Abstract
The ITI (inter-trypsine inhibitor) gene family includes five genes (ITIH1 to ITIH5) that encode proteins involved in the dynamics of the extracellular matrix (ECM). ITIH5 was found inactivated by partial deletion in a case of congenital uterovaginal aplasia, a human rare disease also called Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. The aim of the present study was to analyze the expression of ITIH5 in the uterus in adult life and during embryogenesis in order to establish the involvement of this gene in both normal and pathological conditions of uterus development. This was achieved in mice by reverse transcription-quantitative PCR, whole-mount hybridization, and Western blot analysis. Itih5 expression was much stronger in female genital tract primordia (Müllerian ducts) and derivatives than elsewhere in the body. This gene was strongly expressed during pregnancy and development of the female genital tract, indicating that the encoded protein probably had an important function in the uterus during these periods. Two different specific isoforms of the protein were detected in Müllerian derivatives during embryogenesis and in adults. Although ITIH genes are expected to be predominantly expressed in the liver, ITIH5 is mainly expressed in the uterus during development and adult life. This tends to indicate an additional and specific role of this gene in the female reproductive tract, and furthermore reinforces ITIH5 as a putative candidate gene for MRKH syndrome.
Key words: ITIH5, Extracellular matrix, Female, Mouse, Congenital uterovaginal aplasia, MRKH syndrome
INTRODUCTION
In higher vertebrates, the Wolffian ducts (WDs) and the Müllerian ducts (MDs)—the anlagen of the male and female inner genital tracts, respectively—coexist in the undifferentiated embryo until genetic sex triggers the differentiation of either ovaries or tes-tes (3,13). After sexual differentiation, the production of anti-Müllerian hormone (AMH) and testosterone by the testes leads to the regression of the MDs and WDs development into epididymides, vasa deferentia, and seminal vesicles in the male, respectively. In the female, MDs differentiate into Fallopian tubes, uterus, cervix, and the upper part of the vagina, while WDs degenerate. The molecular cascade of MDs differentiation can be altered at various stages of embryonic development, resulting in malformations of various degrees of severity (26). MRKH syndrome is one of the most frequent abnormalities occurring in early development. It is characterized by congenital aplasia of the uterus and the vagina (17) and is the second leading cause of primary amenorrhea after gonadal dysgenesis (4,27).
It is now clear that MRKH syndrome has a genetic origin, hence the many genetic studies of this condition already carried out and currently under way (18). The chromosomal rearrangements associated with MRKH syndrome include a recently described small interstitial deletion on the short arm of chromosome 10, affecting only the ITIH5 gene (19). This gene encodes a protein involved in extracellular matrix (ECM) dynamics and is a candidate gene for involvement in MRKH syndrome.
ITIH5 is the most recently described member of the inter-α-trypsin inhibitor (ITI) protein family. The members of the ITI family are proteoglycans (ITIH-1 to -5) that are able to bind hyalyronic acid (HA), frequently referred to as hyaluronic acid-binding proteins (HABPs) (2,31). This interaction with HA was initially deciphered in the cumulus oocyte complex in mice, and was shown to stabilize the extracellular matrix (ECM) (6). The ITIH1, ITIH2, and ITIH3 glycoproteins were shown to assemble from a light chain (bikunin) and a heavy chain: HC1 (in ITIH1), HC2 (in ITIH2), or HC3 (in ITIH3) (9). ITIH4 consists exclusively of HC4 (22). The ITIH1 to ITIH4 genes are transcribed principally in the liver, but the ITIH2 and ITIH3 genes are also weakly expressed in the brain (5,22,23). Geisert et al. (10) described the expression of the ITIH1, ITIH2, ITIH3, and ITIH4 genes in the endometrium of cyclic and pregnant gilts and suggested that these proteins might play an important role during pregnancy in pigs. The ITIH5 protein was initially identified and described as a prognostic marker for breast cancer (12,28). More recently, it has also been shown to be downregulated in bladder cancer (16) and poorly differentiated thyroid carcinomas (21).
The aim of this study was to analyze the expression of ITIH5 in the embryonic and adult female genital tract. This could help to understand its putative involvement in both normal and pathological conditions of uterus development. These studies were carried out in mice.
MATERIALS AND METHODS
Animals
Animals were used in compliance with European Commission guidelines and with the approval of the “Haut Conseil des Biotechnologies” (#5450), and of the “Direction Départementale des Services Vétérinaires” (#35-44). We used female CD1-Swiss mice reared in the Experimental Animal Department of the University of Rennes. For embryo staging, the morning on which a vaginal plug was first observed was designated embryonic day 0 (E0). Vaginal smears were used to stage the estrus cycle.
RT-PCR and RT-qPCR
RNA Extraction and cDNA Synthesis
Total RNA was extracted from tissue samples with the RNeasy® Mini kit (Qiagen S.A, Courtaboeuf, France), according to the manufacturer’s protocol. We reverse-transcribed total RNA with the Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega, Charbonnière, France) to generate cDNA, which was subsequently used for RT-PCR and RT-quantitative PCR.
PCR Amplification
Complementary DNAs were amplified with Taq DNA polymerase (GoTaq® DNA polymerase, Promega), according to standard procedures. Primers were designed with Primer 3′ software (http://frodo.wi.mit.edu/primer3/). All the primers had a similar (60°C) melting temperature (T m). Various pairs of primers were designed to bind to various sites along the length of the cDNA sequence, to strengthen our results and to facilitate the detection of mRNA isoforms. The sequences of these primers are available on request.
Reverse Transcription-Quantitative PCR (RT-qPCR)
We used the Power SYBR Green PCR master mix (Applied Biosystems, Villebon-sur-Yvette, France) for qPCR with an ABI Prism 7000 Sequence Detection System (Applied Biosystems), as recommended by the manufacturer. Each sample was tested in triplicate. The yield of each cDNA produced was analyzed by the comparative Ct (threshold cycle) method of qPCR. The values were normalized with respect to the Ct obtained for amplification of the internal standard, the Hprt1 gene (hypoxanthine phosphoribosyl-transferase 1). The expression of this gene was considered to be stable and similar in all cell types. Primer sequences are available on request.
Whole-Mount In Situ Hybridization (WISH)
Preparation of Samples
Female genital tracts were dissected out from embryos at stages E16.5 and E18.5. Tissues were fixed by incubation overnight at 4°C in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) and dehydrated in a series of methanol solutions of increasing concentration.
Synthesis of Riboprobes
A 463-bp cDNA probe corresponding to exons 14 and 15 of the Itih5 gene was inserted into pSPT18 (Roche, Mannheim, Germany). Primer sequences and the recombinant plasmid are available on request. The recombinant plasmid was linearized by digestion with EcoRI. The antisense riboprobe was synthesized from the linearized plasmid in the presence of digoxigenin-labeled UTP, with a DIG-RNA labeling kit (Roche) and the SP6 RNA polymerase promoter, according to the manufacturer’s instructions. Sense riboprobe synthesis required linearization of the plasmid by HindIII digestion and the use of the T7 RNA polymerase promoter.
Hybridization
Tissues were rehydrated and treated with proteinase K according to standard procedures. Hybridization was performed at 65°C for 12 h in hybridization buffer (50% formamide, 5 × SSC pH 7, 100 μg ml−1 tRNA, 100 μg ml−1 sodium heparin, 0.5 M EDTA, 10% CHAPS, 20% Tween 20) to which we added 200 ng/ml of one of the sense or antisense riboprobes. Samples were washed and incubated overnight at 4°C with anti-digoxigenin alkaline phosphatase Fab fragments (Roche), at a dilution of 1:5,000. They were then thoroughly washed and incubated with BM purple solution (Roche) for signal detection. Once optimal signal intensity had been achieved, the samples were washed in PBT (0.1 % Tween 20 in PBS) supplemented with 1 mM EDTA and fixed in 4% PFA in PBS. They were then dehydrated in a series of methanol solutions of increasing concentration and stored in 100% methanol at −20°C.
Western Blot Analysis
Protein Purification
Proteins were extracted from frozen (−80°C) genital tracts and livers from E18.5 mouse embryos, and from the genital tracts from pregnant and nonpregnant mice. Tissues were homogenized in F9 lysis buffer (0.1 M Tris pH 7.5, 0.15 M NaCl, 0.1% SDS, 1% sodium deoxycholate, 1% Triton) and sonicated on ice (4°C) for 5 min. Lysates were centrifuged at 10,000 × g for 5 min at 4°C, and the supernatants were collected in fresh tubes. Protein concentration in the supernatants was determined with a bicinchoninic acid protein assay kit (QuantiProTM BCA assay, Sigma-Aldrich, Lyons, France), according to the manufacturer’s protocol. Protein solutions were mixed with an equal volume of 2 × Laemmli buffer containing 100 mM Tris-HCl pH 6.8, 20% glycerol, 3% SDS, 5% β-mercaptoethanol, heated for 5 min at 95°C, and stored at −20°C until analysis.
Production of the GST-Itih5 Fusion Protein
A pCMV-SPORT plasmid (Invitrogen, Cergy Pontoise, France) containing the full-length cDNA encoding mouse Itih5 was obtained from RZPD (ImaGenes Gmbh, Berlin, Germany). A cDNA fragment corresponding to amino acids 219–952 of the Itih5 protein was inserted into pGEX-3X (GE Healthcare, Saclay, France) and the resulting recombinant plasmid was used to produce a GST-fusion protein, GST-Itih5. E. coli cells were grown at 37°C in 10 ml of LB medium supplemented with ampicillin (50 μg ml−1), until the culture reached an OD660 of 0.8 (about 3 h). Synthesis of the GST-Itih5 fusion protein was induced by adding isopropyl β-d-thiogalactoside (IPTG) to a final concentration of 1 mM. The cells were incubated for a further 3 h at 37°C and then harvested by centrifugation at 2,000 × g for 10 min at 4°C. Proteins were released by resuspending the wet cell pellets in Laemmli lysis buffer and subjecting them briefly to sonication. The proteins were denatured by heating for 5 min at 95°C and then subjected to SDS-PAGE. The GST-Itih5 fusion protein was approximately 105 kDa in size, consistent with its deduced molecular weight.
Antibody Production
A rabbit polyclonal anti-Itih5 antibody was generated at Eurogentec (Seraing, Belgium), with a synthesized peptide corresponding to amino acids 475–490 of the mouse protein (YDEIRTPLLSDIRIDY).
Western Blot
Proteins were separated by electrophoresis in an 8.5% polyacrylamide gel supplemented with 0.1% SDS, in a MiniProtean II electrophoresis system (Bio-Rad, Ivry sur Seine, France). They were transferred onto a nitrocellulose membrane (Millipore S.A.S, Molsheim, France), which was then saturated by incubation in PBT supplemented with 5% nonfat milk powder for 1 h at room temperature. The membrane was subsequently incubated at 4°C, for 90 min with rabbit polyclonal anti-Itih5 antibody at a dilution of 1:500 and washed five times in PBT. It was then incubated with PBT supplemented with a secondary antibody [horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody] at a dilution of 1:20,000, for 1 h at 4°C, and washed five times in PBS. Immunoreactive proteins were detected by chemiluminescence, with an ECL kit (Amersham ECL™ Western Blotting Detection kit, GE Healthcare).
RESULTS
Itih5 Gene Expression in the Mouse Female Genital Tract During Development
RT-PCR and RT-qPCR
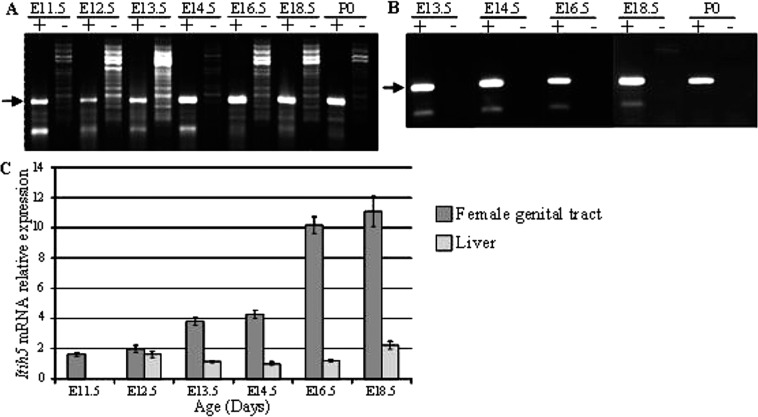
We first analyzed Itih5 mRNA expression by RT-PCR between E11.5 and P0, in the female genital tract and in the liver. Itih5 was expressed in both tissues at each embryonic stage studied. Similar results were obtained with all primer pairs used (Fig. 1A and B shows RT-PCR products obtained with the Ex6-8 primers). RT-qPCR was used to measure changes in Itih5 mRNA levels during embryogenesis in the female genital tract and, for comparison, in the liver. The amount of Itih5 mRNA in the female genital tract increased considerably during embryogenesis, with relative levels increasing by a factor of about five between E11.5 and E18.5 (Fig. 1C). Furthermore, Itih5 mRNA was markedly more abundant in the female genital tract than in the liver, particularly after E16.5 (10 times higher): Itih5 gene expression remained stable, at low levels, throughout embryogenesis in liver (Fig. 1C).
Figure 1.
Itih5 mRNA levels in the female genital tract and liver during development. RT-PCR analysis of Itih5 mRNA (A) in the female genital tract and (B) in fetal liver. Equal amounts of total RNA from each tissue, without the addition of reverse transcriptase (−), were used as negative controls. The position of the expected transcript (316 bp) is indicated by an arrow. (C) RT-qPCR analysis of Itih5 mRNA levels. The values for the expression of the gene was normalized to Hprt1, used as an internal control, and are plotted on the y-axis as multiples of the lowest value for this gene (that in the liver at E14.5). The figure shows one experiment among three independent ones leading to similar results. Error bars reflect variations between triplicates of PCR. E, embryonic day; ITIH, inter-α-trypsin inhibitor heavy chain; P0, day 0 post partum.
WISH Analysis
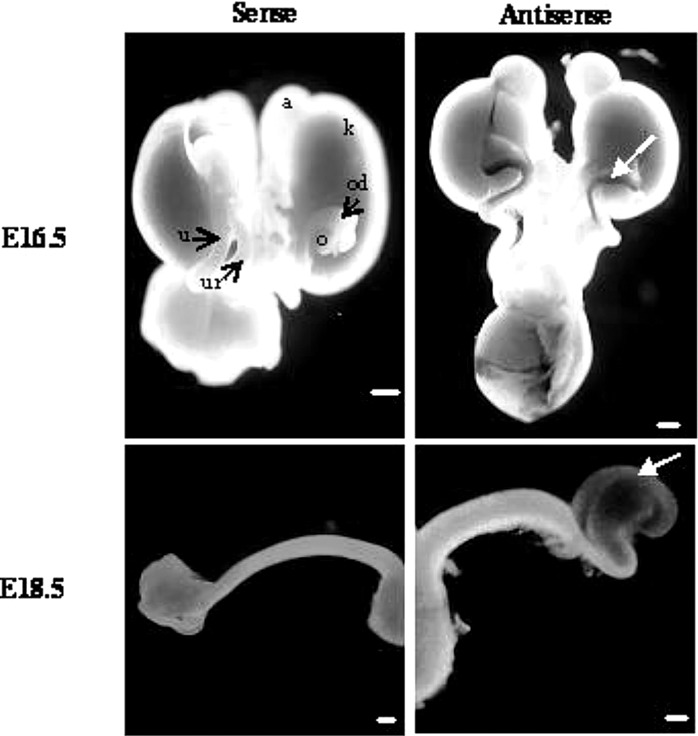
We used whole-mount in situ hybridization at E16.5 and E18.5 to investigate the pattern of Itih5 gene expression in the female genital tract. Itih5 expression was clearly detected, at both embryonic stages, in the cranial part of the uterine horns (Fig. 2).
Figure 2.
In situ hybridization analysis of Itih5 mRNA in the female genital tract of embryonic mice at E16.5 and E18.5 (ventral views). Itih5 expression was detected only in the cranial part of the Müllerian ducts (white arrow). a, adrenal gland; k, kidney; o, ovary; od, oviduct; u, uterine horns; ur, ureter. Scale bars: 500 μm.
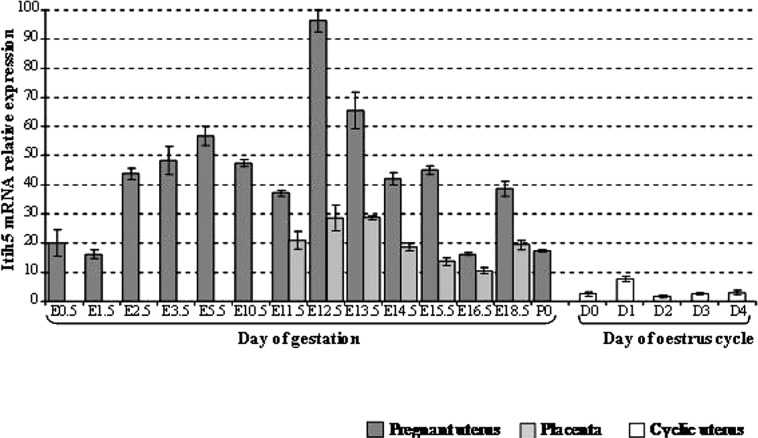
Itih5 Gene Expression in the Adult Mouse Uterus During Pregnancy and the Estrus Cycle, as Analyzed by RT-qPCR Analysis of Itih5 mRNA (Fig. 3)
Figure 3.
RT-qPCR analysis of Itih5 mRNA in the adult uterus during pregnancy and the estrus cycle: comparison with mRNA levels in the placenta. The values for the expression of the gene was normalized to Hprt1, used as an internal control, and are plotted on the y-axis as multiples of the lowest value for this gene [that in the second day (D2) of the estrus cycle]. The figure shows one experiment out of two independent ones leading to similar results. Error bars reflect variations between triplicates of PCR. D, day; E, embryonic day; ITIH, inter-α-trypsin inhibitor heavy chain; P0, day 0 post partum.
Itih5 was found to be expressed in the uterus during gestation and during the estrus cycle. Itih5 mRNA was more abundant in the uterus of pregnant animals than in the placenta (1.5 to 4 times more abundant, depending on the stage of gestation). Itih5 mRNA levels in the uterus of pregnant mice peaked twice: one peak was at the E5.5 stage, with a larger peak observed between E12.5 and E13.5. By contrast, Itih5 mRNA was much less abundant in the cyclic uterus than in the placenta, with little variation during the course of the estrus cycle.
The Genital Tract of Female Embryos and Adult Mice Produce Different Isoforms of Itih5 Protein
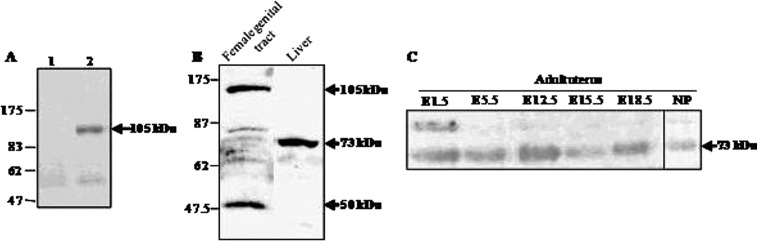
We first checked the specificity of the polyclonal antiserum, by Western blot experiments with the GST-Itih5 fusion protein (Fig. 4A). We then used this antise-rum to characterize the Itih5 protein isoforms produced at E18.5, of both female genital tract and liver samples (Fig. 4B) and in adult uterus during pregnancy and in the quiescent organ (Fig. 4C). Two strong, specific bands were detected for the female embryonic genital tract: the 105-kDa band corresponding to the expected size of the Itih5 precursor and a lower molecular weight (about 50 kDa) band possibly resulting from tissue-specific posttranslational processing and corresponding to an isoform smaller than the 73-kDa form found in the liver (Fig. 4B). By contrast, only the mature form of Itih5 protein (73 kDa) was detected in the uterus of adult mice, whether or not they were pregnant (Fig. 4C). Note that these results can only be considered on a qualitative point of view, due to poor affinity to Itih5 protein of the polyclonal antiserum.
Figure 4.
Western blot analysis of Itih5 protein with the α-Itih5 antibody. (A) Characterization of the α-Itih5 antibody. Western blot analysis of protein extracts from E. coli strain BL21 producing the pGEX-3X/Itih5 recombinant protein. Lane 1: Protein extract without IPTG induction; lane 2: Protein extract with IPTG induction. The arrow indicates the molecular size of the specific signal for the GST-Itih5 protein (approximately 105 kDa). (B) Western blot characterization of the Itih5 isoforms produced in the female genital tract and the liver at E18.5. Arrows indicate the molecular sizes of the specific bands found in the embryonic female genital tract (105 and 50 kDa) and in the liver (73 kDa). (C) Size characterization of the Itih5 isoform produced in the uterus of pregnant and nonpregnant adult mice. The arrow indicates the molecular size of the specific band found in the uterus during gestation and in nonpregnant animals (73 kDa). E, embryonic day; NP, nonpregnant.
DISCUSSION
We found that the Itih5 gene was expressed in the mouse uterus during pregnancy and during female genital tract development. Furthermore, whereas endometrial levels of expression of the Itih1 to Itih4 genes remain constant throughout all stages of the estrus cycle and early pregnancy (10), our results clearly demonstrate that levels of Itih5 gene expression in the female genital tract vary considerably with the physiological state of the uterus.
Itih5 mRNA was much more abundant in the uterus of pregnant adult mice than in the uterus of adult mice that were not pregnant. Our results are consistent with those of a previous study in humans showing ITIH5 expression levels in the placenta to be about 12 times those in the uterus (12). Interestingly, we found that the level of Itih5 expression in the uterus of pregnant mice was up to 50 times the maximal levels observed during the estrus cycle. This strongly suggests that Itih5 plays a key role during gestation, probably in implantation and uterus growth, as previously shown for other ECM components (25). In particular, we observed two peaks of expression, when the embryo was at stages E5.5 and E12.5, corresponding to specific stages of pregnancy in the mouse. Indeed, the period around E5.5 is regarded as the “implantation window”: it corresponds to the physiological preparation of the endometrium for implantation and coincides with the arrival of the embryo for implantation (7). During this period, uterine cells proliferate and/or differentiate in a particular spatiotemporal manner (7). Furthermore, implantation seems to be regulated locally by interactions involving ECM components (1,29). Invasive trophoblasts adhere to, spread, and migrate on ECM substrates and penetrate three-dimensional ECM structures (8). Following implantation, the uterus grows significantly from stage E12.5 onwards. Indeed, pregnancy involves the growth and differentiation of myometrial cells (30) and myometrial growth occurs mostly after midgestation (corresponding to stage E12.5 in mice) (24). In humans, the uterus increases in weight from about 50 g in the nonpregnant state to about 1,200 g at term, thus increasing in mass by a factor of 24 during the course of pregnancy (14). This myometrial growth is dependent on an increase in the synthesis of various ECM proteins, including collagens (type I, III, and IV), elastin, fibronectin, and laminin β2; each of these ECM components displays a specific temporal pattern of expression during gestation (25). The considerable variation of Itih5 mRNA levels in the mouse uterine during the course of gestation is particularly interesting in this respect. The ITIH5 protein thus appears to be one of the set of ECM proteins involved in myometrial growth.
We also analyzed the Itih5 gene products in embryogenesis and adult life. A 73-kDa protein was found in the adult uterus, during both the estrus cycle and pregnancy. This molecule seems to correspond to the mature form of the protein. Indeed, only one form of the mouse Itih5 protein (NP_766059.1) is currently found in databases. This form has a sequence very similar to that of the human NP_85046 isoform, corresponding to a polypeptide precursor of 952 amino acid residues (105 kDa). This precursor undergoes posttranslational processing, including a trimming of the N-terminal end, with the removal of 18 amino acid residues (signal peptide), and of the C-terminal end at the conserved cleavage site (DDPHFVV), resulting in the removal of 271 amino acid residues (12). The final mature protein is approximately 73 kDa in size and, therefore, corresponds to the protein observed in the adult mouse.
We also studied Itih5 expression in the mouse female genital tract during development, from E11.5 onwards, because the Müllerian ducts are known to arise in the mesonephros at E11.5, subsequently differentiating into the female genital tract (15,20). The expression of this gene increased with the progression of genital tract development, from E11.5 to birth. Furthermore, Itih5 expression was markedly stronger in the developing female genital tract than in the liver at the same stage, even though the liver is the main source of ITIH proteins (5,22,23). These observations suggest that ITIH5 plays a major role in the development of the female genital tract. We detected a protein of about 50 kDa in size during the differentiation and development of the Müllerian ducts, suggesting the presence of another Itih5 iso-form not previously described in the mouse. This iso-form may be equivalent to another known human isoform, NP_116206, which is about 52 kDa in size after posttranslational modifications. These observations are consistent with the known balance and combination of proteins forming the ECM, which may be highly variable and tissue-specific (11), resulting from the use of alternative promoters, diverse splicing patterns and various posttranslational modifications (11).
We report here the temporal pattern of expression of the Itih5 gene in the female mouse genital tract during adulthood and embryogenesis. We detected two isoforms of Ithi5, of 73 and 50 kDa in size, in adult animals and embryos, respectively. Further investigations are required to elucidate the mechanisms of action of these two isoforms. These results indicate that ITIH5 probably plays a specific physiological role during cell development and differentiation in the female genital tract in mammals. Even if further functional studies, such as conditional inactivation of the gene, are required to assess the role of ITIH5, both in normal and pathological development of the female reproductive tract, our present results are already consistent with the deletion of the ITIH5 gene found in a case of MRKH syndrome (19), supporting the identification of this gene as a candidate gene for the syndrome.
ACKNOWLEDGMENTS
We thank Pascale Bellaud (Plateforme d’histopathologie (H2P2), Biosit, Biogenouest, Université de Rennes 1, Rennes, France) for technical assistance with histology. Animals were bred and kept at the Central Animal House, Biosit, Université de Rennes1, Rennes, France (Agrement #35-238-40). This work was supported by the CNRS and by grants from “Rennes Métropole,” “Conseil Régional de Bret-agne,” and “La Fondation Langlois.” D.G. is a permanent researcher at the “Institut National de la Recherche Médicale” (INSERM).
REFERENCES
- 1. Armant D. R.; Wang J.; Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal-embryonic dialogue. Semin. Reprod. Med. 18:273–287; 2000. [DOI] [PubMed] [Google Scholar]
- 2. Bost F.; Diarra-Mehrpour M.; Martin J. P. Inter-alpha-trypsin inhibitor proteoglycan family-a group of proteins binding and stabilizing the extracellular matrix. Eur. J. Biochem. 252:339–346; 1998. [DOI] [PubMed] [Google Scholar]
- 3. Carroll T. J.; Park J. S.; Hayashi S.; Majumdar A.; McMahon A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 9:283–292; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Carson S. A.; Simpson J. L.; Malinak L. R.; Elias S.; Gerbie A. B.; Buttram V. C.; Sarto G. E. Heritable aspects of uterine anomalies. II. Genetic analysis of Mullerian aplasia. Fertil. Steril. 40:86–90; 1983. [PubMed] [Google Scholar]
- 5. Chan P.; Risler J. L.; Raguenez G.; Salier J. P. The three heavy-chain precursors for the inter-alpha-inhibitor family in mouse: New members of the multicopper oxidase protein group with differential transcription in liver and brain. Biochem. J. 306:505–512; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L.; Mao S. J.; McLean L. R.; Powers R. W.; Larsen W. J. Proteins of the inter-alpha-trypsin inhibitor family stabilize the cumulus extracellular matrix through their direct binding with hyaluronic acid. J. Biol. Chem. 269:28282–28287; 1994. [PubMed] [Google Scholar]
- 7. Cross J. C.; Werb Z.; Fisher S. J. Implantation and the placenta: Key pieces of the development puzzle. Science 266:1508–1518; 1994. [DOI] [PubMed] [Google Scholar]
- 8. Dey S. K.; Lim H.; Das S. K.; Reese J.; Paria B. C.; Daikoku T.; Wang H. Molecular cues to implantation. Endocrinology 25:341–373; 2004. [DOI] [PubMed] [Google Scholar]
- 9. Diarra-Mehrpour M.; Bourguignon J.; Sesboue R.; Mattei M. G.; Passage E.; Salier J. P.; Martin J. P. Human plasma inter-alpha-trypsin inhibitor is encoded by four genes on three chromosomes. Eur. J. Biochem. 179:147–154; 1989. [DOI] [PubMed] [Google Scholar]
- 10. Geisert R. D.; Ashworth M. D.; Malayer J. R. Expression of inter-alpha-trypsin inhibitor heavy chains in endometrium of cyclic and pregnant gilts. Reproduction 126:621–627; 2003. [PubMed] [Google Scholar]
- 11. Gorski J. P.; Olsen B. R. Mutations in extracellular matrix molecules. Curr. Opin. Cell. Biol. 10:586–593; 1998. [DOI] [PubMed] [Google Scholar]
- 12. Himmelfarb M.; Klopocki E.; Grube S.; Staub E.; Klaman I.; Hinzmann B.; Kristiansen G.; Rosenthal A.; Durst M.; Dahl E. ITIH5, a novel member of the inter-alpha-trypsin inhibitor heavy chain family is downregulated in breast cancer. Cancer Lett. 204:69–77; 2004. [DOI] [PubMed] [Google Scholar]
- 13. Jacob M.; Konrad K.; Jacob H. J. Early development of the mullerian duct in avian embryos with reference to the human. An ultrastructural and immunohisto-chemical study. Cells Tissues Organs 164:63–81; 1999. [DOI] [PubMed] [Google Scholar]
- 14. Johansson B. Different types of smooth muscle hypertrophy. Hypertension 6:III64–68; 1984. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi A.; Shawlot W.; Kania A.; Behringer R. R. Requirement of Lim1 for female reproductive tract development. Development 131:539–549; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Lu Y.; Liu P.; Wen W.; Grubbs C. J.; Townsend R. R.; Malone J. P.; Lubet R. A.; You M. Cross-species comparison of orthologous gene expression in human bladder cancer and carcinogen-induced rodent models. Am. J. Transl. Res. 3:8–27; 2010. [PMC free article] [PubMed] [Google Scholar]
- 17. Morcel K.; Camborieux L.; Guerrier D. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Orphanet J. Rare Dis. 2:13; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morcel K.; Dallapiccola B.; Pasquier L.; Watrin T.; Bernardini L.; Guerrier D. Clinical utility gene card for: Mayer-Rokitansky-Küster-Hauser syndrome. Eur. J. Hum. Genet. 20(2); 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morcel K.; Watrin T.; Pasquier L.; Rochard L.; Le Caignec C.; Dubourg C.; Loget P.; Paniel B. J.; Odent S.; David V.; Pellerin I.; Bendavid C.; Guerrier D. Utero-vaginal aplasia (Mayer-Rokitansky-Kuster-Hauser syndrome) associated with deletions in known DiGeorge or DiGeorge-like loci. Orphanet J. Rare Dis. 6:9; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orvis G. D.; Behringer R. R. Cellular mechanisms of Mullerian duct formation in the mouse. Dev. Biol. 306:493–504; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pita J. M.; Banito A.; Cavaco B. M.; Leite V. Gene expression profiling associated with the progression to poorly differentiated thyroid carcinomas. Br. J. Cancer 101:1782–1791; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saguchi K.; Tobe T.; Hashimoto K.; Sano Y.; Nakano Y.; Miura N. H.; Tomita M. Cloning and characterization of cDNA for inter-alpha-trypsin inhibitor family heavy chain-related protein (IHRP), a novel human plasma glycoprotein. J. Biochem. 117:14–18; 1995. [DOI] [PubMed] [Google Scholar]
- 23. Salier J. P.; Chan P.; Raguenez G.; Zwingman T.; Erickson R. P. Developmentally regulated transcription of the four liver-specific genes for inter-alpha-inhibitor family in mouse. Biochem. J. 296:85–91; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shynlova O.; Kwong R.; Lye S. J. Mechanical stretch regulates hypertrophic phenotype of the myometrium during pregnancy. Reproduction 139:247–253; 2010. [DOI] [PubMed] [Google Scholar]
- 25. Shynlova O.; Mitchell J. A.; Tsampalieros A.; Langille B. L.; Lye S. J. Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol. Reprod. 70:986–992; 2004. [DOI] [PubMed] [Google Scholar]
- 26. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil. Steril. 49:944–55; 1988. [DOI] [PubMed] [Google Scholar]
- 27. Timmreck L. S.; Reindollar R. H. Contemporary issues in primary amenorrhea. Obstet. Gynecol. Clin. North Am. 30:287–302; 2003. [DOI] [PubMed] [Google Scholar]
- 28. Veeck J.; Chorovicer M.; Naami A.; Breuer E.; Zafrakas M.; Bektas N.; Durst M.; Kristiansen G.; Wild P. J.; Hartmann A.; Knuechel R.; Dahl E. The extracellular matrix protein ITIH5 is a novel prognostic marker in invasive node-negative breast cancer and its aberrant expression is caused by promoter hyper-methylation. Oncogene 27:865–876; 2008. [DOI] [PubMed] [Google Scholar]
- 29. Wang J.; Armant D. R. Integrin-mediated adhesion and signaling during blastocyst implantation. Cells Tissues Organs 172:190–201; 2002. [DOI] [PubMed] [Google Scholar]
- 30. Young R. C. Myocytes, myometrium, and uterine contractions. Ann. NY Acad. Sci. 1101:72–84; 2007. [DOI] [PubMed] [Google Scholar]
- 31. Zhao M.; Yoneda M.; Ohashi Y.; Kurono S.; Iwata H.; Ohnuki Y.; Kimata K. Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J. Biol. Chem. 270:26657–26663; 1995. [DOI] [PubMed] [Google Scholar]