Abstract
Background
We have developed a simple lipoaspirate washing method using a coffee filter to eliminate liposuction noxious material before isolating adipose tissue derived mesenchymal stem cells (AT-MSCs), and used them in clinical trials. Before administration to patients, MSCs are usually suspended in physiologic saline. However, MSCs only survive for a limited time in physiologic saline. Therefore, alternative solution that can preserve MSC survival will be beneficial. Therefore, the purpose of this study was to compare the use of physiologic saline and Dulbecco’s modified Eagle’s Medium (DMEM) as temporary storage solution for our AT-MSCs.
Methods
We did viability assessments of AT MSCs after 0, 3, 6, 24, 48, 72, and 96 hours suspended in physiologic saline compared to DMEM, and stored at 4 ℃. Further proliferation capacities of the cells after various suspension times were assessed. All viability and proliferation capacity assessments were done in four replications. Differences between the various suspension time in terms of viability and proliferation capacity were compared and tested by ANOVA or Kruskal-Wallis test.
Results
Viability was >70% after 48 hours in physiologic saline and 24 hours in DMEM, which showed that physiologic saline was superior compared to DMEM. Increase in PDT began to be significant compared to initial PDT after 24 hours in both physiologic saline, and DMEM.
Conclusions
For our AT-MSCs, physiologic saline was superior to DMEM, and storage should not exceed 24 hours.
Keywords: Adipose tissue, mesenchymal stem cell (MSC), storage solution, viability, population doubling time (PDT)
Introduction
Adipose tissue as a source for multipotent mesenchymal stem cells has many advantages over bone marrow. Adipose tissue collection from patient for autologous use is easier, less invasive and painful compared to bone marrow puncture. Moreover, lipoaspirate waste from obese person who wish to lose their excess fat can be used as allogeneic source of MSCs. Adipose tissue derived MSCs (AT-MSCs) showed subtle differences from bone marrow derived MSCs (BM-MSCs) (1). However, the same as BM-MSCs, they can differentiate into various types of cells, such as chondrocytes, osteocytes and adipocytes (1,2). Moreover, MSCs might release paracrine factors that might help in the healing of various disease conditions (3).
We have developed a simple lipoaspirate washing method using a coffee filter to eliminate the solutions that are used to facilitate liposuction procedure. Before further processing and isolating adipose tissue derived mesenchymal stem cells (AT-MSCs), lipoaspirate should be free from liposuction solution as the solution is toxic to cells (4). We have used the simple washing method processed AT-MSC in ongoing clinical trials. Before administration to patients, MSCs are usually suspended in physiologic saline. However, MSCs only survive for a limited time in physiologic saline (5). Therefore, an alternative solution that can preserve MSC survival will be beneficial. Therefore, the purpose of this study was to compare the use of physiologic saline and Dulbecco’s modified Eagle’s Medium (DMEM) as temporary storage solution for our AT-MSCs.
Methods
This was an in vitro analytical study, which was conducted in Stem Cell Medical Technology Integrated Service Unit, RSCM/Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia, from June through December 2015. Ethical clearance was obtained from Faculty of Medicine Universitas Indonesia Ethical Committee in 2014 and was amended to get prolonged (ethical clearance number: 157/H2. F1/ETIK/2014), which work conformed to the provisions of in accordance with the Helsinki Declaration as revised in 2013.
Sample
The sample (one) was cryopreserved pre-characterized human AT-MSCs, which were processed and isolated using the simple washing method using a coffee filter (4), and stored at P1 in Stem Cell Medical Technology Integrated Service Unit, RSCM/Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia. The sample was derived from femoral fat that was donated by a 35-year male, who underwent an orthopedic surgery due to femoral fracture.
Procedures
Cryopreserved AT-MSCs were thawed and recultured as previously described (6), in five T25 flasks. The cells were attached on plastic culture ware and showed fibroblastic morphology (Figure 1). After confluence, the cells were harvested, pooled, and counted. Cell suspension was homogenized and divided in two equal numbers and transferred into two Eppendorf tubes. Then the tubes were centrifuged at 2,500 rpm, and the cell pellets were each resuspended in 1 mL of either physiologic saline (B Braun, Ecosol NaCl), or DMEM (Gibco, cat. 31600-034 1X), kept in 4 ℃, and used to assess the viability and proliferation capacity.
Figure 1.
Morphology of cultured mesenchymal stem cells.
Viability assessments
For both physiologic saline and DMEM suspended AT-MSCs, viability assessments were done by Trypan blue (Gibco, cat. 15250-061) exclusion method in four replications. Assessments were done at 0, 3, 6, 24, 48, 72, and 96 hours. Viability was calculated by dividing viable with total cell number.
Proliferation capacity assessments
Proliferation capacities were assessed by computing the population doubling time (PDT) (7). PDT was assessed at 0, 3, 6, 24, 48, 72, and 96 hours, in four replications, for both physiologic saline and DMEM suspended AT-MSCs.
Data collection and analysis
Data collected were viability and PDT at 0, 3, 6, 24, 48, 72, and 96 hours, for both physiologic saline and DMEM suspended AT-MSCs. Data were tabulated and means and standard deviations of viability and PDT at 0, 3, 6, 24, 48, 72, and 96 hours were calculated and presented as viability and PDT tables. Further, decrease in viability, and increase in PDT at 3, 6, 24, 48, 72, and 96 hours from their initial viability and PDT (100%) were compared between physiologic saline and DMEM suspended AT-MSCs and were presented as graphs.
Differences between the various time points in terms of viability and PDT were compared. ANOVA was used when the data was normally distributed and the variance was homogenous, while Kruskal-Wallis test was used when the data was unsuitable for ANOVA. Further, differences in viability decrease and PDT increase at various time points between physiologic saline and DMEM were compared. Student t-test was used when the data was normally distributed and the variance was homogenous, while Mann-Whitney test was used when the data was unsuitable for Student t-test. Data analysis was done using SPSS 20.0.
Results
Viability and proliferation capacity of AT-MSCs at 0, 3, 6, 24, 48, 72, and 96 hours after they were suspended in physiologic saline and DMEM can be seen in Tables 1,2 respectively. Significant differences in cell viability after they were suspended in physiologic saline and DMEM compared to their initial viability were shown by asterix.
Table 1. Viability of AT-MSCs at various time points in physiologic saline and DMEM.
| Time points | Viability (%) | ||||||
|---|---|---|---|---|---|---|---|
| Physiologic saline | DMEM | ||||||
| Mean | SD | P | Mean | SD | P | ||
| Initial | 91.29 | 2.17 | – | 92.79 | 2.21 | – | |
| 3 hours | 92.30 | 3.86 | 1.00 | 92.03 | 3.94 | 1.00 | |
| 6 hours | 80.18 | 5.32 | 0.02 | 90.36 | 4.78 | 0.55 | |
| 24 hours | 81.78 | 7.32 | 0.04 | 70.79 | 6.02 | 0.02 | |
| 48 hours | 70.20 | 2.97 | 0.02 | 62.78 | 3.13 | 0.02 | |
| 72 hours | 59.94 | 2.44 | 0.02 | 74.13 | 15.05 | 0.02 | |
| 96 hours | 28.80 | 3.07 | 0.02 | 48.32 | 12.12 | 0.02 | |
SD, standard deviation; P, level of significance compared to initial viability; AT-MCSs, adipose tissue derived mesenchymal stem cells.
Table 2. Population doubling time of AT-MSCs at various time points in physiologic saline and DMEM.
| Time points | Population doubling time (hours) | ||||||
|---|---|---|---|---|---|---|---|
| Physiologic saline | DMEM | ||||||
| Mean | SD | P | Mean | SD | P | ||
| Initial | 26.59 | 4.70 | – | 30.26 | 4.88 | – | |
| 3 hours | 35.22 | 16.19 | 0.39 | 24.75 | 3.88 | 0.08 | |
| 6 hours | 24.85 | 2.29 | 0.77 | 46.70 | 27.69 | 1.00 | |
| 24 hours | 46.93 | 7.97 | 0.04 | 48.75 | 8.33 | 0.02 | |
| 48 hours | 47.05 | 12.14 | 0.14 | 207.81 | 100.38 | 0.02 | |
| 72 hours | 69.45 | 6.53 | 0.06 | NA | NA | NA | |
| 96 hours | 248.22 | 16.20 | 0.02 | NA | NA | NA | |
SD, standard deviation; P, level of significance compared to initial population doubling time; NA, not applicable due to no proliferation capacity; AT-MCSs, adipose tissue derived mesenchymal stem cells.
Comparison of viability and proliferation capacity between physiologic saline and DMEM suspended AT-MSCs at 3, 6, 24, 48, 72, and 96 hours can be seen in Figures 2,3. P values showed the significant differences.
Figure 2.
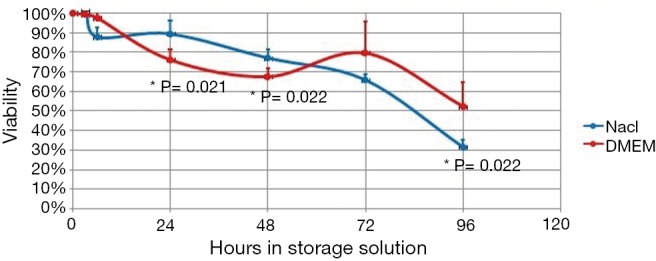
Comparison of viability between physiologic saline and DMEM suspended AT-MSCs after various time points. *, Student t-test P values showed significant differences. AT-MCSs, adipose tissue derived mesenchymal stem cells.
Figure 3.
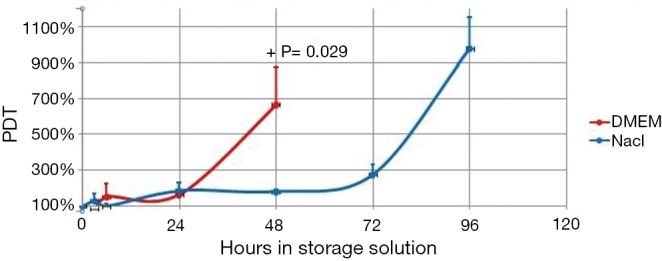
Comparison of proliferation capacity between physiologic saline and DMEM suspended AT-MSCs after various time points. +, A non-parametric test P value showed a significant difference only at 48 hours. AT-MCSs, adipose tissue derived mesenchymal stem cells.
Discussion
Our result showed that viability decrease began to be statistically significant compared to initial viability after 6 hours in physiologic saline and 24 hours in DMEM, though viability was still more than 70% after 48 hours in physiologic saline and 24 hours in DMEM (Table 1). To be used in cell therapy, FDA has set a criteria that cell viability should be at least 70% (8). Therefore, AT-MSCs that are stored in DMEM or physiologic saline should be administered before 24 or 48 hours, respectively, and after 48 hours physiologic saline is superior compared to DMEM as storage solution (Table 1). Moreover, Figure 1 showed that at 24 and 48 hours physiologic saline was significantly better compared to DMEM as storage solution. However, Table 1 showed that at 6 hours, DMEM was better than physiologic saline, though the difference was not significant (Figure 1).
Our previous study on umbilical cord MCSs (UC-MSCs), which compared physiologic saline, phosphate buffered saline (PBS) and high glucose DMEM (DMEM-HG) showed that viability was >70% up to 72 hours in physiologic saline, and up to 96 hours in DMEM-HG, so the best storage solution up to 96 hours was DMEM-HG. However, after 168 hours, viability in DMEM-HG dropped considerably and was below the viability in physiologic saline (5). Moreover, PDT in DMEM-HG could not be assessed due to failure to attach and proliferate, while in physiologic saline and PBS the cells still proliferate though the PDT increased considerably. We supposed that after 168 hours, there was exhaustion of cell metabolism due to the high glucose content (5). Therefore, in this study we changed DMEM-HG to DMEM, but the result of this study showed that DMEM was inferior to physiologic saline, after 24 hours.
Other studies on physiologic saline as storage solution in the same temperature as our study showed variable results (9-11). Ra et al. (9) found that average AT-MSC viability was 85.4% after 72 hours, which was better than our study. Veronesi et al. (10) found that after 18 hours in physiologic saline, viability of bone marrow MSCs (BM-MSCs) was 83% that was more or less the same as our study. Sohn et al. (11) found that after 6 hours, viability of BM-MSCs was >85% that was slightly better than our study. Moreover, they assessed viability by Annexin V/PI, which also detect early apoptotic cells as dead cells and resulted in lower viable cell result compared to trypan blue exclusion method, which was used in this study.
Various other studies on various diseases administered various kind of cells that were suspended in various solutions, but those studies did neither check the viability nor the proliferation capacity of the cells at various time points in those solutions (12-17). Therefore, comparison with our result was not possible.
Compared to initial PDT, PDT began to increase significantly after 24 hours in both physiologic saline and DMEM (Table 2). However, the difference between initial PDT and after 48 and 72 hours in physiologic saline was not statistically significant, though the PDTs were increased considerably. This fact was due to the large standard deviation (Table 2).
In our study, for cells stored in DMEM viability corresponded with PDT result, where both showed significant decrease in viability and increase in PDT after 24 hours. However, for those in physiologic saline, viability did not correspond with PDT, where viability dropped significantly at 6 hours and beyond, though after 48 hours the viability was still >70%, while PDT increased significantly after 24 hours (Table 2). However, significant difference in PDT between physiologic saline and DMEM only occurred at 48 hours (Figure 2). The inconsistent result in our study might be due to the low replication number that in some cases caused high standard deviations, which was the limitation of our study.
Another study by Sohn et al. (11) corroborated our study where viability did not correspond with attachment and proliferation capacity. In our study attachment and proliferation capacity was assessed by PDT, while in Sohn et al. study it was assessed by colony forming unit assay (11).
The discrepancy between the timing of PDT increase and viability decrease might be due to the viability assessment method in this study, which was by Trypan blue dye exclusion method. Trypan blue dye exclusion method can distinguish viable from dead cells, but not cells at early apoptosis, where the cell membrane is still intact. Another method of viability assessment is by using Annexin and propidium iodide (PI) staining, which included dead cells and cells in the process of dying, and therefore give a lower viability value compared to Trypan blue dye exclusion method (18). However, viable cells that were enumerated by Anexin V/PI also showed decrease in proliferation capacity (11), which shows that the viable cells might be non-functional.
Based on FDA guidelines (8), AT-MSCs still met FDA criteria after storage in DMEM or physiologic saline for 24 or 48 hours respectively. However, as PDT was significantly increased after 24 hours in both storage solutions, storage in DMEM or physiologic saline should not exceed 24 hours.
In conclusion, for cell therapy, viability requirement showed that our AT-MSCs could be stored for 48 hours in physiologic saline and 24 hours in DMEM respectively. However, to be functional, storage should not exceed 24 hours.
Acknowledgments
Funding: This work was supported by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Inovasi Perguruan Tinggi di Industri (grant number: 298/UN2.R3.2/HKP.05.00/perjanjian 2017).
Ethical Statement: Ethical clearance was obtained from Faculty of Medicine Universitas Indonesia Ethical Committee in 2014 and was amended to get prolonged (ethical clearance number: 157/H2. F1/ETIK/2014), which work conformed to the provisions of in accordance with the Helsinki Declaration as revised in 2013.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pawitan JA. Future research in adipose stem cell engineering. In: Illouz YG, Sterodimas A, editors. Adipose stem cells and Regenerative Medicine. Heidelberg: Springer, 2011; 257-72. [Google Scholar]
- 2.Pawitan JA. Prospect of adipose tissue derived stem cells in regenerative medicine. Cell Tissue Transplant Ther 2009;2:7-9. 10.4137/CTTT.S3654 [DOI] [Google Scholar]
- 3.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014;2014:965849. 10.1155/2014/965849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawitan JA, Liem IK, Suryani D, et al. Simple lipoaspirate washing using a coffee filter. Asian Biomed 2013;7:333-8. [Google Scholar]
- 5.Krishnanda SI, Agarwal R, Yausep OE, et al. Comparison of Various Solutions for Temporary Storage of Umbilical Cord Derived Mesenchymal Stem Cells. Annu Res Rev Biol 2017;21:1-8. 10.9734/ARRB/2017/38233 [DOI] [Google Scholar]
- 6.Pawitan JA, Goei N, Liem IK, et al. Effect of Cryopreservation and cumulative population doublings on Senescence of Umbilical Cord Mesenchymal Stem Cells. Int J Pharmtech Res 2017;10:109-13. [Google Scholar]
- 7.Suryani D, Pawitan JA, Lilianty J, et al. Comparison of FBS and PRP containing medium effects on human lipoaspirate-derived mesenchymal stem cell proliferation. Med J Indones 2013;22:146-51. 10.13181/mji.v22i3.583 [DOI] [Google Scholar]
- 8.US FDA. Guidance for FDA reviewers and sponsors: Content and review of chemistry, manufacturing, and control (CMC) information for human somatic cell therapy investigational new drug applications (INDs) (Draft). US FDA; 2008 April. Available online: http://www.fda.gov/OHRMS/DOCKETS/98fr/03d0349gdl.pdf
- 9.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011;20:1297-308. 10.1089/scd.2010.0466 [DOI] [PubMed] [Google Scholar]
- 10.Veronesi E, Murgia A, Caselli A, et al. Transportation conditions for prompt use of ex vivo expanded and freshly harvested clinical-grade bone marrow mesenchymal stromal/stem cells for bone regeneration. Tissue Eng Part C Methods 2014;20:239-51. 10.1089/ten.tec.2013.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn HS, Heo JS, Kim HS, et al. Duration of in vitro storage affects the key stem cell features of human bone marrow-derived mesenchymal stromal cells for clinical transplantation. Cytotherapy 2013;15:460-6. 10.1016/j.jcyt.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 12.Cox CS, Jr, Hetz RA, Liao GP, et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 2017;35:1065-79. 10.1002/stem.2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creane M, Howard L, O'Brien T, et al. Biodistribution and retention of locally administered human mesenchymal stromal cells: Quantitative polymerase chain reaction–based detection of human DNA in murine organs. Cytotherapy 2017;19:384-94. 10.1016/j.jcyt.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Manuguerra-Gagné R, Boulos PR, Ammar A, et al. Transplantation of Mesenchymal Stem Cells Promotes Tissue Regeneration in a Glaucoma Model Through Laser-Induced Paracrine Factor Secretion and Progenitor Cell Recruitment. Stem Cells 2013;31:1136-48. 10.1002/stem.1364 [DOI] [PubMed] [Google Scholar]
- 15.Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Invest Ophthalmol Vis Sci 2010;51:2051-9. 10.1167/iovs.09-4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead B, Hill LJ, Blanch RJ, et al. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016;18:487-96. 10.1016/j.jcyt.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Ye EA. Investigation of mesenchymal stem cells and the development of experimental strategies for rescuing glaucomatous eyes using a stem cell-based therapy. Dissertation. Paper 13496. 2013. Iowa State University, Ames, Iowa, USA. Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=4503&context=etd [Google Scholar]
- 18.François M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012;14:147-52. 10.3109/14653249.2011.623691 [DOI] [PMC free article] [PubMed] [Google Scholar]


