Highlights
-
•
The prevalence of pPCL was 1.2%.
-
•
Treatment with novel agents and transplantation may yield a better prognosis.
-
•
Hypercalcemia at diagnosis was suggested to predict worse outcomes.
Keywords: Primary plasma cell leukemia, Novel agent, Hypercalcemia, Stem cell transplantation
Abstract
We retrospectively analyzed twenty-six patients with primary plasma cell leukemia (pPCL) registered from May 2005 until April 2015 by the Kansai Myeloma Forum. Twenty patients received novel agents (bortezomib or lenalidomide), and their median survival of was 34 months. The median survival of patients who underwent autologous stem cell transplantation (SCT) was 40 months, those undergoing allogeneic SCT 55 months, and those undergoing both types of SCT (auto–allo) 61 months; whereas for those who did not undergo SCT it was 28 months (p = 0.845). The only statistically significant risk factor identified by multivariate analysis was hypercalcemia.
1. Introduction
Primary plasma cell leukemia (pPCL), a rare aggressive form of plasma cell dyscrasia, is characterized by a fulminant clinical course and poor prognosis. It is defined by the presence of >2 × 109/μL peripheral blood plasma cells or plasmacytosis accounting for >20% of the differential white cell count. pPCL is also defined as de novo PCL without previous evidence of multiple myeloma (MM), whereas secondary PCL is defined as leukemic transformation in patients with end-stage MM. pPCL accounts for 1.3%–3.4% of plasma cell dyscrasias[1].
According to Surveillance, Epidemiology and End Results (SEER) analysis, the median overall survival (OS) of patients with pPCL is 6 months. However, OS was reportedly 4–6 months for patients diagnosed between 1973 and 2005, and 12 months for those diagnosed between 2006 and 2009. The introduction of novel agents (bortezomib or lenalidomide) as first-line therapy for pPCL has significantly improved the prognosis [2].
Because of the rarity of the disease, no prospectively randomized controlled trials have been performed; thus, the biological, clinical, and prognostic features of this disease have not been clearly determined. The clinical outcomes of pPCL in real-world settings also remain unclear and there is no consensus regarding how to treat it.
We therefore here retrospectively analyzed treatment strategies and outcomes of a real-world cohort of patients with pPCL registered with the Kansai Myeloma Forum (KMF), which is a registry study group for plasma cell dyscrasias.
2. Patients and methods
The Kansai Myeloma Forum (KMF), a study group comprising 73 facilities in the Kansai region of Japan, was established in 2012 to register clinical data of patients with all types of plasma cell dyscrasias with the aim of retrospectively analyzing treatment strategies and their outcomes. By April 2015, KMF had registered the clinical data of 2022 patients with plasma cell dyscrasias, of whom 26 had been diagnosed as having pPCL between May 2005 and April 2015 and registered in the KMF data base. We have here retrospectively analyzed their data.
OS was calculated as the period from the diagnosis to the time of death or last follow-up. Survival curves were created using the Kaplan–Meier method, and differences evaluated using the log-rank test. Multivariate analysis of survival was performed using the Cox proportional hazards model. All statistical tests were two-sided and statistical significance was set at p < 0.05. Additionally, 95% confidence intervals were calculated. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 2.13.0 (R Foundation, Vienna, Austria). More precisely, a modified version of R Commander (version 1.6–3), it is designed to incorporate the statistical functions frequently used in biostatistics. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and was approved by the Institutional Review Boards of all the institutes participating in the KMF.
3. Results
3.1. Patients’ characteristics
From May 2005 until April 2015, 26 of the 2022 patients with plasma cell dyscrasias registered in the KMF data base had diagnoses of pPCL. Thus, the prevalence of pPCL was 1.2% in our cohort. Median follow up period was 22 (0.2–108) months. The clinical characteristics of the study patients are shown in Table 1. The median age of the 26 patients was 63.5 years (range: 21–82); 62% were men and 38% women. The frequencies of M-protein types were IgG (35%), IgA (11%), Bence-Jones protein (BJP) (35%), and IgD (11%). According to the International Staging System (ISS), 79% had ISS Stage 3 disease. Osteolytic lesions and extramedullary plasmacytoma were present in 38% and 15% of patients, respectively.
Table 1.
Patients’ characteristics.
| No. of patients | 26 |
| Median age, range (y/o) | 63.5(21–82) |
| Male sex (%) | 62 |
| PS ≥ 2(%) | 30 |
| M protein(%) | |
| BJP(kappa/lamuda ratio) | 35(2:1) |
| IgG | 32 |
| IgA | 11 |
| IgD | 11 |
| ISS(%) | |
| III | 79 |
| osteolytic bone lesion(%) | 38 |
| extramedullary plasmacytoma (%) | 15 |
| Chromosome abnormality (n) | |
| del(13q) | 4 |
| del(17p) | 3 |
| t(11;14) | 1 |
| t(14;16) | 1 |
PS: Performance Status.
BJP: Bence Jones protein.
ISS: International Staging System.
3.2. Cytogenetic abnormalities
Fluorescence in situ hybridization (FISH) analysis was performed to identify cytogenetic abnormalities in 9 patients, out of which the commonest abnormality was deletion 13q (n = 4), following by deletion 17p (n = 3), t(11;14) (n = 1), and t(14;16) (n = 1).
3.3. Treatment
Conventional therapies were initially administered to 47%, of the study patients and novel agents (bortezomib- or lenalidomide-based therapies) to 42%; 11% of the patients were not treated because of deterioration of general condition. All the novel agent regimens contained bortezomib. The most frequently used regimen was bortezomib plus dexamethasone (VD) (19%), followed by bortezomib, cyclophosphamide and dexamethasone (VCD) (12%), bortezomib, doxorubicin and dexamethasone (PAD)(8%), and bortezomib, lenalidomide and dexamethasone (VRD)(3%). The most frequently used conventional regimen was dexamethasone (23%), followed by vincristine, doxorubicin and dexamethasone (VAD)(12%), and melphalan and prednisolone (MP)(12%).
The response rates were 92% in patients receiving the novel agent-containing regimens (CR: 0%, PR: 75%, SD: 17%) and 75% in those receiving the conventional agent regimens (CR: 8%, PR: 25%, SD: 42%).
3.4. Survival
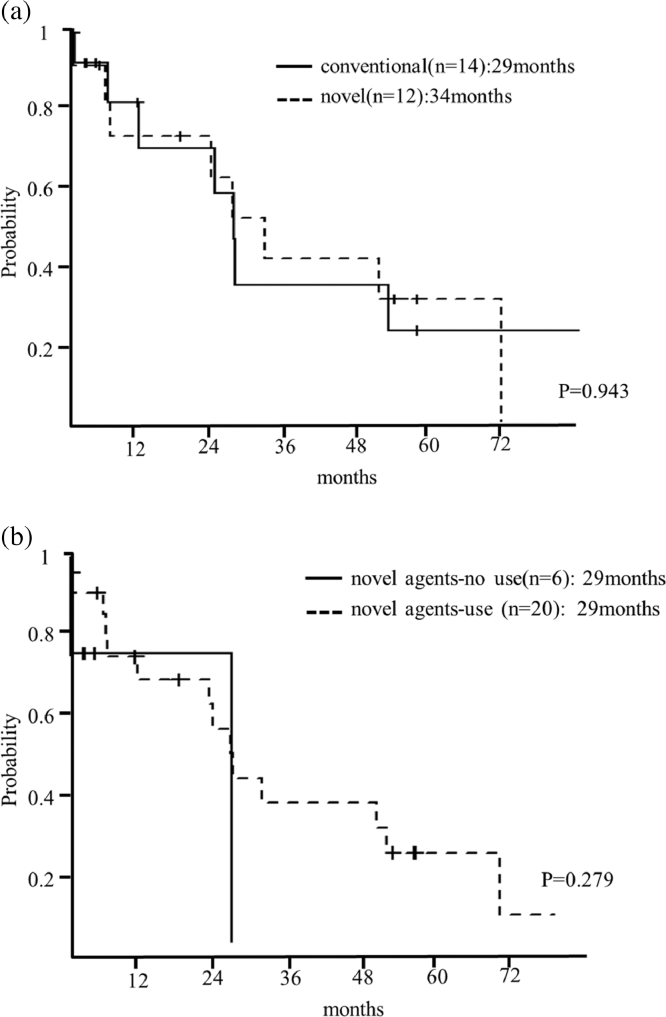
The median survival from diagnosis to time of death or last follow-up of all patients was 29 months. The median OS of the patients who were not treated was 15 days. The median OS of the patients who were initially treated with conventional therapies was 29 months (95%CI: 6-NA), whereas it was 34 months for those treated with novel agents (95%CI: 5-NA) (p = 0.943) (Fig. 1(a)). The median OS of patients who were treated with novel agents (bortezomib or lenalidomide) throughout the entire treatment period was 29 months (95%CI: NA-NA) and was also 29 months for those who did not receive novel agents (95%CI: 6–57) (p = 0.279) (Fig. 1(b)).
Fig. 1.
(a). Survival curve of patients with pPCL according to initial treatment. Median survival of patients who were initially treated with conventional therapies was 29 months (95%CI: 6-NA), whereas it was 34 months (95%CI: 5-NA) in those who received novel agents was (p = 0.943). (b). Survival curve of patients with pPCL according to use of novel agents throughout the treatment period. Median survival of patients who were treated with novel agents throughout their treatment was 29 months (95%CI: NA-NA) and was also 29 months (95%CI: 6–57) for those who did not receive novel agents (p = 0.279).
3.5. Outcomes in patients who underwent transplantation
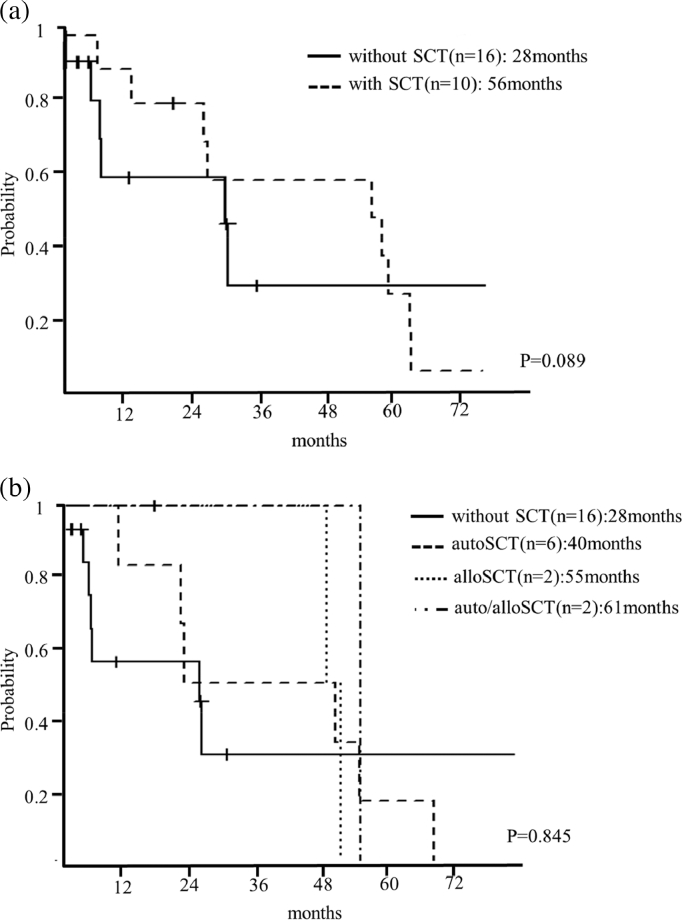
The median OS after all types of SCT was 56 months (95%CI: 12–61), whereas it was 28 months in patients who did not undergo SCT (95%CI: 5-NA) (p = 0.089) (Fig. 2(a)). Autologous SCT, allogeneic SCT, and both were performed in 23%, 8%, and 8% of patients, respectively. Median OS after autologous SCT was 40 months (95%CI: 12-NA), after allogeneic SCT 55 months (95%CI: 54-NA), and after both types of SCT 61 months (95%CI: NA-NA), whereas it was 28 months in patients who did not undergo SCT (95%CI: 5-NA) (p = 0.845) (Fig. 2(b)).
Fig. 2.
(a). Survival curves of patients with pPCL according to stem cell transplantation. Median survival after all types of SCT was 56 months (95%CI: 12–61), whereas it was 28 months in patients who did not undergo SCT (95%CI: 5-NA) (p = 0.089). SCT: stem cell transplantation. (b). Survival curves of patients with pPCL according to type of stem cell transplantation. Median survival with autologous SCT was 40 months (95%CI: 12-NA), with allogeneic SCT 55 months (95%CI: 54-NA), and with both therapies 61 months (95%CI: NA-NA), whereas without SCT it was 28 months (95%CI: 5-NA) (p = 0.845). autoSCT: autologous stem cell transplantation. alloSCT: allogeneic stem cell transplantation.
3.6. Prognostic factors
Univariate analysis was performed to identify risk factors associated with poor survival. Beta-2-microglobulin, lactate dehydrogenase (LDH), or white blood cells, and decreases in hemoglobin or platelet counts had no significant influence on OS. However, hypercalcemia was associated with poor survival (Table 2). Multivariate analysis identified only hypercalcemia remained as a significant factor affecting survival (hazard ratio: 11.85, 95%CI: 1.45–96.59, p = 0.020), whereas other factors were dropped out by backward stepwise selection.
Table 2.
Univariate analysis.
| Variable | Category | Number of Cases | HR (95%CI) | p-value |
|---|---|---|---|---|
| B2M | >5.5 mg/l | 15 | 1.13 (0.39–3.29) | 0.817 |
| ≤5.5 mg/l | 11 | 1 | ||
| LDH | >250 IU/l | 10 | 1.61 (0.56–4.62) | 0.377 |
| ≤250 IU/l | 16 | 1 | ||
| Ca | >11 mg/dl | 8 | 5.80 (1.37–24.52) | 0.017 |
| ≤11 mg/dl | 18 | 1 | ||
| eGFR | <40 ml/min | 13 | 1.26 (0.43–3.67) | 0.669 |
| ≥40 ml/min | 13 | 1 | ||
| WBC | >13,000/μl | 16 | 0.98 (0.35–2.78) | 0.977 |
| ≤13,000/μl | 10 | 1 | ||
| Hb | <8.0 g/dl | 10 | 1.53 (0.54–4.29) | 0.421 |
| ≥8.0 g/dl | 16 | 1 | ||
| PLT | <10 × 104/μl | 11 | 1.37 (0.47–3.98) | 0.561 |
| ≥10 × 104/μl | 15 | 1 |
B2M: Beta-2-microglobulin.
LDH: lactate dehydrogenase.
Ca: Calcium.
eGFR: glomerular filtration rate.
WBC: white blood cell.
Hb: hemoglobin.
PLT: platelet.
4. Discussion
In this study, registry data were analyzed to determine the clinical features, therapeutic approaches, and clinical outcomes of pPCL in real-world settings. The prevalence of pPCL was 1.2%, the median age was 63.5 years, 62% were men, and the most frequent M-protein was light chain type (κ:λ = 2:1). These characteristics are similar to those reported for previous studies [3], [4].
Cytogenetic abnormalities were sought and found in only nine patients, 13q deletion being the most frequently identified, consistent with previous reports [5].
The standard therapy for pPCL has not yet been established. However, some studies have indicated that the novel agents (bortezomib or lenalidomide) are associated with a better prognosis. Lobovic et al. reported that in 25 patients with pPCL who were treated with bortezomib-based (n = 12) or lenalidomide-based regimens (n = 8), the median OS was 23.6 months [6]. D'Arena et al. reported a 2-year OS of 55% in patients treated with bortezomib-based regimens [7]. Musto et al. reported a 28 month median OS in patients treated with lenalidomide-based regimens [8]. These reports suggest that the novel agents significantly extend OS and improve the prognosis of pPCL compared with conventional regimens. Recently, Iriuchishima et al. reported that novel agents significantly prolonged OS compared to other treatments (2.85 versus 1.65 years, p = 0.049) [9].
In our study, patients who were treated with novel agents (bortezomib or lenalidomide) showed a tendency to have longer OS; however, the difference was not statistically significant (Fig. 1). In our study, all regimens using novel agents contained bortezomib.
Drake et al. reported a 3-year OS of 39.5% after autologous SCT [10]; whereas Mahindra et al. reported a 3-year OS of 66% after autologous SCT and 39% after allogeneic SCT [11]. Loiseau et al. compared patients with conventional treatment versus with double autologous SCT. The median overall survival was 16 months with conventional treatment and 31 months with SCT (p < 0.0001) [12].
Those results indicate that the OS is better with SCT than with conventional treatments. However, the results of SCT have not yet been satisfactorily established and further development of treatment strategies is needed. In our study, only 10 patients underwent SCT, which is too few to draw definite conclusions. However, SCT may improve prognosis. We identified hypercalcemia as the only prognostic factor significantly associated with an inferior OS. Although a previous study reported that high LDH and older age were poor prognostic factors [2], our findings did not support this.
Our study had several limitations. First, there were relatively few patients analyzed and it was a retrospective study. Second, although chromosomal abnormalities are thought to affect prognosis, they were only sought, and identified, in nine patients. Limitations in the patients’ insurance policies prevented us from performing detailed chromosomal tests, including FISH, in the remaining patients; this problem needs to be addressed.
Here, we have reported the clinical features and outcomes of twenty-six patients with pPCL in the Kansai Myeloma Forum registry. We found that novel agents (bortezomib or lenalidomide) may improve the prognosis of pPCL, as may allogeneic and/or autologous SCT, in these real-world patients. However, the prognosis of pPCL remains dismal. Thus, further investigations to assess clinical features and develop new treatment strategies are needed.
Conflict of interest
This work was supported by grants from Celgene Co., Ltd., Takeda Pharmaceutical Co., Fujimoto Pharmaceutical Co., and Ono Pharmaceutical Co.
Authors' contributions
AN and HS designed the study. AN performed statistically analysis and interpretation of data, drafted the article. HS and YK revised the article. HY, HK, SK, TK, YA, TK, YK, SF, NU, EK, HU, YS, TT, FU, KO, TH, KM, MK, MS, HT, CS, MH, JK, ATK, SN, IM provided patient data.
Acknowledgments
The authors would like to thank all myeloma patients registered in KMF and all KMF investigators for their scientific support. We also thank Ms. Okuyama R for her assistance and Dr. Nakatani E for giving us great advice regarding this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2018.07.001.
Appendix. Supplementary materials
References
- 1.Fernández de Larrea C.1., Kyle R.A., Durie B.G. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonsalves W.I., Rajkumar S.V., Go R.S. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124:907–912. doi: 10.1182/blood-2014-03-565051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiedemann R.E., Gonzalez-Paz N., Kyle R.A. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22:1044–1052. doi: 10.1038/leu.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colović M.1., Janković G., Suvajdzić N. Thirty patients with primary plasma cell leukemia: a single center experience. Med. Oncol. 2008;25:154–160. doi: 10.1007/s12032-007-9011-5. [DOI] [PubMed] [Google Scholar]
- 5.Van de Donk N.W., Lokhorst H.M., Anderson K.C. How I treat plasma cell leukemia. Blood. 2012;120:2376–2389. doi: 10.1182/blood-2012-05-408682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebovic D., Zhang L., Alsina M. Clinical outcomes of patients with plasma cell leukemia in the era of novel therapies and hematopoietic stem cell transplantation strategies: a single-institution experience. Clin. Lymphoma Myeloma Leuk. 2011;11:507–511. doi: 10.1016/j.clml.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 7.D'Arena G., Valentini C.G., Pietrantuono G. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA multiple myeloma working party. Ann. Oncol. 2012;23:1499–1502. doi: 10.1093/annonc/mdr480. [DOI] [PubMed] [Google Scholar]
- 8.Musto P., Simeon V., Martorelli M.C. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28:222–225. doi: 10.1038/leu.2013.241. [DOI] [PubMed] [Google Scholar]
- 9.Iriuchishima H., Ozaki S., Konishi J., Matsumoto M., Murayama K., Nakamura F. Primary plasma cell leukemia in the era of novel agents: a multicenter study of the Japanese Society of Myeloma. Acta Haematol. 2016;135:113–121. doi: 10.1159/000439424. [DOI] [PubMed] [Google Scholar]
- 10.Drake M.B., Iacobelli S., van Biezen A. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95:804–809. doi: 10.3324/haematol.2009.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahindra A., Kalaycio M.E., Vela-Ojeda J. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia. 2012;26:1091–1097. doi: 10.1038/leu.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H., Roussel M., Campion L., Leleu X., Marit G., Jardel H. Cytogenetic and therapeutic characterization of primary plasma cell leukemia: the IFM experience. Leukemia. 2012;26:158–159. doi: 10.1038/leu.2011.176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.