Abstract
Background:
There are little data on the usefulness of trastuzumab (TZM) retreatment as the first-line treatment for patients with HER2 (human epidermal growth factor receptor 2)–positive breast cancer recurrence after perioperative treatment with TZM.
Aim:
To clarify the outcome and safety of TZM retreatment in patients with recurrent HER2-positive breast cancer.
Method:
An observational study was conducted on patients who relapsed after primary systemic therapy with TZM using the central registration system. The primary end point was progression-free survival (PFS). Secondary end points consisted of the response rate, overall survival (OS), and safety.
Result:
In total, 34 patients were registered between July 2009 and June 2012. The median follow-up time was 23.7 months (2-24 months). The 1- and 2-year PFS rates were 46.9% (95% confidence interval (95% CI): 29.2%-62.9%) and 29.8% (95% CI: 15.0%-46.3%), respectively (median 10.6 months). The median PFS time for patients receiving TZM combined with CTx was 13.9 months. The 1-and 2-year OR rates were 93.9 (95% CI: 77.9%-98.4%) and 84.8% (95% CI: 67.4%-93.4%). Trastuzumab-induced grade 3/4 adverse events were not observed.
Conclusions:
This study suggests that the PFS and OS in Japanese patients who relapsed after perioperative TZM therapy improved or were similar to those in previous reports. Differences in patient backgrounds and treatments must be considered when interpreting the results. Trastuzumab should be used combination with CTx and/or HTx for retreatment. Retreatment with TZM is safe.
Trial registration: UMIN000002738.
Keywords: breast cancer, HER2, metastatic/recurrence, trastuzumab, retreatment
Introduction
In the 1980s, HER2 (human epidermal growth factor receptor 2) was identified as a prognostic factor.1,2 Slamon et al3 compared a group of HER2-positive patients with metastatic breast cancer (MBC) in whom only chemotherapy with paclitaxel or anthracycline and cyclophosphamide was performed as primary treatment with a group of patients in whom chemotherapy was combined with trastuzumab therapy, demonstrating the usefulness of trastuzumab. Since then, anti-HER2 therapy for HER2-positive metastatic/recurrent breast cancer, pertuzumab (CLEOPATRA study),4 and T-DM1 (trastuzumab emtansine) (EMILIA study, TH3RESA study)5,6 have been developed. These HER2-targeting agents markedly improved the outcome of HER2-positive advanced and recurrent breast cancer.
In the guidelines prepared by the ASCO (American Society of Clinical Oncology) and ESMO (European Society for Medical Oncology),7,8 HER2-targeting regimens for HER2-positive advanced and recurrent breast cancer, such as pertuzumab + trastuzumab + taxans, and T-DM1 and trastuzumab combined with chemotherapy, are recommended.
However, there are countries and/or regions where pertuzumab or T-DM1 may not be used or are restricted. In such circumstances, trastuzumab and chemotherapy is inevitable as the first choice, but paucity of data is available on trastuzumab retreatment for patients with breast cancer who relapse after perioperative trastuzumab therapy.
The purpose of this observational study was to clarify the efficacy and safety of retreatment with trastuzumab for Japanese patients with recurrence after perioperative treatment with trastuzumab.
Patients and Study Design
Using the central registration system, a clinical observational study was conducted involving patients with HER2-positive breast cancer in whom recurrence was confirmed after perioperative treatment with trastuzumab for early breast cancer. Among the days of specimen collection for cytological or histological diagnosis and imaging procedures, the day on which recurrence was initially diagnosed was regarded as the day of recurrence.
Eligibility criteria consisted of (1) age on registration: 20 years or older, (2) trastuzumab administration for 10 months or more as perioperative treatment, (3) presence of evaluable lesions (measurable lesions are not essential), (4) left ventricular ejection fraction (LVEF) within 28 days before the start of treatment for recurrence: ≥50%, and (5) adequate Informed consent (IC). Exclusion criteria consisted of (1) stage IV patients at initial diagnosis or (2) presence of brain metastasis.
The study protocol was approved by each institutional review board. Data management was conducted by the Department of EBM Research, Institute for Advancement of Clinical and Translational Science, Kyoto University Hospital. The data were collected using an electronic data capture system (Satellite; Densuke Systems Co., Ltd., Tokyo, Japan). This study has been registered with the University Hospital Medical Information Network (UMIN000002738).
Primary and Secondary End Points
The primary end point in this study was progression-free survival (PFS). This refers to the interval from the first day of trastuzumab administration for recurrence until the confirmation of disease progression. In all registered patients, the earliest appearance of disease progression (include brain-only lesion), primary disease-related death, or other disease-related death was regarded as an event. In the other patients, the day on which the absence of disease progression was finally confirmed was regarded as the point of completion.
Secondary end points in this study included response rate, overall survival (OR), and safety. Tumor assessment was based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 with appropriate imaging study including computed tomography, magnetic resonance imaging, or other. The proportion of patients with complete response (CR) or partial response (PR) was regarded as the response rate. The interval from the first day of trastuzumab administration for recurrence until the day of death was regarded as OS. Events included death from any cause. In surviving patients and those in whom survival was unclear, the day of final survival confirmation was regarded as the point of completion. Regarding safety and adverse events, we adopted data on events that were considered to be related to trastuzumab by clinicians or of which the relationship with trastuzumab could not be ruled out. Safety was evaluated according to the NCI-Common Terminology Criteria for Adverse Events v4.0 (CTCAE v4.0) (Japanese version, JCOG version).
Statistics
All eligible patients were analyzed. For the background of the patient population, categorical variables were summarized with the frequency and rate and continuous variables with fundamental statistics. The PFS and OS were estimated using the Kaplan-Meier method. Similarly, the PFS was estimated in subgroups. Log-rank tests for time-to-event end points provided 2-sided P values, and P values <.05 were considered statistically significant. Cox regression analysis was used to estimate hazard ratios and 95% confidence intervals (CIs). For analysis, SAS software ver. 9.3 was used.
Results
Patient characteristics and treatment
A total of 34 patients were registered between July 2009 and June 2012. The mean age was 53.5 years. The median follow-up time was 23.7 months (2-24 months). The age at the time of recurrence, state of menopause, T and N stage, histologically invasive diameter, number of lymph node metastases at initial diagnosis, histological grade, HER2 status, estrogen receptor (ER) status, progesterone receptor (PgR) status (priority order was defined as status at recurrence → that at the time of surgery → that at the initial diagnosis), interval from the resection of the primary lesion until recurrence (<12, 12-24, and ≥24 months), and site of recurrence are shown in Table 1.
Table 1.
Patient characteristics.
| Number | 34 | (100.0%) | |
| Age (at diagnosis of recurrence) | Mean (SD) | 53.5 | (13.6) |
| Min/max | 25 | 77 | |
| Menopausal state | Pre | 14 | (41.2%) |
| Post | 20 | (58.8%) | |
| T | TX | 1 | (3.0%) |
| T1 | 3 | (9.1%) | |
| T2 | 20 | (60.6%) | |
| T3 | 7 | (21.2%) | |
| T4 | 2 | (6.1%) | |
| N.A. | 1 | ||
| N | NX | 0 | (0.0%) |
| N0 | 7 | (21.2%) | |
| N1 | 19 | (57.6%) | |
| N2 | 5 | (14.7%) | |
| N3 | 2 | (6.1%) | |
| N.A. | 1 | ||
| Histological grade | 1 | 1 | (3.0%) |
| 2 | 6 | (18.2%) | |
| 3 | 26 | (78.8%) | |
| N.A. | 1 | ||
| HER2 status | IHC ≤2/FISH+ | 3 | (8.8%) |
| IHC 3 | 30 | (88.2%) | |
| IHC unknown/FISH+ | 1 | (2.9%) | |
| ER/PgR status | Either positive | 18 | (52.9%) |
| Both negative | 16 | (47.1%) | |
| From resection of primary tumor to recurrence | <12 mo | 2 | (5.9%) |
| 12-24 mo | 11 | (32.4%) | |
| >24 mo | 21 | (61.8%) | |
| Mean (SD) | 32.0 | (18.2) | |
| Max, min | 7 | 98 | |
| Site of recurrence Multiple selection |
Preserved breast, Ax | 8 | (23.5%) |
| Chest wall, Sp, Ps | 10 | (29.4%) | |
| Counter side Ax | 3 | (8.8%) | |
| Liver | 7 | (20.6%) | |
| Lung | 10 | (29.4%) | |
| Bone | 7 | (20.6%) | |
| Other | 8 | (23.5%) | |
| Disease type | Visceral | 16 | (47.1%) |
| Nonvisceral | 18 | (52.9%) |
There was no history of heart failure in any patient. Hypertension requiring treatment was noted in 3 patients (8.8%). All patients had received chemotherapy with anthracyclines, taxans, or both concurrently and sequentially combined with trastuzumab before and/or after surgery. Hormone therapy had been performed for all hormone receptor–positive patients as primary systemic treatment.
First-line treatment after recurrence is shown in Table 2. Trastuzumab monotherapy was performed for 2 patients (5.9%), trastuzumab + chemotherapy for 26 (76.5%), trastuzumab + hormone therapy for 4 (11.8%), and trastuzumab + chemotherapy + hormone therapy for 2 (5.9%). Trastuzumab was administered to 14 patients (41.2%) weekly and to 20 (58.8%) triweekly. For chemotherapy, taxans were administered to 17 patients (60.7%) and to 11 others (39.3%).
Table 2.
First-line treatment for recurrence in this study.
| Number | 34 | (100.0%) | |
| Combination with trastuzumab | Trastuzumab monotherapy | 2 | (5.9%) |
| Trastuzumab + chemotherapy | 26 | (76.5%) | |
| Trastuzumab + hormonal therapy | 4 | (11.8%) | |
| Trastuzumab + chemotherapy + hormonal therapy | 2 | (5.9%) | |
| Trastuzumab schedule | Weekly | 14 | (41.2%) |
| Triweekly | 20 | (58.8%) | |
| CTx | DTX | 4 | (14.3%) |
| PTX | 11 | (39.3%) | |
| TC | 2 | (7.1%) | |
| Capecitabine | 3 | (10.7%) | |
| TS-1 | 2 | (7.1%) | |
| VNR | 6 | (21.4%) | |
| Hormonal therapy | TAM | 1 | (16.7%) |
| LET | 4 | (66.7%) | |
| ANA + LH-RH | 1 | (16.7%) |
Abbreviations: DTX, docetaxel; PTX, paclitaxel; TC, docetaxel/cyclophosphamide; VNR, vinorelbine; TAM, tamoxifen; LET, letrozole; ANA, anastrozole; LHRH, LHRH analogue.
Response rate
For time to event analysis, patients with disease progression/death after 24 months were regarded as surviving patients without disease progression at 24 months. The best response was evaluated, and the response rate was calculated as the sum of CR and PR patients. The response rate (CR + PR) was 44.1% (CR: 9 patients, PR: 6 patients, stable disease [SD]: 12 patients, progressive disease [PD]: 5 patients, and not evaluable [NE]: 2 patients). The response rate stratified by treatment was 50% in combination with trastuzumab and chemotherapy and 100% in combination with trastuzumab, chemotherapy, and hormonal therapy as shown in Table 3.
Table 3.
Response rate in this study.
| Response | 34 | (100.0%) | |
| Best response | CR | 9 | (26.5%) |
| PR | 6 | (17.6%) | |
| SD | 12 | (35.3%) | |
| PD | 5 | (14.7%) | |
| NE | 2 | (5.9%) | |
| Response rate | |||
| All patients (n = 34) | CR/PR | 9/6 | (44.1%) |
| Trastuzumab + chemotherapy (n = 26) | CR/PR | 8/5 | (50.0%) |
| Trastuzumab + chemotherapy + hormonal therapy (n = 2) | CR/PR | 1/1 | (100.0%) |
| Trastuzumab + hormonal therapy (n = 4) | CR/PR | 0/0 | (0.0%) |
| Trastuzumab monotherapy (n = 2) | CR/PR | 0/0 | (0.0%) |
| Disease progression after first-line treatment | No | 13 | (38.2%) |
| Yes | 21 | (61.8%) | |
Abbreviations: CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Progression-free survival
In all patients, PFS was analyzed using the Kaplan-Meier method. The 1- and 2-year PFS rates were 46.9 (95% CI: 29.2%-62.9%) and 29.8% (95% CI: 15.0%-46.3%), respectively, with a median PFS period of 10.6 months (95% CI: 6.4-18.9 months) (Figure 1A). Progression-free survival, with respect to the subgroups age, state of menopause, T factor for the primary lesion, N factor, histological grade, HER2 status, ER, PgR, ER/PgR, surgical procedure, interval from the primary surgery until recurrence, site of recurrence (locoregional, sites other than locoregional), presence or absence of pre/postoperative chemotherapy, treatment for recurrence, type of chemotherapy for recurrence, and response to treatment, was analyzed. The log-rank test and Cox regression analysis with dummy variables were conducted. None of the variables reached significance but the use of chemotherapy combination with trastuzumab (Figure 1B). The median PFS of patients receiving trastuzumab and chemotherapy was 13.9 months.
Figure 1.
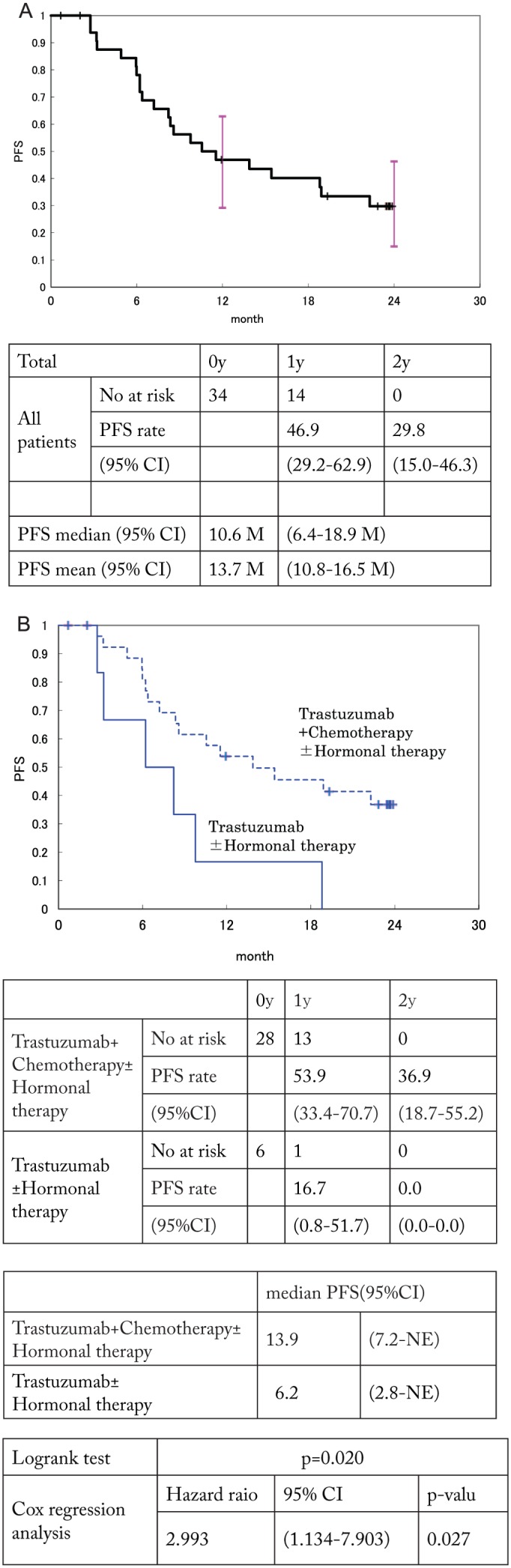
(A) PFS in this cohort and (B) PFS stratified using chemotherapy. PFS indicates progression-free survival.
Overall survival
In all patients, the OS was analyzed using the Kaplan-Meier method. The 1- and 2-year cumulative survival rates and 95% CI were calculated. The 1- and 2-year survival rates were 93.9 (95% CI: 77.9%-98.4%) and 84.8% (95% CI: 67.4%-93.4%), respectively (Figure 2). The median survival rate and time were not reached.
Figure 2.
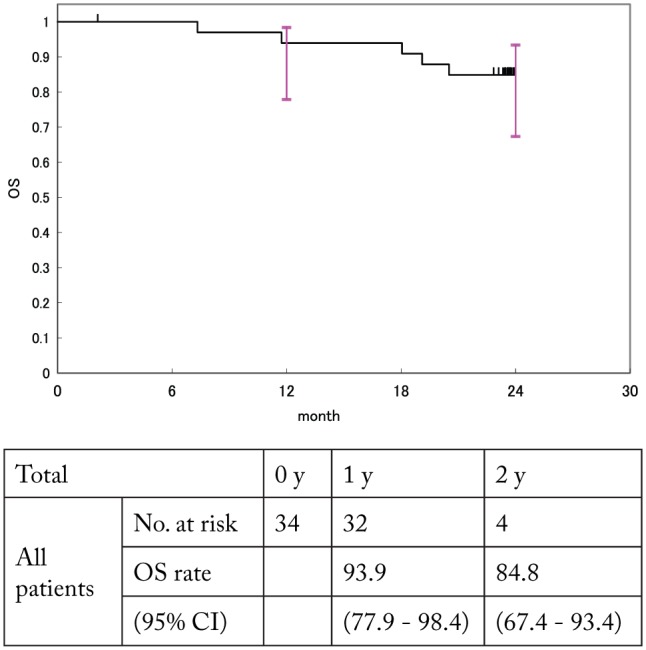
OS in this cohort. CI indicates confidence interval; OS, overall survival.
Five patients died of breast cancer (primary disease). Among the five patients who died in this cohort, there were 2 cases of PD, 2 cases of PR, and 1 case of best response SD. The 2 patients who died within 1 year after relapse had PD.
Safety
Adverse events were observed in 8 patients (23.5%): infusion reactions in 2, nausea in 1, and others in 5. There were no grade 3/4 adverse events. According to the NYHA (New York Heart Association) classification, 2 patients had grade I events. In all, 17 and 11 patients underwent ultrasonic cardiography 1 and 2 years after starting trastuzumab retreatment, respectively. One patient exhibited an absolute LVEF of 48%.
Discussion
Several randomized trials with trastuzumab have reported improved outcomes in patients with HER2-positive early breast cancer.9–13 We here report the outcome of treatment of patients with HER2-positive early-stage breast cancer who received perioperative trastuzumab treatment. The 3-year relapse-free and OS rates were 94.2% and 98.9%, respectively.14 Under these circumstances, it is becoming difficult to conduct clinical trials for patients with recurrent HER2-positive breast cancer. Few data are available on patients who relapse after perioperative trastuzumab therapy.
Currently, as first-line therapy for HER2-positive recurrent breast cancer, combination therapy with pertuzumab, trastuzumab, and chemotherapeutic drugs is recommended according to the CLEOPATRA study.4 In CLEOPATRA study, proportion of patients without a history of chemotherapy was 53%, and patients who had received trastuzumab accounted for only 10%. The median PFS was 18.5 months in the pertuzumab group and 12.4 months in the placebo (trastuzumab + docetaxel) group. However, in subgroups consisting of 88 patients who had been exposed to trastuzumab, the median PFS in pertuzumab and placebo group was 16.9 and 10.4 months, respectively. In our study, all patients had received adjuvant trastuzumab therapy and the median PFS for patients receiving trastuzumab combined with chemotherapy was 13.9 months. Although our results are inferior to the pertuzumab group, it seems to be better than placebo group (trastuzumab + taxans).
In EMILIA study,5 patients with HER2-positive advanced or recurrent breast cancer, who had previously been treated with trastuzumab and taxans, were randomly assigned to T-DM1 or lapatinib plus capecitabine. The median PFS as assessed by independent review was 9.6 months with T-DM1 versus 6.4 months with lapatinib plus capecitabine. The objective response rate with T-DM1 was 43.6%. In HER2-positive recurrent breast cancer, the disease-free interval (DFI) was estimated as a predictive factor for the efficacy of trastuzumab retreatment,15 however. In the CLEOPATRA and EMILIA study, the definitions of DFI differed from 12 months (CLEOPATRA) to 6 months (EMILIA), respectively, which may have influenced the results. In this study, DFI did not influence PFS.
In a phase 2 study involving patients similar to the subjects of this study, RHEA (Retreatment after HErceptin Adjuvant trial),16 combination therapy with docetaxel or paclitaxel and trastuzumab was performed for 43 HER2-positive recurrent breast cancer patients who had received perioperative trastuzumab therapy for 10 months or more, as conducted in this cohort. The response rate was 61%. The median PFS was 8 months, and the median OS was 25 months. Our study may have included patients in whom not only taxans but also other chemotherapeutic regimens were combined with trastuzumab. In addition, distant metastases were present in all patients in the RHEA study, whereas visceral metastases were present in 16 patients (47%) in our study. Another observational study reported that the PFS of trastuzumab retreatment for patients who relapsed after perioperative trastuzumab treatment to be consistent for approximately 7 to 12 months.15,17–19
From our study, lack of chemotherapy with trastuzumab was identified as a poor prognostic factor.
Trastuzumab had reported synergistic with chemotherapy3,10 and that monotherapy has quite limited activity. All patients with CR had received trastuzumab + chemotherapy ± hormone therapy in our study; chemotherapy should be used in combination with trastuzumab.
Concerning safety, we collected data on adverse events associated with trastuzumab or for which the association with trastuzumab could not be ruled out in this cohort. There were no grade 3/4 adverse events. No patient exhibited heart hypofunction requiring the discontinuation of trastuzumab administration. There is a possibility that the patients with cardiac function deterioration due to perioperative trastuzumab treatment was not registrated, and the fact that very few patients with hypertension in this cohort were affected, retreatment was safely performed in clinical practice.
Including our study, data on OS for anti-HER2 therapy for patients with HER2-positive metastatic/recurrent breast cancer are lacking. The optimal sequence for anti-HER2 therapy, including pertuzumab and T-DM1, needs to be set in the future. However, the number of patients with HER2-positive recurrent breast cancer may further decrease with advances in HER2-targeting perioperative treatment. The data from this study may aid in future research.
Conclusions
Although there are significant limitations of our study, in particular, the small sample size and short median follow-up, this study suggests that outcomes for trastuzumab retreatment in Japanese patients who relapsed after perioperative trastuzumab therapy were similar compared with those in previous reports. Differences in the patient background and treatments must be considered when interpreting the results. Trastuzumab should be used in combination with chemotherapy and/or hormonal therapy for retreatment. Retreatment with trastuzumab is safe.
Acknowledgments
The authors are grateful to all of the coinvestigators and patients for their cooperation in the JBCRG C-02 study. The authors also thank the following additional investigators for their contributions to this study: Tetsuo Takeuchi ans Yosuke Sasaki for statistical work, Sachiko Inoue and Miyoko Hasebe for data management, and Kiyomi Kashiwa, Nobuko Aoki, and Tomoko Kotani for secretarial work.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was conducted using our own funds from JBCRG.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.Y. received honoraria from Chugai, Novartis, Takeda, Eisai, Daiichi Sankyo, and AstraZeneca, outside this study. N.M. received honoraria from Chugai, AstraZeneca, Pfizer, Takeda, Eisai, and Kyowa Kirin, outside this study. T.T. received grants and personal fees from Novartis, Chugai, and Taiho; personal fees from Daiichi Sankyo, Kyowa Hakko Kirin, and Eisai; grants from Takeda, Ono, and MSD and Merck Serono, outside this study. Y.S. received honoraria from Chugai, Eisai, Takeda, AstraZeneca, Daiich Sankyo, and Taiho during the conduct of this study. Y.K. receives personal fees from JBCRG during the conduct of the study, personal fees from Chugai and Kyowa Hakko Kirin, outside this study. K.K. is a board member of Japan Breast Cancer Research Group Association. S.M. received personal fee from Chugai, outside this study. S.O. received personal fees from AstraZeneca, Chugai, Eisai, Taiho, Pfizer, Novartis, Kyowa Hakko Kirin and Sanofi, outside this work and is a board member of Japan Breast Cancer Research Group Association. M.T. received grants from Chugai, Taiho, and C&C Research Laboratories, during the conduct of the study; honoraria from Chugai, Takeda, AstraZeneca, Eisai, Genomic Health, Taiho, Eli Lilly, Kyowa Hakko Kirin, and C&C Research Laboratories, outside the submitted work; in addition, M.T. has a patent USPTO Application #: #20140350358 pending, and a patent USPTO Application #: #20140073533 Chuga pending; and he is also a member of Board of Directors, Japan Breast Cancer Research Group Association. All remaining authors have declared no conflicts of interest.
Author Contributions: HY contributed to concept development, study design, interpretation of the results, manuscript writing and funding for the project; MS, NM, YO, TT, ET, TS YS, KY, YK, TL, SO, KY, NY, KK, HS, SO and TI to conducting study, interpretation of the results and manuscript writing; SY to study design, data management, interpretation of the results and manuscript writing; SM to study design, interpretation of the results and statistical analyses; SO and MT to experimental design, interpretation of results and manuscript writing.
References
- 1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 2. Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. [DOI] [PubMed] [Google Scholar]
- 3. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 4. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–699. [DOI] [PubMed] [Google Scholar]
- 7. Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23:489–502. [DOI] [PubMed] [Google Scholar]
- 8. Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. [DOI] [PubMed] [Google Scholar]
- 10. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. [DOI] [PubMed] [Google Scholar]
- 13. Spielmann M, Roche H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. [DOI] [PubMed] [Google Scholar]
- 14. Yamshiro H, Iwata H, Masuda N, et al. Outcomes of trastuzumab therapy in HER2-positive early breast cancer patients. Int J Clin Oncol. 2015;20:709–722. [DOI] [PubMed] [Google Scholar]
- 15. Lambertini M, Ferreira AR, Poggio F, et al. Patterns of care and clinical outcomes of first-line trastuzumab-based therapy in HER2-positive metastatic breast cancer patients relapsing after (neo)adjuvant trastuzumab: an Italian multicenter retrospective cohort study. Oncologist. 2015;20:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang I, Bell R, Feng FY, et al. Trastuzumab retreatment after relapse on adjuvant trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer: final results of the Retreatment after HErceptin Adjuvant trial. Clin Oncol (R Coll Radiol). 2014;26:81–89. [DOI] [PubMed] [Google Scholar]
- 17. Murthy RK, Varma A, Mishra P, et al. Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2-positive metastatic breast cancer. Cancer. 2014;120:1932–1938. [DOI] [PubMed] [Google Scholar]
- 18. Krell J, James CR, Shah D, et al. Human epidermal growth factor receptor 2-positive breast cancer relapsing post-adjuvant trastuzumab: pattern of recurrence, treatment and outcome. Clin Breast Cancer. 2011;11:153–160. [DOI] [PubMed] [Google Scholar]
- 19. Negri E, Zambelli A, Franchi M, et al. Effectiveness of trastuzumab in first-line HER2+ metastatic breast cancer after failure in adjuvant setting: a controlled cohort study. Oncologist. 2014;19:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]


