Abstract
It is a well-established fact that unfavourable social and economic conditions have a negative impact on health and longevity. Recent findings suggest that this is also true of age-related dementias. Yet most common indicators of socioeconomic status (SES) say very little about the actual mechanisms at play in disease development. The present paper explores five work exposure characteristics, all of which have a clear social gradient, that could potentially shed further light on the relationship between SES and dementia. Specifically, it investigates whether these exposures could moderate the impact of a well-known genetic risk factor: the APOE ɛ4 allele. The empirical analyses are based on data from a Swedish population study (n = 1019). Main occupation was linked to The Job Exposure Matrix to estimate the individuals’ exposure to the following work environment factors: work control, support, psychological demands, physical demands and job hazards. All analyses were conducted using binary logistic regression and focused specifically on gene-work exposure interactions. A significant main effect of work control on dementia risk was detected for males (OR = 0.68; p< 0.05), but not for females. However, control was found to significantly moderate the effect of APOE ɛ4 in both genders, albeit in different ways. These findings do not only underscore the importance of considering interactions between social and genetic risk factors to better understanding multifactorial diseases such as dementia. They also propose that gender- and class-based inequities interact, and hence must be considered simultaneously, also in relation to this particular disease.
Keywords: Dementia, Population studies, Gender, APOE ɛ4, Work environment conditions, Job Exposure Matrix
Highlights
-
•
There are steep social gradients in exposure to various work environment conditions.
-
•
Work control alters the effect of APOE ɛ4 in both genders, albeit in different ways.
-
•
High work control protects male, but not female, APOE ɛ4-carriers.
-
•
‘Male’ high control jobs differ greatly from ‘female’ high control jobs.
-
•
Gender- and class-based inequities interact also in the development of dementia.
1. Introduction
The present study seeks to shed further light on the complex relationship between socioeconomic status (SES) and health or, more specifically, old-age dementia. It draws on the idea that SES differences in health are, at least partly, attributable to differences in work environment (Borg and Kristensen, 2000, Marmot et al., 1997, Nilsen et al., 2014) and adds to the current state of research by exploring whether work exposures can in fact moderate genetic risk. With the increase in longevity all across the world, dementia has become a public health issue of major concern to all ageing societies (Winblad et al., 2016; World Health Organization, 2012). The most common cause of dementia is Alzheimer’s disease (AD), accounting for 50–70 per cent of all cases, and the majority of elderly people have multimorbidity involving Alzheimer-related changes in combination with other pathologies, especially cerebrovascular changes (Attems and Jellinger, 2013, James et al., 2016). The major genetic risk factor for AD is the apolipoprotein E (APOE) ɛ4 allele – a non-causative mutation known to increase disease risk by between three and 15 times (Blennow, de Leon & Zetterberg, 2006; Scheltens et al., 2016). Further, dementia follows a social gradient (Marmot, 2004). Low educational attainment stands out as a prominent risk factor (Meng & D’Arcy, 2013; Ngandu et al., 2007; Wang et al., 2012; Winblad et al., 2016), and previous findings suggest that low occupational class is associated with increased disease risk (Hasselgren et al., 2018; Qiu et al., 2003; Sattler, Toro, Schoenknecht, & Schroeder, 2012). Still, current knowledge is limited concerning whether and how social inequities interact with individual genetic endowments in the development of disease. Additionally, most common indicators of SES, including those mentioned above, say very little about the actual mechanisms at play in disease development. Given that most individuals spend a vast amount of time at work throughout life, it is hardly surprising that work environment exposures seem to partly explain SES differences in a variety of health outcomes, including cognitive decline (Borg and Kristensen, 2000, Marmot et al., 1997, Nilsen et al., 2014, Toivanen and Hemström, 2006). In the present paper, we explore a range of work exposures that could potentially shed further light on the relationship between SES and dementia. Additionally, we set out to investigate if any of these exposures could moderate the impact of the APOE ɛ4 allele, and whether gender differences exist in this respect.
1.1. Dementia and work environment exposures
Some of the most important psychosocial work exposures include demand characteristics of work tasks, job demands (e.g., work load), decision latitude (e.g., control over the work process/intellectual discretion) and social support (Johnson and Hall, 1988, Karasek, 1979, Stansfeld and Candy, 2006, Then et al., 2014). Many of these factors have previously been linked to cognitive decline in general (Andel et al., 2011, Elovainio et al., 2009) as well as to prospective cognitive complaints (Stenfors, Hanson, Oxenstierna, Theorell, & Nilsson, 2013). In relation to dementia specifically, a number of studies suggest that psychosocial factors such as high levels of control, social demands/support (Andel et al., 2012, Seidler et al., 2004) and challenge at work (Seidler et al., 2004) serve a potentially protective function, also when controlling for APOE ɛ4 allelic status (Wang, Wahlberg, et al., 2012). Likewise, high job strain1 and work-related stress in midlife have been suggested to increase the risk of dementia late in life (Sindi et al., 2016). However, it still remains unclear whether work environment exposures could moderate genetic risk in dementia, as they have been suggested to do in relation to, e.g., atherosclerosis (Hintsanen et al., 2008, Hintsanen et al., 2007). With regard to having a physically demanding job, the evidence is still limited, although associations between physical demands and occurrence of AD (Smyth et al., 2004), as well as indicators of AD pathology (Stern et al., 1995), have been reported. In relation to job hazards, this is, to our knowledge, the first study to explore the potential association between dementia and hazardous work using an index that combines a range of adverse exposures (see Section 2.3). Two suggested physiological mechanisms seem to dominate previous studies dealing with the association between psychosocial work exposures and dementia. First, with regard to occupations in which decision latitude, skill discretion and/or psychological demands are high, the cognitive reserve hypothesis (Stern, 2002, Stern, 2012) proposes that activities containing intellectual stimulation could increase compensatory abilities in the neuronal networks and hence improve the brain’s resilience to degenerative changes (Qiu et al., 2009, Then et al., 2017). The second explanation is related to stress and vascular mechanisms (Johansson et al. 2010; Johansson et al., 2013; Wang et al., 2012). For example, stress is known to be associated with cardiovascular risk factors such as hypertension (Kivimäki et al., 2002, Vrijkotte et al., 2000) that, in turn, increases the risk of developing dementia (Kivipelto et al., 2001; Skoog et al., 1996). Further, it is hypothesized to heighten levels of glucocorticoid hormones in the blood, which could cause damage to the hippocampus (Bremner, 2006, Lupien et al., 1999, Sapolsky, 1996) and, possibly, increase buildup of beta-amyloid (Aβ) peptide and tau-protein in the brain (both of which are involved in the development of AD) (Dong et al., 2004, Green et al., 2006).
1.2. On the importance of ‘contextualizing risk’
As suggested by Stansfeld and Candy (2006, p. 442), ‘social hierarchies and the implicit power relationships contained therein influence the distribution of work-related stressors’. In the context of the present study, it should therefore be underlined that there are considerable gender and SES differences in exposure to various work characteristics. It has repeatedly been reported that white-collar workers have a higher degree of work control than blue collar workers, and that women in both of these occupational classes report lower levels of control compared to their male counterparts. Similarly, high occupational physical demands are associated with lower SES, and men are, on average, more exposed than women in this respect (Hall, 1989, Matthews et al., 1998, Rovio et al., 2007, Swedish Work Environment Authority, 2016). Furthermore, stressors such as emotional demands and violence/threats of violence are known to be widespread in human service professions where women predominate (Brotheridge and Grandey, 2002, Swedish Work Environment Authority, 2016 Hochschild, 2003), and a number of studies have reported higher risks of affective and stress related complaints in these occupational groups (see for example; Johnson et al., 2005; Stansfeld, Rasul, Head, & Singleton, 2011; Wieclaw, Agerbo, Mortensen, & Bonde, 2006). For women, the possible ‘double burden’ of professional and domestic engagements must also be acknowledged. During the 20th century, women’s labour market participation has increased substantially in large parts of the world, including Sweden (Statistics Sweden, 2011). While men have gradually come to spend more time on domestic chores, there have been, and still are, large gender differences in this respect (Statistics Sweden, 1992, Statistics Sweden, 2012). In terms of health outcomes, the combination of high workloads in both paid and unpaid work among women has previously been linked to various indicators of psychological strain and ill-health (Floderus et al., 2009, Krantz et al., 2005), also in older cohorts (Arber et al., 1985, Hall, 1992).
2. Material and methods
2.1. Study population
The study sample is derived from two longitudinal studies from Gothenburg, Sweden, that were merged to become one in 2000; the H70 Birth Cohort Study and the Prospective Populations Study of Women (PPSW) (Karlsson et al., 2009). The present sample consists of 1019 individuals (229 men and 790 women), all living in Sweden on September 1, 2000 (Table 1). Of the 790 women, 691 had previously been part of the Prospective Populations Study of Women (PPSW). All participants were sampled from the Swedish population register and systematically selected on the basis of birth dates. The women were born in 1908, 1914, 1918, 1922 or 1930, while all men were born in 1930. Of these individuals, 923 (90.6%) consented to donate blood for genetic analyses. A more detailed description of the baseline sample can be found elsewhere (Karlsson et al., 2009; Karlsson et al., 2010). Follow-up examinations were carried out in 2005-06 (n = 724) and 2009-10 (n = 368). Informed consent was acquired from all participants or their relatives, and the studies were approved by the regional Ethics Review Board for medical research in Gothenburg (Skoog et al., 2015).
Table 1.
Characteristics of the study population, n(%) / mean(sd).
| All | Males | Females | |
|---|---|---|---|
| Presence of APOE ɛ4 | 262 (28.4) | 65 (29.2) | 197 (28.1) |
| Education | |||
| Primary | 597 (62.2) | 130 (57.0) | 467 (63.8) |
| Lower secondary | 220 (22.9) | 39 (17.1) | 181 (24.7) |
| Upper secondary/university | 143 (14.9) | 59 (25.9) | 84 (11.5) |
| Occupational class | |||
| Blue collar | 408 (48.9) | 93 (40.8) | 315 (52.0) |
| Lower white collar | 220 (26.8) | 31 (13.6) | 189 (31.2) |
| White-collar/self-employed | 206 (24.7) | 104 (45.6) | 102 (16.8) |
| Sex | |||
| Male | 229 (22.5) | – | – |
| Female | 790 (47.5) | – | – |
| Year of birth | |||
| 1908 | 8 (0.8) | – | 8 (1.0) |
| 1914 | 44 (4.3) | – | 44 (5.6) |
| 1918 | 171 (16.8) | – | 171 (21.7) |
| 1922 | 216 (21.2) | – | 216 (27.3) |
| 1930 | 580 (56.9) | 229 (100.0) | 351 (44.4) |
| Job exposures | |||
| Work control | 5.0 (1.1) | 5.6 (1.3) | 4.7 (1.0) |
| Psychological demands | 4.7 (1.2) | 4.9 (1.4) | 4.6 (1.2) |
| Support | 8.5 (0.9) | 9.0 (0.9) | 8.3 (1.0) |
| Job hazards | 2.2 (1.1) | 3.0 (1.5) | 1.8 (0.5) |
| Physical demands | 3.0 (1.5) | 3.6 (1.9) | 2.8 (1.3) |
| Diagnosed with dementia at baseline | 94 (9.2) | 5 (2.2) | 89 (11.3) |
| Diagnosed with dementia 2000–2012 | 246 (24.1) | 27 (11.8) | 219 (27.7) |
| Age at baseline | 75.3 (5.8) | 70.5 ( 0.2) | 76.7 (5.9) |
Comment: N = 1019.
2.2. Neuropsychiatric examinations, diagnoses and genotyping
The clinical examination, conducted at an outpatient department or in the participant’s home, comprised comprehensive social, functional, physical, neuropsychiatric and neuropsychological assessments as well as an interview with a close informant. The semi-structured neuropsychiatric examinations included ratings of common symptoms and signs of dementia (e.g., assessments of memory, orientation, general knowledge, apraxia, visuospatial function, understanding proverbs, following commands, naming ability and language) and were performed by trained psychiatric research nurses. For a more detailed description of the procedures, see Guo et al. (2007) and Skoog, Nilsson, Palmertz, Andreasson & Svanborg (1993). In addition, semi-structured interviews with a close informant were performed and comprised questions regarding changes in behaviour and intellectual function, psychiatric symptoms, activities of daily living, and, in cases of dementia, age of onset and disease course (Karlsson et al., 2009, Skoog et al., 2015). Dementia was diagnosed by geriatric psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition Revised (DSM-III-R) (APA, 1987). The diagnoses were based on symptoms rated during the neuropsychiatric examinations as well as on information from the close informant interviews, as previously described in detail (Guo et al., 2007, Skoog, Nilsson, Palmertz, Andreasson & Svanborg, 1993). For individuals lost to follow-up, incident dementia cases (until 2012) were diagnosed on the basis of information from medical records, evaluated by geriatric psychiatrists, or from the Swedish Hospital Discharge Register (Guo et al., 2007). Blood samples were collected and the SNPs (single nucleotide polymorphisms) rs7412 and rs429358 in APOE (gene map locus 19q13.2) were genotyped using the KASPar® PCR SNP genotyping system (LGC Genomics, Hoddesdon, Herts, UK) or by mini-sequencing, as previously described in detail (Blennow et al., 2000). Genotype data for these two SNPs were used to define ε2, ε3, and ε4 alleles. Because ε4 is the only allele associated with an increased risk of AD, the subsequent analyses focused solely on this variant.
2.3. Assessment of work environment exposures
Information on occupation was obtained through interviews at baseline and/or in conjunction with the follow-up examinations. All participants were asked to specify their main occupation in free text. Individuals (women only, approx. 12.5 per cent) who stated that they had primarily engaged in domestic work/had not been employed during working age were excluded from further analyses. Using these data, in combination with the validated Job Exposure Matrix (JEM) (Johnson et al., 1990, Johnson and Stewart, 1993), we were able to estimate each participant’s exposure to the following work environment factors: work control, support, psychological demands, physical demands and job hazards. The data on which the JEM is based were originally sampled through the annual Swedish Survey of Living Conditions (ULF) for 1977 and 1979, respectively. In order to construct the JEM, Johnson et al. (1990) used factor analysis to combine sets of items. Five factors/scales, corresponding to the work environment exposures listed above, were retained. With regard to work control, the scale comprises items such as degree of influence over the planning of work, flexible working hours, possibility of learning new things and degree of variation in task content. The support scale was constructed from questions regarding the possibility of social interaction with co-workers, at the workplace as well as outside work. Psychological demands refer to whether the job is perceived as hectic and/or psychologically demanding, while the physical demands scale includes items such as exposure to accident risk and heavy lifting. Finally, the job hazards scale indicates the individual’s level of exposure to, e.g., noise, heavy shaking and inadequate ventilation. Based on the matrix, each individual received a score, ranging from 0 to 10, for each of the five factors, where higher scores indicate higher levels of the factor. For ease of interpretation, the exposure indicators were later mean centred. Given the substantial gender differences in work content, the JEM includes separate scales for men and women (see also Hall, 1989; Johnson et al., 1990). In practice, this means that a man and a woman holding the same occupation are assigned different JEM scores. A final remark on retrospectively estimating work exposure is necessary. As noted by Johnson et al. (1990), the activities and exposures associated with a certain occupation are likely to change over time. In this respect, it is advantageous that the version of the JEM used in the present paper was constructed from data sampled during the 1970s, i.e., when the majority of our study population was still active on the labour market.
2.4. Covariates
In order to distinguish between different occupational groups, all occupations in free text were coded in accordance with the Swedish SEI standards for socioeconomic classification (Statistics Sweden, 1982), which has many commonalities with the Eriksson-Goldthorpe scheme (Goldthorpe, 2007). Based on the initial, two-digit classifications, three aggregated socioeconomic groups were specified: Blue collar (BC) corresponds to manual workers, Lower white collar (LWC) to assistant, non-manual employees and Upper white collar (UWC) includes intermediate/higher non-manual workers and professionals in occupations as well as upper-level executives, self-employed and farmers (for a more detailed description of these groups, see Hasselgren et al., 2018). The education variable was mainly constructed from responses gathered in conjunction with the baseline examination in 2000-01, when all respondents were asked to specify the level/type of their educational attainment. In cases where information was missing at baseline, it was, if available, obtained from the follow-up examinations. The variable has three values: where Primary corresponds to elementary school/vocational school, Lower secondary to girls’ school/junior secondary school/folk high school and Secondary/university to high school/university. Although the most commonly used SES indicators, e.g., income, education and occupational class, are largely overlapping, they are also likely to be linked to health via partly different mechanisms (Lahelma et al., 2004, Torssander and Erikson, 2010). Therefore, in the subsequent analyses, we chose to control for both education and occupational class, albeit in separate models.
2.5. Statistical analyses
Due to issues of non-normality, the non-parametric Mann-Whitney U test and the The Kruskal-Wallis H test, with a Bonferroni correction for multiple group comparisons (Dunn, 1964, Laerd Statistics, 2015, Laerd Statistics, 2016), were used to confirm previous findings on the bivariate associations between SES/gender and work environment exposures. Because the distributions of scores were not similar across the compared groups, as assessed by visual inspection, all comparisons refer to differences in mean ranks, where higher ranks indicate greater exposure (Laerd Statistics, 2015, Laerd Statistics, 2016). In order to assess the potential associations between dementia and the five job exposure indicators, we used bivariate logistic regression, because the dependent variable is dichotomous, indicating whether an individual had been diagnosed with dementia prior to, or during, 2012 (Long & Freese, 2006). Logistic regression was hence used in the multivariate analyses as well, and we report odds ratios with 95% CIs. All models were stratified by gender, as the JEM contains separate scales for men and women (see Section 2.3). To facilitate the exploration of potential interaction effects, separate models were computed for each job exposure indicator. Because interaction models differ from additive regression models in the sense that the coefficient of any constitutive term X cannot be interpreted as an un-conditional marginal effect, it is not possible to draw any substantial conclusions based on the estimates shown in the results table. Hence, for the interaction models, we computed, and graphically illustrate, the conditional marginal effect of APOE ɛ4 at different levels of each work exposure indicator (Brambor, Clark, & Golder, 2006).
3. Results
3.1. Bivariate and descriptive analyses
As expected, significant differences were observed between the different SES groups for all of the five job exposure indicators: control, support, psychological demands, physical demands and job hazards (Table 2a). In general, lower SES corresponded to lower levels of control and psychological demands, but higher physical demands and more exposure to hazardous conditions. Further, men scored significantly higher than women in all these dimensions (Table 2b). With regard to dementia, a significant (negative) association was observed only for work control (OR = 0.76; p< 0.01) (Table 2c). A summary of the main occupational categories, stratified by gender, in which control was reported to be high revealed that, among men, occupations such as engineer, private sector manager and academic professional were most common. In contrast, the most common professions among women who reported high control were clerical worker, teacher and public-sector care worker (Table 4).
Table 2.
Bivariate analyses of (a) job exposures by SES, (b) job exposures by gender and (c) dementia by job exposure.
| JOB EXPOSUREa | BC (n=398) | LWC (n=214) | UWC (n=197) | Kruskal-Wallis H | BC vs. LWC | BC vs. UWC | LWC vs. UWC |
|---|---|---|---|---|---|---|---|
| Mean rank | Mean rank | Mean rank | Chi2 (df), sig | Adj. sig | Adj. sig | Adj. sig | |
| Work control | 245.4 | 497.1 | 627.4 | 398.9 (2) *** | *** | *** | *** |
| Psychological demands | 328.1 | 389.9 | 576.8 | 151.0 (2) *** | ** | *** | *** |
| Support | 312.4 | 498.1 | 490.9 | 123.5 (2) *** | *** | *** | ns. |
| Job hazards | 489.4 | 282.5 | 367.6 | 116.1 (2) *** | *** | *** | *** |
| Physical demands | 579.5 | 178.1 | 298.9 | 466.1 (2) *** | *** | *** | *** |
| JOB EXPOSUREb and c | Males (n=222) | Females (n=587) | Mann-Whitney U | Dementia (n=809) | |||
|---|---|---|---|---|---|---|---|
| Mean rank | Mean rank | U, sig | OR [CI] | Sig. | |||
| Work control | 525.0 | 359.6 | 38508.5*** | 0.76 [0.65-0.89] | ** | - | - |
| Psychological demands | 439.0 | 392.1 | 57609.0*** | 0.97 [0.84-1.11] | ns. | - | - |
| Support | 551.4 | 349.6 | 32649.0*** | 0.86 [0.73-1.02] | ns. | - | - |
| Job hazards | 545.9 | 351.7 | 33876.0*** | 0.97 [0.82 -1.15] | ns. | - | - |
| Physical demands | 473.0 | 379.3 | 50055.0*** | 1.11 [0.99-1.24] | ns. | - | - |
Comment: *p<0.05. BC = Blue collar; LWC = Lower white collar; UWC = Upper white collar.
p<0.01;
p<0.001.
Table 4.
Main occupational categories (high/low control) for males and females (%).
| Males | Females | |
|---|---|---|
| Control > meana | Engineers (28.9) | Clerical workers (41.9) |
| Private sector managers/executives (16.7) | Care workers, e.g., nurses, home- and childcare workers (18.1) | |
| Academic professionals, e.g., doctors, architects, researchers, lecturers (11.4) | Teachers (7.6) | |
| Control < meanb | Craftsmen/mechanics/machine-menders (38.0) | Retail workers/shop assistants (29.2) |
| Factory-/construction-/dock workers (18.5) | Assistant nurses (14.4) | |
| Transportation workers, e.g., drivers, railroad workers (9.3) | Cleaners (8.9) |
N (males) = 120, N (females) = 315.
N (males) = 108, N (females) = 271.
3.2. Multivariate analysis
Results from the multivariate analyses are displayed in Table 3, where we report odds ratios with 95% CIs. In Fig. 1 (males) and Fig. 2 (females), we report the results for the interaction models.
Table 3.
Logistic regression for dementia risk. Odds ratios [95% CI].
| Model 1 |
Model 2 |
Model 3b |
Model 4c |
|||||
|---|---|---|---|---|---|---|---|---|
| Males | Femalesa | Males | Femalesa | Males | Femalesa | Males | Femalesa | |
|
CONTROL | ||||||||
| control | 0.68* [0.48, 0.97] | 0.92 [0.73, 1.15] | 0.95 [0.61, 1.46] | 0.76 [0.58, 1.00] | 0.81 [0.47, 1.40] | 0.76 [0.55, 1.06] | 0.84 [0.50, 1.39] | 0.86 [0.64, 1.17] |
| apoe | 2.35 [0.98, 5.63] | 1.82* [1.13, 2.95] | 1.56 [0.56, 4.30] | 1.88** [1.16, 3.05] | 1.55 [0.56, 4.29] | 1.88* [1.16, 3.05] | 1.93 [0.68, 5.48] | 1.96** [1.17, 3.26] |
| control*apoe | 0.37* [0.16, 0.85] | 1.88* [1.12, 3.13] | 0.37* [0.16, 0.86] | 1.88* [1.12, 3.13] | 0.39* [0.17, 0.89] | 2.02* [1.15, 3.57] | ||
| constant | 0.09 | 1.10e-06 | 0.10 | 6.60e-07 | 0.07 | 7.10e-07 | 0.07 | 8.37e-07 |
| Pseudo R2 | 0.05 | 0.13 | 0.09 | 0.14 | 0.09 | 0.14 | 0.12 | 0.14 |
| N | 217 | 536 | 217 | 536 | 217 | 536 | 216 | 517 |
| PSYCHOLOGICAL DEMANDS | ||||||||
| dempsych | 0.78 [0.58, 1.05] | 1.12 [0.93, 1.36] | 0.84 [0.56, 1.25] | 1.06 [0.84, 1.34] | – | – | – | – |
| apoe | 2.32 [0.98, 5.53] | 1.83* [1.13, 2.95] | 2.23 [0.93, 5.37] | 1.82* [1.12, 2.94] | – | – | – | – |
| dempsych*apoe | 0.86 [0.48, 1.56] | 1.20 [0.79, 1.82] | – | – | – | – | ||
| constant | 0.09 | 8.23e-07 | 0.10 | 7.96e-07 | – | – | – | – |
| – | – | – | – | |||||
| Pseudo R2 | 0.04 | 0.13 | 0.04 | 0.14 | – | – | – | – |
| N | 217 | 536 | 217 | 536 | – | – | – | – |
| SUPPORT | ||||||||
| support | 0.89 [0.55, 1.44] | 0.96 | 1.16 [0.57, 2.35] | 0.99 | – | – | – | – |
| apoe | 2.08 [0.89, 4.89] | 1.81* | 1.99 [0.82, 4.79] | 1.81* | – | – | – | – |
| support*apoe | 0.53 [0.19, 1.49] | 0.89 | – | – | – | – | ||
| constant | 0.10 | 9.29e-07 | 0.10 | 9.70e-07 | – | – | – | – |
| – | – | – | – | |||||
| Pseudo R2 | 0.02 | 0.13 | 0.03 | 0.13 | – | – | – | – |
| N | 216 | 536 | 216 | 536 | – | – | – | – |
| JOB HAZARDS | ||||||||
| hazards | 1.20 [0.92, 1.57] | 1.22 [0.81, 1.85] | 1.15 [0.80, 1.66] | 1.40 [0.87, 2.27] | – | – | – | – |
| apoe | 2.18 [0.92, 5.14] | 1.81* [1.12, 2.93] | 2.12 [0.89, 5.08] | 1.86* [1.15, 3.01] | – | – | – | – |
| hazards*apoe | 1.09 [0.64, 1.86] | 0.61 [0.25, 1.53] | – | – | – | – | ||
| constant | 0.10 | 1.13e-06 | 0.10 | 9.88e-07 | – | – | – | – |
| – | – | – | – | |||||
| Pseudo R2 | 0.03 | 0.13 | 0.03 | 0.14 | – | – | – | – |
| N | 217 | 536 | 217 | 536 | – | – | – | – |
| PHYSICAL DEMANDS | ||||||||
| demphys | 1.19 [0.96, 1.48] | 1.18 [0.99, 1.39] | 1.18 [0.89, 1.57] | 1.32** [1.07, 1.63] | – | – | – | – |
| apoe | 2.21 [0.93, 5.21] | 1.76* [1.09, 2.85] | 2.19 [0.91, 5.27] | 1.89** [1.16, 3.07] | – | – | – | – |
| demphys*apoe | 1.02 [0.65, 1.59] | 0.72 [0.50, 1.03] | – | – | – | – | ||
| constant | 0.09 | 1.17e-06 | 0.09 | 8.62e-07 | – | – | – | – |
| – | – | – | – | |||||
| Pseudo R2 | 0.03 | 0.14 | 0.03 | 0.14 | – | – | – | – |
| N | 217 | 536 | 217 | 536 | – | – | – | – |
Comment: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Adjusted for age (only applicable for women).
Adjusted for occupational class.
Adjusted for education.
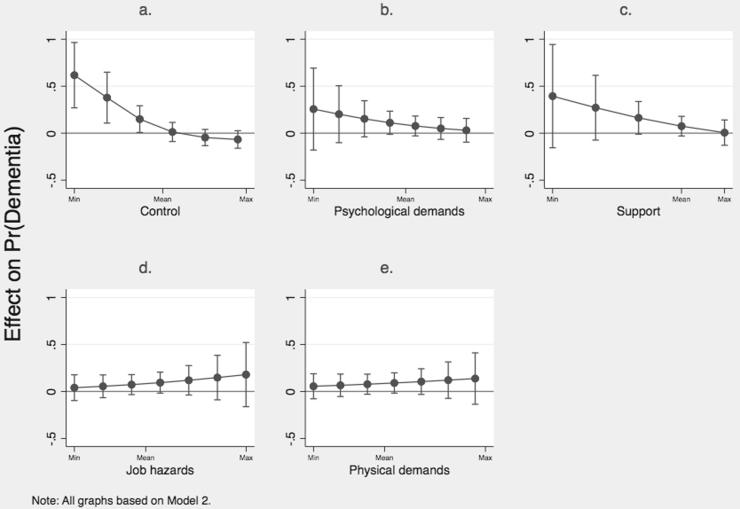
Fig. 1.
Conditional marginal effects of APOE e4 at different levels of (a) control, (b) psychological demands, (c) support, (d) job hazards and (e) physical demands, for males. 95% CIs.
Fig. 2.
Conditional marginal effects of APOE e4 at different levels of (a) control, (b) psychological demands, (c) support, (d) job hazards and (e) physical demands, for females. 95% CIs.
A significant relationship between work control and dementia was detected for males (OR = 0.68, i.e., the more work control, the lower risk of dementia), but not for females (OR = 0.92) (Table 3, Model 1). With regard to the interaction models, the APOE ɛ4-control interaction term was significant among both men and women (Table 3, Model 2). In order to draw the correct substantial conclusions from these models, we computed the conditional marginal effect of APOE ɛ4 at different levels of control, which confirmed the presence of significant interaction effects. Among males, the estimated effect of APOE ɛ4 on the probability of dementia was only significant (the CIs did not overlap zero) at lower levels of control. However, at higher levels of control (> mean), the corresponding effect was found to be non-significant (Fig. 1a). The pattern observed among women was directly opposed to that among men. The effect of APOE ɛ4 was only significant at higher levels of control (Fig. 2a). Finally, for both women and men, these results remained significant when controlling for both occupational class (Model 3) and education (Model 4).
For psychological demands, social support and job hazards (Table 3, Model 1), no significant effects on dementia risk were observed among either men or women. As regards the interaction models, the CIs overlapped zero and/or each other at all levels of the exposure variables (Fig. 1, Fig. 2). This held true also with a confidence level of 84% (MacGregor-Fors and Payton, 2013, Payton et al., 2003) (results not shown here but can be requested from the first author). Additionally, Pseudo R2 remained virtually unchanged after inclusion of the interaction terms. Consequently, the presence of interaction effects could not be confirmed in either of the two groups, and we did not proceed to compute models with further adjustments.
For both men and women, the estimated effects of physical demands were non-significant (Table 3, Model 1). For males, no significant interaction effect between support and APOE ɛ4 was observed (Fig. 1e). For females, the CIs only overlap zero at high levels of physical demands, which indicates that some sort of interaction could exist (Fig. 2e). Further, with a confidence level of 84%, there was a small non-overlap in the effect of APOE ε4 between the highest and lowest level of physical demands (results not shown here but can be requested from the first author). However, the difference in effect was much smaller than for work control, and Pseudo R2 remained unchanged after the interaction term was added. Thus, the fully adjusted models were not considered. Finally, drawing on Karasek’s (1979) job strain model, we combined the control and psychological demand variables into continuous strain variable where high values indicate high strain. As this indicator did not reveal any significant results, neither main effects nor interactions, the analyses are not reported here (but can be requested from the first author).
4. Discussion
In the present paper, we investigated whether a number of work environment exposures, all known to be closely related to SES, could moderate the effect of the APOE ε4 allele, which is the major genetic risk factor for dementia. The overall results suggest that work control is the most influential indicator in moderating the effect of the gene variant, although it seems to do so differently among men than among women.
The initial bivariate analyses (Table 2) revealed positive and significant associations between work control and dementia. Further, they confirmed previous research on socioeconomic and gender differences in all of the five work exposure factors, which underlines that the distribution of work-related stressors is closely related to occupational hierarchies as well as to gender segregation in the labour market (Eriksson and Karlsson, 2009, Hall, 1989, Matthews et al., 1998, Rovio et al., 2007, Stansfeld and Candy, 2006, Swedish Work Environment Authority, 2016).
The findings from the multivariate analyses could not corroborate previously detected associations between physical demands (Smyth et al., 2004, Stern et al., 1995), social support (Andel et al., 2012) and occurrence of dementia. Likewise, no significant effects were observed for job hazards. In contrast to the findings of Sindi et al. (2016), yet in line with those of Seidler et al. (2004), high psychological demands were not found to significantly increase the risk of developing dementia late in life.2 Moreover, none of these four work exposure characteristics was found to moderate the increased risk of dementia implied by APOE ε4. For high work control, on the other hand, the results of our analysis supported previous findings on its protective effect (Andel et al., 2012, Seidler et al., 2004; Wang, Wahlberg, et al., 2012). More specifically, it was revealed that, for men, the estimated effect of APOE ɛ4 on the probability of dementia was relatively sizeable, but only significant at lower levels of control (approx. increase = 50 per cent) (Fig. 1a). This implies that control could actually moderate the effect of APOE ɛ4, such that men who carry this gene variant are ‘protected’ if they have held professions in which they could exert control over their own work situation. The pattern observed among women was directly opposed to that among men, indicating that the effect of APOE ɛ4 was only significant, and again quite sizeable (approx. increase = 50 per cent), at higher levels of control. Finally, for both women and men, these effects remained significant while controlling for both occupational class (Model 3) and education (Model 4). With reference to previously suggested pathological pathways between work exposures and dementia, it is not surprising that work control stands out, at least among men, as the most protective factor. This is the case, we argue, because the control indicator derived from the JEM includes two separate components: skill discretion and decision authority (Van der Doef & Maes, 1999), both of which could be related to the main pathological mechanisms proposed in the literature as well as to other known risk factors. First, the control indicator includes items such as ‘possibility of learning new things’ and ‘variation in task content’, which, in line with the cognitive reserve hypothesis (Stern, 2002, Stern, 2012), are likely to provide intellectual stimulation that could improve the brain’s resilience to degenerative changes (Qiu et al., 2009, Then et al., 2017). Second, it comprises items such as ‘influence over the planning of work’ and ‘flexible working hours’, i.e., factors that could reduce stress and, consequently, the occurrence of cardiovascular risk factors (Kivimäki et al., 2002; Kivipelto et al., 2001; Skoog et al., 1996; Theorell et al., 2016; Vrijkotte et al., 2000) and/or augmented glucocorticoid hormone levels (Bremner, 2006, Dong et al., 2004, Green et al., 2006, Lupien et al., 1999, Sapolsky, 1996). Finally, lack of work control is a known risk factor for depressive symptoms (for a review, see; Theorell et al., 2015), which in turn is hypothesized increase dementia risk (Livingston et al., 2017, Ownby et al., 2006). Interpreting the interaction model for women is less straightforward, although a few things are clear at this stage. First, ‘male’ high control jobs are likely to be very different from ‘female’ high control jobs, especially in previous generations. In the present sample, human service workers make up a large proportion of the women in the ‘high control’ group’ (see Table 4). According to previous findings, affective and stress related disorders are particularly common in such professions (Johnson et al., 2005, Stansfeld et al., 2011, Wieclaw et al., 2006). However, reportedly important stressors contributing thereto, e.g., emotional demands, are not included in the JEM. It is thus possible that even though these women were exposed to certain favourable workplace conditions, they were also exposed to unmeasured negative ones. By extension, this could explain why they actually seem to be ‘worse off’ compared to their low control counterparts. Finally, for women, the ‘double burden’ of professional and domestic engagements could cause a sort of role strain that adversely affects health. Given historical differences in the gendered division of labour, it is reasonable to assume that the women in the present sample (born in or before 1930) may have encountered more traditionalist expectations on themselves as women. Thus, even if they worked full-time in a professional occupation, they might still have been expected to shoulder the main responsibility for childcare and domestic chores. Ultimately, this would result in a greater total workload and hence in elevated stress levels (Arber et al., 1985, Floderus et al., 2009, Hall, 1992, Krantz et al., 2005). Naturally, the present study has a number of limitations. For reasons related to selective survival/mortality, it is often preferable to use years-to-diagnosis as the dependent variable, but such a design was not chosen because differences in ‘age when diagnosed’ exist between cohorts in the present sample. Instead, we measured dementia at one specific point in time. This choice also maximized the number of cases, which was important because the number of dementia cases was relatively small. Owing to the small number of cases, we did not distinguish between dementia subtypes. This is a significant limitation in the sense that APOE ɛ4 is generally considered to be a major risk factor for AD, while for other dementia subtypes, associations are less well-established. However, AD is the most common form of dementia, accounting for approximately 50–70 per cent of all cases, and recent studies suggest that the ɛ4 allele could also be associated with other forms of dementia such as VaD, which is the second most common form (Liu et al. 2012; Rohn, 2014). Finally, information on lifetime occupation was collected retrospectively, which is a potential source of measurement uncertainty.
5. Conclusions
The present study suggests that work control is the most influential indicator in moderating the effect of the APOE ɛ4 allele on dementia, although it seems to do so differently among men than among women. While holding a high control profession appears to ‘protect’ men, in the sense that it buffers the increased risk implied by the gene variant, the opposite pattern was observed for women. These findings not only underscore the importance of considering interactions between social and genetic risk factors in better understanding multifactorial diseases such as dementia. They also highlight that factors which are more ‘proximate’ to the individual, such as work environment characteristics, must not be studied as if distinct from the underlying systems that ‘put[] people at risk of risks’ (Link & Phelan, 1995, p. 80), e.g., gender- and class-based inequities. In line with this, we encourage intersectional studies targeting the potential health-effects of job exposures other than those examined here, as well as home-work interactions and historical changes in work content.
Financial disclosure statement
The authors confirm that all funding sources have been acknowledged and that none of them were involved in: 1) the design of the study, 2) the collection, analysis and interpretation of data or 3) writing the manuscript.
Conflict of interest statement
The authors have no conflicting interests to report.
Statement of ethical approval
Informed consent was acquired from all participants or their relatives. The regional Ethical Review Board for medical research in Gothenburg, Sweden approved the H70/PPSW examination waves in 2000 (ref: Ö402-99), 2004 (ref: T453 04) and 2009 (ref: 075-09).
Acknowledgements
This study was funded by: The Stena Foundation, the Swedish Research Council [2015-02830, 2013-8717], the Swedish Research Council for Health, Working Life and Welfare [2004-0145, 2006-0596, 2008-1111, 2010-0870, 2013-1202, 2013-2300, 2013-2496], the Alzheimer's Association Zenith Award [ZEN-01-3151], the Alzheimer’s Association Stephanie B. Overstreet Scholars [IIRG-00-2159], Sahlgrenska University Hospital (ALF), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Stiftelsen Professor Bror Gadelius’ Minnesfond, the Swedish Brain Foundation (Hjärnfonden), the Swedish Alzheimer Foundation (Alzheimerfonden), the Torsten Söderberg Foundation and the Swedish Society of Medicine. HZ is a Wallenberg Academy Fellow and acknowledges support from the UK Dementia Research Institute and KB holds the Torsten Söderberg Professorship in Medicine. The study was accomplished while CH was affiliated with the Swedish National Graduate School for Competitive Science on Ageing and Health (SWEAH), which is funded by the Swedish Research Council. A sincere thank you to Karen Williams for her diligent proof reading of the article.
Footnotes
Job strain is a composite measure and widely used definition of psychosocial stress at work, characterized by the combination of high psychological demands and low decision latitude (control) (Karasek, 1979).
The lack of significant associations in this respect could possibly be attributed to the fact that the indicator of psychological demands found in the JEM is ‘weaker’ (constructed from two items only) than, e.g., the one assessing control (12 items).
References
- Andel R., Crowe M., Hahn E.A., Mortimer J.A., Pedersen N.L., Fratiglioni L., Gatz M. Work‐related stress may increase the risk of vascular dementia. Journal of the American Geriatrics Society. 2012;60(1):60–67. doi: 10.1111/j.1532-5415.2011.03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andel R., Crowe M., Kåreholt I., Wastesson J., Parker M.G. Indicators of job strain at midlife and cognitive functioning in advanced old age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(3):287–291. doi: 10.1093/geronb/gbq105. [DOI] [PubMed] [Google Scholar]
- APA . (3rd ed. revised ed.) American Psychiatric Association; Washington DC: 1987. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Arber S., Gilbert G.N., Dale A. Paid employment and women’s health: A benefit or a source of role strain? Sociology of Health Illness. 1985;7(3):375–400. [Google Scholar]
- Attems J., Jellinger K. Neuropathological correlates of cerebral multimorbidity. Current Alzheimer Research. 2013;10(6):569–577. doi: 10.2174/15672050113109990002. [DOI] [PubMed] [Google Scholar]
- Blennow K., Ricksten A., Prince J., Brookes A., Emahazion T., Wasslavik C., Bogdanovic N., Andreasen N., Båtsman S., Marcusson J. No association between the α2-macroglobulin (A2M) deletion and Alzheimer's disease, and no change in A2M mRNA, protein, or protein expression. Journal of Neural Transmission. 2000;107(8-9):1065–1079. doi: 10.1007/s007020070052. [DOI] [PubMed] [Google Scholar]
- Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. The Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Borg V., Kristensen T.S. Social class and self-rated health: Can the gradient be explained by differences in life style or work environment? Social Science Medicine. 2000;51(7):1019–1030. doi: 10.1016/s0277-9536(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Brambor T., Clark W.R., Golder M. Understanding interaction models: Improving empirical analyses. Political Analysis. 2006;14(1):63–82. [Google Scholar]
- Bremner D.J. Stress and brain atrophy. CNS Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS Neurological Disorders) 2006;5(5):503–512. doi: 10.2174/187152706778559309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotheridge C.M., Grandey A.A. Emotional labor and burnout: Comparing two perspectives of “people work”. Journal of Vocational Behavior. 2002;60(1):17–39. [Google Scholar]
- Dong H., Goico B., Martin M., Csernansky C., Bertchume A., Csernansky J. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in appsw (tg2576) mutant mice by isolation stress. Neuroscience. 2004;127(3):601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dunn O.J. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–252. [Google Scholar]
- Elovainio M., Ferrie J.E., Singh-Manoux A., Gimeno D., De Vogli R., Shipley M.J., Kivimäki M. Cumulative exposure to high-strain and active jobs as predictors of cognitive function: The Whitehall II study. Occupational and Environmental Medicine. 2009;66(1):32–37. doi: 10.1136/oem.2008.039305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Karlsson J.C. A package of flexibility? In: Skorstad E., Ramsdal H., editors. Flexible Organizations and the New Working Life: A European Perspective. Ashgate; Farnham: 2009. pp. 97–109. [Google Scholar]
- Floderus B., Hagman M., Aronsson G., Marklund S., Wikman A. Work status, work hours and health in women with and without children. Occupational and Environmental Medicine. 2009;66(10):704–710. doi: 10.1136/oem.2008.044883. [DOI] [PubMed] [Google Scholar]
- Goldthorpe J.H. Vol. 2. Stanford University Press; Stanford: 2007. (On Sociology. Vol 2, Illustration and Retrospect). [Google Scholar]
- Green K.N., Billings L.M., Roozendaal B., McGaugh J.L., LaFerla F.M. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Waern M., Sjögren K., Lissner L., Bengtsson C., Björkelund C., Östling S., Gustafson D., Skoog I. Midlife respiratory function and incidence of Alzheimer's disease: A 29-year longitudinal study in women. Neurobiology of Aging. 2007;28(3):343–350. doi: 10.1016/j.neurobiolaging.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Hall E.M. Gender, work control, and stress: A theoretical discussion and an empirical test. International Journal of Health Services. 1989;19(4):725–745. doi: 10.2190/5MYW-PGP9-4M72-TPXF. [DOI] [PubMed] [Google Scholar]
- Hall E.M. Double exposure: The combined impact of the home and work environments on psychosomatic strain in Swedish women and men. International Journal of Health Services. 1992;22(2):239–260. doi: 10.2190/7VW4-GE0D-WRKU-Q62V. [DOI] [PubMed] [Google Scholar]
- Hintsanen M., Elovainio M., Puttonen S., Kivimäki M., Lehtimäki T., Kähönen M., Raitakari O.T. Val/met polymorphism of the comt gene moderates the association between job strain and early atherosclerosis in young men. Journal of Occupational and Environmental Medicine. 2008;50(6):649–657. doi: 10.1097/JOM.0b013e318165c7ec. [DOI] [PubMed] [Google Scholar]
- Hasselgren C., Ekbrand H., Fässberg M.M., Zettergren A., Zetterberg H., Blennow K., Skoog I., Halleröd B. APOE ε4 and the long arm of social inequity: Estimated effects of socio-economic status and sex on the timing of dementia onset. Ageing & Society, published online ahead of print. 2018:1–25. [Google Scholar]
- Hintsanen M., Elovainio M., Puttonen S., Kivimäki M., Raitakari O.T., Lehtimäki T., Viikari J. Neuregulin-1 genotype moderates the association between job strain and early atherosclerosis in young men. Annals of Behavioral Medicine. 2007;33(2):148–155. doi: 10.1007/BF02879896. [DOI] [PubMed] [Google Scholar]
- Hochschild A.R. University of California Press; Berkeley, Calif: 2003. The Managed Heart: Commercialization of Human Feeling. [Google Scholar]
- James B.D., Wilson R.S., Boyle P.A., Trojanowski J.Q., Bennett D.A., Schneider J.A. Tdp-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.V., Hall E.M. Job strain, work place social support, and cardiovascular disease: A cross-sectional study of a random sample of the Swedish working population. American Journal of Public Health. 1988;78(10):1336–1342. doi: 10.2105/ajph.78.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.V., Stewart W., Fredlund P., Hall E.M., Theorell T. Department of Stress Research, Karolinska Institute; Stockholm: 1990. Psychosocial job exposure matrix: An occupationally aggregated attribution system for work environment exposure characteristics. (Stress Reserach Reports, no. 221) [Google Scholar]
- Johnson J.V., Stewart W.F. Measuring work organization exposure over the life course with a job-exposure matrix. Scandinavian Journal of Work, Environment Health. 1993:21–28. doi: 10.5271/sjweh.1508. [DOI] [PubMed] [Google Scholar]
- Johnson S., Cooper C., Cartwright S., Donald I., Taylor P., Millet C. The experience of work-related stress across occupations. Journal of Managerial Psychology. 2005;20(2):178–187. [Google Scholar]
- Johansson L., Guo X., Waern M., Ostling S., Gustafson D., Bengtsson C., Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Johansson L., Guo X., Hällström T., Norton M.C., Waern M., Ostling S., Bengtsson C., Skoog I. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ Open. 2013;3(9):1–7. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasek R.A. Job demands, job decision latitude, and mental strain: Implications for job redesign. Administrative Science Quarterly. 1979;24(2):285–308. [Google Scholar]
- Karlsson B., Klenfeldt I.F., Sigström R., Waern M., Östling S., Gustafson D., Skoog I. Prevalence of social phobia in non-demented elderly from a Swedish population study. American Journal of Geriatric Psychiatry. 2009;17(2):127–135. doi: 10.1097/jgp.0b013e3181860051. [DOI] [PubMed] [Google Scholar]
- Karlsson B., Sigström R., Waern M., Östling S., Gustafson D., Skoog I. The prognosis and incidence of social phobia in an elderly population. A 5-year follow-up. Acta Psychiatrica Scandinavica. 2010;122(1):4–10. doi: 10.1111/j.1600-0447.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- Kivimäki M., Leino-Arjas P., Luukkonen R., Riihimäi H., Vahtera J., Kirjonen J. Work stress and risk of cardiovascular mortality: Prospective cohort study of industrial employees. British Medical Journal. 2002;325(7369):857. doi: 10.1136/bmj.325.7369.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E.-L., Laakso M.P., Hänninen T., Hallikainen M., Alhainen K., Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. British Medical Journal. 2001;322(7300):1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz G., Berntsson L., Lundberg U. Total workload, work stress and perceived symptoms in Swedish male and female white-collar employees. The European Journal of Public Health. 2005;15(2):209–214. doi: 10.1093/eurpub/cki079. [DOI] [PubMed] [Google Scholar]
- Laerd Statistics (2015). Mann-Whitney U test using SPSS statistics. Statistical tutorials and software guides. Retrieved from 〈https://statistics.laerd.com/〉.
- Laerd Statistics (2016). Kruskal-wallis h test using spss statistics. Statistical tutorials and software guides. Retrieved from 〈https://statistics.laerd.com/〉.
- Lahelma E., Martikainen P., Laaksonen M., Aittomäki A. Pathways between socioeconomic determinants of health. Journal of Epidemiology and Community Health. 2004;58(4):327–332. doi: 10.1136/jech.2003.011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B.G., Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;35:80–94. [PubMed] [Google Scholar]
- Liu X., Li L., Liu F., Deng S., Zhu R., Li Q., He Z. ApoE gene polymorphism and vascular dementia in Chinese population: A meta-analysis. J. Neural Transmission. 2012;119(3):387–394. doi: 10.1007/s00702-011-0714-6. [DOI] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Cohen-Mansfield J. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Long J.S., Freese J. Vol. 2. Stata Press; College Station, Tex: 2006. (Regression models for categorical dependent variables using stata). [Google Scholar]
- Lupien S., Nair N., Briere S., Maheu F., Tu M., Lemay Μ, Meaney M. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia in later life. Reviews in the Neurosciences. 1999;10(2):117–140. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- MacGregor-Fors I., Payton M.E. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS One. 2013;8(2):e56794. doi: 10.1371/journal.pone.0056794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M. Bloomsbury; London: 2004. Status Syndrome. [Google Scholar]
- Marmot M., Ryff C.D., Bumpass L.L., Shipley M., Marks N.F. Social inequalities in health: Next questions and converging evidence. Social Science Medicine. 1997;44(6):901–910. doi: 10.1016/s0277-9536(96)00194-3. [DOI] [PubMed] [Google Scholar]
- Matthews S., Hertzman C., Ostry A., Power C. Gender, work roles and psychosocial work characteristics as determinants of health. Social Science Medicine. 1998;46(11):1417–1424. doi: 10.1016/s0277-9536(97)10141-1. [DOI] [PubMed] [Google Scholar]
- Meng X., D’Arcy C. Apolipoprotein e gene, environmental risk factors, and their interactions in dementia among seniors. International Journal of Geriatric Psychiatry. 2013;28(10):1005–1014. doi: 10.1002/gps.3918. [DOI] [PubMed] [Google Scholar]
- Ngandu T., von Strauss E., Helkala E.L., Winblad B., Nissinen A., Tuomilehto J., Kivipelto M. Education and dementia: What lies behind the association? Neurology. 2007;69(14):1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- Nilsen C., Andel R., Fors S., Meinow B., Mattsson A.D., Kåreholt I. Associations between work-related stress in late midlife, educational attainment, and serious health problems in old age: A longitudinal study with over 20 years of follow-up. BMC Public Health. 2014;14(1):878. doi: 10.1186/1471-2458-14-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby R.L., Crocco E., Acevedo A., John V., Loewenstein D. Depression and risk for alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton M.E., Greenstone M.H., Schenker N. Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? Journal of Insect Science. 2003;3:1–6. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Karp A., von Strauss E., Winblad B., Fratiglioni L., Bellander T. Lifetime principal occupation and risk of Alzheimer’s disease in the kungsholmen project. American Journal of Industrial Medicine. 2003;43(2):204–211. doi: 10.1002/ajim.10159. [DOI] [PubMed] [Google Scholar]
- Qiu C., Kivipelto M., von Strauss E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues in Clinical Neuroscience. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S., Kareholt I., Viitanen M., Winblad B., Tuomilehto J., Soininen H., Kivipelto M. Work-related physical activity and the risk of dementia and Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2007;22(9):874–882. doi: 10.1002/gps.1755. [DOI] [PubMed] [Google Scholar]
- Rohn T.T. Is apolipoprotein E4 an important risk factor for vascular dementia. International Journal of Clinical and Experimental Pathology. 2014;7(7):3504–3511. [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Why stress is bad for your brain. Science. 1996;273(5276):749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sattler C., Toro P., Schoenknecht P., Schroeder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Research. 2012;196(1):90–95. doi: 10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer's disease. The Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- Seidler A., Nienhaus A., Bernhardt T., Kauppinen T., Elo A., Frölich L. Psychosocial work factors and dementia. Occupational and Environmental Medicine. 2004;61(12):962–971. doi: 10.1136/oem.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindi S., Hagman G., Håkansson K., Kulmala J., Nilsen C., Kåreholt I., Soininen H., Solomon A., Kivipelto M. Midlife work-related stress increases dementia risk in later life: The caide 30-year study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2016;72(6):1044–1053. doi: 10.1093/geronb/gbw043. [DOI] [PubMed] [Google Scholar]
- Skoog I., Nilsson L., Palmertz B., Andreasson L.A., Svanborg A. A population-based study of dementia in 85-year-olds. New England Journal of Medicine. 1993;328(3):153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- Skoog I., Nilsson L., Persson G., Lernfelt B., Landahl S., Palmertz B., Andreasson L., Odén A., Svanborg A. 15-year longitudinal study of blood pressure and dementia. The Lancet. 1996;347(909):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Skoog I., Waern M., Duberstein P., Blennow K., Zetterberg H., Börjesson-Hanson A., Östling S., Guo X., Kern J., Gustafson D., Gudmundsson P., Marlow T., Kern S. A 9-Year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biological Psychiatry. 2015;78(10):730–736. doi: 10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Smyth K.A., Fritsch T., Cook T.B., McClendon M.J., Santillan C.E., Friedland R.P. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63(3):498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- Stansfeld S., Candy B. Psychosocial work environment and mental health—a meta-analytic review. Scandinavian Journal of Work, Environment Health. 2006:443–462. doi: 10.5271/sjweh.1050. [DOI] [PubMed] [Google Scholar]
- Stansfeld S.A., Rasul F., Head J., Singleton N. Occupation and mental health in a national UK survey. Social Psychiatry and Psychiatric Epidemiology. 2011;46(2):101–110. doi: 10.1007/s00127-009-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Sweden . Statistics Sweden; Stockholm: 1982. MIS 1982:4 SEI - socioekonomisk indelning [socioeconomic classification system] [Google Scholar]
- Statistics Sweden . Statistics Sweden; Stockholm: 1992. Tidsanvändningsundersökningen 1990/91 [the Swedish Time Use Survey 1990/91] [Google Scholar]
- Statistics Sweden (2011). Arbetsmarknaden under 50 år – några karaktäristiska drag [the labour market during 50 years - some characteristic features]. Retrieved from 〈https://www.scb.se/sv_/Hitta-statistik/Statistik-efter-amne/Arbetsmarknad/Arbetskraftsundersokningar/Arbetskraftsundersokningarna-AKU/23265/23272/Behallare-for-Press/321232/〉.
- Statistics Sweden . Statistics Sweden; Stockholm: 2012. Nu för tiden - en undersökning om svenska folkets tidsanvändning år 2010/11 [the Swedish Time Use Survey 2010/11] [Google Scholar]
- Stenfors C.U., Hanson L.M., Oxenstierna G., Theorell T., Nilsson L.-G. Psychosocial working conditions and cognitive complaints among swedish employees. PLoS One. 2013;8(4):e60637. doi: 10.1371/journal.pone.0060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Alexander G.E., Prohovnik I., Stricks L., Link B., Lennon M.C., Mayeux R. Relationship between lifetime occupation and, parietal flow - implications for a reserve against Alzheimers-disease pathology. Neurology. 1995;45(1):55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Swedish Work Environment Authority . Swedish Work Environment Authority; Stockholm: 2016. Arbetsmiljön 2015 [The Work Environment 2015] (Arbetsmiljöstatistik Rapport [Work environment statistics report)( 2016:2) [Google Scholar]
- Then F.S., Luck T., Heser K., Ernst A., Posselt T., Wiese B., AgeCoDe Study G. Which types of mental work demands may be associated with reduced risk of dementia? Alzheimers Dementia. 2017;13(4):431–440. doi: 10.1016/j.jalz.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Then F.S., Luck T., Luppa M., Thinschmidt M., Deckert S., Nieuwenhuijsen K., Riedel-Heller S.G. Systematic review of the effect of the psychosocial working environment on cognition and dementia. Occupational and Environmental Medicine. 2014;71(5):358–365. doi: 10.1136/oemed-2013-101760. [DOI] [PubMed] [Google Scholar]
- Theorell T., Hammarström A., Aronsson G., Träskman Bendz L., Grape T., Hogstedt C., Hall C. A systematic review including meta-analysis of work environment and depressive symptoms. BMC Public Health. 2015;15(1):738–751. doi: 10.1186/s12889-015-1954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell T., Jood K., Järvholm L.S., Vingård E., Perk J., Östergren P.O., Hall C. A systematic review of studies in the contributions of the work environment to ischaemic heart disease development. The European Journal of Public Health. 2016;26(3):470–477. doi: 10.1093/eurpub/ckw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen S., Hemström Ö. Income differences in cardiovascular disease: Is the contribution from work similar in prevalence versus mortality outcomes? International Journal of Behavioral medicine. 2006;13(1):89–100. doi: 10.1207/s15327558ijbm1301_11. [DOI] [PubMed] [Google Scholar]
- Torssander J., Erikson R. Stratification and mortality: A comparison of education, class, status and income. European Sociological Review. 2010;26(4):465–474. [Google Scholar]
- Van der Doef M., Maes S. The job demand-control (-support) model and psychological well-being: A review of 20 years of empirical research. Work Stress. 1999;13(2):87–114. [Google Scholar]
- Vrijkotte T.G., Van Doornen L.J., De Geus E.J. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension. 2000;35(4):880–886. doi: 10.1161/01.hyp.35.4.880. [DOI] [PubMed] [Google Scholar]
- Wang H.-X., Gustafson D.R., Kivipelto M., Pedersen N.L., Skoog I., Windblad B., Fratiglioni L. Education halves the risk of dementia due to apolipoprotein ε4 allele: A collaborative study from the Swedish Brain Power initiative. Neurobiology of Aging. 2012;33(5):1007.e1–1007.e7. doi: 10.1016/j.neurobiolaging.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Wang H.-X., Wahlberg M., Karp A., Winblad B., Fratiglioni L. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimer’s & Dementia. 2012;8(2):114–120. doi: 10.1016/j.jalz.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Wieclaw J., Agerbo E., Mortensen P.B., Bonde J.P. Risk of affective and stress related disorders among employees in human service professions. Occupational and Environmental Medicine. 2006;63(5):314–319. doi: 10.1136/oem.2004.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H., Cedazo-Minguez A., Dubois B., Edvardsson D., Feldman H., Fratiglioni L., Frisoni G.B., Gauthier S., Georges J., Graff C., Iqbal K., Jessen F., Johansson G., Jonsson L., Kivipelto M., Knapp M., Mangialasche F., Melis R., Nordberg A., Rikkert M.O., Qiu C., Sakmar T.P., Scheltens P., Schneider L.S., Sperling R., Tjernberg L.O., Waldemar G., Wimo A., Zetterberg H. Defeating Alzheimer's disease and other dementias: A priority for European science and society. Lancet Neurology. 2016;15(5):455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2012. Dementia - A public Health Priority. [Google Scholar]