed nonsignificant changes between active and placebo conditions.10, 11, 12
In general, studies exhibit considerable variability. Many methodological and practical problems remain to be solved before rTMS therapy can be developed for tinnitus in clinical practice. In particular, these problems concern the method and center(s) of targeting, as well as the side of stimulation.
The side contralateral to the tinnitus was used as a target for stimulation or application in the left hemisphere in most studies.9 However, the auditory pathway ends in both hemispheres, and no functional changes in the auditory cortex have been found in patients with tinnitus compared to healthy controls when measuring brain metabolism.4 In addition, no differences have been found in studies comparing the effect of stimulation delivered contralaterally or ipsilaterally to the symptomatic ear.9, 13 Despite this, very few studies have used bilateral stimulation.11, 14 This is also why mostly only patients with unilateral tinnitus were included.
On the other hand, there is nearly agreement regarding stimulation frequency. In general, TMS protocols with <1 Hz frequency are considered inhibitory protocols and used mostly for the treatment of tinnitus.9 An increasing amount of data also suggest that the efficacy of rTMS therapy in tinnitus can be enhanced by stimulating frontal or prefrontal cortical areas in addition to the temporoparietal cortex.10, 15, 16, 17, 18, 19 These results are in line with increased functional connectivity between frontal and temporal cortical areas in tinnitus patients on imaging.20
The aim of this prospective study was to determine the effect of rTMS with unique settings on the treatment of primary subjective nonpulsatile tinnitus. Therefore, the study was set up as a parallel double‐blinded randomized controlled trial considering that rTMS results from crossover studies must be considered with care because patient blinding may not be adequate (the difference between real and placebo rTMS could be obvious for a subject undergoing both forms) and carryover effects may exist. A group treated by medicament therapy alone was also added to the comparison. There is currently no effective pharmacological treatment for chronic tinnitus. Therefore, this group can be considered another placebo group.
We tried to adjust the study setting according to the most recent data the way we believed rTMS could provide the maximal effect for tinnitus treatment with an awareness of eventual higher risks of side effects. The generally acknowledged inhibitory stimulation (1 Hz frequency) was targeted to the primary auditory cortex bilaterally. In addition, the stimulatory frequency (25 Hz) was targeted to the left dorsolateral prefrontal cortex.
Materials and Methods
This randomized double‐blinded controlled trial was approved by the Ethics Committee of the University Hospital Ostrava and performed in accordance with the Declaration of Helsinki and applicable regulatory requirements with good clinical practice. The study was registered at ClinicalTrials.gov under the identifier NCT03425045. Written informed consent was obtained from the patients before initiating any procedure. The study was performed between March 2015 and May 2017 in a tertiary referral hospital. All authors reviewed and approved the final manuscript.
Patients
Adult patients suffering from unilateral or bilateral chronic subjective nonpulsatile primary tinnitus for at least 6 months were included in the study. The definition of tinnitus was based on subjective complaints of noise, ringing, and/or buzzing with no external source. Exclusion criteria were as follows: head injury or brain surgery, epilepsy, organic brain lesion, Meniere's disease or fluctuating hearing loss, cochlear or bone‐anchored hearing device implantation, history of attempted suicide, pregnancy, consumption of anticonvulsants or antipsychotic medication, pacemaker, or previous rTMS.
Randomization and blinding
Using random number generation, patients were assigned into the rTMS group, sham stimulation group, or medicament therapy only group. Both the patients and outcome assessor were blinded to the intervention group to which the patients belonged. Stimulation was performed in different hospital building by investigator, who was not in contact with outcome assessor or patients except for the course of stimulation. Patients receiving medicament therapy were not blinded.
Positioning
To achieve optimal coil positioning at the patient's primary auditory cortex, image‐guided stereotaxy was performed with the aid of a frameless stereotactic device using structural imaging data to guide TMS coil placement. The location of the patient's primary auditory cortex and dorsolateral prefrontal cortex was determined on a structural T1‐weighted magnetic resonance image with gadolinium contrast that was performed during the diagnostic stage (Magnetom Avanto Siemens 1.5‐Tesla, Siemens Healthcare GmbH, Erlangen, Germany).
The patients were seated in a desk chair with their chin in a jaw support and their forehead secured with a band against a support bar. Using a template, the coil was positioned above the marked location with the handle pointing upwards, perpendicular to the skull. The coil was held in place against the patient's head by a mechanical arm. The location of the targeted cortex was marked with ink on the scalp, and a neurosurgical marker was placed in order to identify the spot in the following days. The patients were provided with ear plugs to minimize the noise dose and possible residual inhibition.
Stimulation
The DuoMAG XT‐100 transcranial magnetic stimulator (Deymed, Payette, ID, USA) was used for magnetic stimulation. The rTMS was performed with a 70‐mm air‐cooled 70BF Butterfly Coil (Deymed). The resting motor threshold (RMT) was determined in every rTMS patient on the first day of treatment using a descending staircase method until the lowest intensity at which 5 of 10 consecutive pulses induced a visible twitch in the contralateral hand was reached. For each hemisphere, the intensity was set according to the motor threshold obtained for that hemisphere. The dorsolateral prefrontal cortex (frequency 25 Hz, 300 pulses, and 80% RMT) on the left side and primary auditory cortex on both sides (1 Hz, 1000 pulses, and 110% RMT) were stimulated in every patient for 5 consecutive days. There was no difference between rTMS group and sham stimulation group. Every patient received 2300 pulses per session (three stimulation sites). A 5–10 min break was used to switch the coil from one position to the other and to allow the patient to relax. All patients were treated by the same investigator.
Placebo treatment was performed with a 70‐mm 70BFP Placebo Butterfly Coil (Deymed) replicating the appearance, sound emission, stimulation of superficial tissue (muscles), and operation of the TMS coil without stimulating the cortical tissue. Motor thresholds were not determined in placebo patients to prevent them from perceiving the difference between real and placebo TMS, protecting the blinding. The neuronavigation procedure and treatment schedule were similar.
Medicament therapy
Medicament therapy consisted of ginkgo biloba extract EGb 761 once a day for 6 months. No medicament therapy was given for tinnitus in the rTMS and sham groups.
Data acquisition
The Tinnitus Reaction Questionnaire (TRQ), Tinnitus Handicap Questionnaire (THQ), Tinnitus Handicap Inventory (THI), Beck Depression Inventory (BDI), pure‐tone audiometry with Fowler scoring of hearing loss, and tinnitus analysis (loudness matching) were used to evaluate tinnitus in all patients. Audiometry and tinnitus analysis were performed by one audiology assistant trained in tinnitus analysis and blinded to treatment type. Testing was performed in a soundproof cabin using a Madsen Orbiter 922 audiometer (Madsen Ltd., Budapest, Hungary) compliant with ISO 389 standards. Pure‐tone audiometry was performed according to international standards (ISO 8253‐1).
Follow‐up
Data were recorded the day patient was included in the study and during follow‐up at 1 and 6 months.
Statistical analysis
Descriptive statistics, such as the arithmetic mean, standard deviation, and absolute and relative frequency tables, were used for data processing. Pearson's chi‐squared test, Fisher's exact test, Kruskal–Wallis test, and analysis of variance were used for comparisons among groups. The statistical tests were assessed using a significance level of 5%. The statistical analysis was performed using Stata 13 software (Stata Corp., College Station, TX, USA). Risk groups were identified using SPSS Answer Tree 3.1 (IBM Corp., Armonk, NY, USA).
Results
A total of 56 patients suffering from unilateral or bilateral chronic subjective nonpulsatile primary tinnitus for at least 6 months were included in the study (Table 1). Compliance with follow‐up was 94.6%. One patient in the rTMS group and two patients in sham group were lost during follow‐up and excluded from the study. Twenty‐six patients suffered from unilateral tinnitus and 27 from bilateral tinnitus. Tinnitus was right sided in 34 cases and left sided in 46 cases. No differences were found among the three intervention groups with regards to average age, gender distribution, tinnitus duration, hearing loss, or education level (Table 1).
Table 1.
Characteristics of the study participants
| rTMS group (n = 20) | Sham group (n = 12) | Medicament group (n = 21) | P‐value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 47.9 ± 14.31 | 51.8 ± 10.34 | 60.6 ± 15.6 | 0.156a | ||||||
| Male | 13 | 10 | 10 | 0.120b | ||||||
| Female | 7 | 2 | 11 | 0.120b | ||||||
| Tinnitus duration (months) | 53.4 ± 61.89 | 76.8 ± 76.85 | 36.5 ± 30.93 | 0.436c | ||||||
| Hearing loss | 9 | 6 | 14 | 0.353b | ||||||
| Education level | Prim. | Sec. | Ter. | Prim. | Sec. | Ter. | Prim. | Sec. | Ter. | 0.295d |
| 6 | 8 | 6 | 4 | 5 | 3 | 13 | 5 | 3 | ||
Data are given as mean ± standard deviation or n. Prim, primary; Sec, secondary; Ter, tertiary.
Kruskal–Wallis test.
Pearson's chi‐squared test.
Analysis of variance.
Fisher's exact test.
No significant effect of rTMS was found in the BDI, THQ, and TRQ scores or tinnitus masking at 1 or 6 months compared to the sham coil group and medicament therapy only when number of improved patients was evaluated (Table 2). No significant effect of rTMS was found in the BDI, THQ, TRQ, and THI scores when analysis of variance was performed (Figures 1, 2, 3, 4, Table 3).
Table 2.
Comparison of improvement in questionnaires’ score and tinnitus masking after 1 and 6 months
| 1 month | 6 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Not improved | Improved | Not improved | Improved | ||||||
| N | % | N | % | N | % | N | % | ||
| BDI | rTMS | 9 | 47% | 10 | 53% | 10 | 50% | 10 | 50% |
| Sham | 8 | 67% | 4 | 33% | 7 | 58% | 5 | 42% | |
| Medicament | 13 | 68% | 6 | 32% | 11 | 52% | 10 | 48% | |
| P = 0.359a | P = 0.900a | ||||||||
| THI | rTMS | 7 | 37% | 12 | 63% | 6 | 30% | 14 | 70% |
| Sham | 5 | 42% | 7 | 58% | 4 | 33% | 8 | 67% | |
| Medicament | 15 | 75% | 5 | 25% | 15 | 71% | 6 | 29% | |
| P = 0.039a | P = 0.016a | ||||||||
| THQ | rTMS | 7 | 37% | 12 | 63% | 7 | 35% | 13 | 65% |
| Sham | 6 | 50% | 6 | 50% | 5 | 42% | 7 | 58% | |
| Medicament | 9 | 45% | 11 | 55% | 12 | 57% | 9 | 43% | |
| P = 0.754a | P = 0.348a | ||||||||
| TRQ | rTMS | 6 | 32% | 13 | 68% | 6 | 30% | 14 | 70% |
| Sham | 5 | 42% | 7 | 58% | 4 | 33% | 8 | 67% | |
| Medicament | 12 | 60% | 8 | 40% | 10 | 48% | 11 | 52% | |
| P = 0.197a | P = 0.477a | ||||||||
| Tinnitus masking (right) | rTMS | 8 | 67% | 4 | 33% | 10 | 77% | 3 | 23% |
| Sham | 6 | 86% | 1 | 14% | 3 | 43% | 4 | 57% | |
| Medicament | 12 | 92% | 1 | 8% | 12 | 86% | 2 | 14% | |
| P = 0.269b | P = 0.149b | ||||||||
| Tinnitus masking (left) | rTMS | 14 | 82% | 3 | 18% | 14 | 82% | 3 | 18% |
| Sham | 7 | 70% | 3 | 30% | 4 | 40% | 6 | 60% | |
| Medicament | 13 | 72% | 5 | 28% | 13 | 68% | 6 | 32% | |
| P = 0.740b | P = 0.083b | ||||||||
BDI, Beck Depression Inventory; rTMS, repetitive transcranial magnetic stimulation; THI, Tinnitus Handicap Inventory; THQ, Tinnitus Handicap Questionnaire; TRQ, Tinnitus Reaction Questionnaire.
Pearson's chi‐squared test.
Fisher's exact test.
Figure 1.
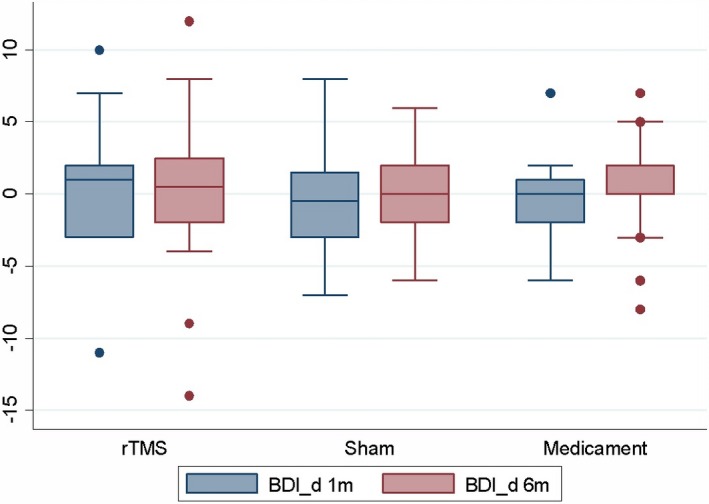
Change in BDI scores after 1 and 6 months (=positive value means improvement).
Figure 2.
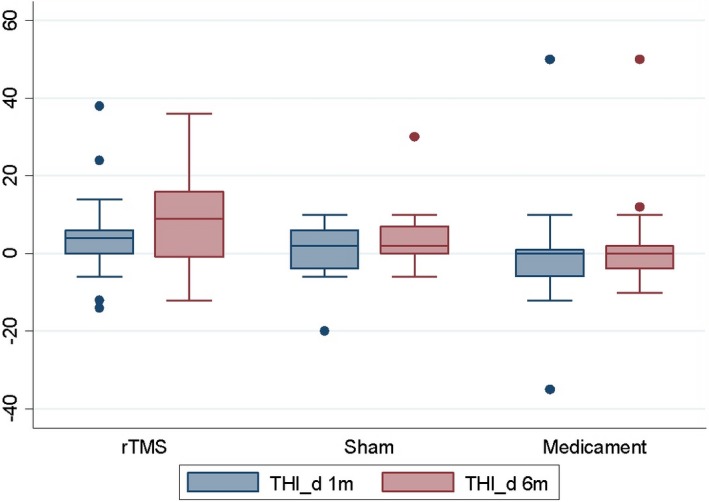
Change in THI scores after 1 and 6 months (=positive value means improvement).
Figure 3.
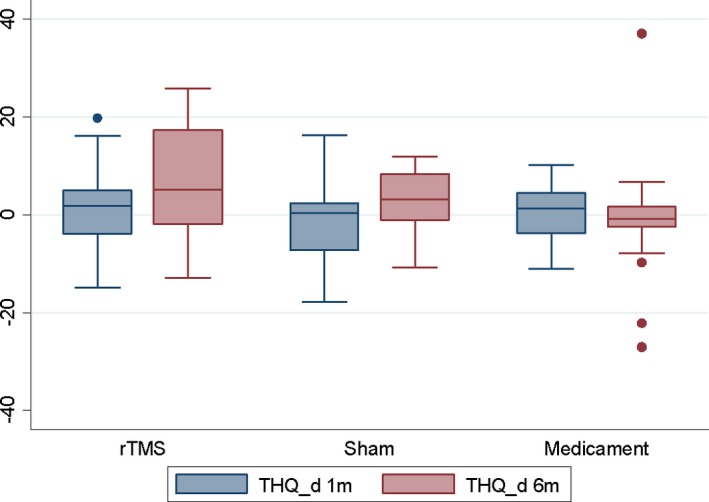
Change in THQ scores after 1 and 6 months (=positive value means improvement).
Figure 4.
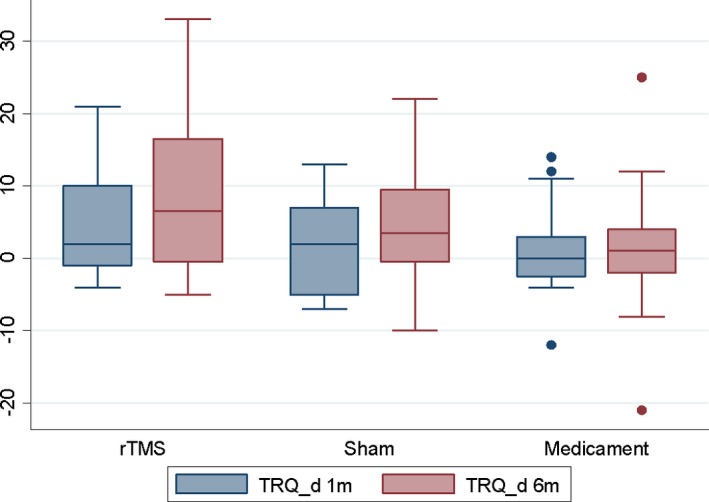
Change in TRQ scores after 1 and 6 months (=positive value means improvement).
Table 3.
Evaluation of changes in questionnaires’ score after 1 and 6 months (=positive value means tinnitus improvement)
| 1 month | 6 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min. | Max. | N | Mean | SD | Min. | Max. | ||
| BDI | rTMS | 19 | 0.5 | 4.40 | −11 | 10 | 20 | 0.1 | 5.50 | −14 | 12 |
| Sham | 12 | −0.6 | 4.29 | −7 | 8 | 12 | 0.0 | 3.59 | −6 | 6 | |
| Medicament | 19 | −0.4 | 2.91 | −6 | 7 | 21 | 0.3 | 3.20 | −8 | 7 | |
| P = 0.681a | P = 0.9731a | ||||||||||
| THI | rTMS | 19 | 4.5 | 11.72 | −14 | 38 | 20 | 9.1 | 11.85 | −12 | 36 |
| Sham | 12 | 0.2 | 7.98 | −20 | 10 | 12 | 4.3 | 9.41 | −6 | 30 | |
| Medicament | 20 | −0.7 | 14.90 | −35 | 50 | 21 | 1.4 | 12.39 | −10 | 50 | |
| P = 0.3945a | P = 0.1111a | ||||||||||
| THQ | rTMS | 19 | 1.5 | 8.52 | −14.8 | 19.8 | 20 | 6.1 | 12.55 | −12.9 | 25.9 |
| Sham | 12 | −1.1 | 8.75 | −17.8 | 16.3 | 12 | 2.8 | 6.34 | −10.8 | 11.9 | |
| Medicament | 20 | 0.1 | 6.23 | −11.1 | 10.3 | 21 | −1.2 | 11.99 | −27 | 37.1 | |
| P = 0.6489a | P = 0.1272a | ||||||||||
| TRQ | rTMS | 19 | 4.9 | 7.04 | −4 | 21 | 20 | 9.1 | 11.55 | −5 | 33 |
| Sham | 12 | 1.5 | 6.69 | −7 | 13 | 12 | 4.7 | 8.24 | −10 | 22 | |
| Medicament | 20 | 1.4 | 6.27 | −12 | 14 | 21 | 1.7 | 8.84 | −21 | 25 | |
| P = 0.1964a | P = 0.0641a | ||||||||||
BDI, Beck Depression Inventory; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation; THI, Tinnitus Handicap Inventory; THQ, Tinnitus Handicap Questionnaire; TRQ, Tinnitus Reaction Questionnaire.
One‐way analysis of variance for repeated measures.
There was significant effect on THI score at 1 and 6 months. Improvement was found after 1 month in 63% of patients in the rTMS group, 58% of patients in the sham coil group, and 25% of patients in the medicament therapy group (P = 0.039). Improvement was also found after 6 months in 70% of patients in the rTMS group, 67% of patients in the sham coil group, and 29% of patients in the medicament therapy group (P = 0.016; Table 2). The effect of therapy was not dependent on education level at 6 months (Table 4).
Table 4.
Education level and effect of therapy after 6 months
| Education level | BDI change | THI change | THQ change | TRQ change | ||||
|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | |
| Primary | 13 | 10 | 13 | 10 | 11 | 12 | 8 | 15 |
| Secondary | 10 | 8 | 7 | 11 | 7 | 11 | 6 | 12 |
| Tertiary | 5 | 7 | 5 | 7 | 6 | 6 | 6 | 6 |
| P‐valuea | 0.677 | 0.485 | 0.793 | 0.606 | ||||
BDI, Beck Depression Inventory; I, improved; NI, not improved; THI, Tinnitus Handicap Inventory; THQ, Tinnitus Handicap Questionnaire; TRQ, Tinnitus Reaction Questionnaire.
Pearson's chi‐squared test.
In general, treatment was tolerated well. Three patients experienced temporal side effects from rTMS (all headache) and three patients experienced temporal side effects from placebo (1 headache, 1 dizziness, and 1 blurred vision).
Discussion
The parallel double‐blinded randomized controlled study was uniquely set according to the most recent data the way we believed rTMS could provide the maximal effect for tinnitus treatment. Although multiple sites stimulation was performed, a higher risk of side effects was not reported. However, no significant effect of rTMS was found at 1 or 6 months based on the BDI, THQ, and TRQ scores or tinnitus masking in our study. Although a significant effect of rTMS on THI score was found after 1 and 6 months when number of improved patients was evaluated, but the effect was nearly the same as with the sham coil. There was improvement at 1 month in 63% and 58% of patients in the rTMS and sham groups, respectively, and the difference was even smaller after 6 months. Improvement was noted in 70% patients in the rTMS group and 67% in the sham coil group. Although a positive trend in the THI score may have been in favor of rTMS, the effect was so small that it should be considered clinically irrelevant. The biggest difference in THI score was when both groups were compared to the medicament therapy group, in which only 25% and 29% of patients noted tinnitus improvement, respectively. The THI is a 25‐item self‐response questionnaire with three possible answers (yes, sometimes, and no) and a score range 0–100. It was developed for busy clinical practice to quickly quantify the impact of tinnitus on daily living.21 Therefore, it could be less precise in scoring tinnitus severity than the more time‐consuming THQ or TRQ and has been evaluated in other studies as only a secondary outcome.22 In general, questionnaires’ scores were very variable at the time of indication. Therefore, evaluation of number of improved patients was preferred as main parameter. However, no significant effect of rTMS was found even if analysis of variance was performed. Results were very variable among patients as shown in box plots.
Some studies have suggested that tinnitus of short duration (<2 years) and normal hearing could be predictors of beneficial treatment outcomes.23, 24, 25 However, this was not confirmed in an analysis of larger samples.26 In our study, there was no difference between groups in terms of tinnitus duration or hearing loss. The effect of therapy could be also dependent on socioeconomic status. However, the effect of therapy was not dependent on education level at 6 months. Result could be influenced by short treatment phase, which belongs among shorter referred treatment phases. Some authors even recommend treatment for several weeks.27 However, number of improved patients is regardless the shorter treatment phase relatively high in our study. Therefore, more likely high number of improved patients in sham/medicament group is an issue from the statistical point of view.
Our results are in agreement with recent analogical class I studies in which no significant changes between active and placebo conditions were found.11, 24 Even when additional stimulation targeted left dorsolateral prefrontal cortex after bilateral primary cortex stimulation, no significant changes (except THI score) were found compared to sham stimulation or medicament therapy only. The results could be explained physiologically by recently reported data showing no changes in neural connectivity following rTMS therapy targeting the left temporal junction in resting‐state functional connectivity on functional magnetic resonance imaging.28
Further research is necessary to identify better targets and better stimulation settings before rTMS therapy could be developed for clinical practice.
Conclusions
This study did not show a significant effect of bilateral low‐frequency rTMS of the primary auditory cortex and additional high‐frequency stimulation of the left dorsolateral prefrontal cortex compared to parallel placebo sham coil treatment and medicament therapy only. Further research is necessary to identify better targets and settings for rTMS treatment in patients with chronic subjective nonpulsatile primary tinnitus before rTMS therapy can be developed for clinical practice.
Author Contributions
M.F. – conception and design of the study, acquisition and analysis of data, and drafting a significant portion of the manuscript; P.M. – acquisition of data; P.K. – acquisition of data; M.B. – conception and design of the study and analysis of data; D.J. – acquisition of data; H.Z. – conception and design of the study; H.T. – analysis of data; K.Z. – conception and design of the study and analysis of data; and P.K. – conception and design of the study.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (FNOs/2015).
Funding Information
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (FNOs/2015).
Funding Statement
This work was funded by Ministry of Health, Czech Republic – conceptual development of research organization grant FNOs/2015.
References
- 1. Bauer C. Tinnitus and hyperacusis In: Flint P, Haughey B, Lund V, Niparko J, Richardson M, Robbins KT, Thomas JR, eds. Cummings otolaryngology: head and neck surgery. Philadelphia: Mosby, 2010:2131–2139. [Google Scholar]
- 2. Axelsson A, Ringdahl A. Tinnitus – a study of its prevalence and characteristics. Br J Audiol 1989;23:53–62. [DOI] [PubMed] [Google Scholar]
- 3. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am 2003;36:239–248. [DOI] [PubMed] [Google Scholar]
- 4. Geven LI, de Kleine E, Willemsen AT, van Dijk P. Asymmetry in primary auditory cortex activity in tinnitus Patients and controls. Neuroscience 2014;256:117–125. [DOI] [PubMed] [Google Scholar]
- 5. Perry BP, Gantz BJ. Medical and surgical evaluation and management of tinnitus In: Tyler RS, ed. Tinnitus handbook. San Diego: Singular Thomson Learning, 2010:221–242. [Google Scholar]
- 6. Møller AR. Neural plasticity and disorders of the nervous system. Cambridge: Cambridge University Press, 2006. [Google Scholar]
- 7. Folmer LR, Carrol JR, Rahim A, et al. Effects of repetitive transcranial magnetic stimulation (rTMS) on chronic tinnitus. Acta Otolaryngol Suppl 2006. Dec;556:96–101. [DOI] [PubMed] [Google Scholar]
- 8. Oberman L, Edwards D, Eldaief M, Pascual‐leone A. Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J Clin Neurophysiol 2011;28:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lefaucheur JP, André‐Obadia N, Antal A, et al. Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–2206. [DOI] [PubMed] [Google Scholar]
- 10. Langguth B, Landgrebe M, Frank E, et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J Biol Psychiatry 2014;15:276–285. [DOI] [PubMed] [Google Scholar]
- 11. Hoekstra CE, Versnel H, Neggers SF, et al. Bilateral lowfrequency repetitive transcranial magnetic stimulation of the auditory cortex in tinnitus patients is not effective: a randomised controlled trial. Audiol Neurootol 2013;18:362–373. [DOI] [PubMed] [Google Scholar]
- 12. Engelhardt J, Dauman R, Arné P, et al. Effect of chronic cortical stimulation on chronic severe tinnitus: a prospective randomized double‐blind cross‐over trial and long‐term follow up. Brain Stimul 2014;7:694–700. [DOI] [PubMed] [Google Scholar]
- 13. Kim BG, Kim DY, Kim SK, et al. Comparison of the outcomes of repetitive transcranial magnetic stimulation to the ipsilateral and contralateral auditory cortex in unilateral tinnitus. Electromagn Biol Med 2014;33:211–215. [DOI] [PubMed] [Google Scholar]
- 14. Plewnia C, Vonthein R, Wasserka B, et al. Treatment of chronic tinnitus with θ burst stimulation: a randomized controlled trial. Neurology 2012;78:1628–1634. [DOI] [PubMed] [Google Scholar]
- 15. Kleinjung T, Eichhammer P, Landgrebe M, et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol Head Neck Surg 2008;138:497–501. [DOI] [PubMed] [Google Scholar]
- 16. Kreuzer PM, Landgrebe M, Schecklmann M, et al. Can temporal repetitive transcranial magnetic stimulation be enhanced by targeting affective components of tinnitus with frontal rTMS? A randomized controlled pilot trial. Front Syst Neurosci 2011;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Ridder D, Song JJ, Vanneste S. Frontal cortex TMS for tinnitus. Brain Stimul 2013;6:355–362. [DOI] [PubMed] [Google Scholar]
- 18. Lehner A, Schecklmann M, Kreuzer PM, et al. Comparing single‐site with multisite rTMS for the treatment of chronic tinnitus – clinical effects and neuroscientific insights: study protocol for a randomized controlled trial. Trials 2013;14:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehner A, Schecklmann M, Poeppl TB, et al. Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network—a pilot study. Brain Topogr 2013;26:501–510. [DOI] [PubMed] [Google Scholar]
- 20. Schlee W, Weisz N, Bertrand O, et al. Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS ONE 2008;3:e3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 1996;122(2):143–148. [DOI] [PubMed] [Google Scholar]
- 22. Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 2003;83:137–153. [DOI] [PubMed] [Google Scholar]
- 23. Kleinjung T, Steffens T, Sand P, et al. Which tinnitus patients benefit from transcranial magnetic stimulation? Otolaryngol Head Neck Surg 2007;137:589–595. [DOI] [PubMed] [Google Scholar]
- 24. Khedr EM, Rothwell JC, Ahmed MA, El‐Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry 2008;79:212–215. [DOI] [PubMed] [Google Scholar]
- 25. Marcondes RA, Sanchez TG, Kii MA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: a double‐blind controlled, clinical and neuroimaging outcome study. Eur J Neurol 2010;17:38–44. [DOI] [PubMed] [Google Scholar]
- 26. Frank G, Kleinjung T, Landgrebe M, et al. Left temporal low‐frequency rTMS for the treatment of tinnitus: clinical predictors of treatment outcome‐a retrospective study. Eur J Neurol 2010;17:951–956. [DOI] [PubMed] [Google Scholar]
- 27. Rossini PM, Burke D, Chen R, et al. Non‐invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an IFCN Committee. Clin Neurophysiol 2015;126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roland LT, Peelle JE, Kallogjeri D, et al. The effect of noninvasive brain stimulation on neural connectivity in Tinnitus: a randomized trial. Laryngoscope 2016;126:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]


