Abstract
Background
Stereotactic ablative radiotherapy (SABR) is a promising option for non-operated early-stage non-small cell lung cancer (NSCLC) compared to conventional fractionated radiotherapy (CFRT). However, results from conclusive randomized controlled trials are not yet available. The aim of our study was to explore the effectiveness of SABR vs. CFRT for non-operated early-stage NSCLC.
Patients and methods
We used a comprehensive population-based database to identify clinical stage I non-operated NSCLC patients in Taiwan diagnosed from 2007 to 2013 who were treated with either SABR or CFRT. We used inverse probability weighting and the propensity score as the primary form of analysis to address the nonrandomization of treatment. In the supplementary analyses, we constructed subgroups based on propensity score matching to compare survival between patients treated with SABR vs. CFRT.
Results
We identified 238 patients in our primary analysis. A good balance of covariates was achieved using the propensity score weighting. Overall survival (OS) was not significantly different between those treated with SABR vs. CFRT (SABR vs. CFRT: probability weighting adjusted hazard ratio [HR] 0.586, 95% confidence interval 0.264–1.101, p = 0.102). However, SABR was significantly favored in supplementary analyses.
Conclusions
In this population-based propensity-score adjusted analysis, we found that OS was not significantly different between those treated with SABR vs. CFRT in the primary analysis, although significance was observed in the supplementary analyses. Our results should be interpreted with caution given the database (i.e., nonrandomized) approach used in our study. Overall, further studies are required to explore these issues.
Key words: stereotactic ablative radiotherapy, conventional fractionated radiotherapy, non-small cell lung cancer
Introduction
Surgery is the cornerstone for treating early-stage non-small cell lung cancer (NSCLC), although radical radiotherapy may be used for medically inoperable cases.1,2 In recent years, stereotactic ablative radiotherapy (SABR, or so-called stereotactic body radiotherapy) has been used to deliver radiotherapy instead of conventional fractionated radiotherapy (CFRT).2,3,4,5 Promising results have been reported for medically inoperable and operable cases and even other cancers.6,7,8,9
However, a recent randomized phase II study (the SPACE trial) challenged the general belief that SABR is superior to CFRT, as also mentioned in a 2017 systematic review.5,10 It showed that disease control and overall survival were similar for SABR and CFRT, although SABR was better considering some side effects and quality of life. However, this study had limited power (67%), and a larger randomized controlled trial (RCT) is required.10
Statement of general knowledge
PubMed for published reports using the keywords ([stereotactic radiotherapy] OR [stereotactic body radiotherapy] OR [stereotactic ablative radiotherapy] OR [SBRT] OR [SABR]) AND ([non-small cell lung cancer] OR [NSCLC]) AND ([survival] OR [OS]) was searched on Sep 2nd 2017, for evidence regarding the efficacy of SABR vs. CFRT. In addition to the aforementioned SPACE trial, we identified another small (n = 50) randomized study showing better treatment efficacy for SABR compared to CFRT in peripheral NSCLC.11 However, patients of various stages (stages I–IV) were included in the study, and the results of stage I patients were not reported. We also found a meta-analysis (published in 2010) that reported better overall survival (OS) for SABR compared to CFRT, but all of the included studies were nonrandomized.12 In addition, none of the included studies directly compared SABR and CFRT.12 We also found four subsequent single institutional nonrandomized studies from Europe or North America and two subsequent population-based studies from North America.13,14,15,16,17,18 However, to the best of our knowledge, no population-based study from Asia has compared SABR vs. CFRT for treating early-stage NSCLC.
Study aim
Given the relatively limited evidence on this topic, we investigated the effectiveness of SABR vs. CFRT for non-operated early-stage NSCLC in a population-based sample from Taiwan.
Patients and methods
Data source
The Health and Welfare Data Science Center (HWDC) database is a set of databases providing complete information regarding the Taiwan cancer registry, death registry, and reimbursement data for the whole Taiwanese population provided by the Bureau of National Health Insurance (NHI).19 The high quality of this cancer registry has been reported.20 NHI is a single-payer, compulsory social insurance program that provides insurance coverage to the majority of citizens in Taiwan.21 All of the above data were included in the HWDC with deidentified personal identifiers.
Identification of study cases and study design
A flowchart showing the identification of study cases appears in Figure 1 as suggested by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.22 Briefly, we identified stage I histology-documented NSCLC patients diagnosed from 2007 to 2013 who received either CFRT or SABR without surgery. We used the date of diagnosis as the index date. We determined the explanatory variable of interest (CFRT vs. SABR) based on the record in the cancer registry using the dose/fractionation recommended by the National Comprehensive Cancer Network (NCCN) NSCLC guideline (CFRT: 60–70 Gy in 1.8–2 Gy/fraction; SABR: 25–34 Gy/1 fraction, or 45–60 Gy/3 fractions, or 48–50 Gy/4 fractions, or 50–55 Gy/5 fractions, or 60–70 Gy/8–10 fractions).1 We also collected other covariate and outcome data from the HWDC. We decided on covariates (age, sex, residency, comorbidity, histology, T stage, period, use of positron emission tomography [PET], use of systemic therapy, and previous cancer) based on our clinical and HWDC-related research experiences as well as previous reports.23,24,25 The covariates were defined as follows. Patient residency was classified as northern Taiwan or elsewhere. We included this covariable because geographic practice variation had been report in the literature26 and we felt it might influence treatment choice in our clinical and research experiences.24 Comorbidity was defined as with or without a modified Carlson comorbidity score ≥1, as used in our previous NHI cancer study.24 Histology was classified as adenocarcinoma or non-adenocarcinoma. T stage was classified as T1 vs. T2. Period was classified as 2007–2009 or 2010–2013 because staging was changed since 2010. Use of PET, systemic therapy, and previous cancer was classified as yes or no. We used the national death registry to determine survival status and used OS as our endpoint, as initially completed in the SPACE trial.10 This study was approved by the Research ethics committee at our institute (CMUH104-REC-002).
Figure 1.
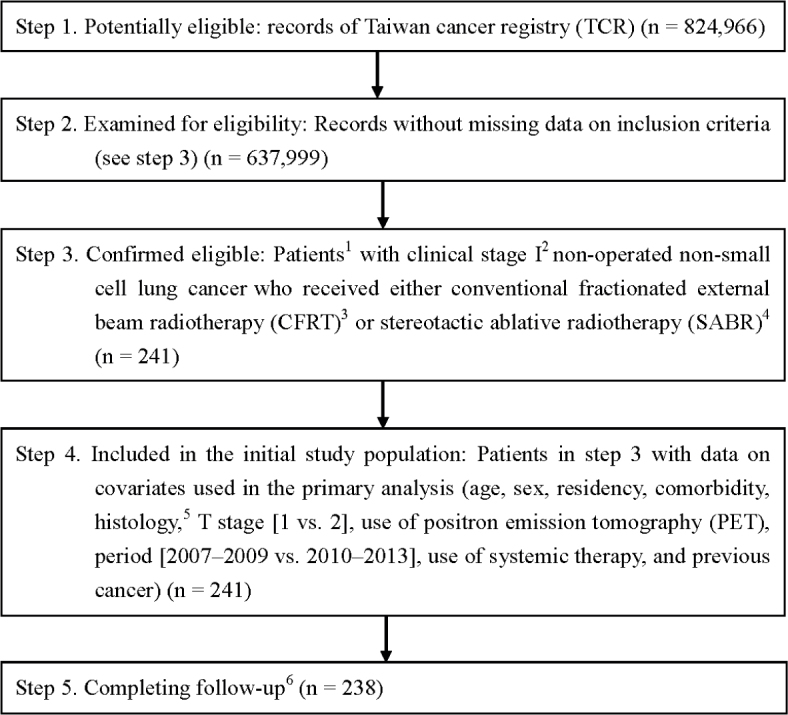
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) study flowchart and the number of individuals at each stage of the study.
1 We only included those treated (class 1–2) at a single institution to ensure data consistency.
2 Sixth (2007–2009) or Seventh (2010–2013) American Joint Committee on Cancer.
3 60–70 Gy in 1.8–2 Gy/fraction, ±10% in dose.
4 Dose/fraction compatible with National Comprehensive Cancer Network non-small cell lung cancer guideline 2017 v8 (i.e., 25–34 Gy/1 fraction, or 45–60 Gy/3 fractions, or 48–50 Gy/4 fractions, or 50–55 Gy/5 fractions, or 60–70 Gy/8–10 fractions), ±10% in dose.
5Adenocarcinoma or non-adenocarcinoma.
6 Without missing information in the Taiwan cancer registry and death registry.
Statistical analysis
We used the Kaplan-Meier method and log-rank test to compare crude OS between patients treated with SABR vs. CFRT. We further used inverse probability weighting (IPW) based on the propensity score (PS) as the primary means of analysis to address the nonrandomization of treatment.27 We modeled the use of SABR vs. CFRT as the dependent variable and the above covariates as independent variables and used logistic regression to model the probability of receiving SABR. Then we used the logit of the probability as the PS, as described previously.27 Tabulation and standardized differences were used to assess the balance of covariates between treatment groups.27,28 We used a weighted Cox model to compare OS between treatment groups for the entire follow-up period (censored on December 31, 2015).27,29 We used bootstrap analysis to obtain confidence intervals and p-values, as described previously.30 For OS results with statistical significance, we further calculated the E-factor to evaluate the robustness of our finding regarding potential unmeasured confounder[s] as suggested in the recent literature.31
Supplementary analyses
In the first supplementary analysis (SA-1), we constructed a subgroup based on PS matching and used a robust variance estimator to compare OS and lung cancer-specific survival of patients treated with SABR vs. CFRT. We also used cause of death to obtain lung cancer-specific survival (LCSS). In the second supplementary analysis (SA-2), we constructed another subgroup by PS matching limited to cases from 2011 to 2013 to use the additional covariate (performance status, classified as Eastern Cooperative Oncology Group [ECOG] 0–2 vs. 3–4) in PS modeling to compare the survival of patients treated with SABR vs. CFRT. We limited to this period [2011–2013] because performance information was available in Taiwan cancer registry since 2011. SAS 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
Identification of study cases
As shown in Figure 1, we found 238 clinical stage I NSCLC patients who received either SABR or CFRT from 2007 to 2013 were included in our primary analysis. The characteristics of these patients are described in Table 1. Although an imbalance in covariate distribution was observed before PS weighting such as higher percentage of patients with comorbidity received SABR [32%] than those without comorbidity [17%], a good balance of covariates and small standardized differences (≤ 0.25) were observed for all covariates after we adjusted for PS weighting.28,32
Table 1.
Patient characteristics for the whole study population
| SABR | CFRT | Standardized difference (rounded)* | |||||
|---|---|---|---|---|---|---|---|
| Number or mean (sd)* | (%)* | Number or mean (sd)* | (%)* | Before IPW | After | ||
| Age | 77.81 (7.85) | 75.40 (9.96) | 0.27 | 0.24 | |||
| Sex | Female | 20 | (29) | 44 | (26) | 0.07 | 0.07 |
| Male | 49 | (71) | 125 | (74) | |||
| Residency | Non-north | 32 | (46) | 93 | (55) | 0.17 | 0.19 |
| North | 37 | (54) | 76 | (45) | |||
| Comorbidity | Without | 9 | (13) | 43 | (25) | 0.32 | 0.25 |
| With† | 60 | (87) | 126 | (75) | |||
| Histology | Adenocarcinoma | 40 | (58) | 82 | (49) | 0.19 | 0.24 |
| Non-adenocarcinoma | 29 | (42) | 87 | (51) | |||
| T stage | T1 | 38 | (55) | 49 | (29) | 0.55 | 0.08 |
| T2 | 31 | (45) | 120 | (71) | |||
| Period | 2007–2009 | 15 | (22) | 65 | (38) | 0.37 | 0.22 |
| 2010–2013 | 54 | (78) | 104 | (62) | |||
| Use of PET | Yes | 37 | (54) | 55 | (33) | 0.44 | 0.09 |
| No | 32 | (46) | 114 | (67) | |||
| Use of systemic therapy | Yes | 10 | (14) | 73 | (43) | 0.67 | 0.17 |
| No | 59 | (86) | 96 | (57) | |||
| Previous cancer | Yes | 9 | (13) | 16 | (9) | 0.11 | 0.06 |
| No | 60 | (87) | 153 | (91) | |||
CFRT = conventional fractionated radiotherapy; IPW = inverse probability weighting; PET = positron emission tomography; SABR = stereotactic ablative radiotherapy; sd = standard deviation;
rounded at the second
modified Carlson comorbidity score ≥ 1
Primary analysis
After a median follow-up of 28 months (range 2–105), 171 patients were found to have died (40 SABR and 131 CFRT). We found that SABR led to higher crude OS compared to CFRT, as shown in Figure 2. The 5-year OS rates for SABR and CFRT were 31% and 20%, respectively (log-rank test, p = 0.0008). After IPW, OS was not significantly different between those treated with SABR vs. CFRT (SABR vs. CFRT: IPW adjusted hazard ratio [HR] 0.586, 95% confidence interval 0.264–1.101, p = 0.102).
Figure 2.
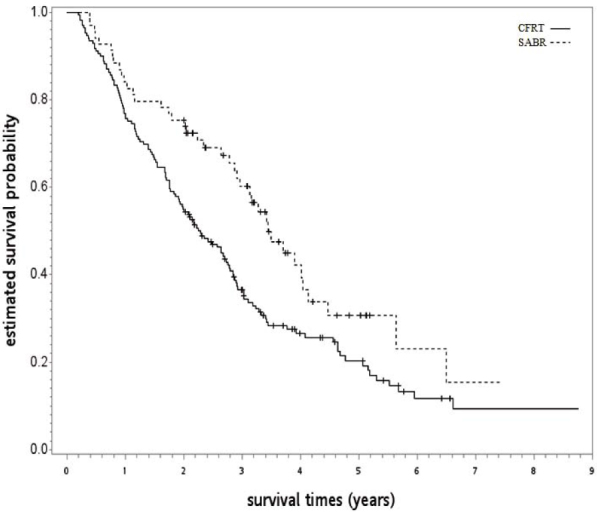
Kaplan-Meier survival curve for the whole study population.
CFRT = conventional fractionated radiotherapy; SABRT = stereotactic ablative radiotherapy
Supplementary analyses
In SA-1, a good balance of covariates was observed with small standardized differences (≤ 0.25) for the PS-matched subgroup (n = 120; see Table 2). Compared to CFRT, the OS (HR 0.672, p = 0.039) and LCSS (HR 0.529, p = 0.007) of patients receiving SABR were superior. The observed HR 0.672 for OS could be explained away by an unmeasured confounder that was associated with both selections of SABR/CFRT and live/death by a risk ratio of 1.96 fold each, but weaker confounding could not do so. The OS curve is shown in Figure 3. In SA-2, well-balanced covariates were observed with small standardized differences (≤ 0.25) when cases were limited to 2011 to 2013 with an available performance status (n = 52; see Table 3), although there were some imbalances before matching such as those with poor performance status [ECOG 3~4] were more likely to receive SABR [60%] than those with acceptable performance status [33%]. We found SABR was associated with further improvement in hazard for death (HR 0.381, p = 0.016) compared to CFRT, as seen in Figure 4. The observed HR 0.381 for OS could be explained away by an unmeasured confounder that was associated with both selections of SABR/CFRT and live/death by a risk ratio of 3.29 fold each, but weaker confounding could not do so.
Table 2.
Patient characteristics in the first supplementary analysis
| SABR | CFRT | |||||
|---|---|---|---|---|---|---|
| Standardized difference (rounded)* | ||||||
| Number or mean (sd)* | (%)* | Number or mean (sd)* | (%)* | |||
| Age | 77.47 (8.26) | 77.75 (9.79) | 0.03 | |||
| Sex | Female | 18 | (30) | 24 | (40) | 0.21 |
| Male | 42 | (70) | 36 | (60) | ||
| Residency | Non-north | 29 | (48) | 30 | (50) | 0.03 |
| North | 31 | (52) | 30 | (50) | ||
| Comorbidity | Without | 9 | (15) | 8 | (13) | 0.05 |
| With† | 51 | (85) | 52 | (87) | ||
| Histology | Adenocarcinoma | 37 | (62) | 41 | (68) | 0.14 |
| Non-adenocarcinoma | 23 | (38) | 19 | (32) | ||
| T stage | T1 | 30 | (50) | 31 | (52) | 0.03 |
| T2 | 30 | (50) | 29 | (48) | ||
| Period | 2007–2009 | 15 | (25) | 15 | (25) | 0.00 |
| 2010–2013 | 45 | (75) | 45 | (75) | ||
| Use of PET | Yes | 30 | (50) | 31 | (52) | 0.03 |
| No | 30 | (50) | 29 | (48) | ||
| Use of systemic therapy | Yes | 10 | (17) | 13 | (22) | 0.13 |
| No | 50 | (83) | 47 | (78) | ||
| Previous cancer | Yes | 8 | (13) | 7 | (12) | 0.05 |
| No | 52 | (87) | 53 | (88) | ||
CFRT = conventional fractionated radiotherapy; PET = positron emission tomography; SABR = stereotactic ablative radiotherapy; sd = standard deviation;
rounded at the second
modified Carlson comorbidity score ≥ 1
Figure 3.
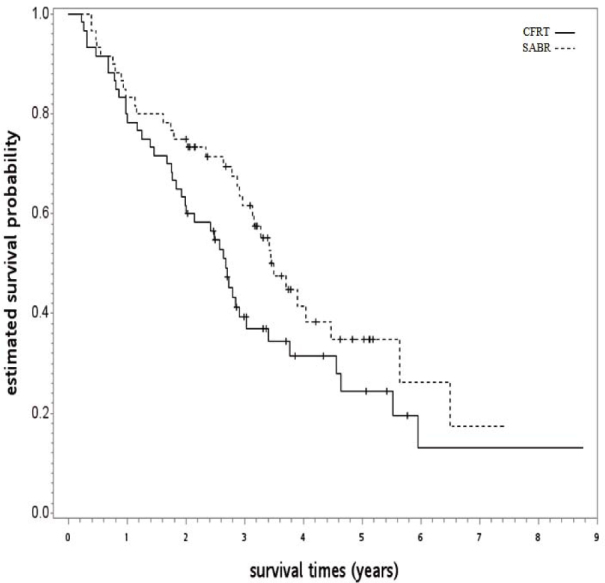
Kaplan-Meier survival curve for the first supplementary analysis.
CFRT = conventional fractionated radiotherapy; SABRT = stereotactic ablative radiotherapy
Table 3.
Patient characteristics in the second supplementary analysis
| SABR | CFRT | Standardized difference (rounded)* | ||||
|---|---|---|---|---|---|---|
| Number or mean (sd)* | (%)* | Number or mean (sd)* | (%)* | |||
| Age | 76.92 (8.84) | 77.73 (9.19) | 0.09 | |||
| Sex | Female | 8 | (31) | 7 | (27) | 0.09 |
| Male | 18 | (69) | 19 | (73) | ||
| Residency | Non-north | 16 | (62) | 18 | (69) | 0.16 |
| North | 10 | (38) | 8 | (31) | ||
| Comorbidity | Without | # | # | 0.13 | ||
| With† | # | # | ||||
| Histology | Adenocarcinoma | 14 | (54) | 15 | (58) | 0.08 |
| Non-adenocarcinoma | 12 | (46) | 11 | (42) | ||
| T stage | T1 | 11 | (42) | 11 | (42) | 0.00 |
| T2 | 15 | (58) | 15 | (58) | ||
| Use of PET | Yes | 13 | (50) | 12 | (46) | 0.08 |
| No | 13 | (50) | 14 | (54) | ||
| Use of systemic therapy | Yes | # | # | 0.13 | ||
| No | # | # | ||||
| Previous cancer | Yes | 3 | (12) | 3 | (12) | 0.00 |
| No | 23 | (88) | 23 | (88) | ||
| Performance status | ECOG (0–2) | # | # | 0.00 | ||
| ECOG (3–4) | # | # | ||||
CFRT = conventional fractionated radiotherapy; ECOG = Eastern Cooperative Oncology Group; PET = positron emission tomography; SABR = stereotactic ablative radiotherapy; sd = standard deviation; # Exact numbers are not reported because the Health and Welfare Data Science Center (HWDC) database center policy is to avoid numbers in single cells ≤ 2
rounded at the second
modified Carlson comorbidity score ≥ 1
Figure 4.
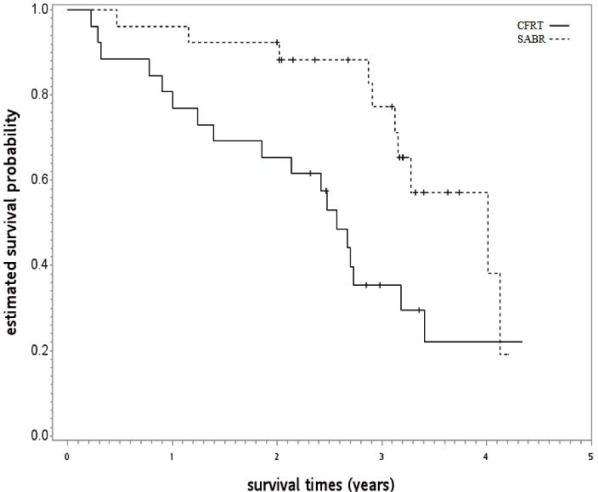
Kaplan-Meier survival curve for the second supplementary analysis.
CFRT = conventional fractionated radiotherapy; SABRT = stereotactic ablative radiotherapy
Discussion
In this population-based PS-adjusted analysis, we provide the first empirical evidence from Asia regarding non-operated early-stage NSCLC patients treated with either SABR or CFRT. We found that OS was not significantly different between those treated with SABR vs. CFRT in the primary analysis, although statistical significance was observed in the supplementary analyses.
Our results may be interpreted as compatible with the SPACE trial in that OS was not significantly different between those treated with SABR vs. CFRT. On the contrary, because the point estimate of HR for death was around 0.6, SABR may lead to better OS, but the statistical significance was limited by the moderate sample size. The statistical significance found in our SA supported this hypothesis, as reported in other studies from Europe and North America, and indirect comparison in a previous meta-analysis showed that SABR led to better survival.12,13,14,15,16,17,18 Therefore, our results should not be interpreted as conclusive.
Our study provides additional evidence for practitioners considering SABR in addition to conventional CFRT for non-operated early-stage NSCLC.33 Although the available randomized data did not support the superior efficacy of SABR compared to CFRT, the power of that study was limited and is not compatible with previous retrospective data.10 Although the results of our primary analysis were not significant, the trend was in favor of SABR (HR 0.59), and similar trends with statistical significance were observed in SA. Furthermore, we observed that patients with comorbidity or poor performance status were more likely to receive SABR in the pre-matched population (i.e., SABR patients were possibly prone to die from competing death), so it is possible that SABR had improved LCSS [HR 0.529] but OS benefit was less obvious [HR 0.72] as seen in our SA-1. Therefore, our study may be used by practitioners to select treatment for non-operated early-stage NSCLC while awaiting results from ongoing RCTs (such as NCT01968941 or NCT01014130).
There are some limitations to our study. First, the sample size was moderate, particularly in both supplementary analyses, which severely limits statistical power [around 0.5 ~ 0.8 in the setting of our SA]. Second, identification of the study population may be inhomogeneous because a higher dose may be more effective, although we used the NCCN criteria to classify SABR vs. CFRT.34 Third, treatment selection was not random or specified. The reason for choosing radiotherapy but not surgery was not available due to data limitation. In addition, the reason for choosing SABR or CFRT remains unclear. Unobservable bias is possible in retrospective studies, and results of the aforementioned ongoing trials are required. For example, the location of the primary tumor (central vs. peripheral) or lung function test results were not known and could have been unbalanced, even after we matched for observable covariates.35 Epidermal growth factor receptor (EGFR) status may also have been unbalanced. Population variation in treatment response is an emerging issue, and highly prevalent EGFR mutations in Asia (including Taiwan) is a well-known example.36 Adjuvant EGFR-directed treatment may even improve the outcomes of resected NSCLC.37 However, we found our result was somehow robust [E-factor 3.29] to potential unmeasured confounder(s). Fourth, other endpoints such as local control were not available due to data limitation, although no difference in local control was reported in the SPACE trial.10
Conclusions
In this population-based PS-adjusted analysis, we provide the first empirical evidence from Asia regarding non-operated early-stage NSCLC patients treated with either SABR or CFRT. We found that OS was not significantly different in the primary analysis between those treated with SABR vs. CFRT, although statistical significance was observed in supplementary analyses. Thus, the results of ongoing randomized controlled studies are required.
Acknowledgements
The data analyzed in this study was provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. This work was supported by China Medial University Hospital [DMR-105-046]. The corresponding author would like to thank Dr. Ya-Chen Tina Shih for her suggestions in study design. The authors thank “Textcheck” for professional writing assistance.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.National Comprehensive Cancer Network Guideline for Non-Small Cell Lung Cancer. 2017 https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf version 8. [Cited 14 Jul 2017]. Available at. [Google Scholar]
- 2.Baker S, Dahele M, Lagerwaard FJ, Senan S.. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat Oncol. 2016;11:115. doi: 10.1186/s13014-016-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricardi U, Badellino S, Filippi AR.. Stereotactic body radiotherapy for early stage lung cancer: history and updated role. Lung Cancer. 2015;90:388–96. doi: 10.1016/j.lungcan.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Guckenberger M, Andratschke N, Alheit H, Holy R, Moustakis C, Nestle U. et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014;190:26–33. doi: 10.1007/s00066-013-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray P, Franks K, Hanna GG.. A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. Br J Radiol. 2017;90:20160732. doi: 10.1259/bjr.20160732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J. et al. Stereotactic body radiation therapy for inoperable early stage lungancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P. et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionisi F, Guarneri A, Dell’Acqua V, Leonardi M, Niespolo R, Macchia G. et al. Radiotherapy in the multidisciplinary treatment of liver cancer: a survey on behalf of the Italian Association of Radiation Oncology. Radiol Med. 2016;121:735–43. doi: 10.1007/s11547-016-0650-5. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo Y, Yoshida K, Nishimura H, Ejima Y, Miyawaki D, Uezono H. et al. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: evaluation by comparison with conventional three-dimensional conformal radiotherapy. J Radiat Res. 2016;57:512–23. doi: 10.1093/jrr/rrw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyman J, Hallqvist A, Lund J, Brustugun OT, Bergman B, Bergström P. et al. SPACE - a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8. doi: 10.1016/j.radonc.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Wang SW, Ren J, Yan YL, Xue CF, Tan L, Ma XW.. Effect of image-guided hypofractionated stereotactic radiotherapy on peripheral non-small-cell lung cancer. Onco Targets Ther. 2016;9:4993–5003. doi: 10.2147/OTT.S101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P.. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol. 2010;95:32–40. doi: 10.1016/j.radonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O.. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552–8. doi: 10.3109/0284186X.2013.813635. [DOI] [PubMed] [Google Scholar]
- 14.Widder J, Postmus D, Ubbels JF, Wiegman EM, Langendijk JA.. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e291–7. doi: 10.1016/j.ijrobp.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S. et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–70. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HW, Gabos Z, Ghosh S, Roberts B, Lau H, Kerba M.. Outcomes in stage I non-small cell lung cancer following the introduction of stereotactic body radiotherapy in Alberta - A population-based study. Radiother Oncol. 2015;117:71–6. doi: 10.1016/j.radonc.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Lanni TB Jr, Grills IS, Kestin LL, Robertson JM.. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol. 2011;34:494–8. doi: 10.1097/COC.0b013e3181ec63ae. [DOI] [PubMed] [Google Scholar]
- 18.Mitera G, Swaminath A, Rudoler D, Seereeram C, Giuliani M, Leighl N. et al. Cost-effectiveness analysis comparing conventional versus stereotactic body radiotherapy for surgically ineligible stage I non-small-cell lung cancer. J Oncol Pract. 2014;10:e130–6. doi: 10.1200/JOP.2013.001206. [DOI] [PubMed] [Google Scholar]
- 19.The Health and Welfare Data Science Center database (in Chinese) http://dep.mohw.gov.tw/DOS/np-2497-113.html [Cited 18 Jul 2017]. Available at. [Google Scholar]
- 20.Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS.. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291–6. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 21.Universal Health Coverage in Taiwan. [Cited 15 Jul 2017]. Available at. [Google Scholar]
- 22. https://www.nhi.gov.tw/Resource/webdata/21717_1_UnversalHealthCoverage-2.pdf [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Hsia TC, Tu CY, Fang HY, Liang JA, Li CC, Chien CR.. Cost and effectiveness of image-guided radiotherapy for non-operated localized lung cancer: a population-based propensity score-matched analysis. J Thorac Dis. 2015;7:1643–9. doi: 10.3978/j.issn.2072-1439.2015.09.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien CR, Pan IW, Tsai YW, Tsai T, Liang JA, Buchholz TA. et al. Radiation therapy after breast-conserving surgery: does hospital surgical volume matter? A population-based study in Taiwan. Int J Radiat Oncol Biol Phys. 2012;82:43–50. doi: 10.1016/j.ijrobp.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Jelercic S, Rajer M.. The role of PET-CT in radiotherapy planning of solid tumours. Radiol Oncol. 2015;49:1–9. doi: 10.2478/raon-2013-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder MC, Tien YY, Wright K, Halfdanarson TR, Abu-Hejleh T, Brooks JM.. Geographic variation in the use of adjuvant therapy among elderly patients with resected non-small cell lung cancer. Lung Cancer. 2016;95:28–34. doi: 10.1016/j.lungcan.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC.. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–58. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Hernán MA.. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC.. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–55. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med. 2017;167:268–74. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 33.Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS. et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–20. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum PR. Rosenbaum PR. Design of observational studies (Springer Series in Statistics) New York: Springer; 2010. Reasons for Effects; pp. 104–7. [DOI] [Google Scholar]
- 35.Koshy M, Malik R, Weichselbaum RR, Sher DJ.. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91:344–50. doi: 10.1016/j.ijrobp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Chang JY, Bezjak A, Mornex F. IASLC Advanced Radiation Technology Committee. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. 2015;10:577–85. doi: 10.1097/JTO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 37.Ma BB, Hui EP, Mok TS.. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol. 2010;11:75–84. doi: 10.1016/S1470-2045(09)70160-3. [DOI] [PubMed] [Google Scholar]
- 38.Wu YL, Zhong W, Wang Q, Xu ST, Mao WM, Wu L. et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): a randomized, Phase III trial (CTONG 1104). [Abstract]. 2017 ASCO Annual Meeting; J Clin Oncol. 2017;35(15):8500. doi: 10.1200/JCO.2017.35.15_suppl.8500. [DOI] [Google Scholar]


