Abstract
Cardiovascular disease (CVD) is the leading cause of death in the world and our approach to the control and management of CVD mortality is limited. Nattokinase (NK), the most active ingredient of natto, possesses a variety of favourable cardiovascular effects and the consumption of Natto has been linked to a reduction in CVD mortality. Recent research has demonstrated that NK has potent fibrinolytic activity, antihypertensive, anti-atherosclerotic, and lipid-lowering, antiplatelet, and neuroprotective effects. This review covers the major pharmacologic effects of NK with a focus on its clinical relevance to CVD. It outlines the advantages of NK and the outstanding issues pertaining to NK pharmacokinetics. Available evidence suggests that NK is a unique natural compound that possesses several key cardiovascular beneficial effects for patients with CVD and is therefore an ideal drug candidate for the prevention and treatment of CVD. Nattokinase is a promising alternative in the management of CVD.
Keywords: Nattokinase, natto, cardiovascular disease, antithrombotic agents, antihypertensive drugs, atherosclerosis
Introduction
Cardiovascular diseases (CVDs) are the most prevalent cause of deaths worldwide. In 2015, the number of CVD-related deaths represented 31% of all deaths globally (www.who.int/cardiovascular_diseases). To date, there are limited approaches available for the control and/or management of CVD-related mortality.1
Natto, a cheese-like food made of soybeans fermented with Bacillus subtilis, has been consumed as a traditional food in Asian countries for more than 2000 years. Natto consumption is believed to be a significant contributor to the longevity of the Japanese population.2 Recent studies demonstrated that a high natto intake was associated with decreased risk of total CVD mortality and, in particular, a decreased risk of mortality from ischaemic heart diseases.2
Before the 1980s, very little was known about the mechanism by which natto consumption led to overall cardiovascular health. In 1987, Sumi et al3 discovered that natto contained a potent fibrinolytic enzyme called nattokinase (NK). Since then, a considerable amount of NK research has been performed on NK in Japan, Korea, China, and the United States, and these studies confirmed that NK, an alkaline protease of 275 amino acid residues with a molecular weight of approximately 28 kDa,4 is the most active ingredient of natto and is responsible for many favourable effects on cardiovascular health. First, NK has potent fibrinolytic/antithrombotic activity.3–6 In addition, in both animal and human studies, NK also has an antihypertensive,7,8 anti-atherosclerotic,9,10 lipid-lowering,9,11 antiplatelet/anticoagulant,12 and neuroprotective actions.13,14 All these pharmacologic actions of NK have relevance to the prevention and treatment of CVD. Indeed, NK supplementation has shown to enhance markers of fibrinolysis and anticoagulation and to decrease blood pressure (BP) and atherosclerosis in human subjects.8,9,15–17
The most unique feature of NK is that, as a single compound, it possesses multiple CVD preventative and alleviating pharmacologic effects (namely, antithrombotic, antihypertensive, anticoagulant, anti-atherosclerotic, and neuroprotective effects). There are no other drugs or drug candidates with multiple pharmacologic properties similar to NK. In addition, NK is a natural product that can be administered orally, has a proven safety profile, is economical to use, and provides distinct advantages over other pharmaceutical products. It therefore has the potential to be developed as a new-generation drug for the prevention, treatment, and long-term care of CVD.7
The present review aims to concisely summarise the key pharmacologic effects and mechanisms of action of NK with a focus on their relevance to, and potential for, the prevention and treatment of CVD. The advantages of NK as an agent for the management of CVD will be outlined. Some remaining issues surrounding NK as a drug candidate for CVD will be critically reviewed and analysed.
Pharmacologic Actions of NK
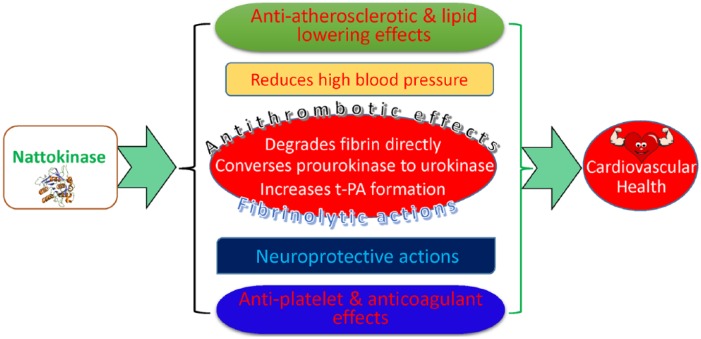
The pharmacologic effects and mechanisms of action of NK will be reviewed and summarised under the following subheadings: fibrinolytic/antithrombotic effects, anti-atherosclerotic and lipid-lowering effects, antihypertensive effects, antiplatelet/anticoagulant effects, and neuroprotective actions as summarised in Figure 1.
Figure 1.
Pharmacologic actions of nattokinase as related to cardiovascular health and disease.
Potent fibrinolytic/antithrombotic effects of NK
Although natto has been consumed in Asia for thousands of years, the fibrinolytic property of NK was only discovered in 1987.3 Since this initial discovery, multiple laboratory and human studies have consistently reported the potent antithrombolytic action of NK.18,19
A considerable amount of work has been performed to evaluate the thrombolytic effects of NK in vitro and in animal models. Using a rat model, Fujita et al5 examined the effect of NK on chemically induced thrombi in the common carotid artery (CCA) and found NK to be 4 times more potent than plasmin in thrombus dissolution. At a concentration of 2836 FU, NK lysed 88% of thrombi within 6 hours,20 and NK exhibited significant prophylactic antithrombotic effects in vivo. The efficacy of NK against thrombosis was confirmed in a carrageenan-induced tail thrombosis model.21 The survival rate of mice with pulmonary thrombosis was increased by NK and the formation of thrombosis in mice was remarkably inhibited by NK, demonstrating significant antithrombotic effects.22 In addition, in a model of rat experimental pulmonary thrombosis, oral administration of NK led to a decrease in thrombus count and plasma euglobulin lysis time (ELT), as well as an increase in tissue plasminogen activator (tPA), indicating that NK is capable of activating plasma fibrinolysis in vivo.23 Omura et al24 further found that a purified protein layer, NKCP, which mainly consisted of NK, had both fibrinolytic and antithrombotic effects, which they described as being similar to that of heparin. In rats, Natto treatment shortened ELT and significantly prolonged partial thromboplastin time compared with a nontreated rat group.25
It is now known that NK not only degrades fibrin directly and effectively but also increases the release of tPA with a subsequent increase in the formation of plasmin.5,17,26–28 Plasminogen activator inhibitor 1 (PAI-1) is the primary inhibitor of tPA and regulates fibrinolytic activity in the fibrinolytic cascade.29 In a study investigating the mechanism by which NK exerted its fibrinolytic effect, NK enhanced fibrinolysis through cleavage and inactivation of PAI-1.4 In this study, NK was shown to cleave active recombinant prokaryotic PAI-1 into low-molecular-weight fragments as well as enhance tissue-type plasminogen activator–induced fibrin clot lysis. The enhanced fibrinolytic activity observed in the absence of PAI-1 appeared to be induced through direct fibrin dissolution by NK.4 Nattokinase also enhanced the production of clot-dissolving agents such as urokinase through the conversion of prourokinase to urokinase.5,19 Furthermore, NK was shown to be capable of blocking thromboxane formation resulting in an inhibition of platelet aggregation without producing the side effect of bleeding.12 Thus, NK was found to be a potent antithrombotic agent, and, by reducing thrombus formation, was able to slow the progression of plaque formation and reverse evolving atherosclerotic lesions.10
Data from human studies also strongly support NK or natto as a potent and promising fibrinolytic agent. In an early human trial, oral administration of NK was shown to produce a gradual enhancement of fibrinolytic activity in the plasma, as indicated by plasma ELT and the production of tPA.6 Following administration of natto bacillus (100 mg/kg) to healthy adult volunteers, ELT was reduced and tPA activity was increased significantly (P < .05).23 In an open-label, self-controlled clinical trial, Hsia et al17 found that after 2 months of administration, fibrinogen, factor VII, and factor VIII levels were decreased significantly implying a promising cardiovascular benefit from NK administration. Even after a single dose of oral NK at 2000 FU, blood fibrin/fibrinogen degradation product levels were significantly increased 4 hours after NK administration (P < .05), confirming elevation of thrombolysis and anticoagulation profiles.16 Hsia’s study supported NK as a useful fibrinolytic/anticoagulant agent to reduce the risk of thrombosis and CVD in humans.
Thrombosis is a common pathology underlying ischaemic heart disease, ischaemic stroke, and venous thromboembolism.30 Thrombolytic/fibrinolytic therapy is used in myocardial infarction, cerebral infarction, and, on occasion, in massive pulmonary embolism. Fibrinolytic therapy can, in many circumstances, reduce mortality. As a result, the use of such treatments has increased rapidly in recent years.31 Commonly used commercial thrombolytic agents, such as plasminogen activators, streptokinase, and anisoylated plasminogen streptokinase-activator complex, all come with serious drawbacks and adverse effects. These thrombolytic drugs are usually expensive, have a short half-life after intravenous administration, and lead to uncontrollable acceleration of fibrinolysis and haemorrhaging. To overcome these risks, a safer thrombolytic agent such as NK is needed for the treatment of thrombolytic disorders or CVD.5
Anti-atherosclerotic and lipid-lowering effects of NK
The underlying pathological change shared by many CVDs is atherosclerosis, which is the primary cause of heart disease and stroke.32 Therefore, drugs that have anti-atherosclerotic effects would have broad clinical relevance; however, these types of drugs are difficult to come by. Nattokinase would appear to be one of those drugs that have such promising anti-atherosclerotic and lipid-lowering effects.
A number of animal studies exploring NK as an anti-atherosclerotic agent have demonstrated that dietary natto extract supplementation suppresses intimal thickening in rats when compared with the control group.10,33 The suppression of intimal thickening after vascular injury may be attributable to the enhanced thrombolytic activities of NK.10,33 There was a striking difference in intimal thickening between the control group and natto fed group where the animals were fed the natto extract or NK for 3 weeks before the injury. Both NK and the natto extract suppressed intimal thickening in rats with endothelial injury. Although Chang et al34 believed that the natto extract suppressed intimal thickening through a synergistic effect attributed to its antioxidant and antiapoptotic properties, other study demonstrated that NK prevented arteriosclerosis by its direct antioxidant effect leading to reduced lipid peroxidation and improved lipid metabolism (inhibition of low-density lipoprotein [LDL] oxidation).35 When used in combination with red ginseng, NK was found to reduce aortic plaque area in hypercholesterol diet–fed rabbits.36
In a recent clinical study conducted in our laboratory,9 daily NK supplementation was an effective way to suppress the progression of atherosclerosis in patients with atherosclerotic plaques. Following NK treatment for 26 weeks, there was a significant reduction in CCA intima-media thickness (CCA-IMT) and carotid plaque size when compared with the baseline before treatment. The carotid plaque size and CCA-IMT reduced from 0.25 ± 0.12 cm2 to 0.16 ± 0.10 cm2 and from 1.13 ± 0.12 mm to 1.01 ± 0.11 mm, respectively. The reduction in the NK group was more significant (P < .01) than that in the group treated with simvastatin (daily dose of 20 mg). Our data suggested that NK was a better alternative to statins, a commonly used drug to reduce atherosclerosis, and furthermore, NK could be a viable alternative therapy for cardiovascular attack and stroke in patients.9
The underlying mechanisms by which NK suppresses atherosclerosis are not known. Early studies indicated that NK enhanced thrombolytic activities.10,33 Available data suggest that the anti-atherosclerotic effect of NK is due to the collective effects of the combination of antithrombotic, anticoagulant, antioxidant, and lipid-lowering effects of NK or natto extract containing NK.9,35–37
In addition to its anti-atherosclerotic effects, NK or natto extract also has a favourable effect on lipids. Using NK or natto extract containing NK, studies from various laboratories25,36–41 confirmed that NK has a hypolipidaemic effect and can significantly reduce the increased serum triglycerides, total cholesterol, and LDL cholesterol (LDL-C) levels in animal models. Our studies found that in patients with hyperlipidaemia, NK treatment (26 weeks at 6500 FU) reduced total cholesterol, LDL-C, and triglycerides. In addition, NK increased the level of high-density lipoprotein cholesterol (HDL-C).9 However, in a small pilot study, Wu et al42 observed a decrease in serum cholesterol, LDL-C, and HDL-C in the NK treatment group following 8 weeks of treatment at a dose of 4000 FU, although the difference was not statistically significant. The insignificant change in serum lipid level observed by Wu et al may be related to the relatively low dosages used and a shorter treatment period. Considering the dosages used in the laboratory studies, showing that hypolipidaemic effects were quite high (eg, Xie et al38 used a daily dose between 90 and 360 mg/kg in their rats, whereas Wu et al used 400 mg daily in their patients), the lipid-lowering action of NK possibly requires a relatively higher dose or longer treatments.
Antihypertensive effects of NK
Natto has been observed to have a beneficial effect on CVDs by lowering BP. In an in vitro study, it was found that natto contained relatively strong inhibitors that suppress angiotensin-converting enzyme (ACE), a key enzyme responsible for the production of a hypertensive peptide hormone, angiotensin II, in the renin-angiotensin system.43
In 2008, Kim et al8 conducted the first randomised double-blinded placebo-controlled trial to investigate the effects of NK supplementation on BP in prehypertension or stage 1 hypertension subjects. Oral administration of NK for 8 weeks resulted in a reduction of both systolic and diastolic BP (net changes were −5.55 and −2.84 mm Hg, respectively, P < .05). The results suggested that NK could play a role in the prevention and treatment of hypertension.8 Very recently, a study by Jensen et al15 suggested that NK consumption for 8 weeks was associated with beneficial changes to BP in patients having hypertension. This is consistent with several laboratory studies demonstrating effective reduction of BP in spontaneous hypertensive rats by NK administration.7,44–47
The mechanism by which NK decreased BP in hypertensive conditions is not clear. Jensen et al15 found that the decline of BP in hypertensive patients in their study was independent of plasma renin activity and the role of ACE in the antihypertensive action of NK is somewhat controversial. Although studies in humans showed that ACE concentrations did not demonstrate a statistically significant difference in patients receiving NK treatment,8 other studies using animal models suggested that the antihypertensive action of NK is related to the inhibition of angiotensin I–converting enzyme.46 In a study using spontaneously hypertensive rats, NK and its fragments reduced hypertension by different mechanisms.7 Nattokinase may decrease BP through cleavage of fibrinogen in plasma, whereas the fragments of NK potentially prevented the elevation of plasma angiotensin II level to suppress hypertension in rats.7 Ibe et al47 found that natto significantly decreased BP 4 hours after oral administration in spontaneously hypertensive rats and the ACE inhibition of natto was dose dependent. Natto extracts from B subtilis–fermented pigeon pea also significantly increased ACE inhibitor activity in hypertensive rats.44
Given the significant side effects associated with the long-term use of antihypertensive drugs,48 NK could be a promising alternative for the management of hypertension in patients with CVD. Thus, daily use of NK could be a successful strategy for the treatment of hypertension.45
Antiplatelet/anticoagulant effects of NK
Low-dose aspirin (85-100 mg daily), as a potent anticoagulant agent, is widely used for the prevention of heart attacks, stroke, and atherothrombotic diseases. Aspirin exerts its antiplatelet action by inhibiting cyclooxygenase (COX) and subsequently reducing the synthesis of thrombogenic thromboxane A2 (TXA2) in platelets. However, the long-term use of aspirin comes with serious gastrointestinal (GI) side effects and bleeding.49 In a study comparing the antiplatelet effects of NK and aspirin, NK was shown to display excellent antiplatelet aggregation and antithrombotic activities in vitro and in vivo, inhibiting thromboxane B2 formation from collagen-activated platelets.12 Wang et al50 found that NK decreased fibrinogen levels in a cerebral ischaemic model and concluded that this was mediated by a pathway similar to that of aspirin. Natto showed an excellent inhibitory effect on platelet aggregation induced by adenosine 5ʹ diphosphate and collagen.25 In addition, NK was found to have positive in vitro haemorheological effects by decreasing red blood cell aggregation and low-shear viscosity.51
All the above data suggest that NK could be a good candidate, without any obvious adverse effects, for the improvement of blood flow and possibly superior to aspirin.
Neuroprotective effects of NK
Nattokinase is capable of degrading amyloid fibrils at neutral pH and normal body temperature, suggesting a role in the treatment of amyloid-related diseases such as Alzheimer disease (AD).52 The ability of NK to dissociate amyloid suggested that NK was a potential drug candidate for amyloid-related disorders and this was confirmed in a recent study involving both in vivo and in vitro models.53 Oral administration of NK in the rat model of AD demonstrated a positive effect in modulation of specific factors in the AD pathway.13 In a rat model of cognitive deficits of AD induced by intoxification of colchicine, nano-nutraceuticals containing NK were demonstrated to enhance the impaired learning and memory capability and to be effective inhibitors in the suppression of amyloid-β and BACE-1 activity, thus suggesting a neuroprotective efficacy of NK.54 Ahmed et al55 demonstrated that NK, at a dose of 360 FU/kg, significantly decreased cholinesterase activity, TGF-β, IL-6, and p53 levels accompanied by a significant increase in Bcl-2 levels as compared with an untreated AD control group. Their data suggested that the neuroprotective effect of NK was due to its proteolytic, anti-inflammatory, and antiapoptotic effects.
In one of the early clinical studies describing the role of NK in the prevention of stroke progression in patients with acute ischaemic stroke, the authors demonstrated a clear neuroprotective effect in patients.56 The neuroprotective effects of NK were demonstrated in a photothrombotic stroke mouse model. Ahn et al57 suggested that the neuroprotective effect in the ischaemic brain was induced through improved blood flow by inhibiting platelet aggregation and thrombus formation by NK, as reported in a conference abstract. Oral intake of NK was also shown to confer neuroprotective effects against focal cerebral ischaemia as evidenced by reduced infarct volume in gerbils through enhanced fibrinolytic activity.50 Regarding the mechanism by which NK protects the brain in ischaemic stroke, a study by Ji et al14 showed that the neuroprotective effect of NK was associated with its antiplatelet activity, antiapoptotic effect, its ability to relax vascular smooth muscle, and its protection of endothelial cells through increased fibrinolytic activity and facilitating spontaneous thrombolysis.
Cardiovascular health is closely linked to brain health. There is a strong causal association between CVD risk factors with the incidence of cognitive decline and AD.58 Therefore, neuroprotective effects of drugs used in patients with CVD have great potential and would add benefits to patients’ overall outcome. Despite more than 100 neuroprotective agents demonstrating neuroprotective effects on focal ischaemic stroke in recent preclinical studies, none has proven to be beneficial in clinical studies.59 Hence, more effective drugs, with proven neuroprotective actions, for the management of cerebrovascular diseases, such as ischaemic heart disease and stroke, are urgently needed.
Clinical Human Studies of NK
Although NK has recently gained popularity as a candidate drug for CVD, clinical investigations of NK in humans are relatively limited. The first NK clinical study conducted in 1990 aimed to assess its fibrinolytic activity in healthy subjects after oral administration.6 A further Japanese study examined the efficacy of NK in the prevention of stroke progression in patients with acute ischaemic stroke and showed clear beneficial effects in these patients after oral administration.56 As shown in Table 1, there are no more than 10 published clinical studies covering the use of NK or the natto extract. Other related NK clinical studies include investigations of antihypertensive effects in patients in North America15 and Asia.8 Further studies also include anti-atherosclerotic and lipid-lowering effects,9,42 fibrinolytic activity and effects on coagulation,6,16 and pharmacokinetics60 and toxicology61 in humans. The low number of NK clinical studies is probably related to the fact that NK is not registered as a drug, but, as a nutritional supplement, and, to date, clinical evidence has not been adequately conclusive.
Table 1.
List of published clinical studies on NK.
| Year | Location of study | Size of study | Clinical condition observed | Summary of findings | References |
|---|---|---|---|---|---|
| 1990 | Japan | 12 | Fibrinolytic activity | 3× NK daily oral administration resulted in enhanced fibrinolytic activity in the plasma and production of tissue plasminogen activator | Sumi et al6 |
| 2004 | Japan | 24 | Ischaemic stroke | NK demonstrated a clear neuroprotective effect in patients with acute ischaemic stroke | Shah et al56 |
| 2008 | Korea | 86 | Hypertension | NK supplementation resulted in a reduction in both systolic and diastolic BP (P < .05) | Kim et al8 |
| 2009 | Taiwan | 45 | Blood coagulation factors | 2 mo of NK treatment significantly decreased fibrinogen, factor VII, and factor confirming a promising cardiovascular benefit | Hsia et al17 |
| 2009 | Taiwan | 30 | Hyperglycaemia | A decrease in serum cholesterol, LDL-C, and HDL-C in the NK group was observed following 8 wk of treatment (4000 FU), but the difference was not statistically significant | Wu et al42 |
| 2013 | USA | 11 | Pharmacokinetics | NK can be measured directly in the human blood after single dosing. Serum levels of NK peaked at approximately 13.3 h ± 2.5 h | Ero et al60 |
| 2015 | Japan | 12 | Thrombolysis and anticoagulation | Blood fibrin/fibrinogen degradation products (thrombolysis and anticoagulation profile) were significantly increased 4 h after NK administration following a single dose of 2000 FU (P < .05), supporting NK as a useful fibrinolytic/anticoagulant agent to reduce the risk of thrombosis and CVDs in humans | Kurosawa et al16 |
| 2016 | USA | 79 | Hypertension and von Willebrand factor | NK consumption for 8 wk led to beneficial changes to BP in hypertensive patients. A decrease in vWF was seen in the female population consuming NK | Jensen et al15 |
| 2016 | USA | 11 | Toxicology/toxicity | NK consumption of 10 mg/kg/day for 4 wk was well tolerated in healthy human volunteers suggesting that the oral consumption of NK is of low toxicological concern | Lampe and English61 |
| 2017 | China | 76 | Atherosclerosis and hyperglycaemia | Daily NK treatment (6500 FU for 26 wk) effectively suppressed the progression of atherosclerosis in patients with atherosclerotic plaques by reducing CCA-IMT and carotid plaque size significantly. NK treatment reduced total cholesterol, LDL-C, and triglyceride and increased HDL-C in hyperlipidaemic patients | Ren et al9 |
Abbreviations: BP, blood pressure; CCA-IMT, common carotid artery; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NK, nattokinase.
It should be pointed out that clinical.gov has, to date, published around 10 clinical trials of NK or natto with the most recent one, entitled “Nattokinase Atherothrombotic Prevention Study” (NCT02080520) being currently conducted by a team of researchers at the University of Southern California (USC; details can be found in https://clinicaltrials.gov). Among these studies, the one from USC (NCT02080520) is a considerably large-sized phase 2 clinical trial and we are awaiting the outcomes of this trial.
More human studies are required to ascertain the efficacy of NK, the indications for its use, and possible drug interactions when used concurrently with other drugs used to treat patients with CVD.
Advantages of NK
As stated above, the advantages of NK include a proven safety profile with a long history of human consumption, the convenience of oral administration (many antithrombotic drugs are injectable), ease of mass production, and it possesses multiple key favourable cardiovascular effects.
The fact that multiple clinical trials on NK have been conducted, or approved to be conducted, provides a good indication of the safety of administration in human subjects. Recent toxicology studies (both in vivo and in vitro) have provided strong corroboration of the safety of oral consumption of NK and these studies concluded that, to-date, there is no toxicologic concern for NK human consumption.61,62 The safety of NK has been comprehensively evaluated in several Good Laboratory Practice (GLP)-compliant studies in rodents and in human volunteers.61 Nattokinase was found to be nonmutagenic and nonclastogenic in vitro, and no adverse effects were observed in 28-day and 90-day subchronic toxicity studies conducted in rats at doses up to 1000 mg/kg/day, which is 100 times higher than the usual dose used in humans. In human volunteers, no adverse effects were observed following 4 weeks of NK consumption at a daily dose of 10 mg/kg for 28 days.61 No sign of toxicity was observed in the Ames test, the bone marrow cell micronucleus tests, or the mouse sperm abnormality tests following NK treatment. In an acute toxicology study conducted by Fu et al,62 no sign of toxicity was observed at daily doses of NK of up to 4 g/kg for a period of 30 days. All these data collectively confirm NK to be a safe product with very little or no toxicologic concerns.
In Japan, China, Korea, and Western countries including Australia, United States, Canada, and Europe, NK products are widely used in the form of tablets or capsules as nutritional supplements for health promotion including blood thinning, blood clot prevention, and improved circulation. The cost of NK supplements is relatively inexpensive, with the cost of daily doses of 200 to 400 mg being approximately US $0.3 to US $0.8 depending on the source of the product. With the advancement of NK production and purification technologies, it is expected that the cost of NK will continue to be low, making it an economically viable drug.28
One of the great advantages of NK is that, as an orally active compound, it possesses many key favourable cardiovascular effects for patients with CVD. Nattokinase has the potential to streamline drug use and to improve patient compliance in CVD prevention and treatment.
Remaining Issues
One key question that has not been adequately addressed is the mechanism by which NK is absorbed into the bloodstream after oral administration. There is no current convincing data available to demonstrate the bioavailability and metabolism of NK administered as an oral dose. The only related study is one showing that, in rats, intraduodenal administration of NK, at a dose of 80 mg/kg leads to degradation of fibrinogen in plasma.63 It should be noted that the NK used was not subjected to digestive gastric fluids and the dose was high in pharmacologic terms. Thus, the relevance of the data is questionable. Recent pilot studies on the pharmacokinetics of NK have not provided an answer to the question of how NK is absorbed into the body.16,60 In fact, the pharmacokinetic data are rather inconsistent and mismatched with pharmacodynamic activities. Ero et al60 claimed that NK was measured directly in their study with a peak concentration at 13.3 hours after oral administration, whereas the thrombolytic activity of NK, measured in another study, was shown to peak 2 to 4 hours after oral dosing.16 These data imply that measurements of NK in the published studies may not be those of the intact NK molecule. Although it has been assumed that NK is stable in the GI tract19,28, it is important to address the key question of the mechanism of NK absorption into the body, with more convincing direct evidence. In fact, several groups have pointed out that the molecular size of NK is considered to be generally too large for oral absorption through the GI tract.19 It is recognised that NK may be susceptible to chemical oxidation and subsequent inactivation, or denaturation, in the GI tract.64 Thus, further studies are required to fully understand the pharmacokinetics of NK.
Potential drug interactions and contraindications to the use of NK in humans are not currently known. There is one report of a patient concurrently using aspirin and NK (400 mg daily), experiencing an acute cerebellar haemorrhage.65 In addition, multiple microbleeds were demonstrated on brain magnetic resonance images suggesting that NK may increase risk of intracerebral haemorrhage in patients who have bleeding-prone cerebral microangiopathy and are taking aspirin concurrently.65 In another report, a patient developed a thrombus in a mechanical valve after nearly a year of NK use without warfarin and underwent a successful repeat valve replacement.66 Use of multiple drugs concurrently is common in patients with CVD. Therefore, it is particularly important to elucidate the potential possible interactions of NK with other drugs acting on the cardiovascular system.
Given the fact that serine proteases, currently in clinical use for stroke patients, have been implicated in tumour growth and metastasis,67,68 it is necessary to design studies to evaluate the potential carcinogenic effects, and effects on cell invasiveness, of NK use in in vitro and in vivo model systems, and to ascertain the presence of any cardiotoxicity associated with this compound.
Concluding Remarks
In recent years, NK has gained popularity due to its beneficial cardiovascular health effects. Research defining the pharmacologic effects of NK and refining the production and purification of NK is increasing. It is particularly exciting to observe that every year contemporary research on NK continues to find more positive and favourable clinical benefits resulting from NK use. For example, NK was recently demonstrated to effectively reduce nasal polyp tissue through fibrin degradation,69 indicating its potential in the treatment of chronic rhinosinusitis. The emerging picture of NK is one of the unique natural compounds with many favourable health effects, especially for patients with CVD. Importantly, NK simultaneously effects several key favourable benefits for thrombosis, hypertension, atherosclerosis, hyperlipidaemia, platelet aggregation, and neuroprotection in patients with CVD. These multiple benefits to patients make the role of NK very unique in the prevention and treatment of CVD with reduced side effects commonly associated with conventional CVD drugs. In addition to the unique multiple functions of NK, other advantages to the patient include ease of oral administration, a robust safety profile and a long in vivo half-life.
In summary, compared with traditional antithrombotic and antihypertensive drugs, NK is characterised by high safety, low cost, simple production process, oral availability, and long in vivo half-life. As such, it is expected to become a new-generation drug for thrombotic disorders or CVDs. Although human trials and clinical studies demonstrate the clinical benefits of NK in the clinical settings, there remain a number of limitations. However, all the available data are encouraging and promising and further clinical trials are needed to fully examine the prospect of NK as an alternative medication to tPA, aspirin, warfarin, or newer anticoagulants in the management of CVD. In the near future, it is possible that patients with CVD may need only a single NK pill to replace multiple drugs administered for the prevention and management of CVD, including tPA, antihypertensives, statins, aspirin, and warfarin.
There are a few obstacles that remain to be overcome for NK to become more widely accepted as a drug, or drug candidate, for CVDs and these include establishment of pharmacokinetic evidence of the absorption and metabolism of NK in humans and the requirement for extensive studies to elucidate potential drug interactions between NK and other cardiovascular drugs, which are commonly, and concurrently, used in the prevention, treatment, and management of CVD in affected patients.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: YL conceived and designed the content and structure of the review; HC, EM, and YL drafted, revised, and edited the review; NR, SL, NN, FS, and XQ revised and edited the manuscript.
Disclosures and Ethics: The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material.
References
- 1. Wu Y, Benjamin EJ, MacMahon S. Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation. 2016;133:2545–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagata C, Wada K, Tamura T, et al. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Am J Clin Nutr. 2017;105:426–431. [DOI] [PubMed] [Google Scholar]
- 3. Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43:1110–1111. [DOI] [PubMed] [Google Scholar]
- 4. Urano T, Ihara H, Umemura K, et al. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276:24690–24696. [DOI] [PubMed] [Google Scholar]
- 5. Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. 1995;18:1387–1391. [DOI] [PubMed] [Google Scholar]
- 6. Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinases. Acta Haematol. 1990;84:139–143. [DOI] [PubMed] [Google Scholar]
- 7. Fujita M, Ohnishi K, Takaoka S, Ogasawara K, Fukuyama R, Nakamuta H. Antihypertensive effects of continuous oral administration of nattokinase and its fragments in spontaneously hypertensive rats. Biol Pharm Bull. 2011;34:1696–1701. [DOI] [PubMed] [Google Scholar]
- 8. Kim JY, Gum SN, Paik JK, et al. Effects of nattokinase on blood pressure: a randomized, controlled trial. Hypertens Res. 2008;31:1583–1588. [DOI] [PubMed] [Google Scholar]
- 9. Ren N, Chen H, Li Y, McGowan E, Lin Y. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Nat Med J China. 2017;97:2038–2042. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki Y, Kondo K, Matsumoto Y, et al. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci. 2003;73:1289–1298. [DOI] [PubMed] [Google Scholar]
- 11. Duan Z, Jiang X, Jiang H, Zhang S, Dong M, Zhao X. Study on the antioxidative activity and effects on experimental hyperlipidemia of natto extract. Acta Nutrimenta Sinica. 2003;26:296–299. [Google Scholar]
- 12. Jang JY, Kim TS, Cai J, et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab Anim Res. 2013;29:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fadl N, Ahmed H, Booles H, Sayed A. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum Exp Toxicol. 2013;32:721–735. [DOI] [PubMed] [Google Scholar]
- 14. Ji H, Yu L, Liu K, et al. Mechanisms of nattokinase in protection of cerebral ischemia. Eur J Pharmacol. 2014;745:144–151. [DOI] [PubMed] [Google Scholar]
- 15. Jensen GS, Lenninger M, Ero MP, Benson KF. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr Blood Press Control. 2016;9:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurosawa Y, Nirengi S, Homma T, et al. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci Rep. 2015;5:11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsia C-H, Shen M-C, Lin J-S, et al. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res. 2009;29:190–196. [DOI] [PubMed] [Google Scholar]
- 18. Tai M-W, Sweet BV. Nattokinase for prevention of thrombosis. Am J Health Syst Pharm. 2006;63:1121–1123. [DOI] [PubMed] [Google Scholar]
- 19. Milner M, Makise K. Natto and its active ingredient nattokinase: a potent and safe thrombolytic agent. Alternat Complement Therap. 2002;8:157–164. [Google Scholar]
- 20. Wang P, Chen J, Chen H. Purification and in vitro thrombolytic effects of nattokinase. Chin Pharm J. 2005;39:1669–1671. [Google Scholar]
- 21. Kamiya S, Hagimori M, Ogasawara M, Arakawa M. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta Haematol. 2010;124:218–224. [DOI] [PubMed] [Google Scholar]
- 22. Yang M, Mei Y, Liang Y. Effect of nattokinase extraction on anti-thrombosis function. Food Sci Technol. 2013;38:197–200. [Google Scholar]
- 23. Sumi H, Yanagisawa Y, Yatagai C, Saito J. Natto Bacillus as an oral fibrinolytic agent: nattokinase activity and the ingestion effect of Bacillus subtilis natto. Food Sci Technol Res. 2004;10:17–20. [Google Scholar]
- 24. Omura K, Hitosugi M, Zhu X, Ikeda M, Maeda H, Tokudome S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J Pharmacol Sci. 2005;99:247–251. [DOI] [PubMed] [Google Scholar]
- 25. Park KJ, Kang JI, Kim TS, Yeo IH. The antithrombotic and fibrinolytic effect of natto in hypercholesterolemia rats. Prev Nutr Food Sci. 2012;17:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yatagai C, Maruyama M, Kawahara T, Sumi H. Nattokinase-promoted tissue plasminogen activator release from human cells. Pathophysiol Haemost Thromb. 2009;36:227–232. [DOI] [PubMed] [Google Scholar]
- 27. Weng Y, Yao J, Sparks S, Wang KY. Nattokinase: an oral antithrombotic agent for the prevention of cardiovascular disease. Int J Mol Sci. 2017;18:E523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabbagh F, Negahdaripour M, Berenjian A, et al. Nattokinase: production and application. Appl Microbiol Biotechnol. 2014;98:9199–9206. [DOI] [PubMed] [Google Scholar]
- 29. Tjarnlund-Wolf A, Brogren H, Lo EH, Wang X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke. 2012;43:2833–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. [DOI] [PubMed] [Google Scholar]
- 31. Trialists FT. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. The Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 32. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki Y, Kondo K, Ichise H, Tsukamoto Y, Urano T, Umemura K. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition. 2003;19:261–264. [DOI] [PubMed] [Google Scholar]
- 34. Chang C-H, Chen K-T, Lee T-H, et al. Effects of natto extract on endothelial injury in a rat model. Acta Medica Okayama. 2010;64:399–406. [DOI] [PubMed] [Google Scholar]
- 35. Iwai K, Nakaya N, Kawasaki Y, Matsue H. Antioxidative functions of natto, a kind of fermented soybeans: effect on LDL oxidation and lipid metabolism in cholesterol-fed rats. J Agric Food Chem. 2002;50:3597–3601. [DOI] [PubMed] [Google Scholar]
- 36. Kang S-J, Lim Y, Kim A-J. Korean red ginseng combined with nattokinase ameliorates dyslipidemia and the area of aortic plaques in high cholesterol-diet fed rabbits. Food Sci Biotechnol. 2014;23:283–287. [Google Scholar]
- 37. Meng F, Xue F, Shi H. Effects of nattokinase on blood lipid and blood rheology in atherosclerosis model rat. Chin J Lab Diagn. 2013;17:1567–1569. [Google Scholar]
- 38. Xie S, Yu Z, Liu X. Preparation of nattokinase and study on its hypolipidemic effect. Chin J Biochem Pharm. 2015;35:17–20. [Google Scholar]
- 39. Zhang Y, Hu X-F, Li W-P. Effects of nattokinase extraction on experimental hyperlipidemia of rabbits. Chin J Trauma Disabil Med. 2010;4:49–51. [Google Scholar]
- 40. Yuan S. Effect of nattokinase on reducing serum lipid in experimental hyperlipidemia rats. Modern Hospitals. 2005;5:10–12. [Google Scholar]
- 41. Duan Z, Jiang X, Jiang H, Zhang S, Dong M, Zhao X. Study on the antioxidative activity and effects on experimental hyperlipidemia of natto extract. Acta Nutrimenta Sinica. 2004;26:296–299. [Google Scholar]
- 42. Wu D-J, Lin C-S, Lee M-Y. Lipid-lowering effect of nattokinase in patients with primary hypercholesterolemia. Acta Cardiologica Sinica. 2009;25:26–30. [Google Scholar]
- 43. Okamoto A, Hanagata H, Kawamura Y, Yanagida F. Anti-hypertensive substances in fermented soybean, natto. Plant Foods Hum Nutr. 1995;47:39–47. [DOI] [PubMed] [Google Scholar]
- 44. Lee B-H, Lai Y-S, Wu S-C. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J Food Drug Anal. 2015;23:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suwanmanon K, Hsieh P-C. Effect of γ-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar–Kyoto rats. J Food Drug Anal. 2014;22:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murakami K, Yamanaka N, Ohnishi K, Fukayama M, Yoshino M. Inhibition of angiotensin I converting enzyme by subtilisin NAT (nattokinase) in natto, a Japanese traditional fermented food. Food Funct. 2012;3:674–678. [DOI] [PubMed] [Google Scholar]
- 47. Ibe S, Yoshida K, Kumada K, Tsurushiin S, Furusho T, Otobe K. Antihypertensive effects of natto, a traditional Japanese fermented food, in spontaneously hypertensive rats. Food Sci Technol Res. 2009;15:199–202. [Google Scholar]
- 48. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community. J Clin Hypertens. 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J-M, Chen H-Y, Cheng S-M, Chen S-H, Yang L-L, Cheng F-C. Nattokinase reduces brain infarction, fibrinogen and activated partial thromboplastin time against cerebral ischemia-reperfusion injury. J Food Drug Anal. 2012;3:686–691. [Google Scholar]
- 51. Pais E, Alexy T, Holsworth J, Ralph E, Meiselman HJ. Effects of nattokinase, a pro-fibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity. Clin Hemorheol Microcirc. 2006;35:139–142. [PubMed] [Google Scholar]
- 52. Hsu R-L, Lee K-T, Wang J-H, Lee LY, Chen RP. Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J Agric Food Chem. 2008;57:503–508. [DOI] [PubMed] [Google Scholar]
- 53. Metkar SK, Girigoswami A, Murugesan R, Girigoswami K. In vitro and in vivo insulin amyloid degradation mediated by Serratiopeptidase. Mater Sci Eng C Mater Biol Appl. 2017;70:728–735. [DOI] [PubMed] [Google Scholar]
- 54. Bhatt PC, Pathak S, Kumar V, Panda BP. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology. 2017;26:105–118. [DOI] [PubMed] [Google Scholar]
- 55. Ahmed HH, Nevein NF, Karima A, Hamza AH. Miracle enzymes serrapeptase and nattokinase mitigate neuroinflammation and apoptosis associated with Alzheimer’s disease in experimental model. WJPPS. 2013;3:876–891. [Google Scholar]
- 56. Shah AB, Rawat S, Mehta S. An open clinical pilot study to evaluate the safety and efficacy of natto kinaseas an add-on oral fibrinolytic agent tolow molecular weight heparin & anti-platelets in acute ischaemic stroke. Japan Pharmacol Therap. 2004;32:437–451. [Google Scholar]
- 57. Ahn Y-J, Kim MH, Kim J, et al. Neuroprotective effect of nattokinase mediated by inhibition of platelet aggregation and thrombosis in photothrombotic stroke. Stroke. 2015;46:APW262. [Google Scholar]
- 58. Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260:211–223. [DOI] [PubMed] [Google Scholar]
- 59. Kaur H, Prakash A, Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology. 2013;92:324–334. [DOI] [PubMed] [Google Scholar]
- 60. Ero MP, Ng CM, Mihailovski T, Harvey NR, Lewis BH. A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern Ther Health Med. 2013;19:16–19. [PubMed] [Google Scholar]
- 61. Lampe BJ, English JC. Toxicological assessment of nattokinase derived from Bacillus subtilis var. natto. Food Chem Toxicol. 2016;88:87–99. [DOI] [PubMed] [Google Scholar]
- 62. Fu Y-S, Li Y-L, Zhang Y. Toxicological safety assessment on safety of nattokinase capsule. Prac Prev Med. 2012;19:1714–1716. [Google Scholar]
- 63. Fujita M, Hong K, Ito Y, et al. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995;18:1194–1196. [DOI] [PubMed] [Google Scholar]
- 64. Weng M, Zheng Z, Bao W, Cai Y, Yin Y, Zou G. Enhancement of oxidative stability of the subtilisin nattokinase by site-directed mutagenesis expressed in Escherichia coli. Biochim Biophys Acta. 2009;1794:1566–1572. [DOI] [PubMed] [Google Scholar]
- 65. Chang YY, Liu JS, Lai SL, Wu HS, Lan MY. Cerebellar hemorrhage provoked by combined use of nattokinase and aspirin in a patient with cerebral microbleeds. Intern Med. 2008;47:467–469. [DOI] [PubMed] [Google Scholar]
- 66. Elahi MM, Choi CH, Konda S, Shake JG. Consequence of patient substitution of nattokinase for warfarin after aortic valve replacement with a mechanical prosthesis. Proc (Bayl Univ Med Cent). 2015;28:81–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother. 2013;67:179–182. [DOI] [PubMed] [Google Scholar]
- 68. Placencio VR, DeClerck YA. Plasminogen activator inhibitor-1 in cancer: rationale and insight for future therapeutic testing. Cancer Res. 2015;75:2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Takabayashi T, Imoto Y, Sakashita M, et al. Nattokinase, profibrinolytic enzyme, effectively shrinks the nasal polyp tissue and decreases viscosity of mucus. Allergol Int. 2017;66:594-602. [DOI] [PubMed] [Google Scholar]