Abstract
“Virtual” memory CD8+ T cells are a subset of immune cells produced by homeostatic mechanisms involving response to self‐antigens, raising the possibility that these cells could mediate autoimmunity. New work by Drobek et al demonstrates that virtual memory T cells are indeed favored by stronger T‐cell receptor signals but exhibit minimal autoreactivity while maintaining self‐tolerance.
Subject Categories: Immunology
In unimmunized mice, a proportion of CD8+ T cells specific for foreign antigens display memory markers instead of the expected “naïve” phenotype. Since this population arises through homeostatic mechanisms rather than a response to infection or immunization, these cells were termed virtual memory (VM) CD8+ T cells (Haluszczak et al, 2009). Previous findings suggested that these cells have heightened self‐peptide/MHC sensitivity. Although VM cells represent 15–20% of the CD8 population in murine lymphoid organs, the basis for their differentiation from the naïve population and their capacity to induce autoimmune pathology are not well understood. In this issue, Drobek et al (2018) investigate the factors that determine which cells enter the VM pool and assess this population's potential for autoreactivity (Fig 1).
Figure 1. Model of virtual memory generation and response to autoantigens.
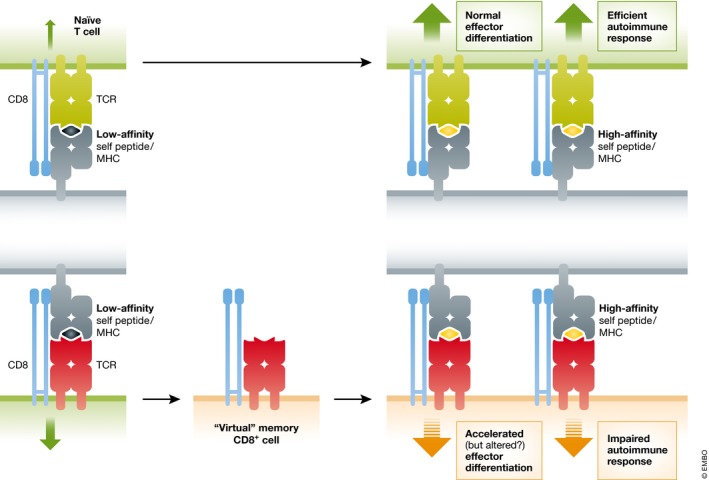
Naïve CD8 T cells with heightened self‐reactivity (size of green arrows on left) differentiate into “virtual memory” CD8 T cells, displaying features that overlap with both “true” memory and naïve CD8+ T cells. Despite elevated inherent self‐reactivity and efficient effector functionality upon activation with high‐affinity ligands, virtual memory cells show less propensity to induce autoimmune pathology than naïve or true memory CD8+ T cells of the same specificity, potentially due to qualitative changes in their effector differentiation.
Virtual memory CD8+ T cells bear similarities to a population of memory‐like T cells produced in response to self‐peptide/MHC ligands and homeostatic cytokines (including IL‐7 and IL‐15) in situations of lymphopenia (Hogquist & Jameson, 2014). This process does not appear to involve foreign antigen exposure, as VM cells arise in germ‐free (GF) and even “antigen‐free” mice (Haluszczak et al, 2009; C.D. Surh, personal communication). VM cells are not identical to “true” memory cells, e.g., those produced by immunization. While VM cells possess memory‐like functional properties, they have notably lower expression of the integrin CD49d (Lee et al, 2013; White et al, 2016). However, while several studies have explored how lymphopenia‐driven memory and VM cells contribute to the immune response against foreign antigens, the implications of their self‐specificity have remained unclear.
CD8 T cells with stronger self‐reactivity are able to receive enhanced homeostatic signals through their T‐cell receptors (TCRs; Sprent & Surh, 2011; Hogquist & Jameson, 2014), but whether all VM cells exhibit this property is currently undefined. In pursuing the hypothesis that self‐reactivity correlates with VM differentiation, Drobek et al (2018) first tested whether enhanced TCR signaling would predispose naïve CD8 T cells to enter the VM lineage. The authors made use of CD8.4 mice, which exhibit stronger TCR signaling upon antigen recognition than wild‐type (WT) mice due to enhanced binding of the tyrosine kinase Lck to the chimeric CD8.4 coreceptor. Among polyclonal cells in unprimed mice, a significantly higher proportion of CD8.4 T cells assumed a VM phenotype relative to WT cells; a similar result was observed in GF mice. However, these memory cells still represented a minority of the CD8 population, indicating that enhanced TCR signaling was not sufficient to enable all cells to assume a memory phenotype and implying that TCR specificity for self is still an important parameter in VM generation. This hypothesis was further substantiated by the finding that the CD8.4 transgene enhanced VM generation in one TCR transgenic strain (OT‐I) but not another (F5). Because the memory cells in CD8.4 OT‐I mice had not encountered cognate antigen, they could be clearly identified as VM cells.
These experiments suggested that VM generation is clone‐specific, but little is known about the TCR repertoires of naïve versus VM cells among the polyclonal pool. Drobek et al addressed this by first defining the TCR usage in cells that developed into the naïve or VM populations, then testing whether T cells bearing those TCRs would make the same developmental “fate decision” when generated in retrogenic mice. Importantly, these studies showed fidelity to the original clonotype: Cells with TCRs derived initially from VM clones differentiated into a sizeable VM population, while those with TCRs from naïve clones yielded only naïve cells. VM clones expressed higher levels of CD5—previously shown to correlate with self‐reactivity (Sprent & Surh, 2011; Hogquist & Jameson, 2014)—than naïve clones, providing further support for a relationship between self‐reactivity and VM generation.
RNA sequencing of VM cells revealed intermediate expression of genes such as cytokine and chemokine receptors relative to memory and naïve cells, which correlates with previous data showing that VM exhibit some but not all functional characteristics of true memory cells. This led the authors to tackle an important but unresolved issue: If cells with high levels of self‐reactivity and enhanced functionality (relative to naïve cells) generate the VM pool, why don't VM cells cause spontaneous autoimmune pathology?
To explore this, the authors first examined the ability of CD8.4 OT‐I cells (the majority of which assume a VM phenotype) to induce experimental autoimmune diabetes following immunization relative to naïve or true memory WT OT‐I cells. These studies used a model in which the OT‐I target foreign antigen, OVA, is expressed in pancreatic β‐cells, allowing the authors to simulate self‐reactivity without depending on ill‐defined low‐affinity self‐antigens. To avoid the possibility that the CD8.4 transgene might compromise straightforward interpretation, these studies were extended to naïve and VM populations from the TCR retrogenic mice on a WT background. Interestingly, the VM cells were less able to provoke autoimmune disease than true memory cells; they were also equivalent or weaker than naïve cells of the same specificity at provoking pathology. The inability of antigen‐specific VM cells to provoke strong autoimmune pathology correlated, in some cases, with impaired upregulation of the high‐affinity IL‐2 receptor chain CD25 and low expression of CD49d. While the mechanistic basis for these differences in autoreactivity requires further study, the finding that the VM population has an attenuated—not heightened—ability to mount an autoaggressive response suggests that the process of VM generation may serve as a novel self‐tolerance mechanism. At the same time, the VM pool has properties (e.g., the ability to rapidly secrete inflammatory cytokines) that mirror those of true memory CD8+ T cells. Hence, these new findings suggest a fine balance in the functional capabilities of VM cells that enables acquisition of certain memory‐like properties while maintaining self‐tolerance.
How these properties of mouse VM cells relate to humans remains incompletely resolved. Prior work (Min et al, 2011; Jacomet et al, 2015; White et al, 2016) suggests the existence of a VM‐like population in humans, but their degree of similarity to murine VM cells and whether they also have impaired autoimmune potential remain to be elucidated by future studies.
The EMBO Journal (2018) 37: e99883
See also: https://doi.org/10.15252/embj.201798518 (July 2018)
References
- Drobek A, Moudra A, Mueller D, Huranova M, Horkova V, Pribikova M, Ivanek R, Oberle S, Zehn D, McCoy KD, Draber P, Stepanek O (2018) Strong homeostatic TCR signals induce formation of self‐tolerant virtual memory CD8 T cells. EMBO J 37: e98518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM (2009) The antigen‐specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 206: 435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC (2014) The self‐obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol 15: 815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomet F, Cayssials E, Basbous S, Levescot A, Piccirilli N, Desmier D, Robin A, Barra A, Giraud C, Guilhot F, Roy L, Herbelin A, Gombert JM (2015) Evidence for eomesodermin‐expressing innate‐like CD8(+) KIR/NKG2A(+) T cells in human adults and cord blood samples. Eur J Immunol 45: 1926–1933 [DOI] [PubMed] [Google Scholar]
- Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC (2013) Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci USA 110: 13498–13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH (2011) MHC class II‐restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol 186: 5749–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Surh CD (2011) Normal T cell homeostasis: the conversion of naive cells into memory‐phenotype cells. Nat Immunol 12: 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O'Connor B, Kedl RM (2016) Virtual memory T cells develop and mediate bystander protective immunity in an IL‐15‐dependent manner. Nat Commun 7: 11291 [DOI] [PMC free article] [PubMed] [Google Scholar]


