Abstract
Cerebrospinal fluid (CSF) is a fluidic part of the brain’s microenvironment that isolates the brain from the rest of the body. CSF dilutes metabolites from neuronal activities and removes them from the brain. Its production and resorption are regulated dynamically and are central to maintaining brain homeostasis. We discovered that the major CSF source, the choroid plexus (CP), harbors the brain’s strongest circadian clock. Here, we consider some implications of the CP circadian clock for metabolite clearance in the brain. If the circadian clock contributes to timed production of the CSF, its synchronization with sleep timing can maximize clearance efficiency and help prevent neurodegenerative diseases such as Alzheimer’s disease.
Keywords: Choroid plexus, circadian clock, metabolite clearance, neurodegenerative disease
Comment on: Myung J, Schmal C, Hong S, et al. The choroid plexus is an important circadian clock component. Nat Commun. 2018;9:1062. doi:10.1038/s41467-018-03507-2. PubMed PMID: 29540683; PubMed Central PMCID: PMC5852131. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5852131/
The choroid plexus (CP) supplies the cerebrospinal fluid (CSF), and by doing so creates a unique liquid environment that surrounds the brain. The CSF, in addition to providing buoyancy to the brain, is a transporting medium that can remove waste metabolites produced during neuronal activities. The CSF has been known since the time of Hippocrates (c. 460-370 BCE); later, Galen (c. 130-200) described it as the “psychic spirit” (pneuma psychikon), which is distinct from blood, or the “vital spirit” (pneuma zootikon), and René Descartes (1596-1650) speculated that it serves as a hydraulic medium produced by the brain that actuates limb movements. It was not until a century ago that Harvey Cushing identified the CP as the source of the CSF,1 and now we know that it provides the microenvironment for the brain, where all brain cells develop and survive.
Similar to the CSF, the CP is normally regarded, both by neuroscience and by medicine, to only play a minor role. It is not directly involved with mental processing and, except for hydrocephalus, there is no specific disorder due solely to CP abnormality. Nonetheless, interest in the CP is slowly increasing: it is a key component for neural development in the embryo and even in adult neurogenesis. Moreover, it is a major source of signaling molecules, such as cytokines, that can cause global state changes in the brain. These recent discoveries suggest that the CP can be an indirect regulator of neuronal activities and that the CP deserves reappraisal.
The microenvironment of the brain changes in synchrony with the external cycles of day and night. The body maintains its own daily activity through an endogenous timing system composed of ~24-hour (circadian) self-sustained clocks, which are synchronized to an exact 24-hour daily rhythm by various cues. This circadian system allows for an organism’s predictive homeostasis. Physiological rhythms occur in multiple timescales, but most of them are entwined with the circadian rhythm. The circadian rhythm is locked to the short timescale of heart rate variability rhythm2 and it gates the long timescale of reproduction in women.3 In eukaryotic cells, a feedback delay between transcription and translation serves as a fundamental mechanism for the circadian clock. This genetic clock is believed to exist in all cells throughout the body. The transcriptional activator CLOCK-BMAL1 complex initiates the transcription of the core clock proteins PER1 & 2, which bind to CRY1 & 2 in the cytosol. The PER-CRY heterodimers translocate to the nucleus and bind to CLOCK-BMAL1, which dislocates the activator complex from DNA and suppresses transcription. The feedback suppression by the PER-CRY complex is an essential ingredient in this clock circuit, but it is the BMAL1 that acts as a substrate for the feedback interactions and a single-gene knockout of Bmal1 completely silences circadian timekeeping.4 Through coordination by other molecules, the feedback delay is fine-tuned and the levels of expression of these genes (“clock genes”) oscillate over the course of a circadian period.
In the brain, the suprachiasmatic nucleus (SCN), located in the basal hypothalamus, is specialized to function as a reference circadian clock for the whole body. It performs this function by modulating electrical firing rates through day and night. Circadian alterations in neuronal firing activities are not unique to the SCN5,6; nonetheless, it is called the master circadian clock because it receives ambient light signals from the retina, thereby entraining its internal circadian cycle to cohere with the external light-dark cycle. The received view of this entrainment pathway is that the SCN emits these clock signals to the rest of the body in a hierarchical, one-way communication stream.
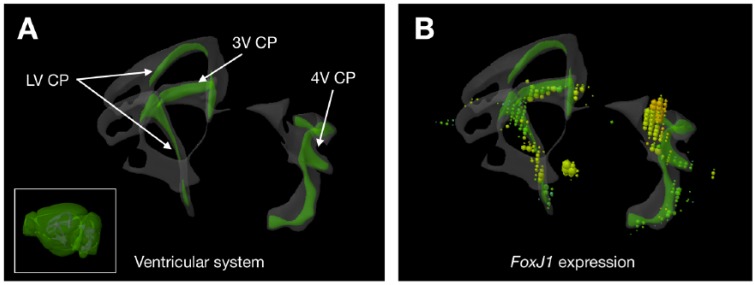
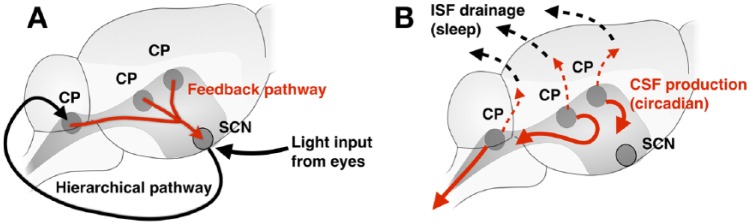
But our work comes as a surprise because it reveals that the communication is, in fact, reciprocal: the CP can also transmit clock signals to the SCN. This was tested in an in vitro system of cocultured CP and SCN explants; we found that in the presence of the CP, the SCN clock accelerated. Next, it was tested in vivo by targeted silencing of Bmal1 expression in the FoxJ1-expressing CP and the ependyma; the transcription factor FOXJ1 regulates development of motile cilia, and its restricted expression in the CP can be visualized from the Allen Mouse Brain Atlas database7 (Figure 1). Although the SCN controls behavioral circadian rhythms expressed through sleep-wake cycles and physiological rhythms of homeostasis, the “ticking” of the SCN clock results from feedback that emanates from the CP clock. This finding does not threaten the “master” role of the SCN clock; rather, it tells us that the reference circadian timing emerges through a process that is, in a sense, “democratic.” In fact, it is a common organizing principle of the neural systems that they have feedback wiring, and the circadian clock system is not an exception (Figure 2A). These brain circadian clocks confer rhythms of homeostasis that occur in a circadian timescale.
Figure 1.
Restricted expression of FoxJ1 in the CP of the mouse brain. (A) The CPs can be found in all of lateral, third, and fourth ventricles (LV, 3V, and 4V). Inset indicates where the CPs are in the whole mouse brain. (B) The transcription factor FoxJ1 is expressed almost exclusively in the CP (expression level represented as spheres on the left hemisphere only). Image credit: Allen Institute. CP indicates choroid plexus.
Figure 2.
Potential new roles for the CP. (A) The circadian clock in the CP provides clock feedback to the master circadian clock, SCN. (B) The circadian clock in the CP likely contributes to rhythmic metabolite clearance in the brain as a sleep-independent process. CP indicates choroid plexus; SCN, suprachiasmatic nucleus.
The strong circadian clock in the CP can imply timed regulation of the CSF production. The CSF has been considered a carrier of waste materials from neurons. It is a dilute medium, containing less than 1/200 protein content compared with blood plasma. This is important for metabolite clearance because the brain lacks a lymphatic system. Recently, it has been proposed that following intense neuronal activity during the wake period, glial cells form a canal around the blood vessel to facilitate clearance of metabolites, known as the paravascular or glymphatic pathway.8 The glymphatic clearance is a sleep-dependent and not circadian clock-dependent process. A vast array of physiological rhythms, however, are under direct control of the circadian clock and not of the sleep-wake cycle. Therefore, it is likely that sleep-independent clearance mechanisms coexist in the brain. As 80% of CSF is produced from the CP, the CP likely plays a key role in this process.
The brain has multiple loci that maintain autonomous circadian rhythmicity. Our research shows that the CP harbors the most robust circadian clock in the mouse brain.9 In our study, strong rhythms were found to be maintained in most of the circumventricular organs (CVOs), which include the CP. This finding is interesting because CVOs are involved with sensing and regulating physiological parameters of water homeostasis in the brain. To engineer homeostasis of the brain environment, production and resorption mechanisms of the CSF must be understood. It is known that compositions of the CSF vary rhythmically in the circadian timescale. The kidney circadian clock controls the circadian changes in osmotic pressure, suggesting that renal water transport is a clock-controlled property.10 Micturition also shows robust circadian rhythmicity and is subject to circadian expression of the gap junction subunit, connexin 43.11 In the CP, the connexin 43-mediated gap junction provides coupling among cellular circadian clocks that synchronizes them and confers robustness to the organ-level clock.
Similarities across these systems suggest a natural hypothesis: CSF production is timed by the CP circadian clock. In the same year that we published this work, Amita Sehgal’s group reported that the permeability of the blood-brain barrier changes circadianly in the fruit fly.12 In as much as the dynamics of CSF production is likely circadian, drainage is also likely to be circadian. What this implies is that the overall homeostasis of the brain’s fluid environment could be under regulation of the circadian clock, in a sleep-independent manner (Figure 2B). Efficient waste clearance requires synchronized CSF production and drainage, i.e., sleep-dependent formation of glymphatic pathway works best when it matches with timing of circadian CSF production. The process can be analogous to the flushing of a toilet: water rushing through the bowl pulls down the waste most efficiently when the sewage pipe is enlarged at the same time. If you skip sleep for one night, even if you later have extended sleep that compensates for the lost sleep time, you still end up with nonrefreshing sleep. The mismatch of sleep timing does have a health consequence and can contribute to a higher mortality risk.13
Circadian rhythms are related to sleep-wake cycles but, importantly, the two are independent processes. The biological circadian rhythms are particularly relevant in medicine as they influence many pathological conditions, such as stroke, asthma, and cardiovascular or metabolic dysfunctions. There is also increasing evidence that some cancers, notably breast cancer, may be linked to circadian disruptions. But, unlike sleep, these biological rhythms are difficult to manipulate. Scheduled application of light, melatonin, and food can entrain or restore these rhythms but not for immediate effects.
One additional thing we discovered about the CP circadian clock is that the clock speed is modulated by the strength of gap junction coupling and a gap junction blocker slows down the clock. This finding suggests the possibility of exploiting this property to manipulate these rhythms such that they can be made to synchronize with sleep rhythms. Internal synchronization of the two rhythms can help maintain normal brain homeostasis that includes metabolite clearance. The CSF production and resorption likely contribute to the clearance system along with the glymphatic pathway. Compelling evidence has shown that the accumulation of amyloid β (Aβ) or senile plaque formation is the earliest change of Alzheimer’s disease (AD) pathophysiology.14 All of the genes responsible to familial AD, including amyloid precursor protein, presenilin-1 and presenilin-2, are involved in the metabolism of Aβ.15 So far, only a few drugs have been approved by the US Food and Drug Administration (FDA) for AD therapy. These medications, however, can only slow down the progression of AD, although their cost-efficiency benefit is positive.16 Amyloid deposition in the brain and its neurotoxicity are considered leading factors of AD pathogenesis and the accumulation of amyloid occurs much earlier than the clinical presentation of cognitive impairments. This finding indicates that clinical intervention should begin before the detrimental effects of amyloid begin to take effect. Therefore, ways to prevent amyloid accumulation and eradication of deposited amyloid are being developed as disease-modifying therapies for AD.17 AD is strongly age dependent, and it often correlates with age-dependent desynchrony between the sleep-wake cycle and internal circadian rhythms.18 The combined action of sleep-dependent and sleep-independent clearance systems is a potential new avenue of research for AD and other neurodegenerative disorders. Coordination of sleep and circadian rhythms (perhaps by modulation of the CP circadian clock) and enhanced amyloid clearance could be critical in reducing the risk of AD.
Acknowledgments
The authors thank Amit Rawal for critical reading.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by Taipei Medical University-Shuang Ho Hospital Collaboration Grant 107TMU-SHH-03 to JM and DW and Taiwan Ministry of Science and Technology Grants 107-2311-B-038-001-MY2 to JM and 104-2420-H-038-001-MY3 to TJL.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JM, DW, VS, and TJL wrote the paper. JM created artwork.
ORCID iD: Jihwan Myung  https://orcid.org/0000-0002-2529-8013
https://orcid.org/0000-0002-2529-8013
References
- 1. Cushing H. Studies on the cerebro-spinal fluid. J Med Res. 1914;31:1–19. [PMC free article] [PubMed] [Google Scholar]
- 2. Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol Regul Integr Comp Physiol. 1994;267:R819–R829. [DOI] [PubMed] [Google Scholar]
- 3. Simonneaux V, Bahougne T. A multi-oscillatory circadian system times female reproduction. Front Endocrinol. 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011;74:175–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. [DOI] [PubMed] [Google Scholar]
- 6. Domínguez-López S, Howell RD, López-Canúl MG, Leyton M, Gobbi G. Electrophysiological characterization of dopamine neuronal activity in the ventral tegmental area across the light-dark cycle. Synapse. 2014:68:454–467. [DOI] [PubMed] [Google Scholar]
- 7. Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. [DOI] [PubMed] [Google Scholar]
- 8. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myung J, Schmal C, Hong S, et al. The choroid plexus is an important circadian clock component. Nat Commun. 2018;9:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hara M, Minami Y, Ohashi M, et al. Robust circadian clock oscillation and osmotic rhythms in inner medulla reflecting cortico-medullary osmotic gradient rhythm in rodent kidney. Sci Rep. 2017;7:7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Negoro H, Kanematsu A, Doi M, et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commun. 2012;3:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell. 2018;173:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort [published online ahead of print April 11, 2018]. Chronobiol Int. doi: 10.1080/07420528.2018.1454458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han SD, Gruhl J, Beckett L, et al. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer’s disease. Brain Imaging Behav. 2012;6:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kennedy JL, Farrer LA, Andreasen NC, Mayeux R, St George-Hyslop P. The genetics of adult-onset neuropsychiatric disease: complexities and conundra? Science. 2003;302:822–826. [DOI] [PubMed] [Google Scholar]
- 16. Fillit H, Hill J. Economics of dementia and pharmacoeconomics of dementia therapy. Am J Geriatr Pharmacother. 2005;3:39–49. [DOI] [PubMed] [Google Scholar]
- 17. Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]