Abstract
Evidence-based contemporary spinal rehabilitation often requires radiography. Use of radiography (X-rays or computed tomography scans) should not be feared, avoided, or have their exposures lessened to decrease patient dose possibly jeopardizing image quality. This is because all fears of radiation exposures from medical diagnostic imaging are based on complete fabrication of health risks based on an outdated, invalid linear model that has simply been propagated for decades. We present 7 main arguments for continued use of radiography for routine use in spinal rehabilitation: (1) the linear no-threshold model for radiation risk estimates is invalid for low-dose exposures; (2) low-dose radiation enhances health via the body’s adaptive response mechanisms (ie, radiation hormesis); (3) an X-ray with low-dose radiation only induces 1 one-millionth the amount of cellular damage as compared to breathing air for a day; (4) radiography is below inescapable natural annual background radiation levels; (5) radiophobia stems from unwarranted fears and false beliefs; (6) radiography use leads to better patient outcomes; (7) the risk to benefit ratio is always beneficial for routine radiography. Radiography is a safe imaging method for routine use in patient assessment, screening, diagnosis, and biomechanical analysis and for monitoring treatment progress in daily clinical practice.
Keywords: radiophobia, radiography, X-ray, low-dose radiation, linear no-threshold (LNT), ALARA
Introduction
The common X-ray is an essential tool for doctors and manual therapists in the treatment of musculoskeletal and neuromusculoskeletal diseases and conditions associated with poor posture and spinal deformity.1–10 There has been an ever-expanding evidence base substantiating the effectiveness of nonsurgical rehabilitative methods for the treatment of posture and spinal deformities, such as forward head posture,11–20 cervical hypolordosis/kyphosis,14–21 thoracic hyperkyphosis,22–29 thoracic hypokyphosis,30,31 lumbar hypolordosis/kyphosis,32–37 and scoliosis.38–41
The common radiograph is an invaluable tool that will continue to be a “go to” procedure to assess and monitor treatment effects related to improving posture with contemporary treatment approaches.11–41 There are, however, many fears concerning the exposure of radiation (ie, radiophobia), particularly for the acquisition of diagnostic medical X-rays (including computed tomography [CT] scans).42–47
Radiophobia stems from decades of scientifically erroneous extrapolations from high-dose atomic bomb survivor data assumed to be linear down to a zero exposure, the so-called “linear no-threshold” (LNT) hypothesis or model. This simple linear model has been the basis for safety standards and theoretical cancer estimates for over 60 years.48,49
There are many reasons why routine radiography is not only feared but also often avoided—unnecessarily—not only by the patient but also by the doctor. We argue that radiography should not be feared and should remain a routine procedure in rehabilitative clinical practice for 7 main reasons:
The LNT model for radiation risk estimates is invalid for low-dose exposures.
Low-dose radiation enhances health via the body’s adaptive response mechanisms (ie, radiation hormesis).
An X-ray with low-dose radiation only induces 1 one-millionth the amount of cellular damage as compared to breathing air for a day.
Radiography is below inescapable natural annual background radiation levels.
Radiophobia stems from unwarranted fears and false beliefs.
Radiography use leads to better patient outcomes.
The risk to benefit ratio is always beneficial for routine radiography.
The purpose of this article is to discuss the rationale behind 7 main reasons why radiography as used in spinal rehabilitative medicine should not be feared or avoided due to unwarranted radiophobia by patients or their provider in daily clinical practice.
The LNT Model for Radiation Risk Estimates Is Invalid for Low-dose Exposures
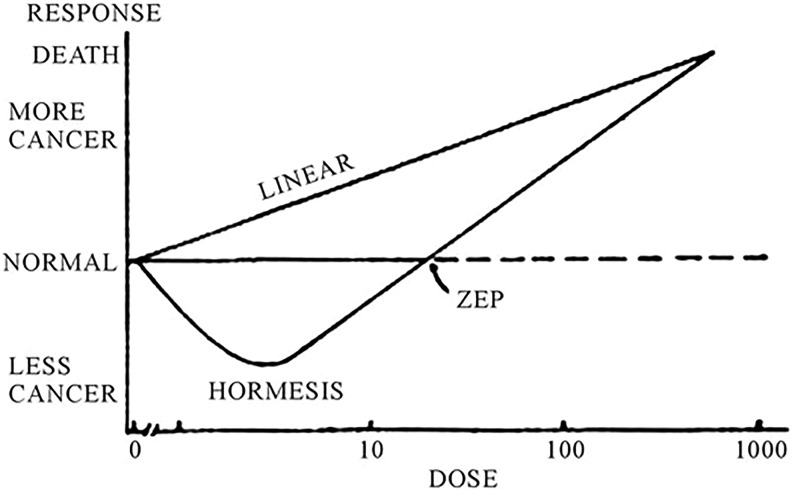
The LNT model for estimating radiation risk assumes that high-dose dose–response data from the Nagasaki/Hiroshima atomic bomb survivors (in the Life Span Study) can be linearly extrapolated down to zero dose (Figure 1).50 Therefore, this model theoretically assumes that all radiation is harmful, no matter the exposure level, even for radiography that is several orders of magnitude less than the high-dose atomic bomb data.
Figure 1.
Linear no-threshold (LNT) model versus hormesis model of cancer incidence. The LNT model depicts linear relationship between dose rate and cancer induction; hormesis model depicts less cancers at lower doses; where at the “zero equivalent point” (ZEP), the model crosses over to mimic the LNT model at higher doses, having more cancers linearly related to dose rate.
Essentially, atomic bomb exposure data are used to theoretically calculate supposed radiogenic cancers from the low doses from radiography (plain film or CT scans).42–47 The problem is, however, that no data have ever supported the LNT model for low-dose radiation exposures as used in radiography.48,49,51 Specifically, according to the Health Physics Society, no model is justified in the estimation of radiogenic health risks at doses less than 100 mSv (10 000 mrem).52 Ironically, and as discussed by Socol et al,53 the International Commission on Radiological Protection (ICRP) admits that cancer deaths estimated using the LNT model for low doses (<100 mSv) are “speculative, unproven, undetectable, and ‘phantom’.”54 Table 1 shows typical radiation exposures from common spinal imaging.
Table 1.
Typical Radiation Exposures of Common X-Ray and CT Imaging and Its Equivalent to Number of Days of Annual Background Radiation for US Average (3.1 mSv), Colorado (6 mSv), and Ramsar, Iran (260 mSv).
| Body Region | Average Effective Dose (mSv) | Equivalent Days of Background | ||
|---|---|---|---|---|
| United States Average: 3.1 mSv | Colorado: 6 mSv | Ramsar, Iran: 260 mSv | ||
| Plain radiography | ||||
| Cervical (AP/Lat) | 0.2 | 24 | 12 | 0 |
| Thoracic (AP/Lat) | 1 | 118 | 61 | 1 |
| Lumbar (AP/Lat) | 1.5 | 177 | 91 | 2 |
| Full-spine series | 2.7 | 318 | 164 | 4 |
| CT imaging | ||||
| Head | 2 | 235 | 122 | 3 |
| Chest | 8 | 942 | 487 | 11 |
| Abdomen–pelvis | 15 | 1766 | 913 | 21 |
Abbreviations: AP/Lat, anteroposterior/lateral; CO, Colorado; CT, computed tomography.
Even the most recent atomic bomb survivor data have been shown to be equally or better described by an S-shaped relationship as opposed to a linear one (LNT).55 As Doss states, “Atomic bomb survivor data (generally regarded as the most important data for estimating health effects of radiation) no longer support the LNT model.”56,57
Since conclusions made for low doses are based on data from only high doses, any proposed support for the LNT for the low-dose region results from “circular reasoning, cherry picking, faulty experimental design, and/or misleading inferences from weak statistical evidence.”51
Recent evidence surrounding the initial adoption of the LNT model has brought to light its dubious history. Calabrese has documented the historical time line of events surrounding the creation of the LNT model and its adoption by the national and international regulatory bodies.58–61 This new body of evidence is likened to the “last nail in the coffin” as it demonstrates flaws in the original research underpinning the LNT and unethical conduct by those involved; it lays to rest any last evidence the LNT had regarding its use in the low-dose range. In fact, there is a strong push from the scientific community for the termination of the LNT model as used in radiation protection standards for the low-dose range (ie, radiography).62–65
With dismissal of the LNT model, of course its corollary the “As Low As Reasonably Achievable” (ALARA) concept as used in medical radiation education and practice collapses with it. There is demand within the scientific community to abandon the ALARA concept.56,57,66–68
Low-Dose Radiation Enhances Health via the Body’s Adaptive Response Mechanisms (ie, Radiation Hormesis)
The body responds to stress (including radiation) by overcompensating for any damage caused during the exposure. This overcompensation is a redundant cascade of physiologic processes that occur on multiple physiologic levels.69–72
Hormesis refers to the J-shaped effect of low doses causing benefit and high doses causing harm from an exposure to an agent; “radiation hormesis” is the hormesis phenomenon applied to living things exposed to radiation (Figure 1). Low radiation doses stimulate health benefits, and high radiation doses are detrimental to health.
Similar to exercise, radiation exposure damages living cells. Exercise, a common act known to be physiologically positive and health promoting, leads the body to respond to muscle tissue damage by overcompensating and repairing muscle cells to be more fit and stronger following the exposure. Radiation exposure causes a similar physiological overcompensation response that in turn makes the exposed cells, tissues, and whole organism more robust and resistant to similar future exposures.
Several have discussed the incredible and sophisticated adaptive responses the body employs to protect itself from radiation and other toxic exposures.69–72 There are different types of defense mechanisms, physical-static and metabolic-dynamic defenses. Physical-static defenses include barriers such as skin or the cell membrane. Metabolic-dynamic defenses include scavenging mechanisms, molecular repair mechanisms (including DNA), and removal of damaged cells such as by programmed cell death (apoptosis), as well as by other means. Another type of metabolic-dynamic protection is the “upregulation” of existing protection mechanisms (also known as “stress response”) that causes overcompensation, so that the organism is better able to withstand future similar exposures (including radiation).
These adaptive responses have been proven to occur in response to many challenges to the body, such as strength training, sunbathing, callus formation, and immunization. The body becomes healthier to the challenges that are within an envelope of exposure that is not sufficient to overwhelming it. The same holds true for radiation exposures from radiography. Löbrich et al determined that DNA double-strand breaks (DSBs) occur after humans receive CT scans; however, these DSBs were repaired between 5 and 24 hours after the scan.73 Most importantly, the innate repair mechanisms repaired more than the damage that had initially occurred from the CT scans; the final DSB count was less than it was prior to the scan. Siegel et al state, “This is evidence of a beneficial (hormetic) effect of low-dose ionizing radiation—and argues against radiogenic causation of either solid cancers or leukemias in children or adults”.65(p866) For the record, a CT scan typically employs an order of magnitude greater radiation exposure than a plain X-ray (Table 1).
An X-Ray With Low-Dose Radiation Only Induces 1 One-Millionth the Amount of Cellular Damage as Compared to Breathing Air for a Day
Cellular damage occurs on a second-by-second basis by normal metabolism. In fact, it has been estimated that every cell in the body has about 10 billion reactive oxygen species (ROS) produced per day.71,74 Since most ROS molecules are produced from normal breathing, it has been stated “the production of oxygen-based radicals is the bane to all aerobic species.”75 This is because ROS molecules (naturally produced byproducts during mitochondrial electron transport of aerobic respiration) act as a double-edged sword as they serve in both intracellular and extracellular signaling as well as contribute to pathological processes.76
How many ROS molecules are produced from an X-ray? Feinendegen et al state that for an average microdose event, such as for a 100 kVp X-ray exposure, approximately 150 ROS molecules in the hit cell will be created within a fraction of a second exposure.70 Thus, the 150 extra ROS molecules per “hit cell” from a radiograph literally represents less than a micropercentage (150/10 000 000 000) of the daily cellular ROS burden that occurs mostly from breathing air. This deems radiogenic cancer risks from the occasional radiograph examination irrelevant.
Siegel et al have stated that if the body lacked the ability to deal with ROS burden, we would have all succumbed to cancer already!64 As discussed above, this does not happen; in fact, the opposite has been demonstrated. Lemon et al have documented increased life span in mice after receiving a single CT scan77 and also in mice receiving multiple CT scans.78 As mentioned, CT scans emit radiation levels in the low-dose range, and these are about an order of magnitude larger than exposures from conventional plain X-ray.
Radiography Is Below Inescapable Annual Background Radiation Levels
Radioactive materials are found throughout nature and include radon, soil, rock, water, food, air, and cosmic radiation from outer space, collectively known as “background” radiation. The International Atomic Energy Agency states that “Exposure to radiation from natural sources is an inescapable feature of everyday life in both working and public environments. This exposure is in most cases of little or no concern to society.”79
The average exposure to background radiation levels in the United States (not including medical imaging) is 3.1 mSv/y.80 Background levels may vary greatly based on geographic location and elevation, for example, the average annual background in Toronto, Canada, home of the first author, is about 1.59 mSv.81 The average worldwide background radiation dose is about 2.4 mSv/y.82
Higher background radiation levels are incurred to residents living at higher altitudes, such as the Colorado plateau being about twice the background versus sea-level states. There are also specific regions in the world where background levels are much higher than average, including Ramsar (Iran), Guarapari (Brazil), Karunagappally (India), Arkaroola (Australia), and Yangjiang (China).
Studies on Ramsar, Iran, show that locals may be exposed up to 80 times the worldwide average natural background radiation exposure (260 mSv/y).83 Of particular note is that of populations residing in these super high background radiation levels, there has never been any ill health effects ever documented to humans anywhere in the world.62,84,85 This is because it has been proven that those living in these high background areas show greater adaptive response than controls.86 Thus, “claims that elevated natural background radiation levels lead to cancer or early childhood deaths are unjustified and misleading.”87
The typical patient exposed to medical radiography will receive anywhere from 0.2 mSv (20 mrem) for a cervical spine series to 2.7 mSv (270 mrem) for a full-spine series (Table 1).88 For comparison, radiation exposure from CT imaging ranges from 2 mSv for a routine head CT, to 8 mSv for a routine chest CT, to 15 mSv for a routine abdomen–pelvic CT scan.89 Although technically comparing radiation from acute X-ray exposures to chronic background exposures is not directly comparable due to the body’s adaptive responses, it suffices to put the perceived risks of radiography into perspective. Thus, plain film radiographs are less than a year's equivalent of background and, depending on where one lives, may equate to only a few days of natural and inescapable background radiation (Table 1).
Radiophobia Stems From Unwarranted Fears and False Beliefs
The LNT was born and adopted by the regulatory agencies during the cold war era (1950s). Great fears were created about any and all radiation ever since the historic atom bomb droppings on Hiroshima and Nagasaki during World War II. These events underpin the radiophobia that still persists today.90
Radiophobia is prevalent and influences a parent’s decision to give consent for the imaging of their child. Patients who are informed about (theoretical) cancer risks associated with CT head scans, for example, are less likely to consent to their child’s imaging versus when they are not informed of the risks (70% vs 90% consent rate).91
There are no existing data supporting the contention that exposure to low-dose radiation as that given by radiographs (or CT scans) contributes to the development of future cancers62—in fact, there are only data that exist that show the opposite—less future cancers by upregulation of the body’s adaptive protective system.87
The continued support of the LNT model by regulatory agencies and scientific advisory bodies, such as the NAS BEIR committee, NCRP, ICRP, and so on, only serves to propagate fear mongering by supporting the ALARA concept and the “Image Gently (children),” and “Image Wisely” (adults) campaigns.63,64 As stated by Siegel et al “Radiophobia is detrimental to patients and parents, induces stress, and leads to avoidance of imaging or suboptimal image quality, both producing misdiagnosis. This can only be overcome by rejection of the LNT fiction and its corollary principle, ALARA, and by termination of the Image Gently Alliance”.65(p867)
Radiography Use Leads to Better Patient Outcomes
It must be stated that radiography remains the most cost-effective and practical imaging method for spine and posture assessment, for diagnosis, and for monitoring treatment progress in clinical practice. This is because magnetic resonance imaging (MRI) has limited availability, is not practical for common practice, and is costly.92 Further, other evolving technologies such as microdose X-ray by slot scanning devices93 are not yet widely available. Conventional radiography, therefore, remains the primary spinal imaging procedure and its use has proven to be essential for achieving better treatment results for several spinal conditions and clinical scenarios.
In the treatment of scoliosis, it has been proven that treatment programs are more effective when tailored specifically to the patient’s spinal deformity rather than employing “cookie-cutter” conventional approaches.38,39 Noh et al determined that although a patient group had a reduction in Cobb angle after a “conventional exercise program” (focusing on core stabilization), superior results were obtained by a comparison group receiving a “corrective spinal technique” (CST) featuring patient-specific Schroth methods.38 The CST group achieved greater improvements in Cobb angle, vertebral rotation, as well as total score, treatment satisfaction, and self-image subscale scores on the Scoliosis Research Society questionnaire 22). Monticone et al determined that traditional spinal exercises were able to maintain the health status (Cobb angle and health-related quality of life [HRQL]) in a group of patients with AIS with mild <25° scoliosis curves; however, the comparison group receiving a customized patient-specific (active self-correction, task-oriented spinal exercises, and education) program got better results achieving improvements in both reduction in Cobb angle and increased HRQL.39
Regarding scoliosis treatment, it must be mentioned that it is essential to differentiate between functional and structural types (eg, hemivertebra) as treatment approaches will vary dramatically.94 Obviously, this differentiation as well as the development of patient-specific, customized exercise programs deems radiography essential. Further, even in standard of care approaches to scoliosis (ie, observation and bracing), radiography is mandatory; in fact, it is unethical to withhold bracing recommendations95 to patients who are qualified candidates.96
Rehabilitation programs aimed at correcting the cervical lordosis or lumbar lordosis require spinal imaging for the determination of appropriate diagnosis. Several randomized clinical trials17,18,21,34–36,97,98 have determined that in patients with either cervical hypolordosis having cervicogenic symptoms (eg, neck pain, headache, etc) or lumbar hypolordosis having lumbosacral symptoms (eg, lower back pain, sciatica, etc), “conventional” physiotherapy treatment programs (ie, stretching exercises, strengthening exercises, infrared irradiation (hot packs), manipulation, myofascial release, transcutaneous electrical nerve stimulation (TENS therapy, mobilization) results in only immediate and short-term improvement in outcomes (ie, reduced pain, increased range of motion, improved nerve function, improvement in HRQL). These initial patient outcome improvements from conventional physiotherapy methods, however, do not last and patient symptoms and outcomes regress toward baseline measures as quickly as 3 months without treatment. Alternatively, patients who receive either cervical or lumbar extension traction (ET) to increase the corresponding lordosis (as well as a cookie-cutter physiotherapy program) achieve better results initially and also remain well up to 1 year following the initial treatment. Patients getting ET also show improvements in lordosis measures, while comparison groups not getting ET do not. It is rationalized that the lasting, long-term improvement results from the structural improvements to the spine (ie, restoration of lordosis).17 Spine alignment can only be diagnosed by radiography.
Manual therapy approaches require radiographic imaging to ensure optimal force vectors for specific spinal joint manipulation.99–101 In the practice of chiropractic, for example, in a sample of 500 patient radiographs, 91%, 70%, and 79% of patients may have radiographic-verified anomalies and pathologies that would alter treatment for the cervical, thoracic, and lumbar spinal areas, respectively.100 Beck et al determined that up to one-tenth patients in a sample of 847 full-spine radiographs demonstrated absolute contraindication to spinal manipulation, including bone fracture, malignant tumor, abdominal aortic aneurism, and atlantoaxial instability.99
Although a secondary consideration, patients are more satisfied when receiving radiographic procedures.102–104 This is probably because it is an expectation of patients to receive a radiographic assessment when presenting with spinal problems.105,106 One study, for example, found that 73% of patients expected X-rays for their lower back problem.106 Thus, radiography use as a part of a comprehensive spinal assessment107 has the added benefit of appeasing the patient.
As shown, radiography use in spine and posture rehabilitation leads to better outcomes when used in determining patient-specific treatment protocols, for example, in treating scoliosis patients as well as patients having cervical and lumbar spine hypolordosis/kyphosis; its use to differentiate functional from structural spinal disorders; and its use for screening for relative and absolute contraindications for introducing various force vectors into the spine via manual spinal manipulation, postural traction, corrective exercises, and so on. Spinal radiography today stands as the most practical method to aid in the delivery of quality and superior, nonsurgical patient treatment for spinal disorders.
Risk to Benefit Ratio Is Always Beneficial for Routine Radiography
It may be more dangerous to not get a diagnostic imaging procedure when indicated than to “err on the side of caution” by avoiding the phantom radiation risk from trivial exposures. In fact,
actual risk arises from radiophobia through patient’s fear-driven imaging avoidance and physician-recommended substitution of alternate procedures . . , true iatrogenic risk arises not only from such alternative procedures but also from misdiagnoses that are secondary either to patient refusal of medically indicated imaging or to nondiagnostic scans resulting from insufficient exposure.48
Brody and Guillerman argue that there may be more risks with alternative imaging such as the need for sedation for pediatrics, geriatrics, or claustrophobic patients referred for MRI.108 Further, exposures to general anesthesia have been deemed potentially detrimental to the cognitive development in the young and may contribute to accelerated cognitive decline in elderly individuals,109 rendering MRI much more risky than radiography for certain patient populations.
Discovery of “incidental findings” (IFs) or previously undiagnosed medical conditions that are discovered unintentionally during radiography alone may confer a favorable risk–benefit balance. Rogers et al found the incidence of IF in children who had a CT scan for blunt head trauma to be 4%; importantly, 1% “warranted immediate intervention or outpatient follow-up.”110
Beck et al determined that the incidence of serious IFs (as found on full-spine radiographs in an outpatient clinic) deemed as “absolute contraindications” for manual therapy were not infrequent; these included fracture (6.6%; 1 in 15), malignant tumor (0.8%-3.1%; 1 in 32 to 1 in 125), abdominal aortic aneurysm (0.8%; 1 in 125), and atlantoaxial instability (0.6%; 1 in 167).99 Also, as stated previously, up to 91% of patients may contain anomalies or pathologies, as determined by X-ray, that would alter originally intended treatment approaches.100
As noted, IFs are common, particularly in their importance for manual therapy approaches to spinal disorders. Even though the incidence of serious IFs may be low, they do exist. This is particularly concerning for the incidence of malignant tumors. Cancer rates are continuing to rise,111 and with this trend will be the increased odds of discovering malignancies as IFs on spinal imaging. This raises the issue of medicolegal implications and liability concerns from failing to identify a serious IF. Therefore, the treating provider should always be comprehensive in their assessment and include radiography when indicated, as goes the old adage “no X-rays, no defense.”112
Further, standing radiography cannot be replaced by alternate methods. For example, CT scans and MRIs are most often performed in the recumbent position and postural data like sagittal balance, and exact spinal curve measurements will not be physiologic. For example, the measurement of lumbar lordosis differs between neutral standing and laying supine113; therefore, typical MRI lordosis data (in recumbent position) will be nonphysiologic and not confer useful information for spine therapists.114
A final argument is that since all mammals possess adaptive response mechanisms to overcompensate for radiation exposures in the levels given by routine radiography (as well as CT scans) and that these levels are not only within and below background radiation levels but also lower than optimal hormetic health-enhancing levels, thus, radiation exposures to our patients only present a zero risk—the equivalent of a “benefit to benefit ratio.”
If there is zero risk, then that leaves only benefit in a risk to benefit ratio. Therefore, as long as an imaging procedure can provide meaningful data in terms of diagnosis, differential diagnosis, monitoring treatment progress, IFs, patient satisfaction, and so on, the benefit will always outweigh a risk of zero.
Conclusion
Contemporary patient-specific spine and posture rehabilitation dictates routine radiography use to achieve superior patient outcomes. Common radiography exposures are below background levels and stimulate innate adaptive protection and are not harmful; they induce only 1 one-millionth the cellular damage than breathing air. All cancer risk estimates and dose minimizing campaigns including the ALARA concept are underpinned by the LNT hypothesis and are deceitful and fear-mongering. Radiography is a safe imaging method for routine use in patient assessment, screening, diagnosis, and biomechanical analysis and for monitoring treatment progress in daily clinical practice.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.E.H. teaches spine rehabilitation methods and sells products to physicians for patient care that requires radiography for biomechanical analysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P.A.O. is paid by CBP NonProfit, Inc for writing the manuscript.
ORCID iD: Paul A. Oakley  http://orcid.org/0000-0002-3117-7330
http://orcid.org/0000-0002-3117-7330
References
- 1. Chun SW, Lim CY, Kim K, Hwang J, Chung SG. The relationships between low back pain and lumbar lordosis: a systematic review and meta-analysis. Spine J. 2017;17(8):1180–1191. [DOI] [PubMed] [Google Scholar]
- 2. Núñez-Pereira S, Hitzl W, Bullmann V, Meier O, Koller H. Sagittal balance of the cervical spine: an analysis of occipitocervical and spinopelvic interdependence, with C-7 slope as a marker of cervical and spinopelvic alignment. J Neurosurg Spine. 2015;23(1):10: 16–23. [DOI] [PubMed] [Google Scholar]
- 3. Lee SH, Son ES, Seo EM, Suk KS, Kim KT. Factors determining cervical spine sagittal balance in asymptomatic adults: correlation with spinopelvic balance and thoracic inlet alignment. Spine J. 2015;15(4):705–712. [DOI] [PubMed] [Google Scholar]
- 4. Scheer JK, Tang JA, Smith JS, et al. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine. 2013;19(2):141–159. [DOI] [PubMed] [Google Scholar]
- 5. Bess S, Protopsaltis TS, Lafage V, et al. Clinical and radiographic evaluation of adult spinal deformity. Clin Spine Surg. 2016;29(1):6–16. [DOI] [PubMed] [Google Scholar]
- 6. Harrison DD, Cailliet R, Janik TJ, Troyanovich SJ, Harrison DE, Holland B. Elliptical modeling of the sagittal lumbar lordosis and segmental rotation angles as a method to discriminate between normal and low back pain subjects. J Spinal Disord. 1998;11(5):430–439. [PubMed] [Google Scholar]
- 7. Harrison DD, Harrison DE, Janik TJ, et al. Modeling of the sagittal cervical spine as a method to discriminate hypolordosis: results of elliptical and circular modeling in 72 asymptomatic subjects, 52 acute neck pain subjects, and 70 chronic neck pain subjects. Spine. 2004;29(22):2485–2492. [DOI] [PubMed] [Google Scholar]
- 8. Oakley PA, Harrison DD, Harrison DE, Haas JW, et al. Evidence-based protocol for structural rehabilitation of the spine and posture: review of clinical biomechanics of posture (CBP) publications. J Can Chiropr Assoc. 2005;49(5):270–296. [PMC free article] [PubMed] [Google Scholar]
- 9. Yu M, Silvestre C, Mouton T, Rachkidi R, Zeng L, Roussouly P. Analysis of the cervical spine sagittal alignment in young idiopathic scoliosis: a morphological classification of 120 cases. Eur Spine J. 2013;22(11):2372–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Huec JC, Saddiki R, Franke J, Rigal J, Aunoble S. Equilibrium of the human body and the gravity line: the basics. Eur Spine J. 2011;20(suppl 5):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moustafa IM, Diab AA. The effect of adding forward head posture corrective exercises in the management of lumbosacral radiculopathy: a randomized controlled study. J Manip Physiol Ther. 2015;38(3):167–178. [DOI] [PubMed] [Google Scholar]
- 12. Kim TW, An DI, Lee HY, Jeong HY, Kim DH, Sung YH. Effects of elastic band exercise on subjects with rounded shoulder posture and forward head posture. J Phys Ther Sci. 2016;28(6):1733–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park HC, Kim YS, Seok SH, et al. The effect of complex training on the children with all of the deformities including forward head, rounded shoulder posture, and lumbar lordosis. J Exerc Rehabil. 2014;10(3):172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wontae Gong W, Hwang Bo G, Lee Y. The effects of Gong’s mobilization on cervical lordosis, forward head posture, and cervical ROM in abnormal posture of the cervical spine of college students. J Phys Ther Sci. 2011;23:531–534. [Google Scholar]
- 15. Wontae Gong W. The effects of cervical joint manipulation, based on passive motion analysis, on cervical lordosis, forward head posture, and cervical ROM in university students with abnormal posture of the cervical spine. J Phys Ther Sci. 2015;27(5):1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wickstrom BM, Oakley PA, Harrison DE. Non-surgical relief of cervical radiculopathy through reduction of forward head posture and restoration of cervical lordosis: a case report. J Phys Ther Sci. 2017;29(8):1472–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moustafa IM, Diab AA, Taha S, Harrison DE, et al. Addition of a Sagittal Cervical Posture Corrective Orthotic Device to a Multimodal Rehabilitation Program improves short- and long-term outcomes in patients with discogenic cervical radiculopathy. Arch Phys Med Rehabil. 2016;97(12):2034–2044. [DOI] [PubMed] [Google Scholar]
- 18. Moustafa IM, Diab AA, Harrison DE. The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: a 1-year randomized controlled study. Eur J Phys Rehabil Med. 2017;53(1):57–71. [DOI] [PubMed] [Google Scholar]
- 19. Harrison DE, Cailliet R, Harrison DD, Janik TJ, Holland B. A new 3-point bending traction method for restoring cervical lordosis and cervical manipulation: a nonrandomized clinical controlled trial. Arch Phys Med Rehabil. 2002;83(4):447–453. [DOI] [PubMed] [Google Scholar]
- 20. Harrison DE, Harrison DD, Betz JJ, et al. Increasing the cervical lordosis with chiropractic biophysics seated combined extension-compression and transverse load cervical traction with cervical manipulation: nonrandomized clinical control trial. J Manip Physiol Ther. 2003;26(3):139–151. [DOI] [PubMed] [Google Scholar]
- 21. Moustafa IM, Diab AAM, Hegazy FA, et al. Does rehabilitation of cervical lordosis influence sagittal cervical spine flexion extension kinematics in cervical spondylotic radiculopathy subjects? J Back Musculoskelet Rehabil. 2017;30(4):937–941. [DOI] [PubMed] [Google Scholar]
- 22. Oakley PA, Jaeger JO, Brown JE, et al. The CBP® mirror image® approach to reducing thoracic hyperkyphosis: a case series. J Phys Ther Sci. 2017;30:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katzman WB, Vittinghoff E, Lin F, et al. Targeted spine strengthening exercise and posture training program to reduce hyperkyphosis in older adults: results from the study of hyperkyphosis, exercise, and function (SHEAF) randomized controlled trial. Osteoporos Int. 2017;28(10):2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katzman WB, Sellmeyer DE, Stewart AL, Wanek L, Hamel KA. Changes in flexed posture, musculoskeletal impairments, and physical performance after group exercise in community-dwelling older women. Arch Phys Med Rehabil. 2007;88(2):192–199. [DOI] [PubMed] [Google Scholar]
- 25. Itoi E, Sinaki M. Effect of back-strengthening exercise on posture in healthy women 49 to 65 years of age. Mayo Clin Proc. 1994;69(11):1054–1059. [DOI] [PubMed] [Google Scholar]
- 26. Ball JM, Cagle P, Johnson BE, Lucasey C, Lukert BP. Spinal extension exercises prevent natural progression of kyphosis. Osteoporos Int. 2009;20(3):481–489. [DOI] [PubMed] [Google Scholar]
- 27. Kamali F, Shirazi SA, Ebrahimi S, Mirshamsi M, Ghanbari A. Comparison of manual therapy and exercise therapy for postural hyperkyphosis: a randomized clinical trial. Physiother Theory Pract. 2016;32(2):92–97. [DOI] [PubMed] [Google Scholar]
- 28. Miller JE, Oakley PA, Levin SB, Harrison DE, et al. Reversing thoracic hyperkyphosis: a case report featuring Mirror Image® thoracic extension rehabilitation. J Phys Ther Sci. 2017;29(7):1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fortner MO, Oakley PA, Harrison DE. Treating ‘slouchy’ (hyperkyphosis) posture with chiropractic biophysics®: a case report utilizing a multimodal Mirror Image® rehabilitation program. J Phys Ther Sci. 2017;29(8):1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell JR, Oakley PA, Harrison DE. Nonsurgical correction of straight back syndrome (thoracic hypokyphosis), increased lung capacity and resolution of exertional dyspnea by thoracic hyperkyphosis Mirror Image® traction: a CBP® case report. J Phys Ther Sci. 2017;29(11):2058–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Betz JW, Oakley PA, Harrison DE. Relief of exertional dyspnea and spinal pains by increasing the thoracic kyphosis in straight back syndrome (thoracic hypo-kyphosis) using CBP® methods: a case report with long-term follow-up. J Phys Ther Sci. 2017;30(1):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrison DE, Cailliet R, Harrison DD, et al. Changes in sagittal lumbar configuration with a new method of extension traction: nonrandomized clinical controlled trial. Arch Phys Med Rehabil. 2002;83(11):1585–1591. [DOI] [PubMed] [Google Scholar]
- 33. Paulk GP, Harrison DE. Management of a chronic lumbar disk herniation with chiropractic biophysics methods after failed chiropractic manipulative intervention. J Manip Physiol Ther. 2004;27(9):579.e1–e7. [DOI] [PubMed] [Google Scholar]
- 34. Moustafa IM, Diab AA. Extension traction treatment for patients with discogenic lumbosacral radiculopathy: a randomized controlled trial. Clin Rehab. 2012;27(1):51–62. [DOI] [PubMed] [Google Scholar]
- 35. Diab AA, Moustafa IM. Lumbar lordosis rehabilitation for pain and lumbar segmental motion in chronic mechanical low back pain. J Manip Physiol Ther. 2012;35(4):246–253. [DOI] [PubMed] [Google Scholar]
- 36. Diab AAM, Moustafa IM. The efficacy of lumbar extension traction for sagittal alignment in mechanical low back pain: a randomized trial. J Back Musculoskelet Rehabil. 2013;26(2):213–220. [DOI] [PubMed] [Google Scholar]
- 37. Oakley PA, Harrison DE. Lumbar extension traction and disk herniation/sequestration: a CBP case report. J Phys Ther Sci. 2017;29(11):2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noh DK, You JS, Koh JH, et al. Effects of novel corrective spinal technique on adolescent idiopathic scoliosis as assessed by radiographic imaging. J Back Musculoskelet Rehabil. 2014;27(3):331–338. [DOI] [PubMed] [Google Scholar]
- 39. Monticone M, Ambrosini E, Cazzaniga D, Rocca B, Ferrante S. Active self-correction and task-oriented exercises reduce spinal deformity and improve quality of life in subjects with mild adolescent idiopathic scoliosis. Results of a randomised controlled trial. Eur Spine J. 2014;23:1204–1214. [DOI] [PubMed] [Google Scholar]
- 40. Harrison DE, Oakley PA. Scoliosis deformity reduction in adults: a CBP® Mirror Image® case series incorporating the ‘non-commutative property of finite rotation angles under addition’ in five patients with lumbar and thoraco-lumbar scoliosis. J Phys Ther Sci. 2017;29(11):2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haggard JS, Haggard JB, Oakley PA, Harrison DE. Reduction of progressive thoracolumbar adolescent idiopathic scoliosis by Chiropractic BioPhysics® (CBP®) Mirror Image® methods following failed traditional chiropractic treatment: a case report. J Phys Ther Sci. 2017;29(11):2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol). 2011;23(4):244–250. [DOI] [PubMed] [Google Scholar]
- 44. Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62(2):75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 47. Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289–296. [DOI] [PubMed] [Google Scholar]
- 48. Siegel JA, Pennington CW, Sacks B. Subjecting radiologic imaging to the linear no-threshold hypothesis: a non sequitur of non-trivial proportion. J Nucl Med. 2017;58(1):1–6. [DOI] [PubMed] [Google Scholar]
- 49. Sacks B, Siegel JA. Preserving the anti-scientific linear no-threshold myth: authority, agnosticism, transparency, and the standard of care. Dose Response. 2017;15(3): doi:10.1177/1559325817717839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Committee to Assess Health Risks from Exposure to Low Levels Of Ionizing Radiation; National Research Council. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 51. Sacks B, Meyerson G, Siegel JA. Epidemiology without biology: false paradigms, unfounded assumptions, and specious statistics in radiation science (with commentaries by Inge Schmitz-Feuerhake and Christopher Busby and a reply by the authors). Biol Theory. 2016;11:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Health Physics Society. Risk assessment. Health Physics Society Position Statement. 1995. Updated April, 1995 http://hps.org/documents/riskassessment_ps008-1.pdf. Accessed February 5, 2018.
- 53. Socol Y, Dobrzyński L, Doss M, et al. Commentary: ethical issues of current health-protection policies on low-dose ionizing radiation. Dose Response. 2013;12(2):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. González AJ, Akashi M, Boice JD, Jr, et al. Radiological protection issues arising during and after the Fukushima nuclear reactor accident. J Radiol Prot. 2013;33(3):497–571. [DOI] [PubMed] [Google Scholar]
- 55. Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. [DOI] [PubMed] [Google Scholar]
- 56. Andronikou S. Letting go of what we believe about radiation and the risk of cancer in children. Pediatr Radiol. 2017;47(1):113–115. [DOI] [PubMed] [Google Scholar]
- 57. Doss M. Should the ALARA concept and the image gently campaign be terminated? Paper presented at: International Pediatric Radiology; May 17, 2016; Chicago, IL http://www.pedrad.org/LinkClick.aspx?fileticket=3E-HiVxngKs%3d&portalid=5. Accessed February 14, 2018. [Google Scholar]
- 58. Calabrese EJ. The mistaken birth and adoption of LNT: an abridged version. Dose Response. 2017;15(4):doi:10.1177/1559325817735478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calabrese EJ. The threshold vs LNT showdown: dose rate findings exposed flaws in the LNT model. Part 1. The Russell-Muller debate. Environ Res. 2017;154:435–451. [DOI] [PubMed] [Google Scholar]
- 60. Calabrese EJ. The threshold vs LNT showdown: dose rate findings exposed flaws in the LNT model. Part 2. How a mistake led BEIR I to adopt LNT. Environ Res. 2017;154:452–458. [DOI] [PubMed] [Google Scholar]
- 61. Calabrese EJ. Obituary notice: LNT dead at 89 years, a life in the spotlight. Environ Res. 2017;155:276–278. [DOI] [PubMed] [Google Scholar]
- 62. Siegel JA, Sacks B. Eliminating use of the linear no-threshold assumption in medical imaging. J Nucl Med. 2017;58(6):1014–1015. [DOI] [PubMed] [Google Scholar]
- 63. Siegel JA, Sacks B, Welsh JS. Time to terminate LNT: radiation regulators should adopt LT. J Radiol Oncol. 2017;1:49–53. [Google Scholar]
- 64. Siegel JA, Sacks B, Welsh JS, Time to eliminate LNT: The NRC needs to adopt LT and eliminate ALARA. Nucl Med Biomed Imaging. 2017;2:1–5. [Google Scholar]
- 65. Siegel JA, Sacks B, Pennington CW, et al. Dose optimization to minimize radiation risk for children undergoing CT and nuclear medicine imaging is misguided and detrimental. J Nucl Med. 2017;58(6):865–868. [DOI] [PubMed] [Google Scholar]
- 66. Doss M. Disavowing the ALARA concept in pediatric imaging. Pediatr Radiol. 2017;47(1):118. [DOI] [PubMed] [Google Scholar]
- 67. Cohen MD. Reply to Dr. Andronikou: disavowing the ALARA concept in pediatric imaging. Pediatr Radiol. 2017;47(1):116–117. [DOI] [PubMed] [Google Scholar]
- 68. Siegel JA, McCollough CH, Orton CG. Advocating for use of the ALARA principle in the context of medical imaging fails to recognize that the risk is hypothetical and so serves to reinforce patients’ fears of radiation. Med Phys. 2017;44(1):3–6. [DOI] [PubMed] [Google Scholar]
- 69. Feinendegen LE, Loken MK, Booz J, Mühlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995;13(suppl 1):7–20. [PubMed] [Google Scholar]
- 70. Feinendegen LE, Pollycove M, Neumann RD. . Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection In: Baum RP, ed. Therapeutic Nuclear Medicine. Heidelberg, Germany: Springer; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22(6):290–306. [DOI] [PubMed] [Google Scholar]
- 72. Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009;7:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Löbrich M, Rief N, Kühne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A. 2005;102(25):8984–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sen K, Sies H, Baeurle P. eds. Redox Regulation of Gene Expression. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 75. Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in signaling pathways. Biochem Soc Trans. 2001;29(2 pt):345–350. [DOI] [PubMed] [Google Scholar]
- 76. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lemon JA, Phan N, Boreham DR. Single CT scan prolongs survival by extending cancer latency in Trp53 heterozygous mice. Radiat Res. 2017;188(4.2):505–511. [DOI] [PubMed] [Google Scholar]
- 78. Lemon JA, Phan N, Boreham DR. Multiple CT scans extend lifespan by delaying cancer progression in cancer-prone mice. Radiat Res. 2017;188(4.2):495–504. [DOI] [PubMed] [Google Scholar]
- 79. International Atomic Energy Agency. Exposure to radiation from natural sources. Update December 9, 2014 http://www-ns.iaea.org/tech-areas/rw-ppss/exposure-to-natural-radiation.asp?s=3. Accessed October 23, 2017.
- 80. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; National Research Council. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 81. Canadian Nuclear Safety Commission. Natural background radiation in Canada. (Last modified 2/3/2014). http://nuclearsafety.gc.ca/eng/resources/maps-of-nuclear-facilities/background-radiation.cfm. Accessed October 23, 2017.
- 82. United Nations Scientific Committee on the Effects of Atomic Radiation 2008. Sources and Effects of Ionizing Radiation. New York, NY: United Nations; 2010:4. [Google Scholar]
- 83. Hendry JH, Simon SL, Wojcik A, et al. Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Protect. 2009;29(2A):A29–A42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dobrzyński L, Fornalski KW, Feinendegen LE. Cancer mortality among people living in areas with various levels of natural background radiation. Dose Response. 2015;13(3):doi:10.1177/1559325815592391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Siegel JA, Welsh JS. Does imaging technology cause cancer? Debunking the linear no-threshold model of radiation carcinogenesis. Technol Cancer Res Treat. 2016;15(2):249–256. [DOI] [PubMed] [Google Scholar]
- 86. Ghiassi-nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health physics. 2002;82(1):87–93 [DOI] [PubMed] [Google Scholar]
- 87. Dobrzyński L, Fornalski KW, Feinendegen LE. Cancer mortality among people living in areas with various levels of natural background radiation. Dose-Response. 2015;13(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Giordano BD, Grauer JN, Miller CP, Morgan TL, Rechtine GR., 2nd Radiation exposure issues in orthopaedics. J Bone Joint Surg Am. 2011;93(12):e69.1–10. [DOI] [PubMed] [Google Scholar]
- 89. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oakley PA, Harrison DD, Harrison DE, Haas JW. On “phantom risks” associated with diagnostic ionizing radiation: evidence in support of revising radiography standards and regulations in chiropractic. J Can Chiropr Assoc. 2005;49(4):264–269. [PMC free article] [PubMed] [Google Scholar]
- 91. Boutis K, Cogollo W, Fischer J, Freedman SB, Ben David G, Thomas KE. Parental knowledge of potential cancer risks from exposure to computed tomography. Pediatrics. 2013;132(2):305–311. [DOI] [PubMed] [Google Scholar]
- 92. Klein MA. Reuse and reduce: abdominal CT, lumbar spine MRI, and a potential 1.2 to 3.4 billion dollars in cost savings. Abdom Radiol (NY). 2017;42(12):2940–2945. [DOI] [PubMed] [Google Scholar]
- 93. Hui SC, Pialasse JP, Wong JY, et al. Radiation dose of digital radiography (DR) versus micro-dose x-ray (EOS) on patients with adolescent idiopathic scoliosis: 2016 SOSORT- IRSSD “John Sevastic Award” Winner in Imaging Research. Scoliosis Spinal Disord. 2016;29;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hawes MC, O’brien JP. The transformation of spinal curvature into spinal deformity: pathological processes and implications for treatment. Scoliosis. 2006;31;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369(16):1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Emans JB, Hedequist D, Miller R, et al. Reference manual for the Boston scoliosis brace. Scoliosis Research Society. 2003. https://www.srs.org/UserFiles/file/bracing-manual/section5.pdf. Accessed January 20, 2018.
- 97. Moustafa IM, Diab AM, Ahmed A, et al. The efficacy of cervical lordosis rehabilitation for nerve root function, pain, and segmental motion in cervical spondylotic radiculopathy. PhysioTherapy. 2011;97(suppl):846–847. [Google Scholar]
- 98. Moustafa IM, Diab AA, Harrison DE. Does improvement towards a normal cervical sagittal configuration aid in the management of lumbosacral radiculopathy: a randomized controlled trial. In: Proceedings of the 13th World Federation of Chiropractic Biennial Congress/ECU Convention; May 13-16, 2015; Athens, Greece 157–158 (Mediterranean Region Award Winning Paper). [Google Scholar]
- 99. Beck RW, Holt KR, Fox MA, Hurtgen-Grace KL. Radiographic anomalies that may alter chiropractic intervention strategies found in a New Zealand population. J Manip Physiol Ther. 2004;27(9):554–559. [DOI] [PubMed] [Google Scholar]
- 100. Pryor M, McCoy M. Radiographic findings that may alter treatment identified on radiographs of patients receiving chiropractic care in a teaching clinic. J Chiropr Ed. 2006;20:93–94. [Google Scholar]
- 101. Bull PW. Relative and Absolute Contraindications to Spinal Manipulative Therapy Found on Spinal X-Rays. In: Proceedings of the World Federation of Chiropractic 7th Biennial Congress; 2003; Orlando, FL:376. [Google Scholar]
- 102. Kendrick D, Fielding K, Bentley E, Kerslake R, Miller P, Pringle M. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M. The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomized (unblinded) controlled trial. Health Technol Assess. 2001;5(30):1–69. [DOI] [PubMed] [Google Scholar]
- 104. Rockey PH, Tompkins RK, Wood RW, Wolcott BW. The usefulness of x-ray examinations in the evaluation of patients with back pain. J Fam Pract. 1978;7(3):455–465. [PubMed] [Google Scholar]
- 105. Sherman R. Chiropractic x-ray rationale. J Can Chiropr Assoc. 1986;30:33–35. [Google Scholar]
- 106. Deyo RA, Diehl AK, Rosenthal M. Reducing roentgenography use. Can patient expectations be altered? Arch Intern Med. 1987;147(1):141–145. [DOI] [PubMed] [Google Scholar]
- 107. Oakley PA, Harrison DE. Radiogenic cancer risks from chiropractic x-rays are zero: 10 reasons to take routine radiographs in clinical practice. Ann Vert Sublux Res. March 10, 2018:48–56. [Google Scholar]
- 108. Brody AS, Guillerman RP. Don’t let radiation scare trump patient care: 10 ways you can harm your patients by fear of radiation-induced cancer from diagnostic imaging. Thorax. 2014;69(8):782–784. [DOI] [PubMed] [Google Scholar]
- 109. Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rogers AJ, Maher CO, Schunk JE, et al. Incidental findings in children with blunt head trauma evaluated with cranial CT scans. Pediatrics. 2013;132(2):e356–e363. [DOI] [PubMed] [Google Scholar]
- 111. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sweat RW, Sweat MH. Guidelines for pre-and post-radiographs for care documentation. Today’s Chiropr. 1995;24:38–39, 60–61. [Google Scholar]
- 113. Meakin JR, Gregory JS, Aspden RM, Smith FW, Gilbert FJ. The intrinsic shape of the human lumbar spine in the supine, standing and sitting postures: characterization using an active shape model. J Anat. 2009;215(2):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oakley PA, Harrison DE. Reply to “Lumbar lordosis: study of patients with and without low back pain.” Clin Anat. 2004;17(4):367. [DOI] [PubMed] [Google Scholar]