Abstract
Treatment of cancers with the cytotoxic agent cisplatin frequently evokes resistance, accompanied by rewiring of metabolic pathways, limiting its clinical use. Recent research by Obrist et al (2018) shows that cisplatin‐resistant growth of lung adenocarcinoma is particularly vulnerable to periodic fasting cycles and starvation‐induced cell death, due to its dependency on glutamine, required for nucleoside biosynthesis, suggesting an opportunity for nutritional anti‐cancer interventions.
Subject Categories: Autophagy & Cell Death, Cancer, Metabolism
Platinum‐based drugs, and in particular cis‐diamminedichloroplatinum or CDDP (best known as cisplatin), are employed for the treatment of a wide array of solid malignancies, including testicular, ovarian, head and neck, colorectal, bladder, and lung cancers. Cisplatin exerts anti‐cancer effects via multiple mechanisms, yet its most prominent and best understood mode of action involves the generation of DNA lesions followed by the activation of the DNA damage response and the induction of mitochondrial apoptosis (Galluzzi et al, 2012). Despite a consistent rate of initial responses, cisplatin treatment often results in the development of chemoresistance, leading to limited therapeutic efficacy. Such alterations are defined as pre‐target resistance, when they affect steps preceding the binding of CDDP to DNA (Karekla et al, 2017), on‐target resistance, when directly related to the molecular damage provoked by CDDP (Sourisseau et al, 2016), post‐target resistance, when they involve defects in apoptotic proteins, etc., (Li et al, 2016), or off‐target resistance when affecting pathways not associated with CDDP‐elicited signals (Leung et al, 2016). Moreover, CDDP‐resistant cells undergo major metabolism rearrangement such as decreased pyridoxine kinase (PDXK) and overactivation of the enzymatic activity of poly (ADP‐ribose) (PAR) polymerase (PARP) (Galluzzi et al, 2014; Michels et al, 2014), but those changes do not occur in all resistant clones, making these biomarkers not clinically useful predictors of resistance. However, given that a metabolic rearrangement is generally associated with CDDP resistance and that the exact mechanisms that account for this process have not been yet unraveled, it is important to identify co‐treatments that may prevent this resistance phenotype or kill resistant cells.
To address this, Obrist et al (2018) used an elegant systematic approach to shed light into the metabolic vulnerabilities of CDDP‐resistant cells. First, to identify potential metabolic vulnerabilities linked to CDDP resistance, they assessed cell death induction in different human and mouse lung cancer cell lines that were either wild type (WT) or CDDP‐resistant in the presence of many drugs including microtubule and metabolic inhibitors but also nutrient‐free conditions (NF). Strikingly, the largest differential susceptibility between resistant clones and WT cells was observed under NF, which contained only a minimal level of glucose (5.6 mM). In line with previous findings that fasting cycles sensitize a variety of cancer cells to chemotherapy (Lee et al, 2012; Bianchi et al, 2015), wild‐type A549, H460, H1650, and TC‐1 chemotherapy‐sensitive cancer cells also underwent a threefold and higher increase in cell death in nutrient‐free media compared to their counterpart in complete medium. However, the effect of starvation conditions was much more pronounced against cisplatin‐resistant cells, reaching toxicity levels that were twofold to fourfold higher than those observed for sensitive cells.
Additionally, thanks to knockdown experiments targeting pro‐apoptotic and anti‐apoptotic proteins, they elegantly demonstrated the involvement of the mitochondrial cell death pathway in starvation‐induced cell death of CDDP‐resistant cancer cells. These findings were confirmed in an in vivo setting where immunodeficient mice injected with A549 CDDP‐resistant cancer cells showed reduced tumor growth and longer survival in response to periodic fasting cycles (24 hours of water only twice a week). In contrast, CDDP‐sensitive tumors were not affected by these 24‐hour fasting cycles. Because previous results indicated that 2–3 consecutive days of fasting were effective against a wide range of cancers, particularly in combination with chemotherapy (Lee et al, 2012; Bianchi et al, 2015), it would be important to test these longer fasting periods on A549 and other CDDP‐resistant cancer mouse models with or without chemotherapy treatment (Fig 1).
Figure 1. Starvation‐induced cell death in cisplatin‐resistant lung cancer cells.
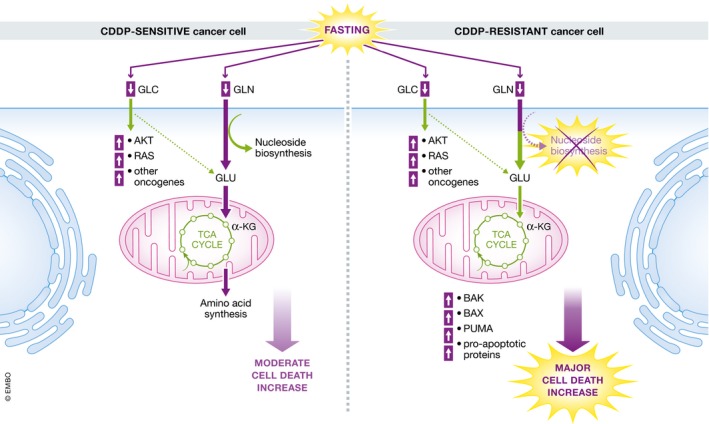
Periodic fasting cycles suppress survival of glutamine‐dependent CDDP‐resistant cells by inhibition of nucleotide synthesis, upregulation of pro‐apoptotic proteins and strongly increased cell death.
Obrist et al (2018) further provided evidence with an elegant set of rescue experiments that the toxic effect of fasting on CDDP‐resistant cancer cells is glutamine (GLN)‐dependent by showing that the addition of glutamine to NF media rescued CDDP‐resistant cancer cells from death. Notably, very low GLN doses (20 μM) were sufficient to reverse toxicity in resistant cancer cells suggesting that it would be difficult to achieve these effects in patients by only limiting specific amino acids. A metabolome analysis showed that in normal culture conditions, resistant clones were characterized by a reduction in Krebs cycle intermediates when compared to sensitive clones. To fuel the Krebs cycle, intracellular GLN must be converted to GLU through an amidohydrolase reaction catalyzed by glutaminase (GLS). However, genetic or pharmacological GLS inhibition could not reverse the effect of GLN in the rescue of CDDP‐resistant cells from starvation‐induced death, demonstrating that GLN does not require GLS to protect resistant cells.
Instead, direct addition of nucleosides rescued all tested CDDP‐resistant cell lines from starvation‐induced killing strongly suggesting that nutrient‐free conditions may be toxic by preventing the generation of high levels of nucleosides. Based on this finding, the authors tested agents that target nucleotide pathways and showed that CDDP‐resistant tumors are selectively sensitive to these drugs. In line with this notion, a recent study showed that fasting cycles potentiate the efficacy of the treatment with the nucleoside analogue gemcitabine in both in vitro and in vivo pancreatic cancer models (D'Aronzo et al, 2015).
In summary, these findings, by Obrist et al (2018), support a wide role for fasting and fasting mimicking diet in targeting not only cancers that are sensitive to cisplatin (Lee et al, 2012; Shi et al, 2012) but also those that have acquired resistance. They also show that a more targeted amino acid restriction can be effective in targeting resistant cells, but it is not clear whether this strategy may be problematic both because of the heterogeneity of metabolic rewiring in resistant cancers and because of the difficulties in preventing amino acids from reaching tumor cells in patients. Because resistance to therapy is one of the dominant limitations of cancer treatment, it will be important to determine whether periodic fasting or its combination with a variety of therapies can be effective in re‐sensitizing a wide range of resistant cancer cells.
The EMBO Journal (2018) 37: e99815
See also: https://doi.org/10.15252/embj.201798597 (July 2018)
References
- Bianchi G, Martella R, Ravera S, Marini C, Capitanio S, Orengo A, Emionite L, Lavarello C, Amaro A, Petretto A, Pfeffer U, Sambuceti G, Pistoia V, Raffaghello L, Longo VD (2015) Fasting induces anti‐warburg effect that increases respiration but reduces ATP‐synthesis to promote apoptosis in colon cancer models. Oncotarget 6: 11806–11819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aronzo M, Vinciguerra M, Mazza T, Panebianco C, Saracino C, Pereira SP, Graziano P, Pazienza V (2015) Fasting cycles potentiate the efficacy of gemcitabine treatment in in vitro and in vivo pancreatic cancer models. Oncotarget 6: 18545–18557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31: 1869–1883 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel‐Bellan A, Castedo M, Kroemer G (2014) Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 5: e1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karekla E, Liao W‐J, Sharp B, Pugh J, Reid H, Quesne JL, Moore D, Pritchard C, MacFarlane M, Pringle JH (2017) Ex vivo explant cultures of non‐small cell lung carcinoma enable evaluation of primary tumor responses to anticancer therapy. Cancer Res 77: 2029–2039 [DOI] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin‐Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, Emionite L, de Cabo R, Longo VD (2012) Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 4: 124ra27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Hung SS, Backstrom I, Ricaurte D, Kwok B, Poon S, McKinney S, Segovia R, Rawji J, Qadir MA, Aparicio S, Stirling PC, Steidl C, Bally MB (2016) Combined use of gene expression modeling and SiRNA screening identifies genes and pathways which enhance the activity of cisplatin when added at no effect levels to non‐small cell lung cancer cells in vitro . PLoS One 11: e0150675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhan M, Chen W, Zhao B, Yang K, Yang J, Yi J, Huang Q, Mohan M, Hou Z, Wang J (2016) Phenylethyl isothiocyanate reverses cisplatin resistance in biliary tract cancer cells via glutathionylation‐dependent degradation of Mcl‐1. Oncotarget 7: 10271–10282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels J, Obrist F, Castedo M, Vitale I, Kroemer G (2014) PARP and other prospective targets for poisoning cancer cell metabolism. Biochem Pharmacol 92: 164–171 [DOI] [PubMed] [Google Scholar]
- Obrist F, Michels J, Durand S, Chery A, Pol J, Levesque S, Joseph A, Astesana V, Pietrocola F, Wu GS, Castedo M, Kroemer G (2018) Metabolic vulnerability of cisplatin‐resistant cancers. EMBO J 37: e98597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Felley‐Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA (2012) Starvation‐induced activation of ATM/Chk2/P53 signaling sensitizes cancer cells to cisplatin. BMC Cancer 12: 571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau T, Helissey C, Lefebvre C, Ponsonnailles F, Malka‐Mahieu H, Olaussen KA, André F, Vagner S, Soria J‐C (2016) Translational regulation of the MRNA encoding the ubiquitin peptidase USP1 involved in the DNA damage response as a determinant of cisplatin resistance. Cell Cycle 15: 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]


