Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is a devastating disorder that is characterized by a progressive decline in renal function as a result of the development of fluid-filled cysts. Defective flow-mediated [Ca2+]i responses and disrupted [Ca2+]i homeostasis have been repeatedly associated with cyst progression in ADPKD. We have previously demonstrated that the transient receptor potential vanilloid type 4 (TRPV4) channel is imperative for flow-mediated [Ca2+]i responses in murine distal renal tubule cells. To determine whether compromised TRPV4 function contributes to aberrant Ca2+ regulation in ADPKD, we assessed TRPV4 function in primary cells that were cultured from ADPKD and normal human kidneys (NHKs). Single-channel TRPV4 activity and TRPV4-dependent Ca2+ influxes were drastically reduced in ADPKD cells, which correlated with distorted [Ca2+]i signaling. Whereas total TRPV4 protein levels were comparable in NHK and ADPKD cells, we detected a marked decrease in TRPV4 glycosylation in ADPKD cells. Tunicamycin-induced deglycosylation inhibited TRPV4 activity and compromised [Ca2+]i signaling in NHK cells. Overall, we demonstrate that TRPV4 glycosylation and channel activity are diminished in human ADPKD cells compared with NHK cells, and that this contributes significantly to the distorted [Ca2+]i dynamics. We propose that TRPV4 stimulation may be beneficial for restoring [Ca2+]i homeostasis in cyst cells, thereby interfering with ADPKD progression.—Tomilin, V., Reif, G. A., Zaika, O., Wallace, D. P., Pochynyuk, O. Deficient transient receptor potential vanilloid type 4 function contributes to compromised [Ca2+]i homeostasis in human autosomal-dominant polycystic kidney disease cells.
Keywords: distal tubule, polycystins, glycosylation
Polycystic kidney disease (PKD) is a group of genetic disorders that are characterized by the development and progressive enlargement of cysts in the kidneys, as well as by nonrenal manifestations, including liver cysts and various cardiovascular pathologies (1, 2). Autosomal-dominant PKD (ADPKD) is the most common, potentially lethal monogenic disorder, affecting 12.5 million people worldwide of all ethnic groups and is a major burden on public health (1, 3). ADPKD is caused by mutations in PKD1 and PKD2, genes that encode polycystin 1 (PC1) and PC2, respectively, which leads to cyst formation predominantly in the collecting ducts (CDs) (3, 4). Autosomal-recessive PKD (ARPKD) is caused by mutations of PKHD1 gene that encodes fibrocystin, which leads to cyst formation exclusively in the CDs (3–6). Evidence demonstrates impaired mechanosensitive [Ca2+]i responses and reduced basal [Ca2+]i levels in primary cultured and freshly isolated cyst cells of both ADPKD and ARPKD (7–11). Of interest, PC1, PC2, and fibrocystin physically interact to form a multiprotein complex that is thought to regulate [Ca2+]i levels (3, 12–15). It has been additionally demonstrated that decreased basal [Ca2+]i levels serve as a molecular switch for cellular responses to cAMP from antiproliferative in normal tubule cells to mitogenic via B-Raf/ERK signaling in cyst cells (11, 16–18). Thus, the identification of specific mechanisms that govern [Ca2+]i homeostasis and mechanosensitivity in cyst cells is essential for understanding PKD pathogenesis and the development of therapeutic approaches to prevent cyst initiation.
By elevating [Ca2+]i, transient receptor potential (TRP) channels can serve as mediators of numerous environmental stimuli, including temperature, chemical agents, mechanical forces, etc. (19). Ca2+-permeable TRP vanilloid type 4 (TRPV4)—one of the most recognized mechanosensitive channels—is activated by elevated flow and hypotonicity (20, 21). TRPV4 exhibits moderate Ca2+ selectivity (PCa/PNa of 6–10), which results in substantial Ca2+ influx across the plasma membrane upon activation (22). Whereas TRPV4 can be detected in many epithelial tissues, including lung, spleen, skin, sweat glands, as well as in other organs (23–27), expression of the channel is much higher in the kidney (26). We have previously demonstrated that TRPV4 is essential for mediating flow-dependent [Ca2+]i elevations in the connecting tubule and CD (28–30). Furthermore, it was reported that TRPV4 predominantly exists as a heterotetramer with PC2 in a 2:2 presumable stoichiometry (31) that forms a 23-pS Ca2+-permeable channel at the apical membrane of CD cells (32, 33). This heterotetrameric channel can be activated by mechanical stimuli, which produces higher Ca2+ influx than the homomeric TRPV4 channel (32, 34); however, the function of TRPV4 and its role in deficient [Ca2+]i balance in ADPKD remains unexplored.
In the current study, we combined Fura-2 ratiometric [Ca2+]i imaging with patch-clamp electrophysiology in primary cells that were cultured from normal human kidneys (NHKs) and ADPKD kidneys to elucidate the role of TRPV4 in human renal epithelial cells during normal physiology and ADPKD pathology. We found that TRPV4 is critical for flow-dependent [Ca2+]i responses in NHK cells and that channel activity positively correlates with resting [Ca2+]i levels. Moreover, we detected markedly decreased single-channel TRPV4 activity and TRPV4-dependent Ca2+ influx in ADPKD cells. This reduced activity was largely attributed to deficient channel glycosylation. Disruption of TRPV4 glycosylation compromised [Ca2+]i homeostasis and abolished flow-dependent [Ca2+]i responses in NHK cells, which eliminated the differences between NHK and ADPKD cells. We propose that direct activation of TRPV4 may restore [Ca2+]i and rescue cystic cells, thus providing a novel therapeutic approach for the treatment of ADPKD.
MATERIALS AND METHODS
Reagents and animals
All chemicals and materials were from MilliporeSigma (St. Louis, MO, USA), VWR (Radnor, PA, USA), and Tocris (Ellisville, MO, USA), unless noted otherwise, and were at least of reagent grade.
Primary cultures of human kidney epithelial cells
Normal regions of human kidneys—confirmed by histologic examination—were collected from nephrectomy specimens that were removed to treat renal carcinomas or withheld from transplantation. ADPKD kidneys were obtained from hospitals that participate in the Polycystic Kidney Research Retrieval Program with the assistance of the PKD Foundation (Kansas City, MO, USA). Samples were deidentified and assigned a unique number in the PKD Biomarkers and Biomaterials Core at the University of Kansas Medical Center. NHK or ADPKD cells were prepared as previously described (11, 35, 36). Use of specimens was approved by the institutional review board at the University of Kansas Medical Center. For experiments, we used 6 different NHK (K337, K340, K342, K373, K376, and K388) and ADPKD (K284, K288, K315, K348, K354, and K429) cell preparations. ADPKD cell preparations that were used in this study were from individuals age <60 yr. As PKD1 mutations account for the majority of clinical cases (∼85%) and individuals with PKD2 mutations have a milder phenotype and later onset of end-stage renal disease (median age, ∼54 yr for PKD1 vs. 74 yr for PKD2 mutations), it is likely that all cell preparations used in the study were from patients with PKD1 mutations (37). At least 4 different NHK and ADPKD cell preparations were used for each experimental set. Cells were grown on glass coverslips to confluency in DMEM/F12 medium that was supplemented with 5% fetal bovine serum, 5 µg/ml insulin, 5 µg/ml transferrin, and 5 ng/ml sodium selenite, as previously described (11, 35, 36). Primary cultures of NHK and ADPKD cells demonstrate prominent staining with Dolichos biflorus lectin, which indicates that cultures are enriched in CD cells (35). Tunicamycin (5 µg/ml) was added directly to the medium for 24 h before the experiments, as necessary.
[Ca2+]i imaging
[Ca2+]i levels were measured in individual NHK and ADPKD cells by using Fura-2 fluorescence ratiometric imaging, as described previously (10, 30, 38). In brief, cells were loaded with Fura-2 by incubation with 2 μM Fura-2/AM in a bath solution for 40 min at room temperature. Cells were subsequently washed and incubated for an additional 10–15 min before experimentation. A coverslip that contained the cells was placed in an open-top imaging study chamber (RC-26GLP; Warner Instruments, Hamden, CT, USA) with a bottom coverslip viewing window. The chamber was attached to the microscope stage of a Nikon Ti-S Wide-Field Fluorescence Imaging System (Nikon Instruments, Melville, NY, USA) that was integrated with a Lambda XL light source (Sutter Instrument, Novato, CA, USA) and a QiClick 1.4 megapixel monochrome charge-coupled device camera (QImaging, Surrey, BC, Canada) via NIS Elements 4.3 Imaging Software (Nikon Instruments). Cells were imaged with a ×40 Nikon Super Fluor objective, and regions of interest were manually drawn for individual cells. Fura-2 fluorescence intensity was determined by excitation at 340 and 380 nm and calculating the ratio of emission intensities at 511 nm in the usual manner every 5 s. No significant Fura-2 bleaching and leakage were detected during the timeline of experiments. Changes in the ratio were converted into changes in [Ca2+]i, as previously documented (39). Experiments were performed under constant perfusion with physiologic saline solution (PSS) that contained (mM) 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 Hepes (pH 7.35) at a rate of 1.5 ml/min. Ca2+-free medium was made from PSS by replacing CaCl2 with 5 mM EGTA. At the end of each experiment, cells were permeabilized with 5 µM ionomycin in PSS that contained 2 mM Ca2+ and Ca2+-free PSS (5 mM EGTA) for calibration of [Ca2+]i. To assess the effect of elevated flow on [Ca2+]i, the rate of perfusion was instantly increased from 1.5 ml/min (∼15 mm H2O) to 15 ml/min (∼80 mm H2O), which produced shear stress of ∼3 dyn/cm2 (28). This value fits well within the physiologic range of shear stress that is present in the distal renal tubule, as previously assessed (21). Changes in perfusion rate and drug applications were made by using a VC-8 valve controller (Warner Instruments).
Single-channel recordings in NHK and ADPKD cells
TRPV4 activity was monitored in cell-attached patches under voltage-clamp conditions (holding voltage Vh = −Vp = −60 mV) using standard procedures (40–43). Current recordings were made in a permanently perfused bath (1.5 ml/min) at baseline and upon application of the TRPV4 agonist, GSK1016790A (30 nM). Recording pipettes had resistances of 8–10 mOhms. Typical bath and pipette solutions were (mM) 150 NaCl, 5 KCl, 2 MgCl2, 1 CaCl2, 10 Hepes, and 5 glucose (pH 7.35); and 150 KCl, 5 NaCl, 2 MgCl2, 5 EGTA, 10 Hepes, and 5 glucose (pH 7.35), respectively. Gap-free, single-channel current data from gOhm seals were acquired and analyzed with the Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA) patch-clamp amplifier interfaced via a Digidata 1440 (Molecular Devices) to a PC running pClamp 10.4 software (Molecular Devices). Currents were low-pass filtered at 1000 Hz with an 8-pole Bessel filter (Warner Instruments). Events were inspected visually before acceptance. We analyzed TRPV4 activity over a span of 60–120 s for each experimental condition. Channel activity in individual patches, defined as NPo, was calculated by using the following equation: NPo = (t1 + 2t2 + …+ntn), where N and Po are the number of TRPV4 in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. For representation, current traces were corrected for slow baseline drifts, as necessary.
TRPV4 expression in Chinese hamster ovary cells
Chinese hamster ovary (CHO) cells were obtained from American Type Culture Collection (Manassas, VA, USA). These cells were maintained on standard culture conditions (DMEM + 10% fetal bovine serum; 37°C, 5% CO2). We performed overexpression of cDNA that contained mouse TRPV4 conjugated with green fluorescent protein (GFP) into CHO cells by transfecting the plasmid using the Polyfect reagent (Qiagen, Valencia, CA, USA), as described previously (44, 45). Transfected cells were identified by GFP fluorescence. The plasmid was a kind gift from Dr. M. Zhu (The University of Texas Health Science Center at Houston, Houston, TX, USA). For studies, 0.8 µg/9.6 cm2 TRPV4-GFP cDNA was used. Currents were recorded under voltage-clamp, whole-cell configuration within 48–72 h after transfection. Whole-cell capacitance (∼9 pF for CHO cells) was routinely compensated. Series resistances—on average 2–5 mOhm—were also compensated. Currents were evoked with 1-s voltage step protocols from −80 to +60 mV. Cells were originally bathed in isotonic (300 mOsm) solution (mM): 55 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 Hepes, 5 glucose, 155 mannitol (pH 7.35). For TRPV4 stimulation, hypotonic (220 mOsm) bath solution was used (mM): 55 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 Hepes, 5 glucose, 80 mannitol (pH 7.35). Pipette solution consisted of (mM) 145 KCl, 5 NaCl, 2 MgCl2, 5 EGTA, 10 Hepes, 2 ATP, and 0.1 GTP (pH 7.35).
Western blot analysis
Confluent monolayers of NHK and ADPKD cells were rinsed with ice-cold PBS and homogenized in hypotonic lysis buffer that contained 50 mM Tris, 1% Triton X-100, 5 mM EDTA (pH 7.4) that was supplemented with Complete Mini-Protease and PhosStop phosphatase inhibitor cocktails (Roche Diagnostics, Indianapolis, IN, USA). Samples were diluted with hypotonic lysis buffer, denatured, and reduced in Laemmli buffer that was supplemented with 5% 2-ME at +75°C for 10 min to obtain the final protein concentration of 4 mg/ml. Samples (10 μg/lane) were separated on 12% polyacrylamide gels at 150 V for 105 min and transferred to a nitrocellulose membrane for 135 min at 100 V. The nitrocellulose membrane was subsequently incubated with anti-TRPV4 Abs (1:1000; Alomone Labs, Jerusalem, Israel) for 1.5 h followed by incubation with secondary anti-actin Abs (1:5000; Abcam, Cambridge, United Kingdom) for 1.5 h at room temperature. Blots were quantified by using ImageJ 1.49s software (National Institutes of Health, Bethesda, MD, USA). Intensities of the total, glycosylated, and nonglycosylated TRPV4 protein bands were normalized to those of the corresponding actin bands and used as loading control.
Statistics
All summarized data are reported as means ± sem. All statistical comparisons were made by using 1-way ANOVA or nonparametric 1-way ANOVA on ranks followed by Dunn’s posttest, as appropriate. Values of P < 0.05 were considered statistically significant.
RESULTS
TRPV4 contributes to [Ca2+]i homeostasis in NHK cells
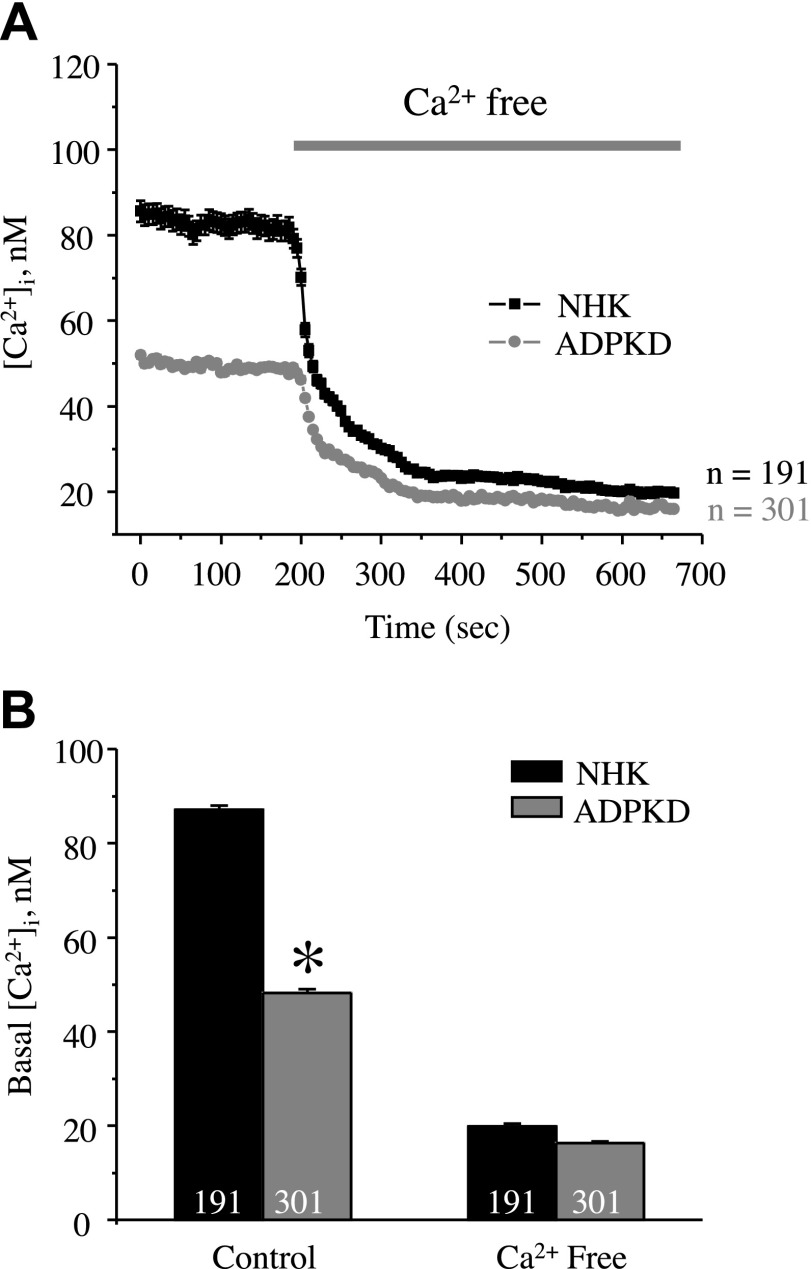
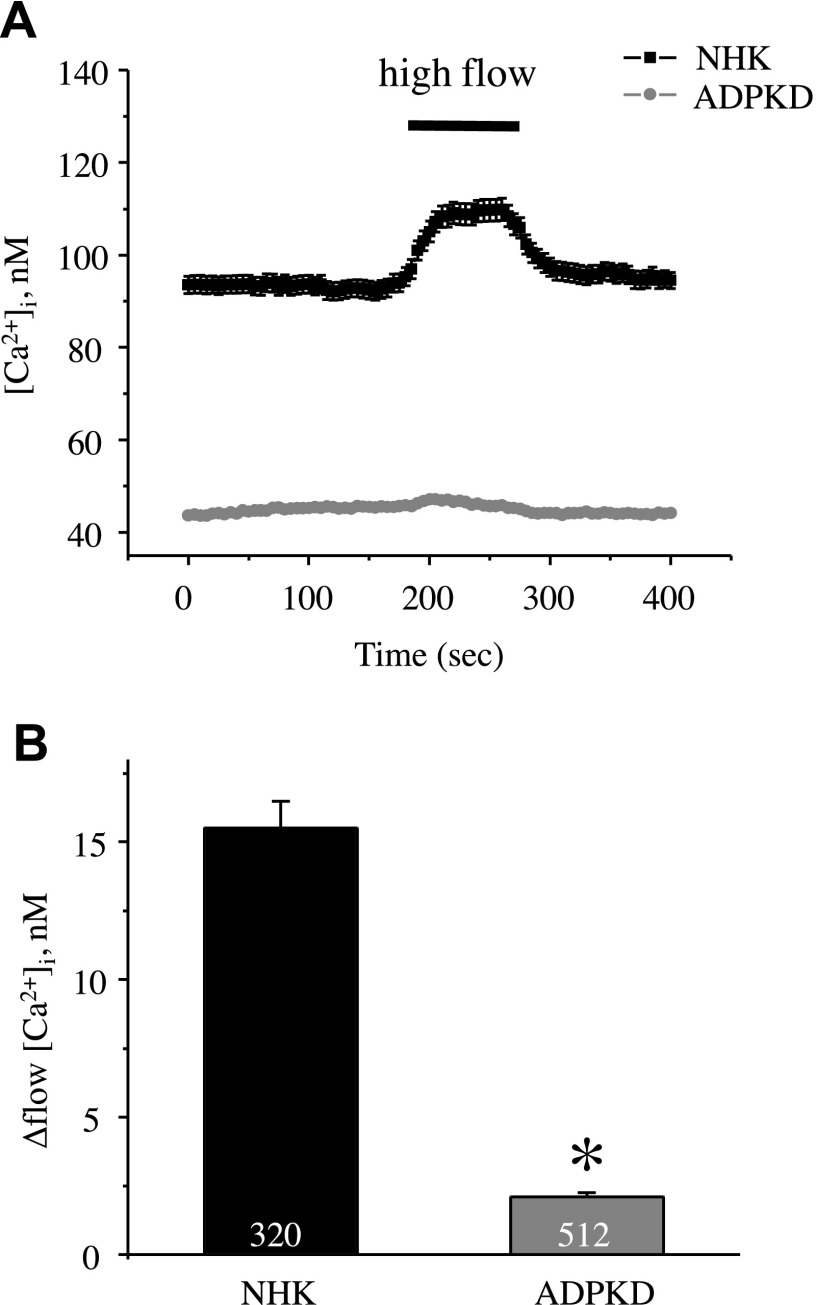
Existing experimental evidence revealed abnormal [Ca2+]i homeostasis in primary cultured human ADPKD cells compared with NHK cells (11). Consistent with this previous report, we observed significantly reduced basal [Ca2+]i levels in ADPKD cells (48 ± 2 nM) compared with NHK cells (87 ± 2 nM; Fig. 1). Removal of extracellular Ca2+ virtually abolished the difference in [Ca2+]i between NHK and ADPKD cells (18 ± 1 nM and 16 ± 1 nM, respectively; Fig. 1). Moreover, ADPKD cells had drastically impaired [Ca2+]i responses to an abrupt 10× elevation of the flow rate in the recording chamber from 1.5 to 15 ml/min (Fig. 2). No response to high flow was detected in the absence of extracellular Ca2+ (data not shown). Altogether, this suggests that a deficiency in direct Ca2+ influx across the plasma membrane underlies the observed difference in [Ca2+]i homeostasis.
Figure 1.
Differences in basal [Ca2+]i levels between NHK and ADPKD cells depend on extracellular Ca2+. A) Summary graph of averaged changes of [Ca2+]i in control and upon the application of Ca2+-free medium (5 mM EGTA, as noted by a bar on the top) in NHK cells (black trace) and ADPKD cells (gray trace). B) Summary graphs of basal [Ca2+]i in NHK and ADPKD cells in the control and in Ca2+-free medium. Asterisk denotes significant decrease vs. NHK cells. *P < 0.05.
Figure 2.
Lack of flow-induced [Ca2+]i responses in ADPKD cells. A) Summary graph of averaged changes of [Ca2+]i in control and upon abrupt 10× elevation of the perfusion rate from 1.5 to 15 ml/min (shown with a bar on top) in NHK cells (black trace) and ADPKD cells (gray trace). B) Summary graph of the magnitude of flow-induced [Ca2+]i elevations in NHK and ADPKD cells. Asterisk denotes significant decrease vs. NHK. *P < 0.05.
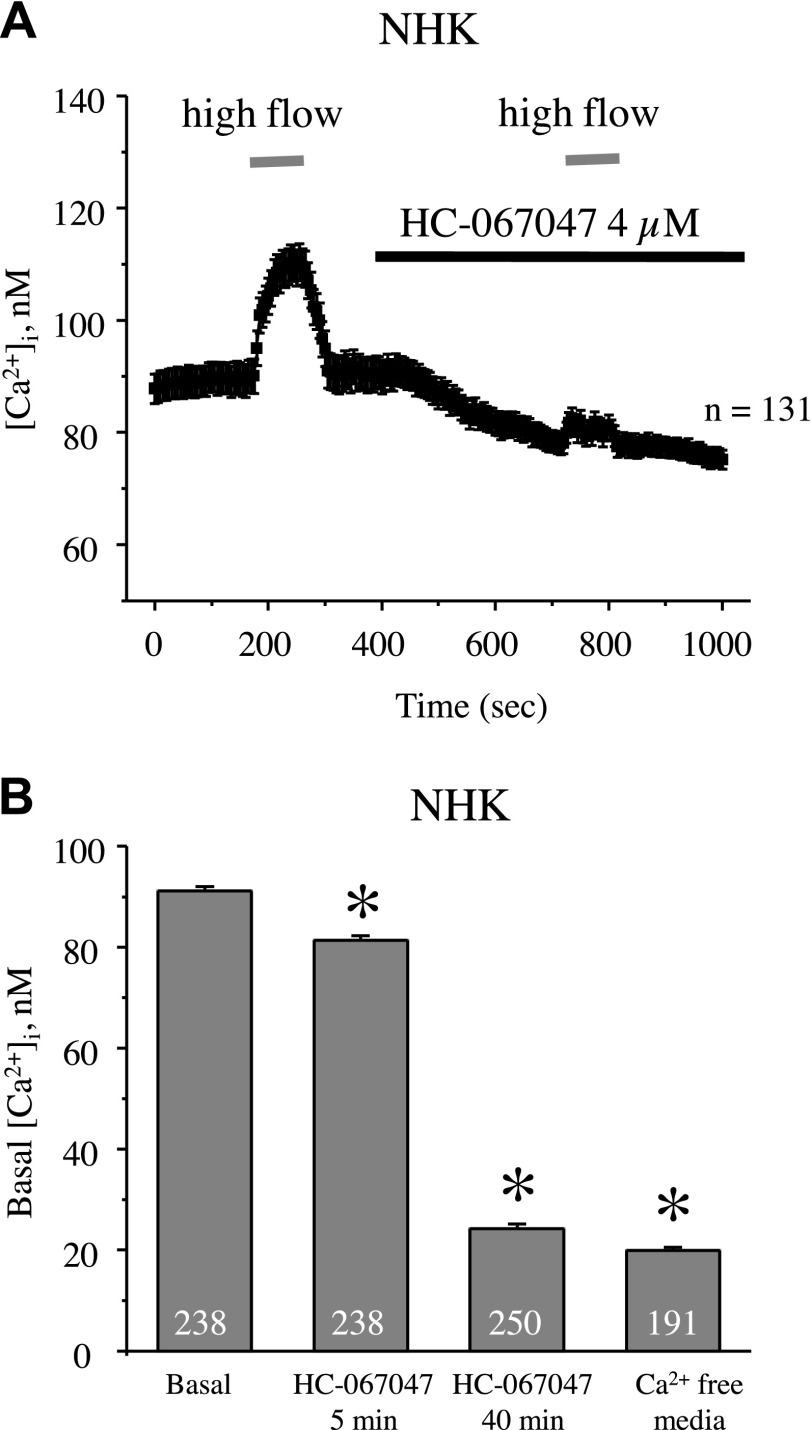
We have previously demonstrated a central role of the mechanosensitive Ca2+-permeable TRPV4 channel in controlling [Ca2+]i levels and mediating flow-induced [Ca2+]i responses in mouse and rat distal tubule cells (28, 46). We thus aimed to determine whether TRPV4 plays a similar role in human cells. As shown in the average time course of [Ca2+]i dynamics in NHK cells, acute inhibition of TRPV4 with a selective antagonist, HC-067047 (4 µM), reduced basal [Ca2+]i levels and abolished the flow-induced response (Fig. 3A). The amplitude of the [Ca2+]i response to flow did not decay spontaneously over time (data not shown). Acute inhibition of TRPV4 had only a mild effect, decreasing basal [Ca2+]i levels from 91 ± 1 nM to 81 ± 1 nM (Fig. 3B), which was not sufficient to explain the differences found in Figs. 1 and 2 in steady-state [Ca2+]i between NHK and ADPKD cells. In contrast, prolonged inhibition of TRPV4 with HC-067047 for 40 min drastically reduced basal [Ca2+]i levels in NHK cells to 24 ± 1 nM (Fig. 3B), which was comparable with levels observed in Ca2+-free medium. Overall, our results demonstrate that TRPV4 plays a central role in controlling [Ca2+]i homeostasis in NHK cells. TRPV4 inhibition blocks flow-induced [Ca2+]i increases and decreases basal [Ca2+]i levels.
Figure 3.
TRPV4 is critical for flow-induced [Ca2+]i elevations and maintaining basal [Ca2+]i levels in NHK cells. A) Summary graph monitoring [Ca2+]i responses to elevated flow (shown with gray bars) in control and upon the application of the TRPV4 blocker, HC-067047 (4 µM, shown with a black bar). B) Summary graphs of resting [Ca2+]i levels in NHK cells in the control, upon the application of HC-067047 for 5 and 40 min, and in Ca2+-free medium (5 mM EGTA). Asterisk denotes significant decrease vs. basal. *P < 0.05.
TRPV4 activity is reduced in ADPKD cells
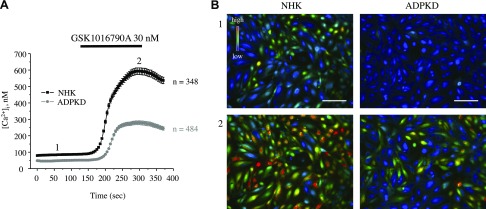
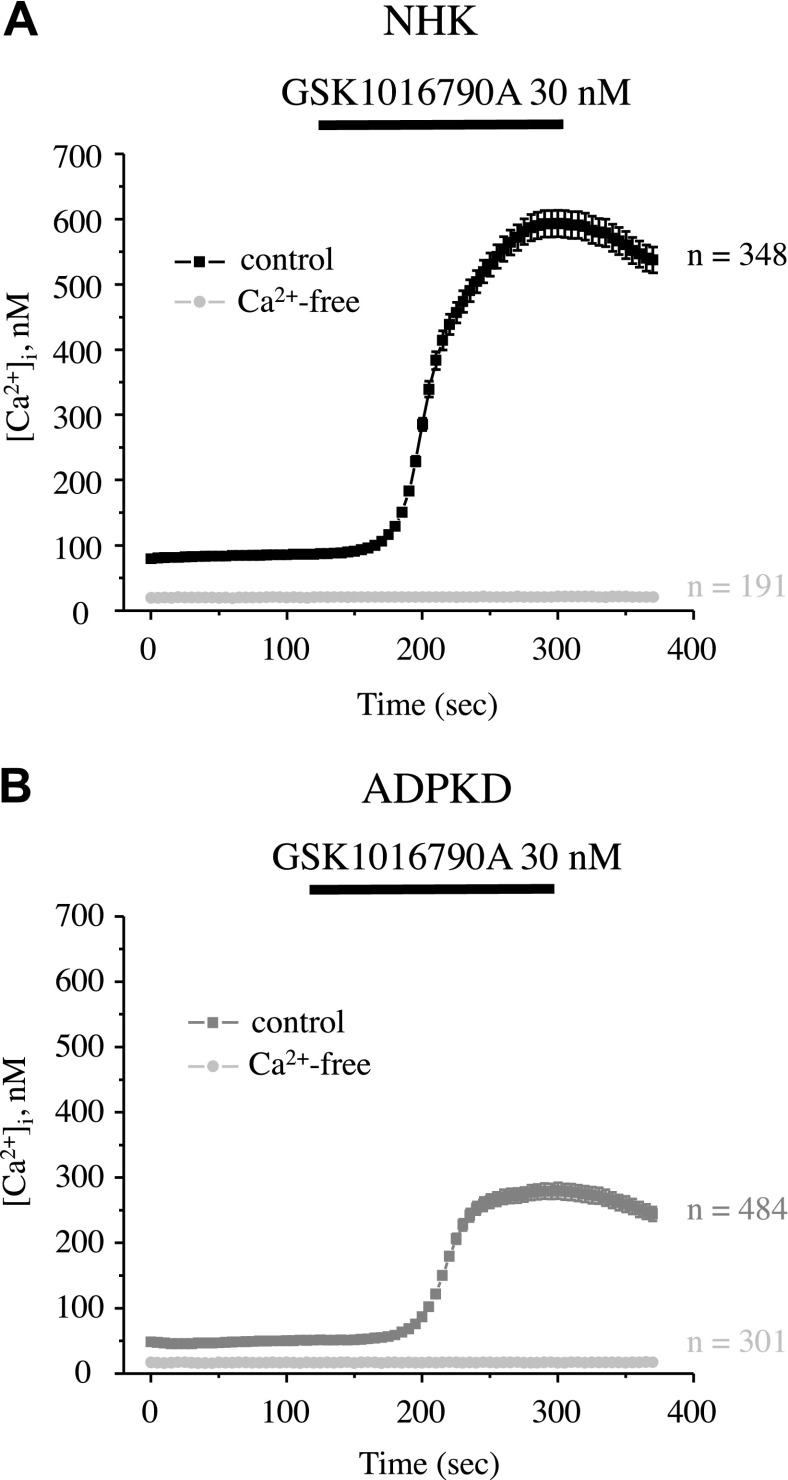
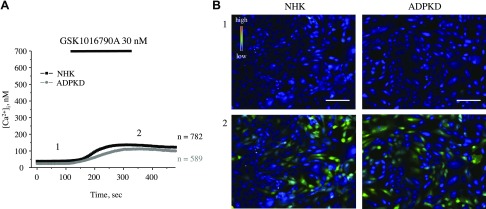
We have previously found that impaired TRPV4 activity is associated with defective [Ca2+]i signaling in cyst cells in an ARPKD rat model (10); therefore, we next tested whether compromised [Ca2+]i balance in ADPKD cells can be explained by a deficient TRPV4 function. As shown in the average time course of [Ca2+]i changes (Fig. 4A) and representative micrographs of primary cultured NHK and ADPKD cells (Fig. 4B), the latter had significantly lower baseline [Ca2+]i levels and significantly impaired [Ca2+]i elevations that were induced by the application of 30 nM GSK1016790A, a highly potent TRPV4 agonist. Detailed analysis of GSK1016790A-mediated [Ca2+]i responses (Supplemental Fig. 1) revealed both a reduced number of ADPKD cells that were responsive to the application of the agonist and a lower magnitude of [Ca2+]i elevations compared with the respective values that were obtained in NHK cells.
Figure 4.
Defective TRPV4-dependent Ca2+ influx in human ADPKD cells. A) Summary graph of averaged changes of [Ca2+]i at baseline and upon the application of the TRPV4 agonist, GSK1016790A (shown with a black bar on the top), in NHK cells (black trace) and ADPKD cells (gray trace). B) Representative pseudocolor images (blue–low and red–high [Ca2+]i) at time point 1 (control) and time point 2 (GSK1016790A) from experiments shown in panel A. Scale bars, 60 µm.
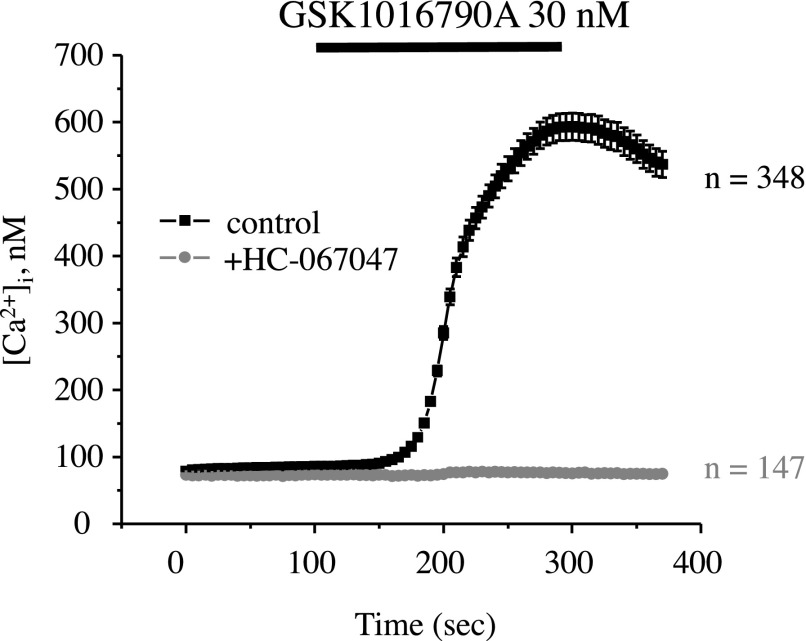
We next verified that GSK1016790A-mediated responses were mediated by direct Ca2+ influx across the plasma membrane. We did not observe any measurable elevations of [Ca2+]i in NHK cells (Fig. 5A) and ADPKD cells (Fig. 5B) when GSK1016790A was applied in Ca2+ free medium. Furthermore, NHK cells failed to respond to GSK1016790A in the presence of the TRPV4 inhibitor, HC-067047 (Fig. 6). Overall, our results strongly suggest that GSK1016790A induces Ca2+ influx by selectively activating TRPV4 and that this response is severely compromised in ADPKD cells.
Figure 5.
TRPV4-mediated [Ca2+]i responses are absent upon the removal of extracellular Ca2+. A) Summary graph of averaged changes of [Ca2+]i at baseline and upon the application of the TRPV4 agonist, GSK1016790A (shown with a black bar on the top), in NHK cells in control (black trace) and Ca2+-free medium (light gray trace). B) Summary graph of averaged changes of [Ca2+]i at baseline and upon the application of the TRPV4 agonist, GSK1016790A (shown with a black bar on the top), in ADPKD cells in control (gray trace) and Ca2+-free medium (light gray trace).
Figure 6.
Inhibition of TRPV4 with HC-067047 precludes GSK1016790A-induced [Ca2+]i responses in NHK cells. Summary graph of averaged changes of [Ca2+]i at baseline and upon the application of the TRPV4 agonist, GSK1016790A (shown with a black bar on the top), in NHK cells in control (black trace) and in the presence of the channel blocker, HC-067047 (gray trace).
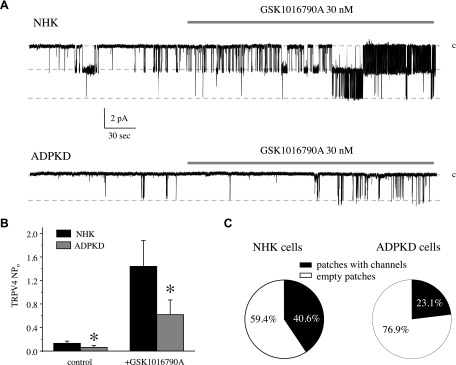
We next used patch-clamp electrophysiology in the cell-attached mode to directly monitor TRPV4 activity at the single-channel level in NHK and ADPKD cells. As shown in the representative experiments (Fig. 7A) and the summary graph (Fig. 7B), we successfully detected a cation-selective channel with an estimated conductance of ∼20 pS (Supplemental Fig. 2), which can be acutely activated by the TRPV4 agonist, GSK1016790A (Fig. 7A, B). Of importance, the probability of observing active channels was higher in NHK cells compared with ADPKD cells (40.6 vs. 23.1%, respectively; Fig. 7C). Furthermore, both basal and GSK1016790A-stimulated channel activity (NPo) were significantly lower in ADPKD cells (Fig. 7A, B). NPo values were 0.13 ± 0.03 vs. 0.06 ± 0.03 (basal) and 1.44 ± 0.44 vs. 0.61 ± 0.24 (GSK1016790A-stimulated) in patches that contained active channels from NHK and ADPKD cells, respectively. In summary, our results demonstrate compromised TRPV4 activity at baseline and in the presence of GSK1016790A in ADPKD cells compared with NHK cells.
Figure 7.
Decreased TRPV4 single-channel activity and impaired responses to GSK1016790A in human ADPKD cells. A) Representative continuous current trace from cell-attached patches monitoring TRPV4 activity in NHK (top) and ADPKD (bottom) cells in control and during the application of 30 nM GSK1016790A (shown with respective bars on the top of each trace). Patches had the holding potential of Vh = −Vp = −60 mV. Inward currents are downward. Dashed lines indicate the respective current state with a c denoting the closed state. B) Summary graph of TRPV4 activity (NPo) in control and in response to GSK1016790A from patch-clamp experiments in NHK and ADPKD cells that contained at least 1 active channel and similar to those shown in panel A. C) Pie charts showing the frequency of observing patches with active TRPV4 channels in NHK and ADPKD cells. Asterisk denotes significant decrease vs. respective NHK. *P < 0.05.
Reduced TRPV4 glycosylation in ADPKD cells
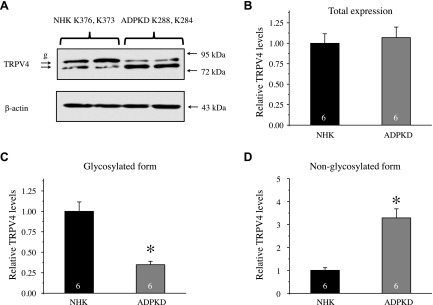
We next investigated whether decreased TRPV4-dependent Ca2+ influx and lower TRPV4 activity were associated with altered TRPV4 expression in ADPKD cells. As shown in the representative Western blot from whole-cell homogenates (Fig. 8A), TRPV4 appears as a doublet of upper glycosylated and lower nonglycosylated forms. We did not observe changes in total TRPV4 levels between NHK and ADPKD cells (1.00 ± 0.11 vs. 1.07 ± 0.12, respectively; n = 6 kidneys each; Fig. 8B); however, we detected a drastic reduction in the abundance of the glycosylated band from 1.00 ± 0.12 to 0.34 ± 0.04 (Fig. 8C) and a respective augmentation of the nonglycosylated TRPV4 band from 1.00 ± 0.11 to 3.29 ± 0.39 (Fig. 8D) in ADPKD cells.
Figure 8.
Diminished TRPV4 glycosylation in human ADPKD cells. A) Representative Western blot of whole-cell lysates from primary cultured NHK and ADPKD cells from different kidneys. Lysates were probed with anti-TRPV4 and anti–β-actin Abs. B–D) Summary graphs comparing total (B), upper glycosylated (C), and bottom nonglycosylated (D) forms of TRPV4 from Western blots similar to that shown in panel A. Intensities of the respective TRPV4-reporting reporting bands were normalized to those of the corresponding actin bands. g, glycosylated form of TRPV4. Asterisk denotes significant difference vs. NHK. *P < 0.05.
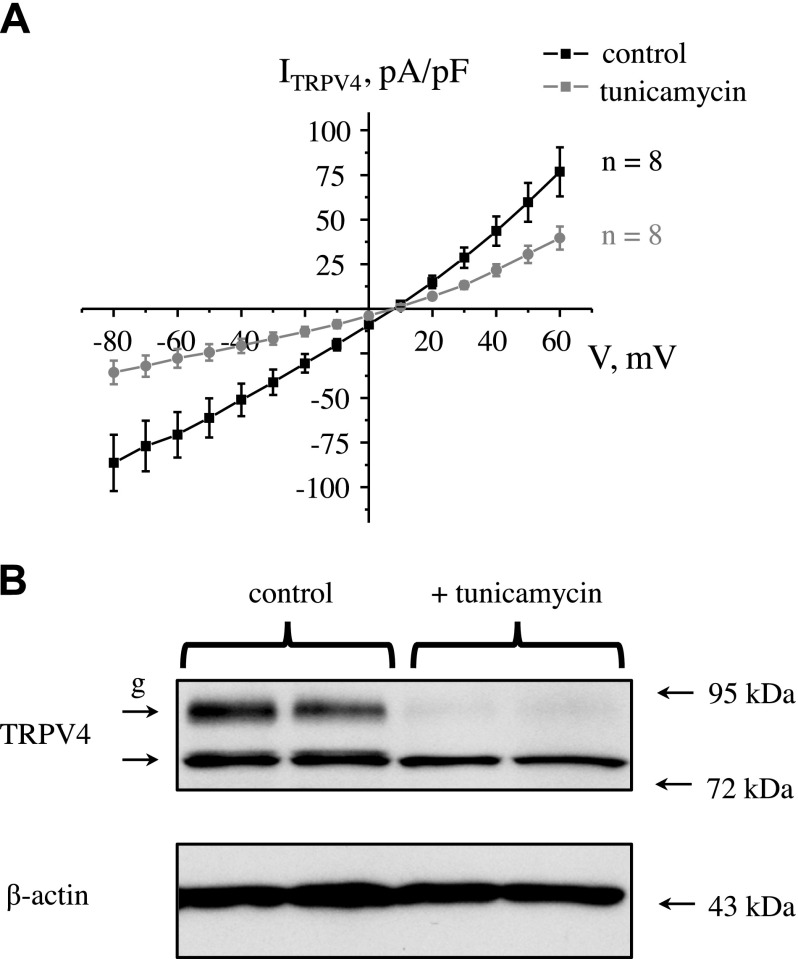
To assess whether deficient TRPV4 glycosylation is associated with decreased channel activity, we overexpressed TRPV4 cDNA into CHO cells and monitored whole-cell currents with patch-clamp (Fig. 9A). Treatment with tunicamycin—5 µg/ml for 24 h—abolished TRPV4 post-translational processing, and only a nonglycosylated TRPV4 band was present (Fig. 9B). Of importance, we detected a dramatic reduction of the amplitude of TRPV4-dependent currents in cells that were pretreated with tunicamycin (Fig. 9A). Only cells with measurable whole-cell currents were taken for analysis. Overall, our results demonstrate a positive correlation between TRPV4 glycosylation and channel activity.
Figure 9.
TRPV4 deglycosylation is associated with reduced channel activity. A) Summary graph of whole-cell TRPV4-dependent current-voltage (I-V) relations upon channel overexpression in CHO cells in control and after pretreatment with tunicamycin (5 µg/ml) for 24 h to block glycosylation. Currents were induced by the application of hypotonic (220 mOsm) medium from isotonic control values (300 mOsm). B) Representative Western blot of whole-cell lysates in CHO cells that overexpress TRPV4 in control and after tunicamycin treatment. Each line represents individual transfection. Lysates were probed with anti-TRPV4 and anti–β-actin Abs. g, glycosylated form of TRPV4.
Reduced TRPV4 glycosylation underlies decreased channel activity in ADPKD cells
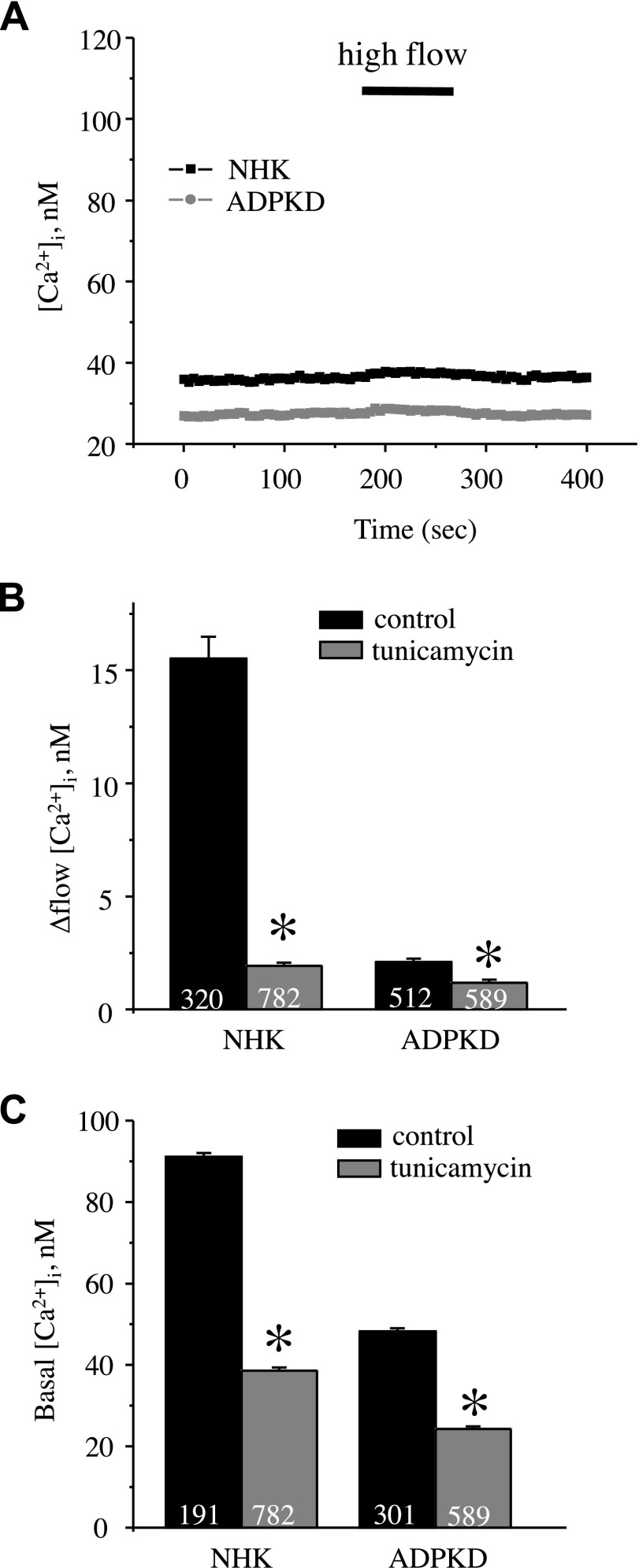
We next determined whether decreased TRPV4 activity in ADPKD cells was the consequence of impaired channel glycosylation. NHK and ADPKD cells were treated with tunicamycin (5 µg/ml for 24 h) to abolish channel glycosylation. In sharp contrast with untreated conditions (Fig. 4), we observed a drastic reduction of cellular responses to the application of the TRPV4 agonist, GSK1016790A (Fig. 10), in cells that were treated with tunicamycin, and this effect was more pronounced in NHK cells than in ADPKD cells (Supplemental Fig. 3). Furthermore, tunicamycin treatment abolished the flow-induced [Ca2+]i increase in NHK cells (Fig. 11A, B) and caused a dramatic decrease in basal [Ca2+]i levels in NHK cells (39 ± 1 nM) and ADPKD cells (24 ± 1 nM; Fig. 11C). Overall, we concluded that the disruption of TRPV4 glycosylation contributes importantly to the dysregulation of Ca2+ homeostasis in ADPKD cells.
Figure 10.
Impaired TRPV4 activity in NHK and ADPKD cells that were treated with tunicamycin. A) Summary graph of averaged changes of [Ca2+]i at baseline and upon the application of the TRPV4 agonist, GSK1016790A (shown with a black bar on the top), in NHK cells (black trace) and ADPKD cells (gray trace) after pretreatment with tunicamycin (5 µg/ml) for 24 h to block glycosylation. B) Representative pseudocolor images (blue–low and red–high [Ca2+]i) at time point 1 (control) and time point 2 (GSK1016790A) from experiments shown in panel A. Scale bars, 60 µm.
Figure 11.
TRPV4 deglycosylation negates differences in [Ca2+]i signaling between NHK and ADPKD cells. A) Summary graph of averaged changes of [Ca2+]i in control and upon abrupt 10× elevation of the perfusion rate from 1.5 to 15 ml/min (shown with a bar on top) in NHK cells (black trace) and ADPKD cells (gray trace) that were pretreated with tunicamycin (5 µg/ml) for 24 h to block glycosylation. B, C) Summary graphs comparing the magnitude of flow-induced [Ca2+]i elevations (B) and basal [Ca2+]i levels (C) in NHK and ADPKD cells in control and after pretreatment with tunicamycin. *P < 0.05.
DISCUSSION
In the current study, we provide direct experimental evidence that demonstrates the essential role that the mechanosensitive Ca2+-permeable TRPV4 channel plays in governing basal [Ca2+]i levels and mediating flow-dependent [Ca2+]i elevations in NHK cells. We also report that decreased TRPV4 activity—likely a result of deficient channel glycosylation—is the major contributing factor to defective [Ca2+]i signaling in human ADPKD cells.
It has long been recognized that both ADPKD and ARPKD are associated with disrupted [Ca2+]i signaling and compromised mechanosensitivity that contribute significantly to the dedifferentiation and aberrant proliferation of cyst cells (3). Nonspecific stimulation of Ca2+ influx across the plasma membrane prevented the cAMP-induced proliferation of cyst cells, which demonstrates the beneficial effect of this strategy in retarding cyst growth (11). Here, we demonstrate, for the first time to our knowledge, that the TRPV4 channel serves as a Ca2+-permeable conduit that is critical for setting basal [Ca2+]i levels and mediating flow-induced [Ca2+]i responses (Figs. 1 and 3) in primary cultured human kidney cells that are of predominantly distal tubule origin (35). We found that TRPV4 function was identical to that reported in freshly isolated distal tubules of mice and rats (10, 28, 29), which points to a high level of conservation of molecular determinants conferring mechanosensitivity to renal tubule cells between rodents and humans. Considering that the kidney exhibits the highest level of TRPV4 expression compared with other tissues and organs (26), targeting channel activity could be a relatively specific strategy by which to regulate [Ca2+]i signaling in renal epithelial cells. Previous experimental evidence suggests that the Ca2+-permeable PC2 channel lacks mechanosensitive properties. Instead, it creates a functional heteromer with TRPV4 to form a mechanomolecular sensor in native and cultured distal tubule cells that is critically dependent on TRPV4 expression (32, 34). Single-channel analysis demonstrated that the heteromer has a distinct biophysical profile and different conductance compared with TRPV4 homotetramer (23 vs. ∼50 pS for inward currents, respectively) (33, 47–49). Of importance, the only GSK1016790A-activated channel in the current study (Fig. 7) has an estimated conductance of 20 pS (Supplemental Fig. 2), which suggests that TRPV4 predominantly coassembles with PC2 in human kidney cells. It should be noted, though, that TRPV4 deletion alone is not sufficient to trigger cytogenesis in mice and zebrafish (32). This indicates that deregulated [Ca2+]i signaling is a critical contributor to PKD progression, but not an original cause.
Abundant literature demonstrates the importance of maintaining the proper physiologic levels of [Ca2+]i to control how renal epithelial cells respond to increased cAMP. Thus, a reduction of [Ca2+]i in NHK and M-1 cells induces the cAMP-dependent activation of the B-Raf/MEK/ERK pathway, which switches cells to a cAMP growth-stimulated phenotype that was observed in ADPKD cells (16, 35, 36). In contrast, an elevation of [Ca2+]i in ADPKD cells restores the normal anti-mitogenic response to cAMP by preventing the activation of B-Raf and ERK (11). Consequently, inhibiting the cAMP-dependent pathways, such as arginine vasopressin type 2 receptor blockade with tolvaptan, or tweaking mechanisms that control cAMP metabolism, such as inhibition of adenylyl cyclases or stimulation of phosphodiesterases, demonstrate notable anticystogenic effects in both basic and clinical studies (17, 50–52); however, interference with cAMP-dependent renal water reabsorption as a result of continuous tolvaptan administration is associated with undesired adverse effects, including polyuria, dehydration, and thirst in patients with PKD, which often leads to the discontinuation of treatment (51). In this regard, stimulation of TRPV4 could be an alternative strategy to counteract cAMP-dependent proliferation and cyst growth. We recently reported that the prolonged systemic administration of the TRPV4 agonist, GSK1016790A, restored mechanosensitive Ca2+ signaling, increased basal [Ca2+]i levels in cyst cells, and significantly blunted cyst growth in a rat model of ARPKD (10). This indicates that the activation of TRPV4 may have therapeutic value. In addition, we speculate that TRPV4 activation to restore [Ca2+]i may allow for lower concentrations of tolvaptan to be used, surpassing the therapeutic effect of arginine vasopressin type 2 receptor inhibition alone, with little or no disturbances in water balance. Future studies are necessary to carefully examine the benefits and drawbacks of targeting TRPV4 for the treatment of ADPKD.
An important observation of the current study is that TRPV4 activity and TRPV4-dependent [Ca2+]i elevations positively correlate with glycosylation of the channel in human kidney cells. Diminished TRPV4 glycosylation in ADPKD cells decreased single-channel activity (Fig. 7), reduced basal [Ca2+]i levels (Fig. 1), and blunted channel activation by the TRPV4 agonist (Fig. 4). Consistently, blockade of TRPV4 glycosylation in NHK cells and CHO cells that overexpress TRPV4 drastically decreased channel activity and compromised [Ca2+]i signaling (Figs. 9–11). TRPV4 is known to be post-translationally modified by complex high mannose sugars at an N-linked glycosylation site (Asn651) that is immediately adjacent to the pore-forming loop (between transmembrane domains 5 and 6), which is sensitive to PNGase F (21, 53). Of interest, glycosylation-deficient mutations of TRPV4, which have implicated in the development of familial digital arthropathy-bachydactyly in humans, have abolished responses to hypotonic mechanical stress and decreased responses to GSK1016790A (54), recapitulating major features observed in ADPKD cells (Figs. 2, 4, and 8). Furthermore, our previous studies have also demonstrated a drastic decrease in TRPV4 glycosylation in a rat model of ARPKD (PCK 453), which leads to impaired channel activity (10). Decreased TRPV4 glycosylation in ADPKD and ARPKD points to a common cellular phenotype and an underlying mechanism for deregulated [Ca2+]i signaling. As TRPV4 is located in the multiprotein complex, including polycystins and fibrocystin, it is possible that their functional status governs post-translational TRPV4 modification. Of note, it was recently shown that Klotho, which has structural similarities with fibrocystin and is highly abundant in the distal convoluted tubule, directly cleaves N-linked oligosaccharides from TRPV5 (another TRP channel from the vanilloid group), which affects channel localization and activity (55). Moreover, multiple reports have demonstrated that aberrant post-translational modification of polycystins, including glycosylation, causes cyst formation (56). For instance, the deletion of AQP11—localized to the endoplasmic reticulum—leads to impaired glycosylation processing and aberrant membrane trafficking of PC-1 in mice (57). Future studies are necessary to carefully investigate whether the same mechanism controls the glycosylation of TRPV4 and polycystins. Currently, there are no specific approaches to augment the glycosylation of plasma membrane proteins, such as TRPV4; however, it is plausible to speculate that the development of such tools in future may correct [Ca2+]i in cystic cells and provide a highly effective therapy for patients with ADPKD.
In summary, this study provides the first direct evidence of a critical role for the Ca2+-permeable TRPV4 channel in controlling [Ca2+]i signaling in human renal tubule cells, and that dysfunctional TRP4 as a result of impaired glycosylation contributes to decreased [Ca2+]i levels and the loss of mechanosensitivity in ADPKD cells. We propose that pharmacologic strategies to directly increase TRPV4 channel activity or glycosylation status will be beneficial in the treatment of ADPKD.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK095029 (to O.P.) and R01-DK081579 (D.P.W.), American Heart Association Grant 17GRNT33660488 (to O.P.), and the American Society of Nephrology Ben J. Lipps Research Fellowship (to V.T.). Human tissues were obtained with the assistance of the University of Kansas Cancer Center’s Biospecimen Repository (P30-CA168524) and hospitals participating in the PKD Foundation’s tissue donation program. Primary cultures of ADPKD and NHK cells were generated by the PKD Biomarkers and Biomaterials Core in the Kansas PKD Research and Translational Core Center at University of Kansas Medical Center (DK106912; to D.P.W.). The authors declare no conflicts of interest.
Glossary
- ADPKD
autosomal-dominant polycystic kidney disease
- ARPKD
autosomal-recessive polycystic kidney disease
- CD
collecting duct
- CHO
Chinese hamster ovary
- GFP
green fluorescent protein
- NHK
normal human kidney
- PC1
polycystin 1
- PC2
polycystin 2
- PKD
polycystic kidney disease
- PSS
physiologic saline solution
- TRPV4
transient receptor potential vanilloid type 4
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. P. Wallace and O. Pochynyuk designed research; V. Tomilin, G. A. Reif, O. Zaika, and O. Pochynyuk performed research; O. Pochynyuk wrote the paper; and V. Tomilin, O. Zaika, and D. P. Wallace edited the draft.
REFERENCES
- 1.Chapman A. B., Devuyst O., Eckardt K. U., Gansevoort R. T., Harris T., Horie S., Kasiske B. L., Odland D., Pei Y., Perrone R. D., Pirson Y., Schrier R. W., Torra R., Torres V. E., Watnick T., Wheeler D. C.; Conference Participants (2015) Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 88, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ecder T. (2013) Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr. Hypertens. Rev. 9, 2–11 [DOI] [PubMed] [Google Scholar]
- 3.Harris P. C., Torres V. E. (2009) Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres V. E., Harris P. C., Pirson Y. (2007) Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- 5.Torres V. E., Harris P. C. (2006) Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2, 40–55, quiz 55 [DOI] [PubMed] [Google Scholar]
- 6.Torres V. E., Harris P. C. (2011) Polycystic kidney disease in 2011: connecting the dots toward a polycystic kidney disease therapy. Nat. Rev. Nephrol. 8, 66–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C., Shmukler B. E., Nishimura K., Kaczmarek E., Rossetti S., Harris P. C., Wandinger-Ness A., Bacallao R. L., Alper S. L. (2009) Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am. J. Physiol. Renal Physiol. 296, F1464–F1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siroky B. J., Ferguson W. B., Fuson A. L., Xie Y., Fintha A., Komlosi P., Yoder B. K., Schwiebert E. M., Guay-Woodford L. M., Bell P. D. (2006) Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am. J. Physiol. Renal Physiol. 290, F1320–F1328 [DOI] [PubMed] [Google Scholar]
- 9.Hovater M. B., Olteanu D., Hanson E. L., Cheng N. L., Siroky B., Fintha A., Komlosi P., Liu W., Satlin L. M., Bell P. D., Yoder B. K., Schwiebert E. M. (2008) Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 4, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaika O., Mamenko M., Berrout J., Boukelmoune N., O’Neil R. G., Pochynyuk O. (2013) TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 24, 604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi T., Hempson S. J., Reif G. A., Hedge A. M., Wallace D. P. (2006) Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J. Am. Soc. Nephrol. 17, 178–187 [DOI] [PubMed] [Google Scholar]
- 12.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Zhang J., Nauli S. M., Li X., Starremans P. G., Luo Y., Roberts K. A., Zhou J. (2007) Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell. Biol. 27, 3241–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retailleau K., Duprat F. (2014) Polycystins and partners: proposed role in mechanosensitivity. J. Physiol. 592, 2453–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekahli D., Parys J. B., Bultynck G., Missiaen L., De Smedt H. (2013) Polycystins and cellular Ca2+ signaling. Cell. Mol. Life Sci. 70, 2697–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi T., Wallace D. P., Magenheimer B. S., Hempson S. J., Grantham J. J., Calvet J. P. (2004) Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 279, 40419–40430 [DOI] [PubMed] [Google Scholar]
- 17.Gattone V. H., II, Wang X., Harris P. C., Torres V. E. (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 9, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T., Nagao S., Kasahara M., Takahashi H., Grantham J. J. (1997) Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am. J. Kidney Dis. 30, 703–709 [DOI] [PubMed] [Google Scholar]
- 19.Song M. Y., Yuan J. X. (2010) Introduction to TRP channels: structure, function, and regulation. Adv. Exp. Med. Biol. 661, 99–108 [DOI] [PubMed] [Google Scholar]
- 20.Nilius B., Vriens J., Prenen J., Droogmans G., Voets T. (2004) TRPV4 calcium entry channel: a paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 286, C195–C205 [DOI] [PubMed] [Google Scholar]
- 21.Wu L., Gao X., Brown R. C., Heller S., O’Neil R. G. (2007) Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am. J. Physiol. Renal Physiol. 293, F1699–F1713 [DOI] [PubMed] [Google Scholar]
- 22.Voets T., Prenen J., Vriens J., Watanabe H., Janssens A., Wissenbach U., Bödding M., Droogmans G., Nilius B. (2002) Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 277, 33704–33710 [DOI] [PubMed] [Google Scholar]
- 23.Venkatachalam K., Montell C. (2007) TRP channels. Annu. Rev. Biochem. 76, 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuyama R., Vriens J., Voets T., Karashima Y., Owsianik G., Vennekens R., Lieben L., Torrekens S., Moermans K., Vanden Bosch A., Bouillon R., Nilius B., Carmeliet G. (2008) TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 8, 257–265 [DOI] [PubMed] [Google Scholar]
- 25.Muramatsu S., Wakabayashi M., Ohno T., Amano K., Ooishi R., Sugahara T., Shiojiri S., Tashiro K., Suzuki Y., Nishimura R., Kuhara S., Sugano S., Yoneda T., Matsuda A. (2007) Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J. Biol. Chem. 282, 32158–32167 [DOI] [PubMed] [Google Scholar]
- 26.Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liedtke W., Friedman J. M. (2003) Abnormal osmotic regulation in Trpv4-/- mice. Proc. Natl. Acad. Sci. USA 100, 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berrout J., Jin M., Mamenko M., Zaika O., Pochynyuk O., O’Neil R. G. (2012) Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J. Biol. Chem. 287, 8782–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamenko M. V., Boukelmoune N., Tomilin V. N., Zaika O. L., Jensen V. B., O’Neil R. G., Pochynyuk O. M. (2017) The renal TRPV4 channel is essential for adaptation to increased dietary potassium. Kidney Int. 91, 1398–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamenko M., Zaika O. L., Boukelmoune N., Berrout J., O’Neil R. G., Pochynyuk O. (2013) Discrete control of TRPV4 channel function in the distal nephron by protein kinases A and C. J. Biol. Chem. 288, 20306–20314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart A. P., Smith G. D., Sandford R. N., Edwardson J. M. (2010) Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophys. J. 99, 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köttgen M., Buchholz B., Garcia-Gonzalez M. A., Kotsis F., Fu X., Doerken M., Boehlke C., Steffl D., Tauber R., Wegierski T., Nitschke R., Suzuki M., Kramer-Zucker A., Germino G. G., Watnick T., Prenen J., Nilius B., Kuehn E. W., Walz G. (2008) TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 182, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z. R., Chu W. F., Song B., Gooz M., Zhang J. N., Yu C. J., Jiang S., Baldys A., Gooz P., Steele S., Owsianik G., Nilius B., Komlosi P., Bell P. D. (2013) TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 8, e73424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J., Wong W. Y., Sun L., Huang Y., Yao X. (2012) Protein kinase G inhibits flow-induced Ca2+ entry into collecting duct cells. J. Am. Soc. Nephrol. 23, 1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi T., Nagao S., Wallace D. P., Belibi F. A., Cowley B. D., Pelling J. C., Grantham J. J. (2003) Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 63, 1983–1994 [DOI] [PubMed] [Google Scholar]
- 36.Belibi F. A., Reif G., Wallace D. P., Yamaguchi T., Olsen L., Li H., Helmkamp G. M., Jr., Grantham J. J. (2004) Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 66, 964–973 [DOI] [PubMed] [Google Scholar]
- 37.Pei Y. (2011) Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin. Pract. 118, c19–c30 [DOI] [PubMed] [Google Scholar]
- 38.Mamenko M., Zaika O., O’Neil R. G., Pochynyuk O. (2013) Ca2+ imaging as a tool to assess TRP channel function in murine distal nephrons. Methods Mol. Biol. 998, 371–384 [DOI] [PubMed] [Google Scholar]
- 39.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 40.Mamenko M., Zaika O., Doris P. A., Pochynyuk O. (2012) Salt-dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension 60, 1234–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamenko M., Zaika O., Ilatovskaya D. V., Staruschenko A., Pochynyuk O. (2012) Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J. Biol. Chem. 287, 660–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamenko M., Zaika O., Prieto M. C., Jensen V. B., Doris P. A., Navar L. G., Pochynyuk O. (2013) Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62, 1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prieto M. C., Reverte V., Mamenko M., Kuczeriska M., Veiras L. C., Rosales C. B., McLellan M., Gentile O., Jensen V. B., Ichihara A., McDonough A. A., Pochynyuk O. M., Gonzalez A. A. (2017) Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in Ang II-induced hypertensive mice. Am. J. Physiol. Renal Physiol. 313, F1243–F1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pochynyuk O., Kucher V., Boiko N., Mironova E., Staruschenko A., Karpushev A. V., Tong Q., Hendron E., Stockand J. (2009) Intrinsic voltage dependence of the epithelial Na+ channel is masked by a conserved transmembrane domain tryptophan. J. Biol. Chem. 284, 25512–25521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pochynyuk O., Tong Q., Medina J., Vandewalle A., Staruschenko A., Bugaj V., Stockand J. D. (2007) Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J. Gen. Physiol. 130, 399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamenko M., Zaika O., Boukelmoune N., O’Neil R. G., Pochynyuk O. (2015) Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am. J. Physiol. Renal Physiol. 308, F275–F286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 [DOI] [PubMed] [Google Scholar]
- 48.Watanabe H., Vriens J., Janssens A., Wondergem R., Droogmans G., Nilius B. (2003) Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium 33, 489–495 [DOI] [PubMed] [Google Scholar]
- 49.Watanabe H., Davis J. B., Smart D., Jerman J. C., Smith G. D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., Flockerzi V., Droogmans G., Benham C. D., Nilius B. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577 [DOI] [PubMed] [Google Scholar]
- 50.Torres V. E., Wang X., Qian Q., Somlo S., Harris P. C., Gattone V. H., II (2004) Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat. Med. 10, 363–364 [DOI] [PubMed] [Google Scholar]
- 51.Torres V. E., Chapman A. B., Devuyst O., Gansevoort R. T., Grantham J. J., Higashihara E., Perrone R. D., Krasa H. B., Ouyang J., Czerwiec F. S.; TEMPO 3:4 Trial Investigators (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinto C. S., Raman A., Reif G. A., Magenheimer B. S., White C., Calvet J. P., Wallace D. P. (2016) Phosphodiesterase isoform regulation of cell proliferation and fluid secretion in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H., Fu Y., Tian W., Cohen D. M. (2006) Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am. J. Physiol. Renal Physiol. 290, F1103–F1109 [DOI] [PubMed] [Google Scholar]
- 54.Lamandé S. R., Yuan Y., Gresshoff I. L., Rowley L., Belluoccio D., Kaluarachchi K., Little C. B., Botzenhart E., Zerres K., Amor D. J., Cole W. G., Savarirayan R., McIntyre P., Bateman J. F. (2011) Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 43, 1142–1146 [DOI] [PubMed] [Google Scholar]
- 55.Chang Q., Hoefs S., van der Kemp A. W., Topala C. N., Bindels R. J., Hoenderop J. G. (2005) The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 [DOI] [PubMed] [Google Scholar]
- 56.Fedeles S. V., Tian X., Gallagher A. R., Mitobe M., Nishio S., Lee S. H., Cai Y., Geng L., Crews C. M., Somlo S. (2011) A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 43, 639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue Y., Sohara E., Kobayashi K., Chiga M., Rai T., Ishibashi K., Horie S., Su X., Zhou J., Sasaki S., Uchida S. (2014) Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an AQP11 knockout model. J. Am. Soc. Nephrol. 25, 2789–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.