Abstract
Both mouse and human mammary glands contain stem/progenitor functional hierarchies that are maintained through the entire life span of the animal. Cells with such functional capacities are potential candidates for tumorigenesis as they are long lived, multipotent, and self-renewing. Using the mouse as a model, this review will discuss what is known about the mammary stem/progenitor hierarchy, the evidence that particular progenitor functions are susceptible to tumorigenic stimuli, how these findings in mice are relevant to the disease in humans, and the role of the local microenvironment in controlling tumorigenesis.
Key words: Breast cancer, Mammary biology, Stem cells, Progenitor cells, Tumorigenesis
INTRODUCTION
The mouse has proven to be an invaluable model for the study of mammary stem cell function and tumorigenesis. Like the human, the animal is born with only a rudimentary epithelial tree, but the gland undergoes extensive postnatal development at the onset of puberty (at approximately 3–4 weeks of age in the mouse). At this time, the ductal tree extends to fill the entire fat pad, penetrating the surrounding stroma through formation of specialized structures known as terminal end buds (31). At the onset of pregnancy, the gland goes through further development, as milk-secreting lobules are formed. Following the cessation of lactation, the gland undergoes involution, marked by extensive apoptosis as the lobules disappear and the gland returns to morphological state that is largely indistinguishable from the nulliparous gland (43). The mammary epithelial population is diverse, with ducts and lobules consisting of a single layer of luminal epithelial cells surrounded by a meshwork of basal myoepithelial cells. The latter express smooth muscle actin (SMA) and can contract to expel the milk produced by the luminal cells during lactation. The luminal layer contains a heterogeneous mixture of cells with different hormonal and growth factor receptor states [e.g., estrogen receptor α (ERα) positive/negative and progesterone receptor (PR) positive/negative].
The study of the mouse mammary gland is facilitated by the ability of the epithelial tree to recapitulate itself when transferred into an epithelial divested fat pad of a recipient animal (12,13,15). Such studies have revealed distinct stem/progenitor functions existing within the gland, controlled by their local environment, and persisting through all stages of development. Cells with such capabilities are attractive targets for tumorigenesis, in that they are long lived, division competent, and relatively undifferentiated, endowing them with properties required to function as so-called “cancer stem cells.” However, how such cells may overcome the protective and suppressive effects of their local niche to acquire genetic and epigenetic changes and to ultimately produce a tumor remains unclear. This review will outline what is known about the mammary stem/progenitor functions within the mouse mammary gland, the evidence that specific progenitor populations are targets of tumorigenesis in the mouse, how these findings in mice are equatable to the disease in humans, and the role of the local microenvironment in controlling tumorigenesis.
STEM/PROGENITOR FUNCTIONS IN THE MOUSE MAMMARY GLAND
DeOme and colleagues (12,13,15) first demonstrated that tissue fragments taken from intact mammary glands could recapitulate the entire epithelial tree when transplanted into the epithelial divested fat pad of a syngenic mouse. Age and reproductive history have no effect on the regenerative capacity of the mammary gland, as cells taken from 26-month-old virgins have the same transplant potential as those taken from 3-week-old mice; both are capable of producing five serial transplant generations before reaching growth senescence (36). Furthermore, transplantation of any portion of the gland is capable of regenerating the entire epithelial tree upon transplantation (36), demonstrating that the mammary regenerative capacity is present throughout the gland.
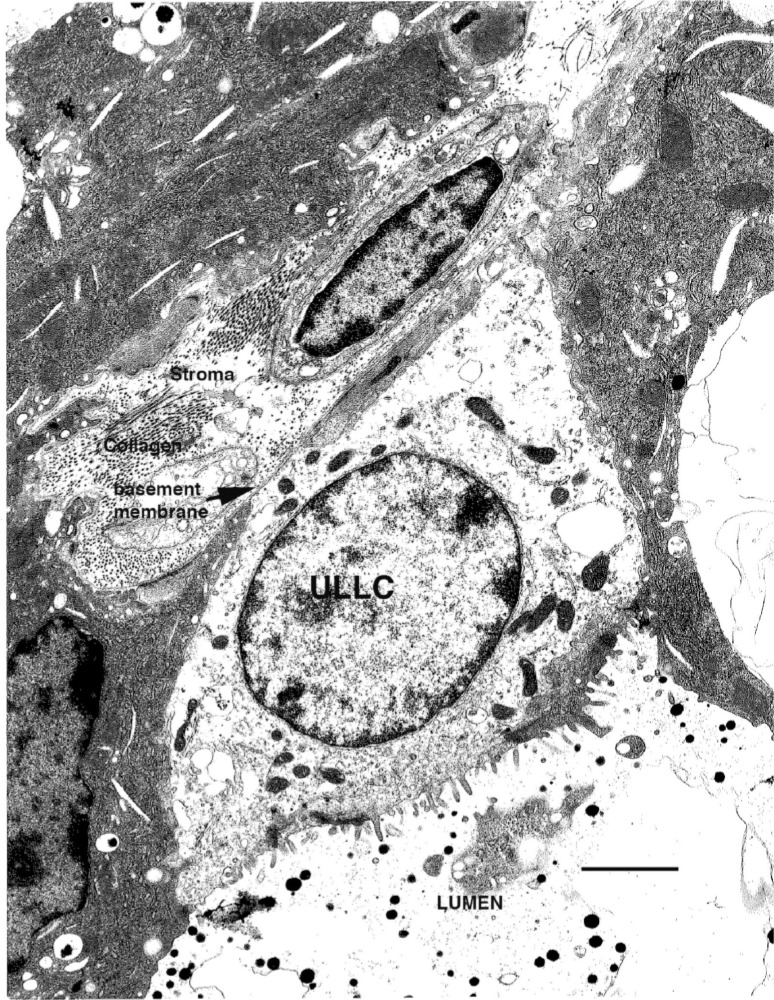
The first direct evidence for the existence of a multipotent stem cell function in the mouse mammary epithelium came from studies using retrovirally [mouse mammary tumor virus (MMTV)] marked tissues, which demonstrated that outgrowths resulting from tissue fragment transplantations were clonal, and that the same MMTV proviral insertions could be detected through five transplant generations (23). In situ evidence for mammary stem/progenitor cell function also exits. Analysis of mammary glands revealed subsets of undifferentiated (pale) cells within the mammary epithelium that had the characteristics of stem/progenitor populations, including the ability to divide and give rise to differentiated secretory cells when treated with hormones that induced lactogenic differentiation ex vivo (36). Ultrastructural analysis further divided these pale cells into small light cells (SLC), undifferentiated large light cells (ULLC), and differentiating large light cells (DLLC) (Fig. 1) (11). These structural categories may represent stem, progenitor, and differentiating cell functions within the mammary gland. These pale cells were present through all stages of development and throughout the gland, but absent from senescent tissues. Taken together, these results demonstrate that stem/progenitor functions are present throughout the mammary tree, and are maintained throughout the life span of the animal, but lost during growth senescence.
Figure 1.
Example of an undifferentiated large light cell (ULLC). The ULLC maintains contact with the lumen and the basement membrane. Note the complex cytoplasm of the surrounding differentiated cells as compared to the ULLC. Scale bar: 5 μm.
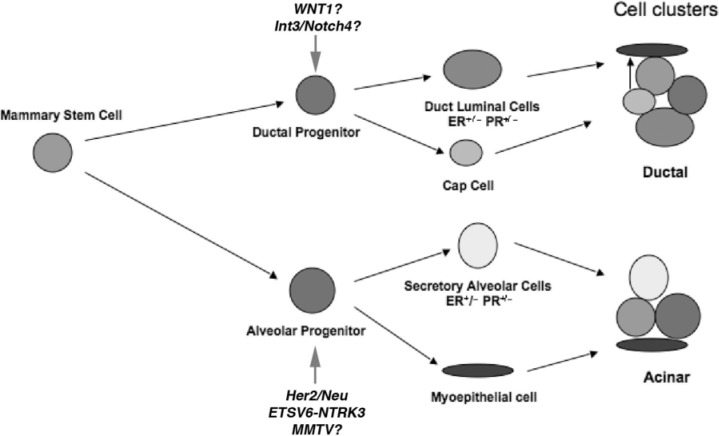
In addition to mammary stem cells, two distinct lineage limited progenitor functions have also been identified in both mouse and rats: lobule limited and duct limited (21,23,33) (Fig. 2). These lineage-limited outgrowths contain both luminal epithelial and basal myoepthelial cell populations. The apparent distinction between the two progenitor functions is that lobule-limited progenitors cannot produce cap cells at the tips of terminal end buds, and are therefore unable to penetrate the fat pad. Duct-limited progenitors, on the other hand, do not produce progeny capable of generating or sustaining alveolar growth and development during pregnancy. Both lineage-limited activities could be identified through serial transplantations of MMTV marked clonal mammary populations, indicating that both lineage-limited progenitors were derived from a single pluripotent mammary epithelial antecedent (23,35). Furthermore, serial transplantation of MMTV marked fragments revealed that ductal elongation and lobulogenesis are independently lost as the gland reaches senescence after multiple transplant generations (35).
Figure 2.
Model of stem/progenitor cellular hierarchy in the mouse mammary gland. The pluripotenet stem cell gives rise to duct-limited and lobule-limited (PI-MEC) progenitors. Both progenitor populations are multipotent. The lobule progenitor gives rise to ER+/− and PR+/− luminal epithelial cells and myoepithelial cells. Duct-limited progenitors also give rise to ER+/− and PR+/− luminal epithelial cells as well as the cap cells during ductal elongation. The cap cells ultimately differentiate into the myoepithelial cells of the ducts. Arrows indicate potential and identified targets of the oncogenic stimuli listed.
A population with the functional attributes of lobule-limited progenitors can be marked in situ utilizing whey acidic protein promoter-cre recombinase (WAP-Cre) and Rosa26-lox-stop-lox-lacZ double transgenic mice (WC/R26) (41). During late pregnancy, the WAP promoter is activated, turning on expression of Cre, which in turn removes the stop sequence from the Rosa26-lacZ transgene and thus permanently turns on expression of β-galactosidase (β-Gal) in the activated cell, and all of its future progeny. Following pregnancy, a small percentage of β-Gal+ cells survive involution. These cells, termed parity-identified mammary epithelial cells (PI-MECs), are long-lived cells that proliferate to produce lobules in subsequent pregnancies (8,41). PI-MECs are multipotent, self-renewing, and capable of retaining their activity through serial transplantations (7,8). In transplants, PI-MEC progeny also are represented in cells that retain nuclear DNA labels for an extended duration [i.e., long label retaining epithelial cells (LREC)] (34). During pregnancy, PI-MECs produce luminal progeny that are positive for ERα or PR, as well as luminal cells that do not express either steroid receptor. In addition, β-Gal+ myoepithelial cells are present in developing secretory acini in parous mice during early pregnancy—before de novo activation of WAP—demonstrating that PI-MECs are capable of producing both luminal and myoepithelial progeny. Originally, PI-MECs were thought to arise from dedifferentiated secretory epithelial cells. However, they were subsequently found to exist in nulliparous glands by treating fragments with growth factors that induced CRE expression, but did not result in lactogenic differentiation (4). These cells possessed all the properties of PI-MECs found in parous hosts, including self-renewal and multipotency. This result is consistent with the previous outlined findings of limiting dilution experiments from nulliparous donors, which produced both duct-limited and lobule-limited outgrowths in pregnant transplant hosts (33). The faculty of lobule-limited progenitors (i.e., PI-MECs) to be marked in situ has afforded extensive research into their role in tumorigenesis.
PI-MECs AS TARGETS OF TUMORIGENESIS IN THE MOUSE MAMMARY GLAND
The first evidence for a role of stem/progenitor cells in mouse mammary tumorigenesis emerged from studies using WAP-TGF-β1 mice. Expression of transforming growth factor-β1 (TGF-β1) from the WAP promoter induces premature senescence of stem/progenitor cells, evidenced by the inability of transgenic glands to recapitulate an epithelial tree upon transplantation into a cleared fat pad (7,22). When WAP-TGF-β1 mice were inoculated with MMTV, tumors formed at a significantly reduced rate as compared to wild-type controls (7). Importantly, WAP-TGF-β1 did not reduce overall proliferation during pregnancy, so the effect on tumorigenesis was likely the result of stem/progenitor cell senescence rather than a general reduction in replication. Later studies revealed that WAP-TGF-β1 induces senescence specifically in PI-MECs, implicating these cells as potential targets of MMTV-induced tumorigenesis (8).
Subsequent studies provided direct evidence that PI-MECs were the targets of tumorigenesis in specific mouse models. When WC/R26 mice were crossed with mice expressing Her2/Neu (ErbB2) from the MMTV-LTR promoter (MMTV-Neu), tumors that formed in parous animals were β-Gal+ (27 out of 28 tumors observed) (19). Importantly, glands from nulliparous littermates were largely devoid β-Gal+ cells, and even microscopic lesions were comprised entirely of β-Gal+ cells that did not express Cre, demonstrating that the tumor antecedents had activated the WAP promoter and survived involution rather than there being a de novo activation of Wap-Cre within the tumor. Furthermore, the authors demonstrated that selective ablation of PI-MECs through the introduction of a WAP-Cre/TSG101fl/fl transgenic system (42) significantly reduced MMTV-Neu-induced tumorigenesis from occurring.
Independently, Jeselson et al. (20) confirmed PI-MEC were targets of transformation of in MMTV-Erb2 mice. By crossing MMTV-Neu mice with Wap-Cre/Rosa26-GFP mice (WC/R26-GFP), the authors demonstrated that cyclin D1 was required for transformation of PI-MECs by the MMTV-Neu transgene. The authors also demonstrate that cyclin D1 is required for alveolar development, providing a link between lobular progenitor (i.e., PI-MEC) function, tumorigenesis, and cyclin D1 activity.
In addition to Her2/Neu, PI-MECs have been identified as the targets of tumorigenesis in a mouse model using the human ETV6-NTRK3 (EN) fusion oncogene (24). EN is formed from a t(12;15)(p13; q25) translocation and the resulting protein consists of the oligomerization domain of ETV6 and the tyro-sine kinase domain of NTRK3 and has been consistently identified in human secretory breast cancer (39). The authors conditionally knocked-in EN through a Wap-Cre-mediated excision of a floxed stopper cassette located within the transgene and found the transgene targeted the lobule-limited progenitor, and that the resulting tumors contained either all luminal, or a mixture of luminal and basal-like cells. This provided evidence that PI-MEC could be targets of oncogenes other than Her2/Neu, and that oncogenic mutations relative to human breast cancer were capable of transforming this important progenitor population.
Several lines of evidence suggest that cell types other than PI-MEC can be transformed by distinct oncogenic events. One well-studied example is the MMTV-Wnt1 model. While MMTV-Neu tumors are devoid of NKcc1 (a sodium/potassium/chloride channel specifically expressed in ductal but not alveolar epithelial subtypes) (30) expression, MMTV-Wnt1 tumors do express the channel marker (19). Furthermore, MMTV-Wnt1-mediated tumorigenesis is unaffected by ablation of cyclin D1 activity (20), verifying that Wnt1 targets a distinct cellular population, potentially the ductal progenitor. Recently, PI-MEC were also found to not be involved in WAP-Int3/Notch4-induced tumorigenesis (9). Therefore, the wealth of evidence suggests that the stem/progenitor population is a target of tumorigenesis, and that distinct oncogenic stimuli initiate malignant transformation in different subsets of cellular populations (Fig. 2).
STEM/PROGENITORS AS TARGETS OF TUMORIGENESIS IN HUMAN BREAST CANCER
While less is known about the stem/progenitor functions present within the human mammary gland, current evidence suggests that a similar cellular organization is present. In situ analysis of human mammary tissue has revealed the presence of SLC and ULLC with characteristics similar to those seen in the murine glands (17,32). Furthermore, clonal patches of cells can be identified in adjacent ducts and lobules by X-chromosome inactivation analysis, suggesting a local stem/progenitor function is present within the gland (40). Recently, analysis of FACS segregated populations has provided evidence that subsets of human breast epithelial cells are capable of generating mammary colonies or structures in vitro and in vivo (14,37,38), further supporting the concept of epithelial cellular hierarchies within the gland.
Human breast cancers are often broadly categorized based on gene expression patterns as “luminal-like” or “basal-like” tumors (28). A long-standing assumption was that these tumors arose from cells within these respective lineages. However, recent work on BRCA1-deficient tissues has demonstrated that this assumption is likely incorrect. Patients harboring mutations in BRCA1 are predisposed to characteristic basal-like tumors (1,16), but seemingly paradoxically two recent publications have identified luminal cells as the targets of tumorigenesis in BRCA1-deficient humans and in mice (27,29). Using lentivector delivery of oncogenes, Proia et al. transformed normal mammary epithelium taken from patients carrying a mutated BRCA1 allele (BRCA1mut/+) and transplanted them into humanized fat pads of immunocompromised mice (29). The resulting tumors had basal-like phenotypes, even if they were enriched for luminal cells prior to transduction. In fact, nearly all of the tumorigenic potential was in the luminal fraction of cells. Furthermore, normal tissue from Brca1mut/+ samples displayed increased expression of basal markers vimentin and CD10, with a corresponding decrease in the amount of cells positive for the luminal associated markers PR and trefoil factor 3. Similarly, Molyneux et al. found that targeted BRCA1 deficiencies in mice only resulted in tumors when directed to a luminal cell population, and never when targeted to basal cells (27). The resulting tumors were phenocopies of those found in humans, suggesting that the mouse model was a reliable surrogate for the disease in humans.
Although not directly tested in these experiments, it is possible the cellular target of transformation in BRCA1 deficient patients is a progenitor cell within the luminal population that is capable of producing both basal and myoepithelial cells. This cell type would be analogous to the PI-MEC population found to be the target of tumorigenesis in MMTV-Neu mice (3,19). Regardless of the specific cell of origin, it is clear the expression profile of a resulting tumor can be misleading, and is at least equally reliant on the resulting cellular microenvironment as it is the particular cell of origin. Furthermore, while little is known about the role of stem/progenitor cells within the human breast, current studies indicate the gland is likely organized in a similar manner as murine models, and that multipotent progenitor populations are likely targets of tumorigenesis.
THE ROLE OF THE MAMMARY NICHE IN CONTROLLING TUMORIGENESIS
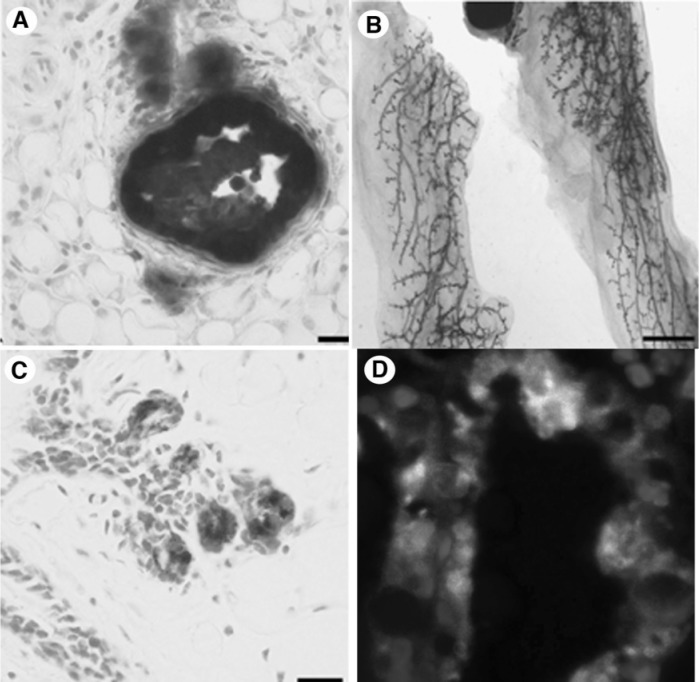
The ability of “normal” microenvironments to suppress tumorigenesis is well documented (2,3,10,18,25,26). Recently, our group demonstrated that re-establishing a normal mammary microenvironment redirected tumor cells derived from WC/R26/ MMTV-Neu mice (which originated from PI-MECs) to contribute to a normal functional mammary outgrowth (3). The differentiation capacity of the tumor cells was demonstrated by transplanting the β-Gal marked tumor cells with wild-type mammary epithelial cells in a 1:50 ratio (Fig. 3). The β-Gal+ transformed cells were found to contribute luminal and basal progeny to both ducts and lobules in the resulting chimeric outgrowths. At pregnancy, the redirected tumor cells secreted milk proteins and later contributed to second-generation outgrowths, demonstrating they were self-renewing (i.e., functioning as progenitors rather than terminally differentiating). This study came on the heels of similar work demonstrating that the mammary microenvironment could reprogram cells of nonmammary origin to adapt a PI-MEC cellular fate (5,6,10). These remarkable studies underscore the dominance of the environment (i.e., the niche) to control cell fate, even that of transformed cells. They also raise an important question: If PI-MECs are the targets of tumorigenesis in MMTV-Neu mice, and tumorigenic cells from these mice can be made to function as normal PI-MEC when a competent niche is restored, how did they become tumors in the first place? It seems a breakdown in their local niche must have led to the malignant progression of these marginally transformed cells to tumor cells. Tumors arising in any transgenic animal happen stochastically at particular sites within the gland (despite the presence of the transgene in every cell within the tissue). How such breakdowns occur in the local architecture or signaling networks of a particular transformed cell’s niche, allowing it to progress to a tumor, is currently a mystery in cancer biology. A greater research effort into this phenomenon is needed, as answers to this complex question would likely yield a greater understanding of tumorigenesis than can be achieved by studying the effects of specific mutations on cell functions per se.
Figure 3.
Cells derived from MMTV-Neu/WC/R26 tumors contribute to a functional mammary gland when mixed with normal mammary epithelium. (A) When inoculated on their own, MMTV-Neu/WC/R26 tumor cells give rise to tumors in cleared mammary fat pads. (B) When mixed with normal mammary epithelium in a 1:50 ratio and inoculated into an epithelial divested fat pad, the β-Gal-marked tumor cells contribute to the resulting normal mammary outgrowth and did not produce tumors. (C) Cross section of a chimeric gland demonstrating presence of β-Gal+ cells. (D) β-Gal and casein coexpression (white) in mammary epithelium in a lactating chimeric gland demonstrates tumor-derived cells capable of differentiating to producing milk proteins.
CONCLUSIONS
Studies in the mouse have revealed PI-MECs can act as targets of tumorigenesis for some oncogenic stimuli. However, different genetic alterations can clearly target different populations of cells, with Wap-Wnt1 and Wap-Int3 mice providing examples of transgenic systems that do not target the PI-MECs. Studies in humans suggest a similar stem/progenitor hierarchy is present, and that the target of tumorigenesis in BRCA1-deficient patients is a progenitor population that lies within the luminal compartment of the normal mammary gland, despite the fact that the resulting tumors have a basal-like phenotype. This cellular target is capable of giving rise to both luminal and basal lineages, as has been seen with mouse progenitor populations. Finally, it is clear that the normal mammary microenvironment (i.e., niche) can control stem/progenitor cell fate, even that of transformed cells. This offers hope for future treatments that could control tumor progression by altering the niche in which the “cancer stem cells” reside. At the same time, these observations raise important questions as to how transformed stem/progenitor populations initiate tumor formation, and impel more research toward understanding this process.
REFERENCES
- 1. Arnes J. B.; Brunet J. S.; Stefansson I.; et al. Placental cadherin and the basal epithelial phenotype of BRCA1-related breast cancer. Clin. Cancer Res. 11:4003–411; 2005. [DOI] [PubMed] [Google Scholar]
- 2. Bissell M. J.; Hines W. C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression Nat. Med. 17:320–329; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Booth B.; Boulanger C.; Anderson L.; Smith G. The mammary microenvironment restricts the tumorigenic phenotype of MMTV-neu-transformed tumor cells. Oncogene 30:679–689; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booth B. W.; Boulanger C. A.; Smith G. H. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J. Cell. Physiol. 212:729–736; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Booth B. W.; Mack D. L.; Androutsellis-Theotokis A.; McKay R. D.; Boulanger C. A.; Smith G. H. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc. Natl. Acad. Sci. USA 105:14891–14896; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulanger C. A.; Mack D. L.; Booth B. W.; Smith G. H. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc. Natl. Acad. Sci. USA 104:3871–3876; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulanger C. A.; Smith G. H. Reducing mammary cancer risk through premature stem cell senescence. Oncogene 20:2264–2272; 2001. [DOI] [PubMed] [Google Scholar]
- 8. Boulanger C. A.; Wagner K. U.; Smith G. H. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene 24:552–560; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Bruno R. D.; Boulanger C. A.; Smith G. H. Notch-induced mammary tumorigenesis does not involve the lobule-limited epithelial progenitor. Oncogene; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bussard K. M.; Boulanger C. A.; Booth B. W.; Bruno R. D.; Smith G. H. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 70:6336–6343; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chepko G.; Smith G. H. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29:239–253; 1997. [DOI] [PubMed] [Google Scholar]
- 12. Daniel C. W.; DeOme K. B. Growth of mouse mammary glands in vivo after monolayer culture. Science 149:634–636; 1965. [DOI] [PubMed] [Google Scholar]
- 13. DeOme K.; Faulkin L. J.; Bern H.; Blair P. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 19:515–520; 1959. [PubMed] [Google Scholar]
- 14. Eirew P.; Stingl J.; Raouf A.; et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat. Med. 14:1384–1389; 2008. [DOI] [PubMed] [Google Scholar]
- 15. Faulkin L. J. Jr.; DeOme K. B. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J. Natl. Cancer Inst. 24:953–969; 1960. [PubMed] [Google Scholar]
- 16. Foulkes W. D.; Brunet J. S.; Stefansson I. M.; et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 64:830–835; 2004. [DOI] [PubMed] [Google Scholar]
- 17. Gudjonsson T.; Villadsen R.; Nielsen H. L.; Ronnov-Jessen L.; Bissell M. J.; Petersen O. W. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16:693–706; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hendrix M. J.; Seftor E. A.; Seftor R. E.; Kasemeier-Kulesa J.; Kulesa P. M.; Postovit L. M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 7:246–255; 2007. [DOI] [PubMed] [Google Scholar]
- 19. Henry M. D.; Triplett A. A.; Oh K. B.; Smith G. H.; Wagner K. U. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23:6980–6985; 2004. [DOI] [PubMed] [Google Scholar]
- 20. Jeselsohn R.; Brown N. E.; Arendt L.; et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell 17:65–76; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamiya K.; Gould M. N.; Clifton K. H. Quantitative studies of ductal versus alveolar differentiation from rat mammary clonogens. Proc. Soc. Exp. Biol. Med. 219:217–225; 1998. [DOI] [PubMed] [Google Scholar]
- 22. Kordon E. C.; McKnight R. A.; Jhappan C.; Hennighausen L.; Merlino G.; Smith G. H. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev. Biol. 168:47–61; 1995. [DOI] [PubMed] [Google Scholar]
- 23. Kordon E. C.; Smith G. H. An entire functional mammary gland may comprise the progeny from a single cell. Development 125:1921–1930; 1998. [DOI] [PubMed] [Google Scholar]
- 24. Li Z.; Tognon C. E.; Godinho F. J.; et al. ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell 12:542–558; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mintz B.; Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA 72:3585–3589; 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mintz B.; Slemmer G. Gene control of neoplasia. I. Genotypic mosaicism in normal and preneoplastic mammary glands of allophenic mice. J. Natl. Cancer Inst. 43:87–109; 1969. [PubMed] [Google Scholar]
- 27. Molyneux G.; Geyer F. C.; Magnay F. A.; et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7:403–417; [DOI] [PubMed] [Google Scholar]
- 28. Peppercorn J.; Perou C. M.; Carey L. A. Molecular subtypes in breast cancer evaluation and management: Divide and conquer. Cancer Invest. 26:1–10; 2008. [DOI] [PubMed] [Google Scholar]
- 29. Proia T. A.; Keller P. J.; Gupta P. B.; et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8:149–163; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shillingford J. M.; Miyoshi K.; Flagella M.; Shull G. E.; Hennighausen L. Mouse mammary epithelial cells express the Na-K-Cl cotransporter, NKCC1: Characterization, localization, and involvement in ductal development and morphogenesis. Mol. Endocrinol. 16:1309–1321; 2002. [DOI] [PubMed] [Google Scholar]
- 31. Silberstein G. B.; Daniel C. W. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev. Biol. 90:215–222; 1982. [DOI] [PubMed] [Google Scholar]
- 32. Smith C. A.; Monaghan P.; Neville A. M. Basal clear cells of the normal human breast. Virchows Arch. A Pathol. Anat. Histopathol. 402:319–329; 1984. [DOI] [PubMed] [Google Scholar]
- 33. Smith G. H. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res. Treat. 39:21–31; 1996. [DOI] [PubMed] [Google Scholar]
- 34. Smith G. H. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132:681–687; 2005. [DOI] [PubMed] [Google Scholar]
- 35. Smith G. H.; Boulanger C. A. Mammary stem cell repertoire: New insights in aging epithelial populations. Mech. Ageing Dev. 123:1505–1519; 2002. [DOI] [PubMed] [Google Scholar]
- 36. Smith G. H.; Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90(Pt. 1):173–183; 1988. [DOI] [PubMed] [Google Scholar]
- 37. Stingl J.; Eaves C. J.; Kuusk U.; Emerman J. T. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63:201–213; 1998. [DOI] [PubMed] [Google Scholar]
- 38. Stingl J.; Eaves C. J.; Zandieh I.; Emerman J. T. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 67:93–109; 2001. [DOI] [PubMed] [Google Scholar]
- 39. Tognon C.; Knezevich S. R.; Huntsman D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2:367–376; 2002. [DOI] [PubMed] [Google Scholar]
- 40. Tsai Y. C.; Lu Y.; Nichols P. W.; Zlotnikov G.; Jones P. A.; Smith H. S. Contiguous patches of normal human mammary epithelium derived from a single stem cell: Implications for breast carcinogenesis. Cancer Res. 56:402–404; 1996. [PubMed] [Google Scholar]
- 41. Wagner K. U.; Boulanger C. A.; Henry M. D.; Sgagias M.; Hennighausen L.; Smith G. H. An adjunct mammary epithelial cell population in parous females: Its role in functional adaptation and tissue renewal. Development 129:1377–1386; 2002. [DOI] [PubMed] [Google Scholar]
- 42. Wagner K. U.; Krempler A.; Qi Y.; et al. Tsg101 is essential for cell growth, proliferation, and cell survival of embryonic and adult tissues. Mol. Cell. Biol. 23:150–162; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson C. J. Involution: Apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 8:203; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]