Abstract
Cerebrovascular reactivity (CVR) reflects the response of brain blood vessels to vasoactive stimuli, such as neural activity. The current research assessed age-related changes in regional CVR to 5% CO2 inhalation in younger (n = 30, range: 21-45 years) and older (n = 29, range: 55-75 years) adults, and the contribution of regional CVR to cognitive performance using blood-oxygen-level dependent contrast imaging (BOLD) functional magnetic resonance imaging (fMRI) at 3T field strength. CVR was measured by inducing hypercapnia using a block-design paradigm under physiological monitoring. Memory and attention were assessed with a comprehensive computerized aging battery. MRI data analysis was conducted using MATLAB® and SPM12. Memory and attention performance was positively associated with CVR in the temporal cortices. Temporal lobe CVR influenced memory performance independently of age, gender, and education level. When analyzing age groups separately, CVR in the hippocampus contributed significantly to memory score in the older group and was also related to subjective memory complaints. No associations between CVR and cognition were observed in younger adults. Vascular responsiveness in the brain has consequences for cognition in cognitively healthy people. These findings may inform other areas of research concerned with vaso-protective approaches for prevention or treatment of neurocognitive decline.
Keywords: Cerebrovascular reactivity, cognition, healthy aging, magnetic resonance imaging (MRI), memory
Introduction
Maintaining good cognitive health is of critical importance in a time where there are increasing numbers of older individuals. Aging is associated with alterations in the structure and function of the brain and the vascular system that supports it. There is growing evidence that age-related declines in cardiovascular and neurocognitive function are intrinsically linked.1–4 Arterial health has emerged as a significant predictor of cognitive performance, and both arterial health and cognitive performance have been shown to decline with increasing age, an indication that there is an age-dependent association between the two.5 Cerebral blood flow (CBF) decreases with advancing age,6 and this reduction is associated with cognitive decline.7 Peripheral arterial stiffness has been shown to predict cognitive performance in a group of healthy middle-aged adults.8 The connection between brain and vascular health is undoubtedly significant; however, the intrinsic nature of this connection is unclear.
Blood-oxygen-level dependent contrast imaging (BOLD) functional magnetic resonance imaging (fMRI) studies of cognitive aging have provided an abundance of information about the brain. Normal physiological aging in the absence of pathology is linked with various alterations in the vasculature of the brain, both functionally and structurally.9 Endothelium-dependent cerebrovascular reactivity (CVR) is a main regulatory mechanism that controls CBR.10,11 CVR refers to a vasodilatory or vasoconstrictor response of the blood vessels to a stimulus, and this measure provides a direct assessment of vascular brain health.12 Impaired CVR indicates microvascular hemodynamic dysfunction, which is implicated in many brain disorders, including stroke13 and Alzheimer’s disease (AD),14–16 as well as mild cognitive impairment.17
The CVR index reflects the response of cerebral blood vessels to extrinsic stimulation by vasodilators such as acetazolamide18,19 or hypercapnic challenge.20,21 Hypercapnia can be induced through inhalation of CO2-enriched gas, which is a harmless way to investigate the effects of vascular functioning on the blood flow parameters in the brain.22–27 Vasodilation in response to CO2 enhances CBR through diameter enlargement in the small arterioles, making it possible to evaluate the function of small intra-cerebral vasomotor vessels.28
Age differences in CVR
Increasing evidence shows that the cerebral vessel contractility and dilation becomes diminished in later life, consequently impacting neurovascular coupling.29 Multiple biochemical elements have been suggested as responsible for this loss of function, including reduced production and release of vasodilatory substances such as nitric oxide (NO), and increasing production of vasoconstrictive factors by the cerebral endothelium.9 The vascular wall is also affected structurally in the normal aging process; thickening of the basement membrane by augmented collagen and losses of smooth muscle and elastin occurs frequently.30 Atherosclerosis is also common, even in healthy aging, and further reduces the capacity for control of vascular diameter and tone. These alterations lead to the progressive stiffening of the cerebral blood vessels, resulting in a reduced capacity for dynamic control of blood flow in response to neural demand.31,32 These differences in vascular responsiveness between younger and older adults have implications for the interpretation of BOLD fMRI data in aging research.33
Age-related differences in CVR have been studied widely, though evidence is mixed. While most reports indicate that blood vessel reactivity in the brain decreases with age,32,34–39 other studies have found that vasomotor reactivity increases,40 or remains the same across the lifespan.41,42
Discrepancies between the type of vasodilatory stimuli, brain regions studied, age ranges, methodologies, and data analysis cloud the interpretation of studies of CVR in aging. While it is likely that vasomotor responsiveness in the brain diminishes due to the known physiological changes that occur in the vasculature with age, age-related alterations in CVR remain to be elucidated.
Regional differences in CVR
Reactivity in the brain is temporally dynamic, differs between vascular territories, and is heterogeneous across regions,43,44 though the findings are inconsistent. Research has shown that regional CVR is higher in the parietal and occipital areas when compared to frontal, temporal and insula cortices43 yet others have found the greatest reactivity in the cerebellum and visual cortex, with the least in the frontal lobes.45 Grey matter has up to three times greater reactivity than white matter.21,23 Furthermore, the effects of normal aging on regional CVR remain inconclusive, and the question of whether some areas are more affected than others remains. The known heterogeneity of age-related changes in regional CBF12 would indicate that CVR also varies between brain regions, and that vascular responsiveness may alter over time differently in differing areas.
One study15 reported that the posterior regions of the brain were more predominantly dysfunctional than other areas in a study of AD patients versus healthy controls, and CVR in these areas was correlated with cognitive performance, suggesting that the relationship between reactivity and cognitive impairment may be region-dependent. Evidently further research is necessary to clarify the regional distribution of vasoreactivity changes in the aging brain, as regions with a lower vasodilatory capacity could be more affected by reduced perfusion than areas that have greater reactivity, potentially contributing to cognitive losses in the elderly.46
Cognition and CVR
Given the predictive relationship of vascular health and cognitive performance,47,48 CVR has been examined for its relationship to cognition. Changes to the integrity of the cerebrovascular system can bring about cognitive change due to dysfunctional neurovascular coupling.43 Vascular disorders have been shown to contribute to neurodegenerative and cognitive illnesses.2,4,49,50 Associations between impaired CVR and severity of dementia have been established.51,52 CVR assessed by the breath-holding index (BHI) was found to be the best predictor of cognitive performance in AD over and above gender, age, education, and vascular risk factors,51 and pathological BHI is also predictive of conversion from MCI to AD.53 One study in cognitively healthy patients with peripheral artery disease reported that CVR was linked to executive function, memory, global cognition, and attention scores.54 While the vessel reactivity-cognition link has often been investigated in cardiovascular and neurodegenerative disorders, particularly AD, the association between cerebrovascular function and cognition in healthy aging is yet to be clarified. If impaired vascular reactivity is found to be implicated in cognitive performance, vaso-protective therapies may be a target for prevention of age-related cognitive decline.
The goal of the present study was to further this research by using MRI to measure the BOLD signal during CO2 inhalation in healthy, normally aging adults to assess differences in CVR across the lifespan. A cognitive assessment was performed to ascertain the relationships between cognition and cerebrovascular function in healthy aging. Second, it was of interest to investigate whether reactivity was the same across different brain regions, that is, was the age-related change in CVR heterogeneous throughout the brain, or are some regions more affected than others. Additionally, the relationships of regional CVR to cognitive performance on measures of memory and attention were examined to explore whether these cognitive domains are differentially affected by CVR in distinct brain regions.
Based on previous research, it was expected that CVR values would vary across regions and CVR would decline with age.7,21,45 Also expected was a positive association between CVR and cognition, such that greater reactivity would be correlated with better performance.15,54
Results
Characteristics of sample are shown in Table 1 below. The younger group was significantly more educated, with lower body mass index (BMI) and larger grey matter volumes than the older group. Reaction times on the memory and attention tasks were significantly faster in the younger group.
Table 1.
Means, standard deviations for characteristics of the study population.
| Whole sample Age range 21-75 (M: 47.22, SD: 18.59) n = 59 |
Younger Age range 21-44 (M: 30.03, SD: 6.22) n = 30 |
Older Age range 55-75 (M: 65, SD: 5.57) n = 29 |
|
|---|---|---|---|
| Years of education | 17.18 (3.69)* | 18.37 (3.22) | 15.81 (3.77) |
| TICS-m | 29.36 (2.40) | ||
| MAC-Q | 23.83 (3.57) | ||
| BMI | 23.98 (4.03)* | 22.61 (3.21) | 25.45 (4.35) |
| Heart rate | 66.98 (9.87) | 67.46 (9.63) | 66.50 (10.26) |
| Systolic pressure, mmHg | 127.11 (16.31) | 124.11 (16.32) | 129.90 (16.09) |
| Diastolic pressure, mmHg | 75.82 (10.76) | 73.56 (10.69) | 77.93 (10.57) |
| gmCBF, mL/100 g/min | 45.04 (9.05) | 45.54 (9.01) | 44.52 (9.21) |
| GMV, cm3 | 703.99 (85.42) ** | 753.44 (73.77) | 652.83 (64.40) |
| WMV, cm3 | 481.68 (56.46) | 487.34 (60.66) | 475.83 (52.18) |
| Baseline etCO2, mmHg | 38.27 (4.55) | 39.06 (4.18) | 37.42 (4.85) |
| Hypercapnia etCO2, mmHg | 46.09 (4.33) | 46.88 (4.03) | 45.24 (4.55) |
| ΔetCO2, mmHg | 7.82 (2.26) | 7.81 (2.32) | 7.82 (2.26) |
| Memory mean RT, ms | 908.19 (113.16) ** | 839.05 (87.08) | 982.10 (88.92) |
| Attention mean RT, ms | 529.65 (84.30)** | 472.78 (62.32) | 586.51 (62.31) |
TICS-m: modified Telephone Interview for Cognitive Status; MAC-Q: Memory Complaint Questionnaire; BMI: body mass index; gmCBF: grey matter cerebral blood flow; GMV: grey matter volume; WMV: white matter volume; etCO2: end-tidal CO2.
Values displayed are M (SD).
P < .01, **P < .001, significant differences between younger and older groups.
CVR is reduced in some regions with age
When using age as a continuous variable, CVR in the temporal lobe was found to significantly decrease with age (r = -0.43, P = .002). An independent t-test revealed that CVR in the temporal lobes was higher in the younger compared to the older group, F(1, 50) = 2.58, P = .006. No other regions-of-interest (ROIs) showed significant change with age in the sample as a whole.
After separating the cohort into age groups, it was observed that CVR was significantly reduced in the cingulum (r = −0.54, P = .008), grey matter (r = −0.54, P = .006) and temporal (r = −0.52, P = .006) areas in the older group. The grey matter and temporal areas still showed a marginally significant reduction after correction for multiple comparisons. A graphical representation of the percentage change in BOLD signal in the temporal lobes plotted with the change in etCO2 is shown in Figure 1, separated by age group. CVR did not change significantly with age in any of the ROI in the younger group. Figure 2 shows the mean regional CVR values for each group. There were no significant differences between genders in CVR in any region studied across the lifespan, nor when separated into age groups.
Figure 1.
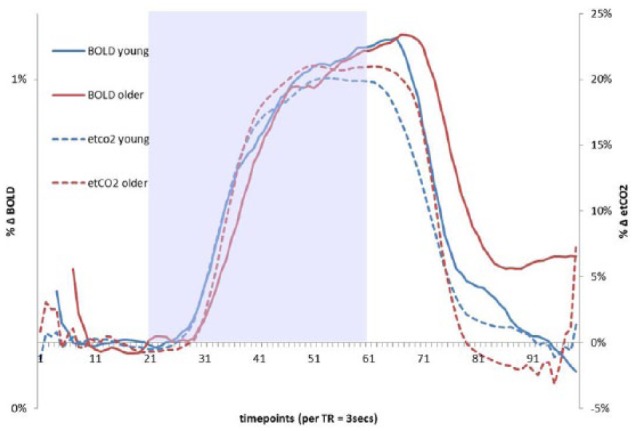
Percentage change in etCO2 and BOLD signal in the temporal lobe ROI in response to the hypercapnic challenge. Each marker on the x-axis represents 3 seconds (1 TR). BOLD traces have been shifted to the right-young by 9 seconds (3 TR), and older by 18 seconds (6 TR), which yielded the maximum cross-correlation between etCO2 and BOLD signals for this ROI. Lines representing younger and older adults are displayed in different colors. Shaded area indicates the hypercapnia period. The etCO2 data points extracted for CVR analysis are indicated by the black lines below the x-axis. To calculate CVR, BOLD data points were extracted and averaged across a 30 second period during hypercapnia and final 30 seconds of the baseline period preceding gas-inhalation separately by group (66-96 seconds after the CO2-inhalation began in the younger group and 72-102 seconds in the older group). After aligning the signals at the maximum cross-correlation, the time from the gas-inhalation onset to 50% of the maximum BOLD signal change was calculated for each group for the temporal lobe ROI, younger 47 seconds, and older 51 seconds, though an independent-samples t-test showed that these differences were not significant (P = .52).
Figure 2.
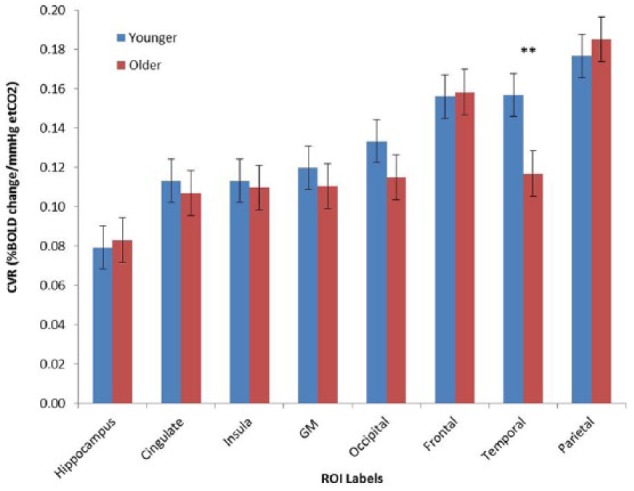
Regional CVR values for younger and older adults. Mean cerebrovascular reactivity values for ROIs averaged across groups.
GM grey matter.
All ROIS were bilateral, masked with a grey matter mask with a 50% probability threshold.
**P < .01.
Temporal lobe CVR predicts cognition across the lifespan
In the sample as a whole, correlations between cognitive variables and CVR were observed. CVR in the temporal lobes was associated with both memory (r = −0.46, P = .001) and attention performance (r = −0.41, P = .003). Table 2 displays results of separate unadjusted linear regressions for temporal lobe CVR and cognitive score. Greater CVR was associated with faster reaction times. Linear regression analyses showed that CVR in the temporal lobes significantly predicted memory score, and this effect was independent of age, gender and years of education. Figure 3 shows the relationship between temporal lobe CVR and memory task reaction times. A second linear regression showed that attention was not significantly predicted by temporal lobe CVR when adjusted for age, gender and education, though the model itself was significant. Table 3 shows the results of separate adjusted linear regression analyses.
Table 2.
Showing results of separate unadjusted linear regressions for temporal lobe CVR contribution to memory and attention scores for the entire sample.
| Memory |
Attention |
|||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| −979.62 | 268.93 | −0.46 | −647.14 | 207.45 | −0.31 | |
| R 2 | 0.21 | 0.17 | ||||
| F | 13.27*** | 9.73** | ||||
P < .01, ***P < .001.
Figure 3.
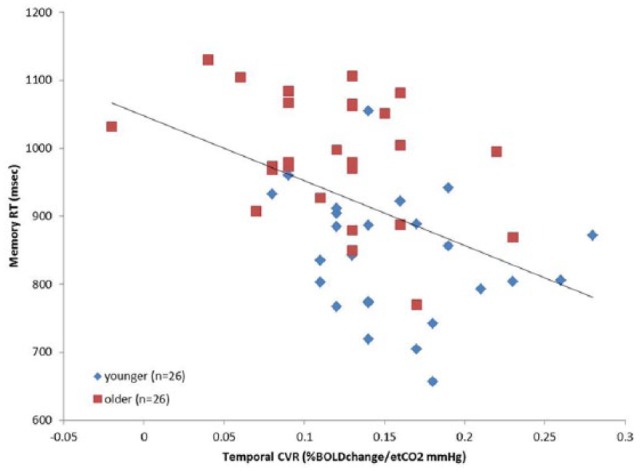
Scatterplot showing the relationship between CVR in the temporal lobes and memory reaction times for the entire sample. Data for younger and older groups are shown in different colors. Black line indicates trend for whole sample data. An adjusted linear regression analysis showed the temporal lobe CVR predicted memory score independent of age, gender and education (P < .01).
Table 3.
Showing results of adjusted linear regression for temporal lobe CVR contribution to memory and attention score for the entire sample.
| Memory |
Attention |
|||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Age | 3.14 | 0.76 | −0.51*** | 3.02 | 0.56 | −0.67*** |
| Gender | −46.07 | 25.12 | −0.20 | −6.31 | 18.74 | −0.04 |
| Education (years) | 1.17 | 3.50 | 0.04 | 5.61 | 2.61 | 0.24* |
| Temporal lobe CVR | −626.24 | 265.20 | −0.29** | −331.54 | −196.87 | −0.20 |
| R 2 | 0.52 | 0.54 | ||||
| F | 11.75*** | 12.16*** | ||||
P < .05, **P < .01, ***P < .001.
Hippocampal CVR predicts memory score in older adults
When separated by age group, separate linear regressions showed that in the younger sample there were no significant associations between CVR in any brain region and either cognitive measure. In the older group however, CVR in the hippocampus significantly predicted memory score, F(1, 20) = 5.83, P = .026, with an R2 of .24. CVR in the hippocampus was negatively associated with memory score such that worse CVR reflected longer response times (ie, slower processing). Attention was not associated with CVR in any brain region in either age group.
Scores on the MAC-Q were significantly correlated with hippocampal CVR (r = −0.439, P = .046), lower CVR was associated with greater subjective memory complaints in the older group. Additionally, there was a marginally significant correlation between hippocampal CVR and TICS-m score in the older group (r = 0.46, P = .056), suggesting a trend toward a relationship between global cognitive impairment and regional reactivity in elderly individuals.
Summary of findings
As a whole, (1) CVR declined with age in the temporal lobes across the lifespan, (2) CVR in the temporal lobes was associated with memory and attention scores, and (3) when adjusted for age, gender and education, temporal lobe CVR predicted memory score across the entire sample.
When the sample was split into age groups, (1) there were significant declines in CVR in several regions with age in the older group, but these declines were not seen in the younger group; (2) there was a significant association between CVR in the hippocampus and memory score in the older sample, but memory was not related to CVR in any region in the younger group; (3) CVR was not associated with attention score in either group; and (4) lower CVR was associated with greater subjective memory concerns and global cognitive impairment in the older group.
Discussion
The present study investigated age-related differences in regional CVR to carbon dioxide in healthy younger and older adults, and the relationships between regional CVR and cognitive performance. It was expected that an age-related CVR decline would be observed between the younger and older groups, and that this decline would vary between brain regions.55 Also expected was a positive association between CVR and cognition, such that greater reactivity would be correlated with better performance.15,54 Temporal lobe CVR predicted memory score independent of age, gender, and education across the entire sample. Hippocampal CVR significantly predicted memory score in the older group and was negatively associated with subjective memory complaints. Consistent with literature, both measures of cognition declined with increasing age. This is the first study to examine the differences in regional CVR between younger and older cognitively healthy individuals in relation to performance on memory and attention tasks. Findings demonstrate that reactivity is linked to cognition in both regionally and task-specific ways in older healthy adults.
Age and regional differences in CVR
While CVR was lower in the older group in several regions studied, these declines were not significant in all areas. The temporal lobes showed significant reductions in reactivity between the younger and older groups. Reactivity declined significantly in the cortical grey matter, the cingulum and the temporal lobes in older adults with age, but no age effects were seen in the younger adults. These findings are in line with previous research that reported heterogeneous declines from younger to older age.12
Mechanisms underlying the decreasing reactivity of cerebral vessels with age can be explained by well-described age-associated vascular stiffening.29 The more distensible, elastic properties of blood vessel walls are known to deteriorate, and regenerate more slowly in aged individuals, while the more rigid wall components, such as collagen and the basement membrane, may be augmented.43 This results in the vessel wall becoming stiffer with time, even in the absence of pathology. Arteriosclerosis can also be prevalent to some degree even in healthy aging, adding to the rigidity of vessels.9 A third insult that occurs with aging is the over-production or release of vasoconstrictive substances such as endothelin-1, and the diminished release of vasodilatory chemicals such as NO.30 Underproduction of vasodilators by the endothelium will result in reduced capacity for dilation or possibly even hyper-constriction, observed as impaired responsiveness. Most MRI-based reports of regional CVR decline suggest that diminishing vasomotor responses are widespread and generally will encompass most areas of the brain, and that the decline in CVR is more abundant and pervasive both spatially and in percentage than the decline in CBF.32
CVR was heterogeneous across brain regions. Reactivity was higher in the parietal, temporal, and frontal areas, and lowest in the hippocampus and cingulate. This is corroborated by previous research indicating that CVR is greater in the cortical grey matter when compared to the deeper grey matter structures.43 Studies investigating the spatial distribution of small vessel reactivity have observed widely varying values between areas, variation even between vascular territories supplied by the same major arteries has been observed.35 Variability between regions is likely due to differences in vascular structure and density, and occurs due to the spatial inhomogeneity and temporally dynamic nature of blood flow, in addition to differing neural and metabolic demands in different parts of the brain.44 ROIs assessed in the current work were intersected with grey matter masks, which we expected would reduce any atrophy-related variability between younger and older adults, thus providing a relative measurement of reactivity not confounded by age-related volume differences.
CVR in the temporal lobes was associated with both memory and attention
To our knowledge, this is the first study to assess the contribution of CVR in specific brain regions to attention and memory in cognitively healthy adults with ages ranging across the adult lifespan. It was observed that better vascular reactivity in the temporal lobes was associated with faster reaction times on both memory and attention tasks. These associations were region-, task- and age-dependent. When the effects of age, gender and education were controlled for, the relationship between temporal CVR and memory remained significant. These findings are corroborated by research aligning vascular reactivity with cognitive scores in patient groups of AD,15,51 vascular dementia,56 and mild cognitive impairment.53 Elucidating the patterns of change in reactivity that occur with cognitive change is important; one study observed that there was a 33% chance of conversion from MCI to AD within a year for those MCI patients who had pathologically low CVR.53
CVR in the hippocampus significantly predicted memory score in older adults
Additionally, we aimed to assess differences between younger and older individuals, and found that when comparing the groups, there were significantly different relationships in regional CVR effects on cognition. Vascular reactivity in the bilateral hippocampus was linked to memory performance in the older group, yet this relationship was not observed in younger adults. Previous research found that vasoreactivity in the hippocampus is reduced in cognitively impaired individuals compared to healthy controls57; however, this is the first study to show a direct correlation between memory function and hippocampal CVR in cognitively healthy individuals. Much evidence has shown the association of hippocampal volume58 and perfusion59,60 to various memory functions, though the impact of integrity of the small vessels supplying this structure are less well understood. This novel finding highlights the contribution of vascular integrity to a distinct cognitive domain both regionally and functionally. This result is not surprising, given the evidence showing that impaired vessel function can lead to changes in perfusion, possibly culminating in damage to brain regions that are strategic for memory functions.43 The hippocampus is particularly vulnerable to damage from ischemia and is notably damaged by AD pathology. Dysfunctional reactivity reduces perfusion, potentially resulting in greater cognitive losses in those at risk.
Another notable finding is the significant relationship between subjective memory complaints and hippocampal CVR. Greater reactivity was associated with less memory concerns. Previous research has linked smaller hippocampal volume to subjective memory problems in cognitively healthy people,61 yet this is the first study to examine this relationship with CVR. Subjective memory concerns may precede dementia and those with subjective complaints are more likely to experience cognitive decline.62,63 Our findings suggest that vascular regional changes may be a part of the early pathological process and serve as a first step in disentangling the relationship between memory deficits and brain atrophy findings.
Highly responsive blood vessels are essential for optimum flow-metabolism coupling. Impaired CVR is an indication of underlying microvascular dysfunction wherein the metabolically active regions of the brain will not be supplied adequately to meet demands. This hypoperfusion can lead to chronic ischemia over time, potentially preceding neurological and cognitive impairments.64 Indeed, research has shown strong evidence of vascular origins in the development and progression of AD.4,49,65
Limitations
The results of this study should be viewed in light of some limitations. Common to all neuroscientific aging research, brain atrophy can present some issues with comparability between age groups. In the present study, CVR values were calculated such that they are absolute measures; however, care was taken to ensure that all ROIs from which the data were extracted were masked with the grey matter probability map to account for individual differences in partial volume and tissue atrophy. This validated procedure has been utilized previously.7
Additionally, it should be noted that inhalation of CO2-enriched gas does not provide a precise standard arterial partial pressure of CO2 (PaCO2) stimulus, due to variability in individual respiratory responses.66 While more exact methods of increasing PaCO2 exist (eg, computer-controlled targeting of end-tidal CO2 partial pressures), these are expensive and require specific equipment and are thus not as accessible. A recent review of MRI-based CVR studies by the authors67 found that inhalation of a fixed concentration of CO2-enriched gas was the most commonly used vasoactive stimuli.
The regional CVR values were calculated from the difference in etCO2 signal from the first 30 seconds of room air breathing to the last 30 seconds of CO2 inhalation, while the BOLD values were calculated on the basis of age group. BOLD signal traces were shifted backward to account for the hemodynamic lag, in order to align the expired gas signal with the increase in BOLD signal. It was found that the younger group had slightly shorter hemodynamic lag than the older group (9 seconds versus 18 seconds), thus the data points sampled for the CVR calculation were different based on age group. While this shifting is valid and has been used in previous research,7 various other sampling methods are available. Future studies may benefit from analyzing CVR using other sampled time-points, for example, averaging the data points from the 30 seconds preceding the maximum BOLD signal value reached post-CO2 inhalation. This would ensure that the maximum signal change is captured for estimating CVR.
Conclusions
In this study, we examined the differences in regional CVR between older and younger healthy adults and investigated the associations of regional CVR to cognitive performance. The current data highlight the association between brain vascular reactivity and cognitive function; better performance was associated with greater blood vessel responsiveness, particularly in regions directly associated with memory function. The identification of cerebrovascular impairment in aging could help to distinguish those who would benefit from vascular-specific therapeutic approaches, and could potentially lead to detection of early dysfunction of the vascular system in people at risk. These findings could inform other areas of research for implications of vascular stiffening in the brain, and provide impetus for vascular-specific therapeutic targets to help maintain cognitive functions over the lifespan.
Experimental Procedure
Participants
In all, 30 younger (aged 21–44, mean age 30 years, SD: 6.22 years, 12 females) and 29 older (aged 55-75, mean age 65 years, SD 5.57 years, 18 females) healthy (ie, active, independently-living, asymptomatic) adults were recruited from the community. Participants underwent telephone screening and had no contraindications for MRI scanning (no pacemaker, metallic implants, or claustrophobia). Participants were of generally good health, with no self-reported history of neurological, psychiatric, cardiovascular or inflammatory diseases, and not suffering from asthma or other respiratory problems. The Modified Telephone Interview for Cognitive Status- (TICS-m68) was used to screen for mild cognitive impairment and dementia for participants aged over 45 years. Participants scoring 23 or below were omitted from the final analysis (n = 3). The Memory Complaint Questionnaire (MAC-Q)69 was used to quantify subjective memory complaints in the older participants. After receiving an explanation of the study procedure, participants provided written informed consent. The research protocol was approved by both the Swinburne University Human Research Ethics Committee and the Alfred Hospital Human Research Ethics Committee, and was conducted in accordance with the Australian Code for the Responsible Conduct of Research. Regional CVR was measured in a total of 54 participants; some data were lost due to motion in the scanner. Table 1 lists the demographic information for the participants.
Experimental design and procedure
Data were acquired in one testing session of approximately 3 hours’ duration. Participant’s blood pressure was taken before and after an initial 5-minute gas-breathing procedure conducted outside the scanner. A registered nurse was present for this familiarization in which each subjects’ SpO2 was constantly monitored. Participants then underwent magnetic resonance imaging (MRI) scanning during which they inhaled CO2 twice over a period of 10 minutes (see Figure 4). After MRI scanning, participants underwent a 30-minute computerized cognitive assessment.
Figure 4.
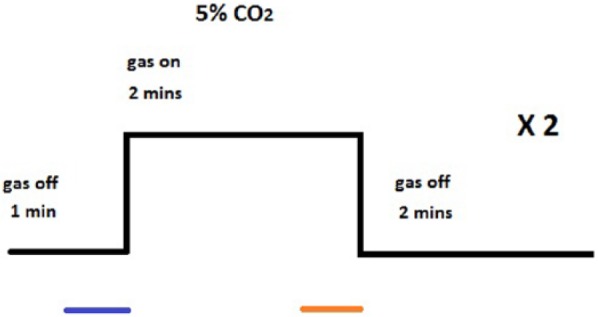
Experimental design of the hypercapnia scans. 5% CO2 inhalation was used to induce hypercapnia. Blue line indicates the period where baseline etCO2 data were collected, orange line indicates the period where hypercapnia etCO2 data was collected. The BOLD data points were extracted from the average point of maximum signal change across all ROIs to the 30 seconds preceding. This equated to the sampled BOLD data points during the hypercapnia period being selected from 66 to 96 seconds after CO2 switched on in the younger group, and 72-102 seconds after CO2 switched on in the older group.
MRI data acquisition
Data were collected using a 3T Siemens Tim Trio MRI scanner (Siemens, Erlangen, Germany) fitted with a 32 channel head coil at Swinburne University of Technology (Hawthorn, Australia). Participants were requested to minimize head movements, and foam padding was inserted around the head to aid this.
Data from two hypercapnia scans were sampled and averaged for analysis. Hypercapnia was induced by inhalation of gas mixture containing 5% CO2, 21% O2, and 74% N2 that was delivered from a 100 L non-diffusing Douglas bag through a mouth-piece with nose-clip to ensure mouth-only breathing. A two-way valve allowed the researcher to manually switch between CO2-enriched gas and room air for the 2 x 5-minute scans, as instructed by a visual signal from the MR operator. Gas delivery was alternated: 1 min room air, 2 min CO2, and 2 min room air. The breathing apparatus is shown in Figure 5. An MRI-safe patient monitor (Medrad Veris system, Bayer HealthCare AG, Leverkusen, Germany) continuously recorded a digital output of participants’ vital signs (heart rate, arterial oxygen saturation, respiratory rate), as well as end-tidal CO2 (etCO2).
Figure 5.
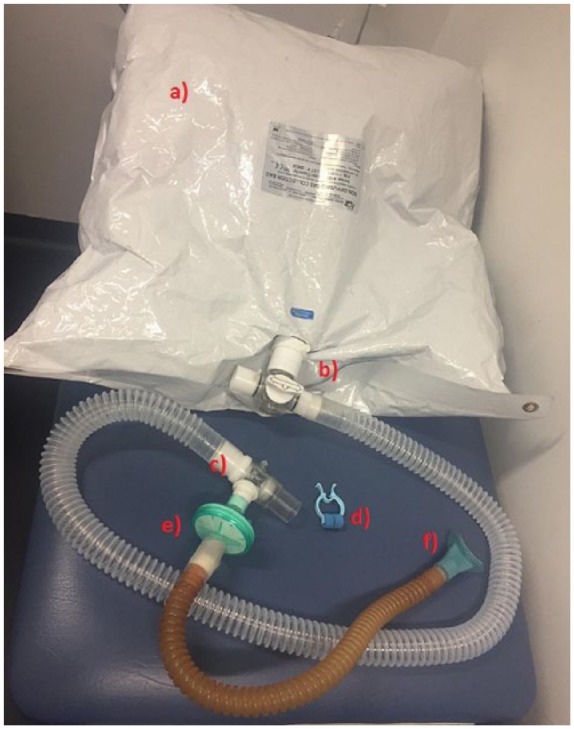
Breathing apparatus (a) 100 L Douglas bag, (b) 3-way valve, (c) 2-way non-rebreathing valve, (d) nose-clip, (e) filter (gas-sampling tube attaches here, not shown), and (f) mouth-piece.
In all, 14 axial slices with in-plane resolution of 3.44 x 3.44 x 5 mm, 2.5 mm apart were acquired oblique to the commissural plane (shown in Figure 6A and B). Hypercapnia scans were acquired using PASL with a PICORE/Q2TIPS tagging sequence, in accordance with similar research on vasoreactivity.70–72 Gradient-echo echoplanar (EPI) readout, TI1 = 700 ms, TI2 = 1500 ms, tag-size = 20 cm, TR = 3000 ms, TE = 13 ms, flip-angle = 90°. T1-weighted structural images were obtained using a high resolution MPRAGE 3D anatomical scan (axial, TR = 2300 ms, TE = 2.52 ms, voxel-size = 1 x 1 x 1 mm3, FOV = 256 mm, 176 slices).
Figure 6.
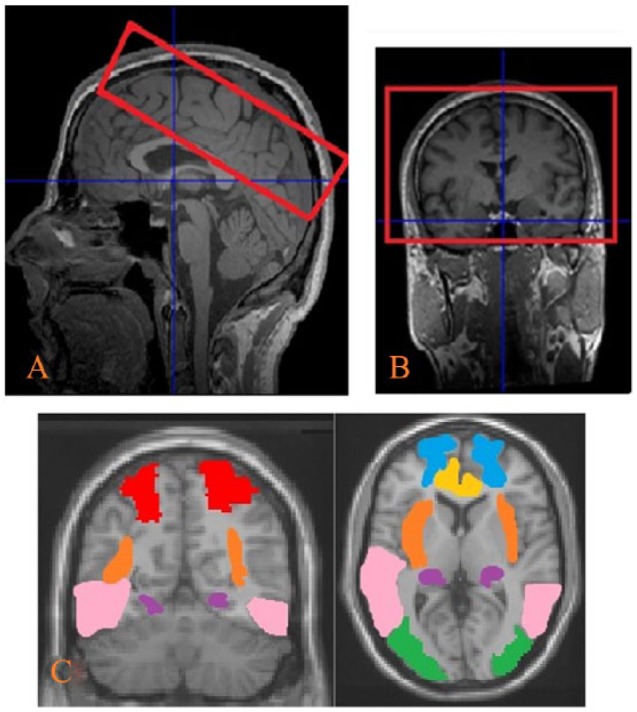
MRI slice positioning and brain regions of interest (ROIs) (A) sagittal view, (B) coronal view, and (C) ROIs included the bilateral parietal, occipital, frontal, temporal, insula, hippocampus and cingulum.
Data analysis
CVR data were processed using SPM12 (SPM12, University College of London, UK) and associated toolboxes, in addition to in-house tools, all run on MATLAB 2014b (The MathWorks). All data were motion-corrected, co-registered to individual T1-weighted images, normalized to the stereotactic space defined by the Montreal Neurologic Institute (MNI space), and then spatially smoothed with 8 mm FWHM Gaussian filter. The data were separated into CBF and BOLD time-series using the surround subtraction and averaging method70 as per Chen and Parrish.71 CVR based on BOLD were calculated. CBF-based CVR values are not reported in this paper.
Age-related changes in CVR were explored using ROI analysis. Seven ROIs were chosen based on previous reports in the literature.7,21 These regions were the bilateral superior parietal, mid-occipital, frontal, mid-temporal, insula, hippocampus and the cingulum (ROIs are shown in Figure 6C) and were applied to the data to extract BOLD time-courses. Mean whole brain grey matter was also used as an ROI. These regions were defined from the AAL package in WFU PickAtlas toolbox73 for SPM12.
Grey and white matter volumes were obtained using standard segmentation in VBM8 software from SPM12. Individual grey matter masks from the VBM analysis were smoothed to the resolution of the BOLD time-series. To account for partial volume and atrophy effects, the grey matter masks were set with a threshold of .5 (>50% probability to be grey matter) and the intersection with each ROI was used for final CVR analysis. CVR in the whole grey matter was also estimated using the thresholded grey matter mask as the ROI for each individual. This adjustment corrects for brain tissue volume differences. CBF in the grey matter at baseline was calculated from the ASL scans, values shown in Table 1.
CVR calculation
The two hypercapnia blocks (Figure 1) were averaged for each subject prior to computing the percentage change in BOLD relative to baseline for each ROI. Baseline etCO2 data were calculated from the final 30 seconds of the room air period at the beginning of each scan, and etCO2 for the hypercapnia period was calculated from the final 30 seconds of each CO2 inhalation block. Due to variable hemodynamic delays, each subject’s BOLD signal for the hypercapnia period were extracted from the point of average maximum signal change across all ROIS and the 30 seconds preceding, which equated to time-points 66–96 seconds for younger and 72–102 seconds for the older group, following the beginning of the CO2-inhalation period. Baseline BOLD data were calculated from the final 30 seconds of the room air period at the beginning of each scan. Data were sampled from different points in order to obtain the maximum CVR change for each group across all ROIs. Both BOLD and etCO2 data were temporally smoothed by a 5-point moving average filter using the “smooth” function in MATLAB. CVR was calculated as the %change in BOLD/ mmHg change in etCO2.
To assess differences in hemodynamic delay between the age groups, the time to 50% of the maximum value from beginning of the CO2 mixture inhalation was computed for each group and each ROI separately.
Cognitive assessment
Screening tool- Modified Telephone Interview for Cognitive Status (TICS-m)
The TICS-m is a reliable and validated brief 13-item test of cognitive functioning that is administered over the telephone.68 The items covered related to questions of orientation, repetition, naming, calculations and immediate and delayed recall with scores ranging from 0 to 35. Participants who scored 23 or below were omitted from the final analysis (n = 3).
Memory Complaint Questionnaire (MAC-Q)
The MAC-Q69 is designed to quantify subjective memory complaints associated with aging. It consists of six questions which ask the participant to compare their current everyday memory to that of earlier life. Possible score range is from 7 to 35. Greater scores indicate greater subjective memory concerns.
Swinburne University Computerized Cognitive Aging Battery (SUCCAB)
The SUCCAB is described previously in Pipingas et al.74 Participants were given instructions on how to perform each task and completed a practice task prior to performing the main task used for analysis. Tasks were designed to test aspects of spatial and object memory, executive processes, attention and processing speed using tasks similar to those used in previous studies examining the effects of aging on cognition.74,75 Tasks were presented in the order of simple reaction time, choice reaction time, immediate recognition, congruent Stroop, incongruent Stroop, spatial working memory, contextual working memory, delayed recognition. The SUCCAB took approximately 30 minutes to complete. The method of composition has been described previously.76 Response times for the immediate and delayed recognition, spatial working memory and contextual memory tasks were averaged to give the memory composite score. Simple and choice reaction time, and the two Stroop task response times were averaged to give the attention composite score. Higher scores reflected slower response time.
Statistical analyses
IBM SPSS statistics v.23 (Chicago, IL, USA) was used to conduct analysis. Pearson’s correlations determined the association between age, CVR in various brain regions and composite cognitive scores. Linear regressions were performed to assess the contribution of CVR in different brain regions to the cognition measures separately. Cognitive score was entered as the dependent variable, age, gender, and years of education were entered as the independent variables in model one, and CVR region entered in model 2. P values of .05 or less were considered statistically significant in all analyses; however, a correction for multiple comparisons (Bonferroni’s correction) was applied to investigate CVR differences between the age groups for the ROI analysis. The level of significance was adjusted on eight ROIs (P ⩽ .00625).
Acknowledgments
Research was supported by a grant from the Barbara Dicker Brain Sciences Association. ME Hughes is supported by the Australian National Imaging Facility.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by a grant from the Barbara Dicker Brain Sciences Foundation.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SJC, AP and TBP conceived and designed the experiments; SJC performed the experiments; SJC, YC, TBP, and MEH analyzed the data; AP, MEH, HM, and SJC worked on funding acquisition; AP, TBP, YC, and MEH contributed materials/analysis tools; and SJC, TBP, YC, MEH, HM, and AP wrote the paper.
ORCID iD: Sarah J Catchlove  https://orcid.org/0000-0003-2314-1706
https://orcid.org/0000-0003-2314-1706
References
- 1. Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. [DOI] [PubMed] [Google Scholar]
- 2. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. [DOI] [PubMed] [Google Scholar]
- 4. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pipingas A, Harris E, Tournier E, et al. Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr Topic Nutraceut Res. 2010;8:79–87. [Google Scholar]
- 6. Pase M, Herbert A, Grima N, et al. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern Med J. 2012;42:808–815. [DOI] [PubMed] [Google Scholar]
- 7. Yezhuvath US, Lewis-Amezcua K, Varghese R, et al. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biome. 2009;22:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pase MP, Pipingas A, Kras M, et al. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens. 2010;28:1724–1729. [DOI] [PubMed] [Google Scholar]
- 9. Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. [DOI] [PubMed] [Google Scholar]
- 10. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 11. Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. [DOI] [PubMed] [Google Scholar]
- 12. Lu H, Cheng Y, Hebrank A, et al. Differential patterns of age-related changes in cerebral blood flow and cerebrovascular reactivity across the lifespan. Proc Intl Soc Mag Reson Med; 2009;17: 677. [Google Scholar]
- 13. Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–467. [DOI] [PubMed] [Google Scholar]
- 14. Glodzik L, Randall C, Rusinek H, et al. Cerebrovascular reactivity to carbon dioxide in Alzheimer’s disease. J Alzheimers Dis. 2013;35:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantin S, Villien M, Moreaud O, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. Neuroimage. 2011;58:579–587. [DOI] [PubMed] [Google Scholar]
- 16. Gao Y-Z, Zhang J-J, Liu H, et al. Regional cerebral blood flow and cerebrovascular reactivity in Alzheimers disease and vascular dementia assessed by arterial spin labeling magnetic resonance imaging. Curr Neurovasc Res. 2013;10:49–53. [DOI] [PubMed] [Google Scholar]
- 17. Richiardi J, Monsch AU, Haas T, et al. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2015;36:33–41. [DOI] [PubMed] [Google Scholar]
- 18. Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiarelli PA, Bulte DP, Wise R, et al. A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage. 2007;37:808–820. [DOI] [PubMed] [Google Scholar]
- 20. Liu YJ, Juan CJ, Chen CY, et al. Are the local blood oxygen level-dependent (BOLD) signals caused by neural stimulation response dependent on global BOLD signals induced by hypercapnia in the functional MR imaging experiment? experiments of long-duration hypercapnia and multilevel carbon dioxide concentration. AJNR. 2007;28:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rostrup E, Law I, Blinkenberg M, et al. Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. Neuroimage. 2000;11:87–97. [DOI] [PubMed] [Google Scholar]
- 22. Liu YJ, Zhu X, Feinberg D, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012;68:912–922. [DOI] [PubMed] [Google Scholar]
- 23. van der Zande FH, Hofman PA, Backes WH. Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology. 2005;47:114–120. [DOI] [PubMed] [Google Scholar]
- 24. Fierstra J, Sobczyk O, Battisti-Charbonney A, et al. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 2013;591:5809–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhogal A, Siero JC, Fisher JA, et al. Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage; 2014;98:296–305. [DOI] [PubMed] [Google Scholar]
- 26. Tancredi FB, Hoge RD. Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab. 2013;33:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wise RG, Pattinson KT, Bulte DP, et al. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2007;27:1521–1532. [DOI] [PubMed] [Google Scholar]
- 28. Heun R, Knappertz PA, Krämer G. Vasomotor reactivity in dementia of Alzheimer type. Int J Geriatr Psychiatry. 1994;9:913–918. [Google Scholar]
- 29. Desjardins M. Vascular correlates of aging in the brain: evidence from imaging data. IRBM. 2015;36:158–165. [Google Scholar]
- 30. Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. [DOI] [PubMed] [Google Scholar]
- 31. Ito H, Kanno I, Ibaraki M, et al. Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab. 2002;22:997–1003. [DOI] [PubMed] [Google Scholar]
- 32. Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. [DOI] [PubMed] [Google Scholar]
- 34. Reich T, Rusinek H. Cerebral cortical and white matter reactivity to carbon dioxide. Stroke. 1989;20:453–457. [DOI] [PubMed] [Google Scholar]
- 35. Tsuda Y, Hartmann A. Changes in hyperfrontality of cerebral blood flow and carbon dioxide reactivity with age. Stroke. 1989;20:1667–1673. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi F, Meyer JS, Sakai F, et al. Normal human aging and cerebral vasoconstrictive responses to hypocapnia. J Neurol Sci. 1979;44:87–94. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto M, Meyer JS, Sakai F, et al. Aging and cerebral vasodilator responses to hypercarbia: responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–496. [DOI] [PubMed] [Google Scholar]
- 38. Barnes JN, Schmidt JE, Nicholson WT, et al. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol. 1985;112:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flück D, Beaudin AE, Steinback CD, et al. Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front Physiol; 2014;5:49.24600398 [Google Scholar]
- 40. Zhu Y-S, Tarumi T, Tseng BY, et al. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab. 2013;33:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davis SM, Ackerman RH, Correia JA, et al. Cerebral blood flow and cerebrovascular CO2 reactivity in stroke-age normal controls. Neurology 1983;33:391–399. [DOI] [PubMed] [Google Scholar]
- 42. Murrell CJ, Cotter JD, Thomas KN, et al. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age (Dordr) 2013;35:905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Novak V. Cognition and hemodynamics. Curr Cardiovasc Risk Rep. 2012;6:380–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu H, Liu P, Yezhuvath U, et al. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Visual Exper. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kastrup A, Krüger G, Glover GH, et al. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. [DOI] [PubMed] [Google Scholar]
- 46. Zhao P, Alsop D, Abduljalil A, et al. Vasoreactivity and peri-infarct hyperintensities in stroke. Neurology. 2009;72:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kearney-Schwartz A, Rossignol P, Bracard S, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 2009;40:1229–1236. [DOI] [PubMed] [Google Scholar]
- 48. Raz N, Rodrigue KM, Kennedy KM, et al. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. [DOI] [PubMed] [Google Scholar]
- 49. De la Torre JC. The vascular hypothesis of Alzheimer’s disease: bench to bedside and beyond. Neurodegen Dis. 2010;7:116–121. [DOI] [PubMed] [Google Scholar]
- 50. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 51. Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37:1010–1015. [DOI] [PubMed] [Google Scholar]
- 52. Lee S-T, Jung K-H, Lee Y-S. Decreased vasomotor reactivity in Alzheimer’s disease. J Clin Neurol. 2007;3:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Viticchi G, Falsetti L, Vernieri F, et al. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol Aging. 2012;33:1127.e1–1127.e9. [DOI] [PubMed] [Google Scholar]
- 54. Haratz S, Weinstein G, Molshazki N, et al. Impaired cerebral hemodynamics and cognitive performance in patients with atherothrombotic disease. J Alzheimers Dis. 2015;46:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leoni RF, Mazzeto-Betti KC, Andrade KC, et al. Quantitative evaluation of hemodynamic response after hypercapnia among different brain territories by fMRI. Neuroimage. 2008;41:1192–1198. [DOI] [PubMed] [Google Scholar]
- 56. Vicenzini E, Ricciardi MC, Altieri M, et al. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol. 2007;58:84–89. [DOI] [PubMed] [Google Scholar]
- 57. Glodzik L, Rusinek H, Brys M, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci. 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heo S, Prakash RS, Voss MW, et al. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schacter DL, Savage CR, Alpert NM, et al. The role of hippocampus and frontal cortex in age- related memory changes: a PET study. Neuroreport. 1996;7:1165–1169. [DOI] [PubMed] [Google Scholar]
- 61. van der Flier WM, van Buchem MA, Weverling-Rijnsburger AWE, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251:671–675. [DOI] [PubMed] [Google Scholar]
- 62. Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. [DOI] [PubMed] [Google Scholar]
- 63. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? a review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. [DOI] [PubMed] [Google Scholar]
- 64. De la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De la Torre J. Is Alzheimer’s disease preceded by neurodegeneration or cerebral hypoperfusion? Ann Neurol. 2005;57:783–784. [DOI] [PubMed] [Google Scholar]
- 66. Fisher JA. The CO2 stimulus for cerebrovascular reactivity: fixing inspired concentrations vs. targeting end-tidal partial pressures. J Cereb Blood Flow Metab. 2016;36:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Catchlove SJ, Pipingas A, Hughes ME, et al. Magnetic resonance imaging for assessment of cerebrovascular reactivity and its relationship to cognition: a systematic review. BMC Neurosci. 2018;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–324. [DOI] [PubMed] [Google Scholar]
- 69. Crook TH, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr. 1992;4:165–176. [DOI] [PubMed] [Google Scholar]
- 70. Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. [DOI] [PubMed] [Google Scholar]
- 71. Chen Y, Parrish TB. Caffeine’s effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage. 2009;44:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luh W, Wong E, Bandettini P, et al. QUIPSS II with thin-slice TI1 periodic saturation (Q2TIPS): a method for improving accuracy of quantitative perfusion imaging. Paper presented at: Proceedings of the ISMRM 6th Annual Meeting, Sydney, NSW, Auatralia, 1998, p. 1191. [Google Scholar]
- 73. Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 74. Pipingas A, Silberstein RB, Vitetta L, et al. Improved cognitive performance after dietary supplementation with a Pinus radiata bark extract formulation. Phytother Res. 2008;22:1168–1167. [DOI] [PubMed] [Google Scholar]
- 75. Scholey AB, Sünram-Lea SI, Greer J, et al. Glucose administration prior to a divided attention task improves tracking performance but not word recognition: evidence against differential memory enhancement? Psychopharmacology (Berl). 2009;202:549–558. [DOI] [PubMed] [Google Scholar]
- 76. Macpherson H, Ellis KA, Sali A, et al. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: a randomized controlled trial. Psychopharmacology (Berl) 2012;220:351–365. [DOI] [PubMed] [Google Scholar]


