Abstract
Hydrogen sulfide (H2S), as an endogenous signaling molecule in mammals, shows a variety of biological effects. Cystathionine gamma-lyase (CSE)/H2S pathway has been implicated in scavenging reactive oxygen species (ROS) in the mammalian cells. Therefore, we first investigated the regulatory effects of exogenously applied hydrogen peroxide (H2O2) on CSE expression in the mammalian cells. African green monkey kidney fibroblast-like cells (COS-7 cells) or human embryonic kidney 293 cells (HEK 293 cells) were transfected with CSE promoter-luciferase reporter constructs and treated with H2O2 of 1, 5, and 10 μM for 0.5 and 1.5 h at 37°C. The transfected cells were assayed for firefly luciferase activities normalized by Renilla luciferase activity. Human lung adenocarcinoma cells (A549 cells) or human liver cancer cells (SMMC-7721 cells) were treated with H2O2 of 1, 5, and 10 μM for 0.5 and 1.5 h at 37°C, and were then harvested and analyzed by Western blotting and quantitative RT-PCR. Our results showed that the treatment of a medium concentration (5 μM) of H2O2 at a longer time (1.5 h) upregulated CSE expression in the mammalian cells at the levels of the promoter, message RNA, and protein. Collectively, exogenously applied H2O2 can not only markedly affect CSE mRNA and protein expression, but also can affect the CSE promoter activity in the mammalian cells. Our observations indicate that that exogenous H2O2 can upregulate the expression of the CSE gene in the mammalian cells, which will provide the possibility of the scavenging effect of the CSE gene indirectly on ROS in the mammalian cells. However, the regulatory mechanism involved in the effects of exogenously applied H2O2 on CSE expression in the mammalian cells need be further studied.
Key words: Hydrogen sulfide, Cystathionine gamma-lyase, Hydrogen peroxide, Oxidative stress
INTRODUCTION
Hydrogen sulfide (H2S), as an endogenous signaling molecule, has been proven to be produced endogenously in the mammalian cells and plays important roles in physiological and pathophysiological conditions (5,9,23,27). Cystathionine gamma-lyase (CSE), one of three key enzymes in the transsulfuration pathway, is responsible for the production of endogenous H2S (19,21,23,26,28–30). Some evidence suggests that H2S exerts protective effects against various stimuli-triggered injuries in many organs including heart, liver, and kidney (7,20,22). One of the most important mechanisms responsible for H2S protection is antioxidation, which exerts its effect not only by increasing reduced glutathione (GSH) in neurons (14), but also by directly scavenging superoxide anions, hydrogen peroxide (H2O2) (10).
A state of moderately increased levels of intracellular reactive oxygen species (ROS) is referred to as oxidative stress such as H2O2. H2O2 may be separately regulated, although it can cause pathological damage; both also function in normal cell regulation (4,18). The antioxidative effect of H2S has been demonstrated in a variety of cell models (10,14,16). Administration of exogenous H2S effectively protects myocytes and contractile activity by its direct scavenging of ROS (10). The H2S administration restored the level of enzyme activities and accelerated the scavenging of H2O2 and superoxide anion generated by homocysteine in isolated mitochondria (2). H2S protects neurons from oxidative stress by increasing the production of the antioxidant glutathione (14), and protects cells against H2O2-induced oxidative injury (16,25).
Since H2S is a reducing agent that readily reacts with H2O2, it is possible that endogenous H2S can scavenge ROS (14). However, it is not known whether or not exogenous H2O2 can regulate CSE expression in the mammalian cells. Therefore, we investigated the regulatory effects of exogenously applied H2O2 on CSE expression in the mammalian cells.
MATERIALS AND METHODS
Construction of a Luciferase Reporter Under the Control of CSE Promoter
A 1.7-kb DNA fragment upstream of the transcriptional start site (−1691 to +25) of the CSE gene was isolated by PCR using the genomic DNA of adult Kunming mice/SLAC (Shanghai SLAC Laboratory Animal Co. Ltd, Shanghai, China) as the template with the two primers: KM1716-forward (5′-CGGGGTACCTCCTGGATTCACATACTCCTTTG-3′) and KM1716-reverse (5′-CCGCTCGAGAGGAGTGCGAGGTGTTGCTTTGGCT-3′). The reaction conditions consisted of the followings: 3 min of initial preheating at 94°C, 30 cycles of 95°C for 30 s, 56°C for 45 s, and 72°C for 2 min 30 s, and a final elongation at 72°C for 10 min. The PCR product was digested with KpnI (TAKARA Biology Co., Ltd, Dalian, China) and XhoI (TAKARA Biotechology Co.), subcloned into the promoterless pGL4.12 vector (Promega, Madison, WI, USA) that contains a firefly luciferase gene driven by the promoter activity of inserted sequences, and sequenced for confirmation, producing the pGL4.12-KM1716 vector. The inserted DNA fragment was confirmed by DNA sequencing (Biosune Biotechnology Co., Ltd. Shanghai, China).
Cell Culture and Treatments of Exogenously Applied H2O2
Human embryonic kidney 293 cells (HEK 293 cells) were cultured in a 5% CO2/balance air incubator at 37°C in a DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and with an additive of 100 U/ml penicillin G, 100 μg/ml streptomycin, and 6.5 mM l-glutamine. African green monkey kidney fibroblast-like cells (COS-7 cells) and human liver cancer SMMC-7721 cells (SMMC-7721 cells) were cultured in a 5% CO2/balance air incubator at 37°C in a RPMI-1640 medium supplemented with 10% heat-inactivated FBS, with an additive of 100 U/ml penicillin G, 100 μg/ml streptomycin, and 2 mM l-glutamine. Human lung adenocarcinoma A549 cells (A549 cells) were cultured in a 5% CO2/balance air incubator at 37°C in a DMEM/F12 medium supplemented with 10% heat-inactivated FBS, with an additive of 100 U/ml penicillin G, 100 μg/mL streptomycin, and 4.5 mM l-glutamine.
According to the different cell lines, the transfected HEK 293 cells, the transfected COS-7 cells, A549 cells, and SMMC-7721 cells were seeded at a density of 0.5–1 × 106 cells in 35-mm dishes (Corning Glass, Corning, NY, USA) prior to treatment. H2O2 (3%, Sigma-Aldrich, Product No.: 323381) was first diluted to 100 μM, 500 μM, and 1 mM of H2O2 with double distilled water by the gradient dilution method. Taking 20 μl solution of H2O2 into 2 ml medium, the concentration of H2O2 in the cell medium was 1, 5, and 10 μM, respectively. Therefore, all tested cells were treated with H2O2 for 1, 5, and 10 μM for 0.5 and 1.5 h at 37°C. The control group received the same volume of saline as that of the H2O2. After treatment, the cells were immediately lysed and assayed. Luciferase assay, quantitative real-time PCR, and Western blotting were as described below.
Luciferase Assay
Because the DNA transfection efficiency would be dependent on the different cells, we decided to regard COS-7 cells and HEK 293 cells as the cell type transfected. The reporter gene assay was conducted in transiently transfected HEK 293 cells and COS-7 cells that were maintained in cell medium. When HEK 293 cells and COS-7 cells cultured in 35-mm dishes (Corning Glass) were 70–80% confluent, 0.032 μg (HEK 293 cells) or 0.0032 μg (COS-7 cells) of the pRL-CMV vector that contains a Renilla luciferase gene and 5 μg of the pGL4.12-KM1716 vector were combined, and then the DNA mixture was incubated with Xfect™ transfection reagent (Clontech Laboratories, Inc., CA, USA) at the ratio of 1.5 μl of Xfect™ polymer/5 μg of DNA. Transfection was performed according to the manufacturer’s instructions. After 12 h, the transfected cells were subcultured in several 35-mm dishes at the proportion of 1:3. After exogenously applied H2O2 was used to treat the transfected COS-7 cells of 0.5 × 106 cells/35-mm dish or HEK 293 cells of 1 × 106 cells/35-mm dish, the transfected cells were assayed for measuring the firefly and Renilla luciferase activities after 48 h of DNA transfection, which was measured using the Dual-Luciferase® Reporter Assay (Promega) according to the manufacturer’s instructions with the SpectraMax® microplate reader (Molecular Devices Corporation).
Quantitative Real-Time PCR
To investigate CSE expression in A549 cells or SMMC-7721 cells, total RNA was isolated using the TransZol Up Reagent (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions after the treatment cells were rinsed with 1× dPBS buffer twice. Obtained RNA samples were quantified by absorbance at 260 nm (Biophoto, Eppendorf) and the integrity, purity, and amount of RNA were verified by visualization of rRNAs after electrophoresis on agarose gel. Total RNA concentration was measured by fluorescence using a Qubit™ RNA Assay Kit (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. First-strand cDNA was synthesized by incubation at 42°C for 30 min using anchored oligo(dT)18 primer. The reaction mixture was in a total volume of 20 μl containing 2 μg of RNA, 1 μl of anchored oligo(dT)18, 10 μl of 2× TS Reaction Mix and TransScript™ RT/RI Enzyme mix as well as RNase-free water (TransGen Biotech, Beijing, China). The reaction was terminated by incubation at 85°C for 5 min and the reaction mixture could be stored at −20°C and used for RT-qPCR.
RT-qPCR was performed in a final volume of 25 μl containing 11 μl cDNA diluted with double-distilled H2O (1:20), 0.5 μl of 0.2 μM different primer, 0.5 μl Passive Reference Dye II, and 12.5 μl of 2× TransStart™ Green qPCR SuperMix (TransGen Biotech, Beijing, China). All reactions were run in triplicate with an Agilent Mx3000P QPCR Systems (Agilent Technologies, USA) and the data were treated by the MxPro QPCR software (Agilent Technologies) using a SYBR green fluorescence quantification system with the following process: an initial denaturation step was made at 95°C for 10 min, and 45 cycles for 30 s at 95°C, 30 s at 60°C, and 10 s at 72°C. The primer pair Q CSE Forward Primer/Q CSE Reversed Primer (Table 1) was designed to determine the relative expression of CSE. The primers specific to our PCR template were treated by Blast biological software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The fluorescence was measured at the end of the extension step at 72°C. Controls for genomic DNA and primer contamination were routinely performed with non-RT or no template PCR reactions, respectively. Dissociation curves were made for each set of oligonucleotides to check primer specificity and to confirm the presence of a unique PCR product. To estimate PCR efficiencies, standard curves were made based on five serial dilutions of a cDNA stock. PCR efficiencies of all primer sets were between 95% and 100%. After verifying that TUBA (α-tubulin gene) and CSE primers had similar amplifying efficiencies, the comparative Ct method 2−ΔΔCt was used to for performing relative quantification analysis of mRNA levels (17). The relative amount of each mRNA was normalized to the housekeeping gene, TUBA. Each sample was run and analyzed in triplicate. The average of the relative amount of each mRNA in control group is defined as 1.0. The quantity of transcripts was estimated from a standard line derived from 20-fold serial dilutions of cDNA pooled from A549 cells or SMMC-7721 cells.
TABLE 1.
PRIMERS USED FOR QUANTITATIVE REAL-TIME PCR ASSAYS
| Gene | Gene Bank Accession No. | Forward/Reverse Primer | Exon | Amplicon Size |
|---|---|---|---|---|
| CSE | NM_001902.5 | 5′-CCTGGGCTGCCCTCTCATCCA-3′ | 2 | 183 bp |
| 5′-TGCCGGAAGCTCAGCAAGGC-3 | 2 | |||
| TUBA | NM_006009.2 | 5′-GCGAAGCAGCAACCATGCGTGA-3′ | 2 | 115 bp |
| 5′-CCATCGGGCTGGATGCCGTG-3 | 2 |
Western Blotting Analysis
For total protein extraction, 1 × 106 cells (A549 cells or SMMC-7721 cells) were incubated in 120 μl of PIPA lysis buffer (mild) (Biomiga, Inc., San Diego, CA, USA), supplemented with 1 mM PMSF proteinase, 0.25 U/μl benzonase, inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Both cells and PIPA lysis buffer were incubated on ice for 30 min, and the lysate was cleared by centrifugation at 12,000 × g at 4°C for 15 min. After centrifugation, glycerol, 2-mercaptoethanol, 10% SDS, 1 M Tris-HCl (pH 6.7), and bromophenol blue were added to the supernatant, and then denatured at 95–100°C for 10 min. The proteins were separated by electrophoresis on a 10% [for the detection of CSE, and α-tubulin (B-7)] sodium dodecylsulfate (SDS)-polyacrylamide gel (Sigma-Aldrich) and transferred onto the polyvinylidene difluoride (PVDF) membrane (0.45 μm, Immobilon-P, Millipore, Billerica, MA, USA). The membranes were blocked with a blocking solution containing 5% skim milk, 137 mM NaCl, 0.1% Tween 20, and 20 mM Tris-HCl (pH 7.6). After a wash, the membrane was incubated overnight at 4°C with anti-CSE (30.7, Santa Cruz, Santa Cruz, CA, USA) monoclonal antibodies (1:1,000 dilution) or anti-α-tubulin (B-7, Santa Cruz) monoclonal antibodies (1:15,000 dilution). After another wash, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (1:10,000) (Santa Cruz) (for the detection of CSE and α-tubulin). Positive bands for CSE or α-tubulin were identified around 43–47 or 52–58 kDa, respectively, by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, IL, USA). The resulting films (Kodak Company Limited, Xiamen, China) were scanned and quantified using density metric analytical software (the Bio-Rad Quantity One software, Bio-Rad Laboratories Co., Ltd., CA, USA).
Statistical Analysis
All data were expressed as mean ± SEM of at least four experiments. Statistical significance was assessed by either one-way ANOVA or two-way ANOVA with repeated measures followed by Tukey’s test. A value of p < 0.05 was considered significant.
RESULTS
Exogenously Applied H2O2 Had Little Effect or Induced the Transcription of the CSE Gene Depending on Cell Type
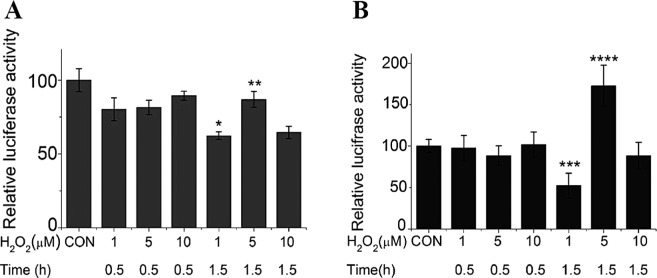
To investigate the effect of exogenously applied H2O2 on the transcription of the CSE gene by the luciferase activity was analyzed. As shown in Figure 1A, H2O2 treatment slightly decreased the luciferase activity at a short time (0.5 h) in the transfected HEK 293 cells. A more significant effect was observed at a longer time (1.5 h). Both low (1 μM) and high (10 μM) concentration of H2O2 decreased the luciferase activity, while the medium concentration (5 μM) of H2O2 had less effect, suggesting that the medium concentration of H2O2 probably induced an upregulation to counteract the downregulation. As shown in Figure 1B, the upregulation effect caused by the medium concentration (5 μM) of H2O2 was more significant in the transfected COS-7 cells at a longer time (1.5 h).
Figure 1.
The effects of exogenous H2O2 on CSE gene expression at the promoter level. (A) Both low (1 μM) and high (10 μM) concentration of H2O2 decreased the luciferase activity in the transfected HEK 293 cells, while the medium concentration (5 μM) of H2O2 had less effect. (B) The upregulation effect caused by the medium concentration (5 μM) of H2O2 was more significant in the transfected COS-7 cells at a longer time (1.5 h). *p < 0.01 versus 5 μM + 1.5 h; **p < 0.05 versus 1 μM + 1.5 h; ***p < 0.05 versus 5 μM + 1.5 h; ****p < 0.05 versus 1 μM + 1.5 h.
Exogenously Applied H2O2 Increases Total CSE mRNA Level
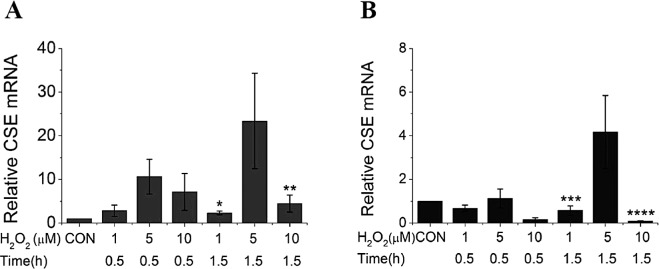
The effect of oxidative stress on CSE expression at the transcription level was also analyzed by RT-qPCR. As shown in Figure 2, the endogenous CSE mRNA level was significantly increased by the treatment of medium concentration (5 μM) of H2O2 in both A549 cells (Fig. 2A) and SMMC-7721 cells (Fig. 2B) at a longer time (1.5 h). In contrast, either a high concentration (10 μM) or low concentration (1 μM) of H2O2 had less effect, suggesting the medium concentration of H2O2 probably induced an upregulation to counteract the downregulation. A high concentration of H2O2 probably damaged the upregulation due to its high toxicity, while a low concentration of H2O2 was not enough to start the activation.
Figure 2.
The effects of exogenous H2O2 on CSE gene expression at the mRNA level. The endogenous CSE mRNA level was significantly increased by the treatment of medium concentration (5 μM) of H2O2 in both A549 cells (A) and SMMC-7721 cells (B) at 1.5 h posttreatment. *p < 0.01 versus 5 μM + 1.5 h; **p < 0.05 versus 5 μM + 1.5 h; ***p < 0.05 versus 5 μM + 1.5 h; ****p < 0.01 versus 5 μM + 1.5 h.
Exogenously Applied H2O2 Induces CSE Protein Level
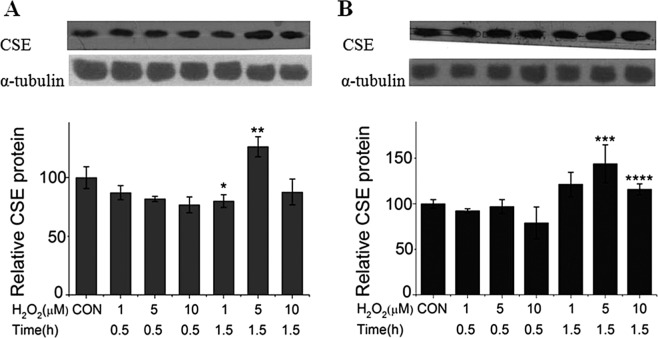
The effect of exogenously applied H2O2 on CSE expression at the protein level was analyzed using the Western Blotting. As shown in Figure 3, the similar as the effect of oxidative stress at the transcription level, the CSE protein level was markedly increased by the treatment of a medium concentration (5 μM) of H2O2 in both A549 cells (Fig. 3A) and SMMC-7721 cells (Fig. 3B) at a longer time (1.5 h). In contrast, either a high concentration (10 μM) or low concentration (1 μM) of H2O2 had less effect, suggesting that the upregulation of CSE expression could be activated only when the H2O2 accumulated to a threshold.
Figure 3.
The effects of exogenous H2O2 on CSE gene expression at the protein level. Similar to the effect of H2O2 at the transcription level, the CSE protein level was also increased by the treatment of medium concentration (5 μM) of H2O2 in both A549 cells (A) and SMMC-7721 cells (B) at a longer time (1.5 h). *p < 0.05 versus 5 μM + 1.5 h; **p < 0.05 versus 1 μM + 1.5 h; ***p < 0.05 versus 1 μM + 1.5 h; ****p < 0.05 versus 5 μM + 1.5 h.
DISCUSSION
The relevant studies indicated that exogenously applied H2S can protect mammalian cells from H2O2-induced oxidative injury (5,7,19,30). In the present study, we first investigated the effects of exogenously applied H2O2 on CSE expression in several mammalian cell lines, and demonstrated that exogenous H2O2 was involved in the regulation of the CSE gene at the promoter, mRNA, and protein levels.
In recent years, both COS-7 cells and HEK 293 cells (two mammalian kidney cell lines) have not only been successfully used in the study of the CSE/H2S signal pathway (11), but also with high DNA transfection efficiencies in gene transfection experiment. Some typical mammalian cell lines such as A549 and SMMC-7721 cells are frequently used in the experiment study of the CSE/H2S signal pathway by many researchers (1,3,6,12,30–32). Therefore, both A549 cells and SMMC-7721 cells are good models for testing the posttranscriptional regulation of the CSE gene by exogenous H2S in the mammalian cells, and both COS-7 and HEK 293 cells in the transcriptional activity.
In particular, it is noteworthy that the treatment of a medium concentration (5 μM) of H2O2 at a longer time (1.5 h) upregulated CSE expression in the mammalian cells at levels of the promoter, mRNA, and protein. Previous studies have reported that exposure of mammalian cells to H2O2 significantly increased ROS generation and H2O2 markedly suppressed intracellular GSH production in the mammalian cells (16). However, exogenous H2S attenuates H2O2-induced ROS generation (16,25) and markedly reduced ROS accumulation in the mammalian cells (14,19,22). Although there is no consensus on the levels of H2S in plasma (8,15,24) and the release of H2S from tissue and cell production at the site of its function has not been measured (13), it can be speculated that the upregulation of CSE expression induced by exogenous H2O2 may be useful for increasing the production and concentration of endogenous H2S, partly scavenging ROS such as H2O2 in the mammalian cells.
Collectively, exogenously applied H2O2 can not only markedly affect CSE mRNA and protein expression, but also can affect the CSE promoter activity in the mammalian cells. Our observations indicate that that exogenous H2O2 can upregulate the expression of the CSE gene in the mammalian cells, which will provide the possibility of the scavenging effect of the CSE gene indirectly on ROS in the mammalian cells. However, the regulatory mechanism involved in the effects of exogenously applied H2O2 on CSE expression in the mammalian cells need be further studied.
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (973 Program, No. 2010CB912604).
Footnotes
The authors declare no conflicts of interests.
REFERENCES
- 1. Bhatia M.; Wong F. L.; Fu D.; Lau H. Y.; Moochhala S. M.; Moore P. K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 19:623–625; 2005. [DOI] [PubMed] [Google Scholar]
- 2. Chang L.; Geng B.; Yu F.; Zhao J.; Jiang H.; Du J.; Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34:573–585; 2008. [DOI] [PubMed] [Google Scholar]
- 3. Choudhary S.; Rosenblatt K. P.; Fang L.; Tian B.; Wu Z. H.; Brasier A. R. High throughput short interfering RNA (siRNA) screening of the human kinome identifies novel kinases controlling the canonical nuclear factor-kappaB (NF-kappaB) activation pathway. J. Biol. Chem. 286:37187–37195; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denu J. M.; Tanner K. G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37:5633–5642; 1998. [DOI] [PubMed] [Google Scholar]
- 5. Dominy J. E.; Stipanuk M. H. New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 62:348–353; 2004. [DOI] [PubMed] [Google Scholar]
- 6. Fang L. P.; Lin Q.; Tang C. S.; Liu X. M. Hydrogen sulfide attenuates epithelial-mesenchymal transition of human alveolar epithelial cells. Pharmacol. Res. 61:298–305; 2010. [DOI] [PubMed] [Google Scholar]
- 7. Fiorucci S.; Antonelli E.; Mencarelli A.; Orlandi S.; Renga B.; Rizzo G.; Distrutti E.; Shah V.; Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42:539–548; 2005. [DOI] [PubMed] [Google Scholar]
- 8. Furne J.; Saeed A.; Levitt M. D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:R1479–1485; 2008. [DOI] [PubMed] [Google Scholar]
- 9. Gadalla M. M.; Snyder S. H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 113:14–26; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geng B.; Chang L.; Pan C.; Qi Y.; Zhao J.; Pang Y.; Du J.; Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 318:756–763; 2004. [DOI] [PubMed] [Google Scholar]
- 11. Ishii I.; Akahoshi N.; Yu X. N.; Kobayashi Y.; Namekata K.; Komaki G.; Kimura H. Murine cystathionine gamma-lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem. J. 381:113–123; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang K.; Zhao M.; Jiang H.; Tan G.; Pan S.; Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 15:1306–1314; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Kimura H. Hydrogen sulfide: Its production and functions. Exp. Physiol. 96:833–835; 2011. [DOI] [PubMed] [Google Scholar]
- 14. Kimura Y.; Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 18:1165–1167; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Levitt M. D.; Abdel-Rehim M. S.; Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox. Signal. 15:373–378; 2011. [DOI] [PubMed] [Google Scholar]
- 16. Lu M.; Hu L. F.; Hu G.; Bian J. S. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic. Biol. Med. 45:1705–1713; 2008. [DOI] [PubMed] [Google Scholar]
- 17. Schmittgen T. D.; Livak K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3:1101–1108; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Schreck R.; Rieber P.; Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 10:2247–2258; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh S.; Padovani D.; Leslie R. A.; Chiku T.; Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284:22457–22466; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su Y. W.; Liang C.; Jin H. F.; Tang X. Y.; Han W.; Chai L. J.; Zhang C. Y.; Geng B.; Tang C. S.; Du J. B.; Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ. J. 73:741–749; 2009. [DOI] [PubMed] [Google Scholar]
- 21. Tokoro M.; Asai T.; Kobayashi S.; Takeuchi T.; Nozaki T. Identification and characterization of two isoenzymes of methionine gamma-lyase from Entamoeba histolytica: A key enzyme of sulfur-amino acid degradation in an anaerobic parasitic protist that lacks forward and reverse trans-sulfuration pathways. J. Biol. Chem. 278:42717–42727; 2003. [DOI] [PubMed] [Google Scholar]
- 22. Tripatara P.; Patel N. S.; Brancaleone V.; Renshaw D.; Rocha J.; Sepodes B.; Mota-Filipe H.; Perretti M.; Thiemermann C. Characterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischaemia/reperfusion injury of the mouse kidney: an in vivo study. Eur. J. Pharmacol. 606:205–209; 2009. [DOI] [PubMed] [Google Scholar]
- 23. Wang R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798; 2002. [DOI] [PubMed] [Google Scholar]
- 24. Wintner E. A.; Deckwerth T. L.; Langston W.; Bengtsson A.; Leviten D.; Hill P.; Insko M. A.; Dumpit R.; VandenEkart E.; Toombs C. F.; Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 160:941–957; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z. S.; Wang X. Y.; Xiao D. M.; Hu L. F.; Lu M.; Wu Z. Y.; Bian J. S. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic. Biol. Med. 50:1314–1423; 2011. [DOI] [PubMed] [Google Scholar]
- 26. Yang G.; Cao K.; Wu L.; Wang R. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J. Biol. Chem. 279:49199–49205; 2004. [DOI] [PubMed] [Google Scholar]
- 27. Yang G.; Wu L.; Jiang B.; Yang W.; Qi J.; Cao K.; Meng Q.; Mustafa A. K.; Mu W.; Zhang S.; Snyde S. H.; Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322:587–590; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang G.; Wu L.; Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 20:553–555; 2006. [DOI] [PubMed] [Google Scholar]
- 29. Yang G.; Yang W.; Wu L.; Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 282:16567–16576; 2007. [DOI] [PubMed] [Google Scholar]
- 30. Yin P.; Zhao C.; Li Z.; Mei C.; Yao W.; Liu Y.; Li N.; Qi J.; Wang L.; Shi Y.; Qiu S.; Fa J.; Zha X. Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell. Signal. 24:1229–1240; 2012. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J.; Xie Y.; Xu Y.; Pan Y.; Shao C. Hydrogen sulfide contributes to hypoxia-induced radioresistance on hepatoma cells. J. Radiat. Res. 52:622–628; 2011. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L.; Charron M.; Wright W. W.; Chatterjee B.; Song C. S.; Roy A. K.; Brown T. R. Nuclear factor-kappaB activates transcription of the androgen receptor gene in Sertoli cells isolated from testes of adult rats. Endocrinology 145:781–789; 2004. [DOI] [PubMed] [Google Scholar]